Abstract
Carbon nanotubes (CNTs) have been a subject of intensive research for a wide range of applications. However, because of their extremely small size and light weight, CNTs are readily inhaled into human lungs resulting in increased rates of pulmonary disorders, most notably fibrosis. Several studies have demonstrated the fibrogenic effects of CNTs given their ability to translocate into the surrounding areas in the lung causing granulomatous lesions and interstitial and sub-pleural fibrosis. However, the mechanisms underlying the disease process remain obscure due to the lack of understanding of the cellular interactions and molecular targets involved. Interestingly, certain physicochemical properties of CNTs have been shown to affect their respiratory toxicity, thereby becoming significant determinants of fibrogenesis. CNT-induced fibrosis involves a multitude of cell types and is characterized by the early onset of inflammation, oxidative stress and accumulation of extracellular matrix. Increased reactive oxygen species activate various cytokine/growth factor signaling cascades resulting in increased expression of inflammatory and fibrotic genes. Profibrotic growth factors and cytokines contribute directly to fibroblast proliferation and collagen production. Given the role of multiple players during the pathogenesis of CNT-induced fibrosis, the objective of this review is to summarize the key findings and discuss major cellular and molecular events governing pulmonary fibrosis. We also discuss the physicochemical properties of CNTs and their effects on pulmonary toxicities as well as various biological factors contributing to the development of fibrosis.
Keywords: Angiogenesis, epithelial mesenchymal transition, inflammation, lung fibrosis, oxidative stress
Introduction
In recent years, a variety of nanomaterials have revolutionized the industrial field with their rapidly emerging applications in the areas of biotechnology, electronics, medicinal drug delivery, cosmetics, material science and aerospace engineering. Among the pool of recently developed nanomaterials, carbon nanotubes (CNTs) have generated great interest commercially with their unique physicochemical properties such as high tensile strength and conductivity (Donaldson et al., 2010; Maynard et al., 2004). With abundant novel applications, the CNT market has been projected to expand substantially within the next decade. However, such massive production is fraught with concerns for environmental and occupational exposure. According to a National Science Foundation (NSF) report, about 6 million workers will be involved with the nanotechnology industry by 2020 including 2 million within the United States, thus indicating a possible prevalence (Patel, 2011). Human exposures to manufactured nanomaterials are most likely to be observed in workers than the general population (Bergamaschi, 2009). A study reported exposure to polyacrylate paint containing nanoparticle within a group of female workers. Affected workers clinically presented with pleural effusions, progressive pulmonary fibrosis, pleural damage and death (Song et al., 2009). Moreover, a risk-assessment study on titanium dioxide nanomaterial observed occupational exposure within factory workers beyond the acceptable limits during the packaging process (DuPont™, 2008). However, nanomaterial exposure and dosimetry data are insufficient for humans owing to the difficulty in detection and accurate measurement tools for this unique and rapidly growing nanomaterial industry. The extraordinary properties of CNTs need to compete with reports of CNT-associated toxicities, thus indicating careful monitoring of human health and safety during their use. Depending upon the type of exposure, CNTs may penetrate the body through various routes such as the lungs and gut. CNTs are high aspect ratio nanomaterials (HARNs) having at least one of their dimensions of the order of 100 nm or less in size, as per the British Standards Institution (BSI) (2007). CNT structure facilitates their entry, deposition and residence in the lungs and pleura, resulting in incomplete phagocytosis and clearance from the lungs (Stella, 2011). Owing to their bio-persistent and non-biodegradable nature, and particularly the resemblance to needle-like asbestos fibers, CNTs are believed to induce biologically harmful effects (Lam et al., 2004). CNTs are similar to asbestos in their fibrous morphology, biopersistence, surface reactivity and the ability to translocate within the alveolar regions and the deeper pleura of the lung. Upon pulmonary exposure, CNTs generate an acute inflammatory response, activate several cell signaling pathways, induce genotoxicity, mesothelioma, diffuse interstitial fibrosis and granulomas similar to that observed in asbestos-exposed animals and humans. However, they differ in their chemical composition, surface charge and the ability to (i) enter mesothelial cells and (ii) induce direct fibrogenic effects (Jaurand et al., 2009; Nagai et al., 2011; Wang et al., 2010b).
Lung toxicity appears to be the major consequence of CNT exposure, ultimately contributing toward granuloma formation, epithelial hypertrophy and early onset of fibrosis (Lam et al., 2004; Muller et al., 2005; Shvedova et al., 2005; Warheit et al., 2004). Accumulating evidence in the literature demonstrates the fibrogenic potential of CNTs. Toxicity reports have indicated the ability of CNTs to translocate into the surrounding areas of the lung causing systemic toxicity, granulomatous lesions, interstitial and sub-pleural fibrosis (Jia et al., 2005; Park et al., 2011; Shvedova et al., 2008). However, the interactions of CNTs with the host at the molecular and cellular levels remain largely unknown. Identification of molecular targets and intracellular signaling is essential to the development of specific biomarkers for risk assessment and early detection of CNT-induced pathogenesis. The pathologic effects of CNTs are likely to be influenced by their physicochemical properties, thus we will first describe the physical and chemical properties of CNTs and their associated cellular toxicities. We will then focus on acute and chronic responses to CNT exposure involving oxidative stress, inflammation, genotoxicity and fibrosis. Finally, cellular mediators and mechanisms of fibrosis as well as biological factors influencing the fibrogenic process will be discussed.
Types and properties of CNTs
As per the British Standards Institute Report (2007), CNTs are HARNs having at least one of their dimensions, i.e. the diameter less than 100 nm whereas the lengths vary from a wide range of micrometers. CNTs are engineered nanomaterials made of graphene sheets that have been rolled into seamless cylindrical structure. They are manufactured mainly via arc discharge, chemical vapor deposition and laser ablation. All the three methods basically involve thermal elimination of carbon atoms from carbon sources including graphite, or gaseous carbon-bearing compounds such as CO, methane, ethylene or other hydrocarbons (Lam et al., 2006). Post-synthesis, CNTs are purified to eliminate residual organics such as soot or amorphous carbon and metals. They are classified into two main types known as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), both demonstrating outstanding chemical and thermal stability. MWCNTs comprise of several single-walled tubes layered onto each other. SWCNTs can be viewed as a single thick-graphite layer rolled into a cylindrical tube (Sinha & Yeow, 2005).
The nanoscale and large surface area of CNTs allow them to interact efficiently with cells, albeit in an undefined manner. Whether CNTs are inherently toxic or is it a wide array of external factors such as length, surface modification, degree of dispersion and the presence of metal impurities playing a role in CNT-induced toxicity is still a subject of intense investigations. Current literature reveals that CNTs based on their type, fiber length, dispersion status and functionality exert considerable variations in toxicities.
Physicochemical properties of CNTs affecting toxicities
Physicochemical factors alter the cytotoxicity of CNTs with respect to their cellular uptake, internalization, phagocytosis and clearance from the body (Borm et al., 2006; Oberdörster et al., 2005). For instance, even though the functionalization of CNTs may aid their solubility and dispersion, it has been speculated to facilitate their uptake and internalization into the systemic circulation. More detailed discussions of the effects of physicochemical properties of CNTs on their biological activities are provided below and are summarized in Tables 1 and 2.
Table 1.
Effects of physicochemical properties of CNTs on their biological activities based on size and surface area.
| Type of CNT | System | Effect | Study |
|---|---|---|---|
| Purified MWCNT, short (220 nm) and long (825 nm) | Human acute monocytic leukemia THP-1 cell line | Longer tubes induce increased inflammation | Sato et al. (2005) |
| SWCNT, long (0.5–100 mm) and short (0.5–2 mm) MWCNT, long (5–9 mm) and short (0.5–2 mm) |
Human epithelial Calu-3 | Longer MWCNTs and SWCNTs cause significant disruption of barrier function | Rotoli et al. (2009) |
| MWCNT, long (13 μm) and (56 μm), tangled (1–5 mm) and (5–20 mm) | In vivo | Length-dependent inflammation and granuloma formation | Poland et al. (2008) |
| MWCNT, short (1–10 mm), long tangled (10–50 mm), long needle-like (450 mm), asbestos (4.6 mm) and carbon black | Primary human macrophages | Enhanced activation of NRLP3 inflammasome and secretion of IL-1β, IL-1α by longer MWCNTs | Palomaki et al. (2011) |
| MWCNT, long (3–14 mm) and short (1.5 mm) | Mammalian immune and epithelial cancer cells RAW264.7 macro-phages and MCF-7 | Increased cytotoxicity with longer MWCNTs | Liu et al. (2012) |
| MWCNT, long, short, tangled, nickel nanowires, long and short | In vivo | Length-dependent retention of CNTs into lung pleura resulting in sustained inflammation and progressive fibrosis | Murphy et al. (2011) |
| MWCNT, dispersed thin (50 nm), aggregative (2–20 nm), thick (150nm) | Human peritoneal mesothelial cells | Thinner MWCNTs induced more potent cytotoxic response in terms of inflammagenocity and carcinogenicity | Nagai et al. (2011) |
| Purified MWCNT, thick (70 nm) and thin (9.4 nm) | Murine alveolar macrophages and in vivo in rats | Thinner MWCNTs more toxic than the thicker ones both in vitro and in vivo | Fenoglio et al. (2012) |
| SWCNT and MWCNT | Bacteria (E. coli K12) cells | SWCNTs are much more toxic to bacteria than MWCNTs based on diameter differences | Kang et al. (2008) |
| SWCNT (138 m2/g), carbon nanofibers, CNF (21 m2/g), asbestos (8 m2/g) | In vivo C57BL/6 mice | SWCNTs with higher surface area induced increased oxidative stress, inflammation, pulmonary damage and fibrosis than CNF and asbestos | Murray et al. (2012) |
| SWCNT, MWCNT, active carbon, carbon black and carbon graphite | Human fibroblast cells | SWCNTs with smaller surface area more toxic than their larger counterparts | Tian et al. (2006) |
| MWCNT, CNF carbon nanoparticles | Human lung tumor cells | Size and aspect ratio dependent cytotoxicity of MWCNT | Magrez et al. (2006) |
Table 2.
Effects of physicochemical properties of CNTs on their biological activities based on (A) functionalization; (B) presence of metal impurities; (C) dispersion status.
| Panel A | |||
| SWCNT, control and acid functionalized (AF-SWCNT) | LA4 mouse lung epithelial cells and in vivo in CD1 mice | AF-SWCNTs more cytotoxic than SWCNTs in vitro; exerted stronger inflammatory response in vivo than control SWCNTs | Saxena et al. (2007) |
| MWCNT, functionalized and non-functionalized | In vivo bone marrow cells of Swiss-Webster mice | Functionalized MWCNTs induced greater clastogenic/genotoxic effects compared to the non-functionalized counterparts | Patlolla et al. (2010a) |
| SWCNT, purified, raw and carboxylated | NRK cell line | Carboxylated SWCNTs more cytotoxic as compared to purified and raw samples | Wang et al. (2011c) |
| SWCNT, SWCNT-phenyl-SO3H, SWCNT-phenyl-SO3Na and SWCNT-phenyl-(COOH)2 | Human dermal fibroblasts | Cytotoxicity dependent on the degree of sidewall functionalization | Sayes et al. (2006) |
| MWCNT, pristine and carboxylated | In vivo mice | The degree of functionalization was inversely proportional to hepatic toxicity | Jain et al. (2011) |
| MWCNT, CNF, carbon nanoparticles | Human lung tumor cells | Functionalized carbon nanoparticles most toxic as compared to MWCNTs and CNFs | Magrez et al. (2006) |
| SWCNT, purified and 6-amino-hexanoic acid-derivatized (AHA-SWCNT) | Human epidermal keratinocytes | Functionalization induced mild cytotoxic responses and maintained cell viability | Zhang et al. (2007) |
| Panel B | |||
| 30 wt% iron-rich SWCNT | Human keratinocytes | Loss of cell viability, oxidative stress due to the catalytic activity of iron content associated within SWCNTs | Shvedova et al. (2003) |
| 26 wt% iron-rich SWCNT | Murine RAW 264.7 macrophages | Loss of intracellular thiols (GSH) and lipid hydroperoxides accumulation within the macrophages | Kagan et al. (2006) |
| Panel C | |||
| SWCNT, poor and well dispersed | In vivo C57BL/6 mice | Poorly dispersed SWCNTs – proximal alveolar regions resulting in granulomatous lesions; well-dispersed CNTs-alveolar interstitial and pleural areas causing parenchymal granulomas and interstitial fibrosis | Mercer et al. (2008) |
| SWCNT, Survanta dispersed (SD-SWCNT) and non-dispersed (ND-SWCNT) | Human lung epithelial BEAS-2B cells | SD-SWCNT induced more potent fibrogenic response both in vitro and in vivo as compared to ND-SWCNT | Wang et al. (2011a) |
Particle dispersion
The biopersistence of CNTs is critical in determining lung toxicity endpoints (Murray et al., 2012; Oberdörster et al., 2005). Factors such as dispersion and aggregation can determine the persistence of CNTs within the lung. Previous studies have shown that the distribution of CNTs within the lung can influence the degree of fibrosis (Muller et al., 2005). Poorly dispersed SWCNTs were found to be restricted to the proximal alveolar regions resulting in granulomatous lesions, whereas well-dispersed CNTs deposited deeper into the alveolar interstitial and pleural areas of the lung causing parenchymal granulomas and interstitial fibrosis (Mercer et al., 2008; Murray et al., 2012). A study comparing inhalation and pharyngeal aspiration of MWCNTs reported a significant increase in fibrosis after pharyngeal aspiration. MWCNT inhalation resulted in collagen deposition in peribronchial and interstitial areas (Mercer et al., 2011).
CNTs can induce direct fibrotic effects without any signs of inflammatory response depending upon their entry/deposition into the deeper lung tissue. For instance, the entry of SWCNTs into the alveolar interstitial space evades their macrophage engulfment (Shvedova et al., 2005). The ability of dispersed SWCNTs to enter the alveolar interstitium and stimulate resident fibroblasts has been suggested to play a key role in SWCNT-induced interstitial fibrosis (Mercer et al., 2008). Subsequent studies identified the mechanism for the direct fibrogenic effects of dispersed SWCNTs. Their findings demonstrated increased cell proliferation, collagen production and upregulation of matrix metalloproteinase (MMP)-2 and MMP-9 in the region surrounding interstitialized CNTs after 2 d of exposure without any signs of persistent inflammation (Wang et al., 2010a, 2011b).
Surface chemistry and functionalization
Surface reactivity of CNTs is a key property enhancing their applicability. During their interaction with biological tissues, surface chemistry plays a key role in determining the toxic responses. Surface charge present on acid-functionalized CNTs elicits inflammatory response. Functionalization of CNTs enhances the degree of oxidative stress and inflammation, thereby governing cytotoxicity in multiple cell lines including lung epithelial cells, lung tumor cells, bone marrow cells and lymphocytes (Bottini et al., 2006; Patlolla et al., 2010b; Saxena et al., 2007; Warheit et al., 2004). In contrast, some studies showed that functionalization of CNTs inversely affected their toxicity (Jain et al., 2011; Sayes et al., 2006; Zhang et al., 2007). It is likely that the effect of CNT functionalization on toxicity is functional group-specific and dependent on the toxicological outcomes such as cell death, DNA damage, fibrosis, which are poorly understood at present.
Particle dimension
Particle length can govern the penetration of CNTs in the interstitium or pleura of the lung. A number of studies have illustrated the effects of CNT length on inflammation and granuloma formation (Poland et al., 2008), cytotoxicity (Liu et al., 2012), oxidative stress (Kagan et al., 2010) and epithelial barrier function (Rotoli et al., 2009). Furthermore, the length can influence the retention and clearance of CNTs from the lung, thereby contributing to the asbestos-like responses in the pleural cavity of the lung leading to acute inflammation and progressive fibrosis (Murphy et al., 2011). The thickness of CNTs contributes to their inflammogenicity and carcinogenicity in mesothelial cells with thinner MWCNTs causing a more pronounced effect (Nagai et al., 2011).
Metal contaminants
The presence of transition metals has been shown to modify the respiratory toxicity of CNTs. Metal contaminants introduced into CNTs during their synthesis affect CNT-induced oxidative stress, inflammation and loss of cell viability (Warheit et al., 2004). The effect of metal contaminants on CNT-induced lung fibrosis has not been thoroughly demonstrated, although it is likely to play a key role. Studies suggest that Ni, Co and Fe are toxic and fibrogenic upon their interaction with CNTs (Lam et al., 2004; Pacurari et al., 2008; Pulskamp et al., 2007; Shvedova et al., 2003, 2005, 2008).
In addition to the physicochemical properties of CNTs described above, dose-metric parameters such as surface area and mass dose have been shown to be important in the toxic risk assessment of CNTs (Shvedova et al., 2012). There is a need for careful monitoring of CNT properties while documenting their toxicity. Hence, it is necessary to accurately assess the potential hazards of CNTs attributable to their properties in order to fully realize their novel applications.
Biodistribution of CNTs
Very few studies have addressed the systemic distribution of CNTs after being deposited in the lungs. Upon clearance from the spleen and liver, SWCNTs are eliminated from the blood circulation via renal excretion (Singh et al., 2006). Intratracheal instillation of MWCNTs results in their uptake via the lymphatic and phagocytic systems similar to asbestos fibers. However, MWCNTs are retained in the lungs 6 months post-exposure indicating their persistent nature (Elgrabli et al., 2008). Extra-pulmonary toxicities of MWCNTs have been reported following their translocation to the liver and kidney (Reddy et al., 2010), although the underlying mechanism is unclear. Alterations in membrane permeability due to pulmonary inflammation can lead to particle distribution extending beyond the lung (Zhu et al., 2009). MWCNT exposure increases the permeability of microvascular endothelium via increased cell migration, reactive oxygen species (ROS) response and actin remodeling (Pacurari et al., 2012). Further investigations of the biodistribution and pharmacokinetics of CNTs are essential in understanding their toxicities.
Biological activities CNTs
Oxidative stress
One of the most frequently reported toxicity endpoints is the formation of ROS, which can be either protective or harmful during biological interactions. Oxidative stress is an imbalance between the production of ROS and their elimination by the host’s defense systems. Oxidative stress amounts to DNA damage, lipid peroxidation and activation of signaling networks associated with loss of cell growth, fibrosis and carcinogenesis (Knaapen et al., 2004; Valko et al., 2006). Following the exposure to CNTs, ROS are induced intrinsically within the cell, extrinsically or indirectly via the effect of internalized CNTs on mitochondrial respiration. The critical factors driving CNT-induced ROS generation include active redox cycling on the surface of nanoparticles (NPs), oxidative functional groups on the NPs and NP-cell interactions, especially in the lungs where there is a rich pool of ROS producers like neutrophils and macrophages (Knaapen et al., 2004). CNT-induced oxidative stress is mainly followed by inflammation, cell injury, apoptosis and activation of cellular signaling pathways such as mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB, which are implicated in the pathogenesis of lung fibrosis (Bonner, 2002, 2007). Several studies have demonstrated the role of CNTs in oxidative stress, one of the mechanisms being mitochondrial damage (He et al., 2011). MWCNT exposure induces ROS production in a variety of cell lines as well as in vivo (Clichici et al., 2012; He et al., 2011; Mitchell et al., 2007; Reddy et al., 2010). Likewise, studies have demonstrated SWCNT-induced ROS generation in multiple cell lines (Azad et al., 2012; Manna et al., 2005; Shvedova et al., 2012) and activation of intracellular signaling pathways including MAPK, Akt, AP-1 and NF-κB in mesothelial cells in a dose-dependent manner (Pacurari et al., 2008). These findings indicate that CNT-induced oxidative stress may serve as an important intermediate endpoint while assessing pulmonary toxicity of CNTs.
Inflammation
Inflammation is commonly observed upon inhalation of CNTs. Characterization of the inflammatory process upon CNT exposure is necessary since inflammation is associated with other pathologic disorders such as fibrosis and cancer. Given the interplay between the inflammatory response and ROS generation, both effects are closely linked and one leads to the other (Donaldson & Poland, 2012). Particle deposition in the lung causes recruitment of inflammatory cells that generate ROS, clastogenic factors and cytokines, either harming or stimulating resident lung cells (Bonner, 2007). Oxidative stress produced from CNT exposure activates pro-inflammatory transcription factors such as NF-κB, AP-1 and MAPK (Pacurari et al., 2008). The inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-1β are well-known mediators of fibrotic lung diseases by activating the pro-fibrotic transforming growth factor (TGF)-β and platelet-derived growth factor (PDGF), respectively. Such stimulation leads to differentiation of fibroblasts to myofibroblasts and their production of extracellular matrix (ECM) proteins (Bonner, 2007; Mangum et al., 2006; Sime et al., 1998). Several reports indicate that MWCNTs and SWCNTs induce a panel of pro-fibrotic inflammatory cytokines and chemokines in human lung cells including TNF-α and IL-8 (Brown et al., 2007), IL-1β, IL-6, IL-10 and monocyte chemoattractant protein (MCP)-1 (He et al., 2011), NLRP3 inflammasome (Pälomaki et al., 2011), IL-13/33 (Beamer et al., 2012) and inflammatory enzymes such as cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) (Lee et al., 2012). Intratracheal and pharyngeal aspiration of CNTs induce robust inflammatory effects in vivo leading to the onset of fibrogenesis (Shvedova et al., 2008). Additionally, ineffective internalization of nano-sized CNTs by macrophages amounts to transient inflammation, suggesting that the rate of CNT elimination may be important in assessing the severity of the associated inflammatory responses (Dong et al., 2012; Kagan et al., 2010).
Genotoxicity
Given the association of inflammatory responses and lung fibrosis as well as the striking similarities between CNTs and asbestos, it is essential to assess the genotoxic potential of CNT exposure (Hubbard et al., 2000). Genotoxicity may be elicited either by the direct interaction of fibrous particles with the genetic material or by secondary damage from particle-induced ROS generation. CNT-induced sustained inflammation and oxidative stress can result in DNA damage and abnormal cell growth, possibly leading to carcinogenesis and fibrogenesis (Jaurand, 1997; Jaurand et al., 2009). A number of studies have provided evidence for the genotoxic effects of CNTs (Kisin et al., 2007, 2011; Patlolla et al., 2010a, b). Oxidative stress-dependent DNA breakage and DNA repair, and the activation of signaling pathways including poly ADP-ribose polymerase (PARP), AP-1, NF-κB, p38 and Akt were reported in human mesothelial cells exposed to SWCNTs (Pacurari et al., 2008). K-ras mutations were observed in mouse lungs upon SWCNT exposure (Shvedova et al., 2008). Chronic SWCNT exposure induced a genotypic change characterized by deregulated p53 amounting to apoptosis-resistant malignant phenotype (Wang et al., 2011a). MWCNTs cause mutations because of their clastogenic (DNA break) and aneuploidic (chromosomal loss) effects (Muller et al., 2008). Mutational changes such as micronuclei formation, DNA damage (Zhu et al., 2007) and p53-independent expression of p21 and cell cycle arrest (Zhang & Yan, 2012) upon MWCNT exposure have been reported in a variety of cell lines.
Permeability barrier function
Alterations in respiratory barrier function is of particular importance to CNT-induced toxicity since respiratory epithelial cells present a protective barrier against inhaled particles and constitute a major determinant of the interaction of the particles with other body compartments. Epithelial cells are responsible for the formation and maintenance of tight junction barrier, only permitting polarized secretory functions and preventing access to xenobiotics and pathogens (Schneeberger & Lynch, 2004). Consequently, respiratory barrier function plays a pivotal role in CNT-related inhalation hazards. MWCNTs have been shown to alter the paracellular permeability of airway epithelial cells by interfering with the formation of tight junctions. The permeability-altering effect of CNTs was shown to be dependent on fiber length and functionalization (Rotoli et al., 2008, 2009). However, the underlying mechanisms of alteration and its effect on CNT-induced pathogenesis have not been clearly elucidated.
CNT-induced pulmonary fibrosis
A variety of environmental and occupational agents including fibrous particles, metals, drugs and microbes are able to induce pulmonary fibrosis. During the development of fibrosis, several common cellular events occur including epithelial cell injury, infiltration of inflammatory cells, proliferation and transformation of fibroblasts into myofibroblasts, and synthesis and deposition of ECM (He et al., 2011). Unique to CNTs is their non-biodegradable and biopersistent nature which likely prolongs the fibrotic process. Multiple factors determine the severity and duration of CNT-induced fibrosis, which are discussed below and are summarized in Figure 1.
Figure 1.
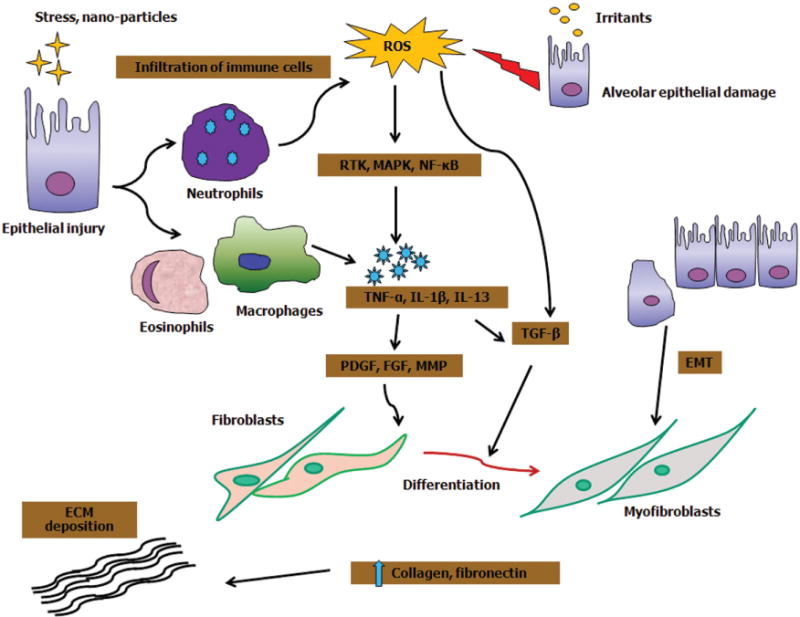
Mechanisms of lung fibrosis: irritants such as nanoparticles induce epithelial injury resulting in infiltration of immune cells such as neutrophils, eosinophils and alveolar macrophages at the site of tissue injury. Activated neutrophils can exaggerate the ROS response. Moreover, ROS generation upon particle–cell interactions activates cytokine growth receptor cascade. ROS-dependent activation of RTKs, MAPK, Akt and NF-κB results in expression of genes related to inflammation and fibrosis. ROS can also activate TGF-β to mediate the fibrogenic effects. Recruitment of leukocytes induces key pro-fibrotic cytokines including TNF-α, IL-1β and IL-13, which can further damage the epithelial cells. TNF-α and IL-1β stimulation upregulates TGF-β and PDGF, respectively, which in turn increase collagen production via fibroblast and myofibroblast proliferation. Alternatively, fibroblasts can directly induce fibrosis via proliferation and differentiation into myofibroblasts. In addition, epithelial cells undergoing EMT expand the pool of fibroblasts and myofibroblasts thereby driving fibrogenesis.
Role of ROS
ROS is widely known to be involved in epithelial cell injury and fibrogenesis (Inghilleri et al., 2006). CNT exposure results in ROS-dependent activation of several transcription factors and signaling pathways including NF-κB, signal transducer and activator of transcription (STAT)-1, MAPK and receptor tyrosine kinases (RTK), which are involved in the regulation of inflammation and fibrosis (Bonner, 2007). For instance, MWCNTs induce fibroblast to myofibroblast differentiation via ROS-dependent NF-κB activation (He et al., 2011), whereas SWCNTs induce collagen production and angiogenesis via ROS-dependent p38-MAPK activation (Azad et al., 2012). MAPK activation is also responsible for the enhancing effect of ROS on Transforming growth factor-beta (TGF-β)-mediated fibrogenic response in human lung cells (Thannickal et al., 2004). Furthermore, ROS has been shown to play a critical role in TGF-β-mediated fibroblast to myofibroblast differentiation with the differentiated cells serving as an additional source of ROS production (Thannickal & Fanburg, 1995). CNT-induced ROS generation is likely to be a result of frustrated phagocytosis which refers to the failure of the macrophage to entirely engulf the long fibers (<15 mm), thus resulting in an inflammatory condition (Donaldson et al., 2010).
Additionally, specific properties of CNTs such as metal contaminants including iron, cobalt, tungsten and vanadium as well as reactive groups on the CNT surface that have been attributed to pulmonary fibrotic response may induce oxidative stress (Antonini, 2003; Bonner et al., 2000; Shvedova et al., 2003; Warheit et al., 2004). Some of the common metal contaminants associated with pulmonary fibrosis include iron, cobalt, tungsten and vanadium.
Role of inflammation
Numerous inflammatory and pro-fibrotic mediators such as TNF-α, IL-1β and TGF-β have been implicated in the pathogenesis of fibrosis. Infiltration of immune cells such as eosinophils, neutrophils and macrophages results in tissue injury and loss of epithelial integrity, thus promoting tissue repair and fibrosis (Wallace et al., 2007). As mentioned earlier, CNT exposure initiates an inflammatory cascade of cytokines in association with oxidative stress. ROS-dependent activation of RTK, MAPK, Akt and NF-κB results in the expression of genes involved in inflammation and fibrosis. Upregulation of TNF-α stimulates TGF-β production, collagen deposition, ECM remodeling and angiogenesis during fibrosis (Sime et al., 1998). TGF-β further enhances fibrogenesis by activating connective tissue growth factor (CTGF). Similarly, IL-1β triggers the secretion of PDGF on lung fibroblasts, enhancing their proliferation (Lindroos et al., 1997). There are reports documenting inflammation-induced fibrosis following CNT exposure. For examples, MWCNTs induce TNF-α resulting in fibrosis in vivo (Muller et al., 2005). SWCNTs upon pharyngeal aspiration induce robust inflammation and an early onset of fibrogenic response in vivo characterized by the secretion of TNF-α, IL-1β and TGF-β as well as several biomarkers of oxidative stress (Shvedova et al., 2005). The time course of TNF-α and IL-1β corresponds to neutrophil influx, whereas that for TGF-β is dependent on macrophage recruitment. The extent of fibrosis is dose and time dependent (Shvedova et al., 2005). Inflammation-driven fibrosis causes granuloma formation associated with epithelial hypertrophy, alveolar thickening and interstitial fibrosis (Muller et al., 2005; Shvedova et al., 2008). Intratracheal instillation of SWCNTs results in early onset of lung fibrosis driven by the secretion of a panel of inflammatory cytokines (Park et al., 2011). In general, inflammation-mediated fibrogenesis can cause deterioration of pulmonary functions.
Role of pre-existing inflammation
Little has been reported about the risk and possibility of a fibrogenic response following CNT exposure in conditions with pre-existing inflammation. Studies have reported how bacterial-derived products modify CNT-related toxicities. In mice with prior bacterial infection, pharyngeal aspiration of SWCNTs promotes inflammatory response, collagen synthesis, reduces phagocytosis of bacteria by macrophages and bacterial clearance from the lungs, thereby increasing host susceptibility to lung fibrosis (Shvedova et al., 2008). Preexposure with lipopolysaccharide, an endotoxin, exaggerates the fibrotic effects of CNTs by (i) intensifying acute inflammatory response (Inoue et al., 2009), (ii) increasing PDGF and its receptor resulting in fibroblast chemotaxis and proliferation (Cesta et al., 2010) and by (iii) elevating the expression of TNF-α and IL-1β resulting in collagen synthesis and ECM deposition (Shvedova et al., 2008). Together, these results support the role of pre-existing inflammation in promoting CNT-induced lung fibrosis.
Role of angiogenesis
Angiogenesis is essential in the formation of new blood vessels, wound healing and tissue repair, thus important in fibrogenesis. Vascular endothelial growth factor (VEGF) regulates the angiogenic response by controlling the migration, proliferation and vasculature of endothelial cells. Initial studies demonstrated neovascularization leading to anastomoses between the systemic and pulmonary microvasculature of patients with pulmonary fibrosis (Turner-Warwick, 1963). Subsequent studies showed structural and functional changes in the vasculature involving the formation of new vessels, remodeling of alveolar capillaries, and imbalance between the angiogenic and angiostatic chemokines (Keane, 2004). These alterations signify the role of altered vascular homeostasis in the pathogenesis of lung fibrosis (Peão et al., 1994; Turner-Warwick, 1963). Interestingly, aberrant expression of VEGF has been reported during idiopathic myelofibrosis and diffuse lung fibrosis (Chaudhary et al., 2007). Besides being a pro-fibrogenic cytokine, TGF-β has the ability to promote angiogenesis. VEGF and TGF-β interact closely with respect to angiogenic and fibrogenic effects (Ferrari et al., 2009). A recent in vitro study showed that SWCNT-induced fibroblast proliferation and collagen production is mediated by VEGF and TGF-β1 (Azad et al., 2012). This study also suggested a positive feedback loop between TGF-β and VEGF during angiogenesis, which promotes the fibrotic process after SWCNT treatment. The observed fibrogenic and angiogenic effects were found to be mediated by ROS-dependent p38 MAPK activation. Inhibitors of ROS, p38 MAPK, VEGF and TGF-β strongly inhibited the pathologic effects of SWCNT. Further in vivo studies are necessary to substantiate the role of these cellular mediators in CNT-induced angiogenesis and fibrosis.
Role of epithelial mesenchymal transition
Epithelial mesenchymal transition (EMT), a process characterized by the transition of fully differentiated epithelial cells to a mesenchymal phenotype, has been suggested to play a key role in fibrosis by serving as a cellular source of resident fibroblasts/myofibroblasts. However, whether or not EMT is a major source of these interstitial lung cells during fibrosis in vivo is still unclear. EMT is brought about by various external stimuli including growth factors, cytokines and hormones (Willis & Borok, 2007). EMT promotes fibrogenesis by causing a loss of epithelial barrier that acts as a defense during advanced stage fibrosis (Chapman, 2011) and by generating mesenchymal cells that expand the pool of fibroblasts/myofibroblasts. In transgenic mice in which the fate of alveolar epithelial cells (AECs) can be tracked, it was found that vimentin-positive mesenchymal cells in injured lungs were mostly of alveolar epithelial origin, suggesting epithelial cells as the main source of mesenchymal expansion (Kim et al., 2006). Epithelial cells from patients with idiopathic pulmonary fibrosis (IPF) also co-express epithelial and mesenchymal markers, supporting the involvement of EMT in fibrosis (Kim et al., 2006; Willis et al., 2005). Furthermore, AECs undergo EMT via stimulation with TGF-β during fibrosis and are an important source of myofibroblasts (Radisky et al., 2007). In contrast to the above findings, a lineage-tracing study in a murine bleomycin-induced lung fibrosis model showed that EMT was not the origin of myofibroblasts as evidenced by the lack of AECs expressing the mesenchymal markers (Rock et al., 2011). However, a recent study showed that such EMT required proper ECM microenvironment, notably type I collagen (DeMaio et al., 2012). Thus, several factors may influence EMT and its fibrotic outcome, including experimental conditions, type of stimuli and the difference between the murine models and human disease.
With regards to CNT-induced EMT, a recent in vivo study demonstrated that SWCNT exposure induced EMT of lung epithelial cells which contributed significantly to fibroblast expansion (Chang et al., 2012). SWCNT exposure also resulted in an activation of TGF-β/p-Smad2 and β-catenin in epithelial cells, which was suggested to play a key role in SWCNT-induced EMT. Given the paucity of data on CNT-induced EMT, future studies are warranted to elucidate the role of EMT and related signaling pathways in CNT-induced lung fibrosis.
Profibrogenic mediators
A variety of fibrogenic cytokines and growth factors are involved in the regulation of pulmonary fibrosis. Key cytokines and growth factors implicated in the pathogenesis of CNT-induced fibrosis are highlighted below.
Transforming growth factor-beta
In the lung, TGF-β is produced mainly from alveolar macrophages and epithelial cells in response to various fibrogenic stimuli (Agostini & Gurrieri, 2006). Both SWCNTs and MWCNTs have been shown to induce TGF-β secretion in vitro (Azad et al., 2012; Mishra et al., 2012; Wang et al., 2010b) and in vivo (Shvedova et al., 2005; Wang et al., 2011b). In addition to its well-established role in EMT and angiogenesis, TGF-β can directly stimulate fibroblasts to produce collagen. Clinical evidence demonstrated that TGF-β is elevated in the lung of IPF patients and rodent models. TGF-β is capable of inducing cytoskeletal rearrangements and differentiation of fibroblasts to myofibroblasts, resulting in increased ECM production and deposition during fibrosis (Leask & Abraham, 2004). Additionally, a positive feedback loop exists between TGF-β and ROS generation. TGF-β stimulates ROS by reducing antioxidant levels in many lung cells, whereas ROS can activate TGF-β to mediate its fibrotic effects. Oxidative stress can thus significantly initiate a cascade of fibrogenic responses via TGF-β signaling (Thannickal & Fanburg, 1995).
Platelet-derived growth factor
PDGF is a key mediator of fibroblast proliferation and chemotaxis (Lindroos et al., 1997). During NP-induced fibrosis, PDGF isoforms induce fibroblast to myofibroblast differentiation and proliferation, thus increasing the immature collagenous tissue in the lung (Bonner, 2002; Mangum et al., 2006). PDGF has been previously implicated in asbestosis and coal dust-induced pneumoconiosis (Vanhee et al., 1994). Exposure of macrophages to MWCNTs induced PDGF secretion, mitochondrial damage and oxidative stress, which promote fibrogenesis (He et al., 2011). PDGF was also shown to be upregulated during SWCNT-induced interstitial fibrosis (Mangum et al., 2006). These studies indicate that coordinated activation of PDGF can drive CNT-induced fibrosis.
Osteopontin
Osteopontin (OPN), a chemotactic cytokine secreted mainly by macrophages, exhibits leukocyte chemotaxis during tissue repair. It induces proliferation and migration of epithelial cells and fibroblasts, and is known to play a key role in ECM remodeling during lung fibrosis (Pardo et al., 2005). Its pro-fibrotic role is implicated by the induction of MMP-7 during SWCNT-induced lung inflammation (Mangum et al., 2006). However, direct evidence for the role of OPN in CNT-induced fibrosis is still lacking.
Matrix metalloproteinases
The MMP family of enzymes plays an important role during wound healing and ECM repair. They are present in moderate levels during normal conditions; however, upon cellular injury and inflammation, their levels rise which helps to recruit immune cells to the site of injury. These fibrotic mediators along with TGF-β can potentially drive NP-induced fibrogenic response (Chakrabarti & Patel, 2005). Recent reports demonstrated the role of MMPs in CNT-induced fibrogenesis. For instance, CNTs induced MMP-9 and in conjunction with TGF-β stimulated fibroblast proliferation, collagen synthesis and secretion of fibroblast growth factor (FGF)-2 which is a potent angiogenic factor and key stimulant for fibroblast proliferation and collagen production (Khalil et al., 2005; Mishra et al., 2012; Wang et al., 2010b). These studies also indicated the early onset of fibrosis brought about by CNTs without persistent inflammation. Thus, activating fibroblast proliferation and collagen synthesis appears sufficient for the atypical rapid interstitial fibrosis caused by CNTs.
Conclusions
This review encompasses the acute and chronic pulmonary responses to CNT exposure and their linkage. The ability of CNTs to cause acute toxicities and chronic fibrotic effects depends on several physicochemical factors such as particle dimension, dispersion status, functionalization and the presence of transition metals. Understanding these factors will enable the design of safer CNT products and their utilization. The cytotoxic and fibrogenic effects of CNTs appear to be associated with their ability to induce oxidative stress and inflammatory and fibrogenic cytokines. There is a close connection between oxidative stress and inflammatory response, as well as cross-talk between inflammation and fibrosis as indicated by the multifunctional roles of the induced cytokines, e.g. TGF-β, PDGF and MMPs. Interestingly, however, there have been reports showing CNT-induced fibrosis with minimum inflammation and oxidative injury, suggesting alternative pathways and mechanisms of fibrosis. Apart from the molecular and cellular changes, other biological factors such as angiogenesis and EMT can also influence fibrosis. EMT may contribute to the increased fibroblasts/myofibroblasts during CNT-induced fibrosis through TGF-β, Smad and β-catenin signaling. Similarly, angiogenesis may promote fibrosis through VEGF-mediated fibroblast proliferation and collagen synthesis. Together, these findings provide a mechanistic framework for the induction of fibrosis by CNTs which could facilitate the development of potential biomarkers and drug targets for diagnosis and treatment of the disease.
Acknowledgments
This work is supported by the National Institutes of Health grant R01-HL076340 and by the National Science Foundation grant EPS-1003907.
Abbreviations
- CNT
Carbon nanotube
- NP
Nanoparticle
- SWCNT
Single-walled carbon nanotube
- MWCNT
Multi-walled carbon nanotube
- MMP
Matrix metalloproteinase
- ROS
Reactive oxygen species
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- AP-1
Activator protein
- ECM
Extracellular matrix
- TNF-α
Tumor necrosis factor-alpha
- IL-1β
Interleukin-1beta
- PDGF
Platelet-derived growth factor
- TGF-β
Transforming growth factor-beta
- COX-2
Cyclooxygenase-2
- iNOS
Inducible nitric oxide synthase
- MCP-1
Monocyte chemoattractant protein
- STAT-1
Signal transducer and activator of transcription
- RTK
Receptor tyrosine kinase
- CTGF
Connective tissue growth factor
- VEGF
Vascular endothelial growth factor
- EMT
Epithelial mesenchymal transition
- AECs
Alveolar epithelial cells
- IPF
Idiopathic pulmonary fibrosis
- FGF
Fibroblast growth factor
- OPN
Osteopontin
Footnotes
Declaration of interest
The authors report no declarations of interest.
Research findings and conclusions are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Agostini C, Gurrieri C. Chemokine/cytokine cocktail in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:357–63. doi: 10.1513/pats.200601-010TK. [DOI] [PubMed] [Google Scholar]
- Antonini JM. Health effects of welding. Crit Rev Toxicol. 2003;33:61–103. doi: 10.1080/713611032. [DOI] [PubMed] [Google Scholar]
- Azad N, Iyer AK, Wang L, et al. Reactive oxygen species-mediated p38 MAPK regulates carbon nanotube-induced fibrogenic and angiogenic responses. Nanotoxicology. 2012 doi: 10.3109/17435390.2011.647929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Girtsman TA, Seaver BP, et al. IL-33 mediates multi-walled carbon nanotube (MWCNT)-induced airway hyper-reactivity via the mobilization of innate helper cells in the lung. Nanotoxicology. 2012 doi: 10.3109/17435390.201.702230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi E. Occupational exposure to nanomaterials: present knowledge and future development. Nanotoxicology. 2009;3:194–201. [Google Scholar]
- Bonner JC. The epidermal growth factor receptor at the crossroads of airway remodeling. Am J Physiol Lung Cell Mol Physiol. 2002;283:L528–30. doi: 10.1152/ajplung.00126.2002. [DOI] [PubMed] [Google Scholar]
- Bonner JC. Lung fibrotic responses to particle exposure. Toxicol Pathol. 2007;35:148–53. doi: 10.1080/01926230601060009. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Moomaw CR, Morgan DL. Airway fibrosis in rats induced by vanadium pentoxide. Am J Lung Cell Mol Physiol. 2000;278:L209–16. doi: 10.1152/ajplung.2000.278.1.L209. [DOI] [PubMed] [Google Scholar]
- Borm PJ, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini M, Bruckner S, Nika K, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–6. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- British Standards Institution (BSI) 2007. Nanotechnologies Part 2: guide to safe handling and disposal of manufactured nanomaterials. BSI PD. 2007:6699–2. [Google Scholar]
- Brown D, Kinloch I, Bangert U, et al. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon. 2007;45:1743–56. [Google Scholar]
- Cesta MF, Ryman-Rasmussen J, Wallace DG, et al. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43:142–51. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res. 2005;31:599–621. doi: 10.1080/019021490944232. [DOI] [PubMed] [Google Scholar]
- Chang CC, Tsai ML, Huang HC, et al. Epithelial-mesenchymal transition contributes to SWCNT-induced pulmonary fibrosis. Nanotoxicology. 2012;6:600–10. doi: 10.3109/17435390.2011.594913. [DOI] [PubMed] [Google Scholar]
- Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Ann Rev Physiol. 2011;73:413–35. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J. 2007;29:976–85. doi: 10.1183/09031936.00152106. [DOI] [PubMed] [Google Scholar]
- Clichici S, Biris AR, Tabaran F, Filip A. Transient oxidative stress and inflammation after intraperitoneal administration of multi-walled carbon nanotubes functionalized with single strand DNA in rats. Toxicol Appl Pharmacol. 2012;259:281–92. doi: 10.1016/j.taap.2012.01.004. [DOI] [PubMed] [Google Scholar]
- DeMaio L, Buckley ST, Krishnaveni MS, et al. Ligand-independent transforming growth factor-β type I receptor signalling mediates type I collagen-induced epithelial-mesenchymal transition. J Pathol. 2012;226:633–44. doi: 10.1002/path.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Poland CA. Inhaled nanoparticles and lung cancer – what we can learn from conventional particle toxicology. Swiss Med Wkly. 2012;142:w13547. doi: 10.4414/smw.2012.13547. [DOI] [PubMed] [Google Scholar]
- Dong PX, Wan B, Wang ZX, et al. Exposure of single-walled carbon nanotubes impairs the functions of primarily cultured murine peritoneal macrophages. Nanotoxicology. 2012 doi: 10.3109/17435390.2012.694487. [DOI] [PubMed] [Google Scholar]
- DuPont™ Nano risk framework output worksheet for: DuPont™ Crystar® 6920 PET poly-(ethylene terephthalate) resin with sepiolite clay, Pangel S-9 as an encapsulated nanodispersed filler. 2008 Available from: http://www.epa.gov/opptintr/nano/dupont2.pdf [last accessed 2 Jan 2013]
- Elgrabli D, Floriani M, Abella-Gallart S, et al. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part Fibre Toxicol. 2008;5:20. doi: 10.1186/1743-8977-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio I, Aldieri E, Gazzano E, et al. Thickness of multiwalled carbon nanotubes affects their lung toxicity. Chem Res Toxicol. 2012;25:74–82. doi: 10.1021/tx200255h. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cook BD, Terushkin V, et al. Transforming growth factor-beta 1 (TGF-beta1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J Cell Physiol. 2009;219:449–58. doi: 10.1002/jcp.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Young S, Schwegler-Berry D, et al. Multiwalled carbon nanotubes induce a fibrogenic response by stimulating reactive oxygen species production, activating NF-κB signaling, and promoting fibroblast-to-myofibroblast transformation. Chemical Res Toxicol. 2011;24:2237–48. doi: 10.1021/tx200351d. [DOI] [PubMed] [Google Scholar]
- Hubbard R, Venn A, Lewis S, Britton J. Lung cancer and cryptogenic fibrosing alveolitis. A population-based cohort study. Am J Respir Crit Care Med. 2000;161:5–8. doi: 10.1164/ajrccm.161.1.9906062. [DOI] [PubMed] [Google Scholar]
- Inghilleri S, Morbini P, Oggionni T, et al. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2006;125:661–9. doi: 10.1007/s00418-005-0116-7. [DOI] [PubMed] [Google Scholar]
- Inoue K, Koike E, Yanagisawa R, et al. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009;237:306–16. doi: 10.1016/j.taap.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Jain S, Thakare VS, Das M, et al. Toxicity of multiwalled carbon nanotubes with end defects critically depends on their functionalization density. Chem Res Toxicol. 2011;24:2028–39. doi: 10.1021/tx2003728. [DOI] [PubMed] [Google Scholar]
- Jaurand MC. Mechanisms of fiber-induced genotoxicity. Environ Health Perspect. 1997;105:1073–84. doi: 10.1289/ehp.97105s51073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurand MF, Renier A, Daubriac J. Mesothelioma: do asbestos and carbon nanotubes pose the same health risk? Particle Fibre Toxicol. 2009;6:16. doi: 10.1186/1743-8977-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Wang H, Yan L, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–83. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- Kagan VE, Konduru NV, Feng W, et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nature Nanotechnol. 2010;5:354–9. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurina YY, Tyurin VA, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macro phages: role of iron. Toxicol Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Kang S, Herzberg M, Rodrigues DF, Elimelech M. Antibacterial effects of carbon nanotubes: size does matter! Langmuir. 2008;24:6409–13. doi: 10.1021/la800951v. [DOI] [PubMed] [Google Scholar]
- Keane MP. Angiogenesis and pulmonary fibrosis: feast or famine? Am J Respir Crit Care Med. 2004;170:207–9. doi: 10.1164/rccm.2405007. [DOI] [PubMed] [Google Scholar]
- Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280:43000–9. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–5. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisin ER, Murray AR, Keane MJ, et al. Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J Toxicol Environ Health Part A. 2007;70:2071–9. doi: 10.1080/15287390701601251. [DOI] [PubMed] [Google Scholar]
- Kisin ER, Murray AR, Sargent L, et al. Genotoxicity of carbon nanofibers: are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol Appl Pharmacol. 2011;252:1–10. doi: 10.1016/j.taap.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: mechanisms. Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- Lam C, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–34. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- Lam CW, James JT, McCluskey R, et al. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36:189–217. doi: 10.1080/10408440600570233. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J: Off Publ Fed Am Soc Exp Biol. 2004;18:816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Lee JK, Sayers BC, Chun KS, et al. Multi-walled carbon nanotubes induce COX-2 and iNOS expression via MAP Kinase-dependent and -independent mechanisms in mouse RAW264.7 macrophages. Particle Fibre Toxicol. 2012;9:14. doi: 10.1186/1743-8977-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindroos PM, Coin PG, Badgett A, et al. Alveolar macrophages stimulated with titanium dioxide, chrysotile asbestos, and residual oil fly ash upregulate the PDGF receptor-alpha on lung fibroblasts through an IL-1beta-dependent mechanism. Am J Respir Cell Mol Biol. 1997;16:283–92. doi: 10.1165/ajrcmb.16.3.9070613. [DOI] [PubMed] [Google Scholar]
- Liu J, Legros S, Ma G, et al. Influence of surface functionalization and particle size on the aggregation kinetics of engineered nanoparticles. Chemosphere. 2012;87:918–24. doi: 10.1016/j.chemosphere.2012.01.045. [DOI] [PubMed] [Google Scholar]
- Magrez A, Kasas S, Salicio V, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–5. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- Mangum JB, Turpin EA, Antao-Menezes A, et al. Single-walled carbon nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Particle Fibre Toxicol. 2006;3:15. doi: 10.1186/1743-8977-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna SK, Sarkar S, Barr J, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5:1676–84. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard AD, Baron PA, Foley M, et al. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health Part A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Hubbs AF, Scabilloni JF, et al. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Particle Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni J, Wang L, et al. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am J Physiol Lung Cell Mol Physiol. 2008;294:L87–97. doi: 10.1152/ajplung.00186.2007. [DOI] [PubMed] [Google Scholar]
- Mishra A, Rojanasakul Y, Chen B, et al. Assessment of pulmonary fibrogenic potential of multiwalled carbon nanotubes in human lung cells. J Nanomater 2012 [Google Scholar]
- Mitchell LA, Gao J, Wal RV, et al. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol Sci: Off J Soc Toxicol. 2007;100:203–14. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- Muller J, Decordier I, Hoet PH, et al. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008;29:427–33. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–31. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Murphy FA, Poland CA, Duffin R, et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AR, Kisin ER, Tkach AV, et al. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Particle Fibre Toxicol. 2012;9:10. doi: 10.1186/1743-8977-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Okazaki Y, Chew SH, et al. Diameter and rigidity of multiwalled carbon nanotubes are critical factors in mesothelial injury and carcinogenesis. Proc Natl Acad Sci USA. 2011;108:E1330–8. doi: 10.1073/pnas.1110013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle Fibre Toxicol. 2005;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacurari M, Qian Y, Fu W, et al. Cell permeability, migration, and reactive oxygen species induced by multiwalled carbon nanotubes in human microvascular endothelial cells. J Toxicol Environ Health Part A. 2012;75:129–47. doi: 10.1080/15287394.2012.625549. [DOI] [PubMed] [Google Scholar]
- Pacurari M, Yin XJ, Zhao J, et al. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ Health Perspect. 2008;116:1211–17. doi: 10.1289/ehp.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomäki J, Välimäki E, Sund J, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–70. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Pardo A, Gibson K, Cisneros J, et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. Plos Med. 2005;2:e251. doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Roh J, Kim S, et al. A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in mice. Archiv Toxicol. 2011;85:1121–31. doi: 10.1007/s00204-011-0655-8. [DOI] [PubMed] [Google Scholar]
- Patel V. Global carbon nanotubes market outlook: industry beckons. Nanotech Insights. 2011;2:31–5. [Google Scholar]
- Patlolla AK, Hussain SM, Schlager JJ, et al. Comparative study of the clastogenicity of functionalized and nonfunctionalized multi-walled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ Toxicol. 2010a;25:608–21. doi: 10.1002/tox.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlolla A, Knighten B, Tchounwou P. Multi-walled carbon nanotubes induce cytotoxicity, genotoxicity and apoptosis in normal human dermal fibroblast cells. Ethn Dis. 2010b;20:S1-65–72. [PMC free article] [PubMed] [Google Scholar]
- Peão MN, Aguas AP, de Sá CM, Grande NR. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Record. 1994;238:57–67. doi: 10.1002/ar.1092380108. [DOI] [PubMed] [Google Scholar]
- Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature Nanotechnol. 2008;3:423–8. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- Pulskamp K, Diabaté S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168:58–74. doi: 10.1016/j.toxlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–9. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AR, Krishna DR, Reddy YN, Himabindu V. Translocation and extra pulmonary toxicities of multi wall carbon nanotubes in rats. Toxicol Mech Meth. 2010;20:267–72. doi: 10.3109/15376516.2010.484077. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:20867–8. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotoli BM, Bussolati O, Barilli A, et al. Airway barrier dysfunction induced by exposure to carbon nanotubes in vitro: which role for fiber length? Human Exp Toxicol. 2009;28:361–8. doi: 10.1177/0960327109105159. [DOI] [PubMed] [Google Scholar]
- Rotoli BM, Bussolati O, Bianchi MG, et al. Non-functionalized multi-walled carbon nanotubes alter the paracellular permeability of human airway epithelial cells. Toxicol Lett. 2008;178:95–102. doi: 10.1016/j.toxlet.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yokoyama A, Shibata K, et al. Influence of length on cytotoxicity of multi-walled carbon nanotubes against human acute monocytic leukemia cell line THP-1 in vitro and subcutaneous tissue of rats in vivo. Mol BioSyst. 2005;1:176–82. doi: 10.1039/b502429c. [DOI] [PubMed] [Google Scholar]
- Saxena R, Williams W, McGee J, et al. Enhanced in vitro and in vivo toxicity of polydispersed acid-functionalized single-wall carbon nanotubes. Nanotoxicology. 2007;1:291–300. [Google Scholar]
- Sayes CM, Liang F, Hudson JL, et al. Functionalization density dependence of single-walled carbon nanotubes cytotoxicity in vitro. Toxicol Lett. 2006;161:135–42. doi: 10.1016/j.toxlet.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Castranova V, Kisin ER, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health Part A. 2003;66:1909–26. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kapralov AA, Feng WH, et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. Plos One. 2012;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–65. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Marr RA, Gauldie D, et al. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153:825–32. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pantarotto D, Lacerda L, et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA. 2006;103:3357–62. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Yeow JT. Carbon nanotubes for biomedical applications. IEEE Trans Nanobioscience. 2005;4:180–95. doi: 10.1109/tnb.2005.850478. [DOI] [PubMed] [Google Scholar]
- Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–67. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- Stella GM. Carbon nanotubes and pleural damage: perspectives of nanosafety in the light of asbestos experience. Biointerphases. 2011;6:P1–17. doi: 10.1116/1.3582324. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Activation of an H2O-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–8. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Toews GB, White ES, et al. Mechanisms of pulmonary fibrosis. Ann Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- Tian F, Cui D, Schwarz H, et al. Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol In Vitro. 2006;20:1202–12. doi: 10.1016/j.tiv.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Turner-Warwick M. Precapillary Systemic-pulmonary anastomoses. Thorax. 1963;18:225–37. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vanhee D, Gosset P, Wallaert B, et al. Mechanisms of fibrosis in coal workers’ pneumoconiosis. Increased production of platelet-derived growth factor, insulin-like growth factor type I, and transforming growth factor beta and relationship to disease severity. Am J Respir Crit Care Med. 1994;150:1049–55. doi: 10.1164/ajrccm.150.4.7921435. [DOI] [PubMed] [Google Scholar]
- Wallace WAH, Fitch PM, Simpson AJ, Howie SEM. Inflammation-associated remodelling and fibrosis in the lung – a process and an end point. Int J Exp Pathol. 2007;88:103–10. doi: 10.1111/j.1365-2613.2006.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Castranova V, Mishra A, et al. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Particle Fibre Toxicol. 2010a;7:31. doi: 10.1186/1743-8977-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luanpitpong S, Castranova V, et al. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011a;11:2796–803. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Mikoryak C, Li S, et al. Cytotoxicity screening of single-walled carbon nanotubes: detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol Pharm. 2011c;8:1351–61. doi: 10.1021/mp2001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Rojanasakul Y, Castranova V, et al. Direct fibrogenic effects of dispersed single-walled carbon nanotubes on human lung fibroblasts. J Toxicol Environ Health Part A. 2010b;73:410–22. doi: 10.1080/15287390903486550. [DOI] [PubMed] [Google Scholar]
- Wang X, Xia T, Ntim SA, et al. Dispersal state of multiwalled carbon nanotubes elicits profibrogenic cellular responses that correlate with fibrogenesis biomarkers and fibrosis in the murine lung. ACS Nano. 2011b;5:9772–87. doi: 10.1021/nn2033055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warheit DB, Laurence BR, Reed KL, et al. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci: Off J Soc Toxicol. 2004;77:117–25. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–34. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-[beta]1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–32. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yan B. Cell cycle regulation by carboxylated multiwalled carbon nanotubes through p53-independent induction of p21 under the control of the BMP signaling pathway. Chem Res Toxicol. 2012;25:1212–21. doi: 10.1021/tx300059m. [DOI] [PubMed] [Google Scholar]
- Zhang LW, Zeng L, Barron AR, Monteiro-Riviere NA. Biological interactions of functionalized single-wall carbon nanotubes in human epidermal keratinocytes. Int J Toxicol. 2007;26:103–13. doi: 10.1080/10915810701225133. [DOI] [PubMed] [Google Scholar]
- Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7:3592–7. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- Zhu MT, Feng WY, Wang Y, et al. Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol Sci: Off J Soc Toxicol. 2009;107:342–51. doi: 10.1093/toxsci/kfn245. [DOI] [PubMed] [Google Scholar]


