Abstract
Purpose
To examine symptom reports and physiological parameters in adolescents using the Eating After Transplant (EAT!) intervention during hematopoietic stem cell transplant (HSCT) recovery.
Design
Repeated measures design.
Setting
HSCT service at a pediatric teaching institution in the southern United States.
Sample
16 adolescents recovering from a first time allogeneic HSCT.
Methods
Use of EAT! was monitored electronically, symptom reports were obtained from a questionnaire, and physiologic parameters were obtained from the medical record at HSCT hospital discharge then 20, 40, and 60 days post discharge.
Research Variables
EAT! use, symptom prevalence, symptom related distress, and physiologic parameters including weight, body mass index (BMI), prealbumin, and albumin
Findings
Symptom prevalence was highest at hospital discharge and steadily declined; however, mean symptom distress scores remained stable. Mean weight and BMI significantly declined during the first 60 days post hospital discharge; prealbumin and albumin markers were unchanged. There was no correlation among use of EAT! and any research variables.
Conclusions
The most frequent symptoms were not always the most distressing symptoms. Weight and BMI significantly declined during HSCT recovery.
Implications for Nursing
Nurses should assess symptom frequency and distress to fully understand patients’ symptom experiences. Nurses should monitor weight and BMI throughout HSCT recovery.
Knowledge Translation
Adolescents experience multiple symptoms throughout HSCT recovery that vary in frequency and distress. Weight and BMI can decline during HSCT recovery and should be monitored closely. Use of a mobile phone application for HSCT symptom management should be investigated further with a variety of outcome measures.
Children undergoing hematopoietic stem cell transplantation (HSCT) have reported treatment-related symptoms as the worst part of their cancer experience, which create difficulties with other life events and are remembered long after treatment ended (Enskar, Carlsson, Golsater, & Hamrin, 1997; Woodgate & Degner, 2003). Nausea, vomiting, fatigue, pain, anorexia, diarrhea, dry mouth, and taste changes develop immediately after HSCT and persist for months (Barker, Anderson, Sauve, & Butzner, 2005; Rodgers et al, 2008), increasing the need for medical care and negatively affecting patients’ development, compliance to treatment, and quality of life (Erickson et al, 2012; Cohen et al, 2012). The Eating After Transplant (EAT!) mobile phone application (app) was developed to provide adolescent HSCT patients with descriptive information and useful strategies regarding common symptoms and eating issues during the first 100 days post HSCT (Rodgers, Krance, Street, & Hockenberry, 2013). To meet the expressed needs of patients recovering from HSCT to participate in self-care activities, manage their symptoms, and have available information delivered in a practical method (Larson, 1995), EAT! provides descriptions of common gastrointestinal (GI) problems and self-care strategies in an easily accessible format for mobile phones. The app has demonstrated acceptability and usability, and HSCT patients were immediately competent with the app following orientation (Rodgers, Krance, Street, & Hockenberry, 2013). The current study extends those findings by assessing whether the EAT! app is associated with decreased symptom prevalence or distress or with improved biomarkers, thereby enhancing well-being.
Significance and Background
HSCT is a common treatment modality for pediatric illnesses including a variety of malignancies, hematologic diseases, immunodeficiency disorders, and genetic disorders. Approximately 1200 allogeneic HSCTs are performed annually in children under the age of 18 years (NMDP, 2012). Patient’s health status can rapidly change throughout the first year following HSCT as a result of the aggressive treatment and related complications; however, the first 100 days following HSCT are associated with the most complications and quality of life issues (Grant, Cooke, Bhatia, & Forman, 2005). Patients often struggle with a variety of physical and psychological symptoms during this time and attempt to learn ways to gain control and relieve uncertainty (Grant, Cooke, Bhatia, & Forman, 2005). Although children and adolescents have distinct developmental differences, it is difficult to report on one single age group due to the limited amount of age specific HSCT research.
Symptoms
Four recent studies have described the physical symptom profile following HSCT among adult patients. Over the first year following HSCT, 118 adults reported that tiredness, poor appetite, taste alterations, dry mouth, and nausea decreased in frequency but persisted throughout the year (Iestra et al, 2002). In addition, a majority of these patients (66%) reported eating difficulties at 50 days post HSCT, and more than one in five at one-year post HSCT. Immediately before HSCT discharge and for two weeks following, 16 adult patients reported appetite loss, nausea, vomiting, diarrhea, and sleep disturbances as significantly affecting their quality of life (QOL), and six of these patients reported ongoing symptoms, such as fatigue, affecting their QOL at 6 weeks post discharge (Hacker & Ferrans, 2003). Throughout the first 100 days following HSCT, 76 adults reported a high prevalence of fatigue, worry, anorexia, nausea/vomiting, pain, and insomnia (Bevans, Mitchell, & Marden, 2008). Last, over the first 100 days after autologous or allogeneic HSCT, 164 adult patients reported physical weakness, sleep disturbance, lack of appetite, fatigue, and drowsiness as the most severe symptoms, with allogeneic HSCT associated with more severe symptoms than autologous HSCT (Cohen et al, 2012).
Pediatric populations have received less attention with only three studies evaluating prevalence and duration of symptoms during HSCT recovery. In the largest study, 132 children and adolescents reported that mucositis, vomiting, abdominal pain and odynophagia were common at 100 days HSCT (Barker et al, 2005). A longitudinal cohort study of GI symptoms and anthropometric measurement changes in 35 children and adolescents through the first 4 months post HSCT found lack of appetite, nausea, vomiting, diarrhea, dry mouth, and taste changes common throughout the study period (Rodgers et al, 2008). Finally, a qualitative study of 13 adolescents’ eating experiences at 50 and 100 days post HSCT described a slow return of the patients’ appetites and eating barriers that consisted of nausea, vomiting, taste changes, dry mouth, and bad smells (Rodgers, Young, Hockenberry, Binder, & Symes, 2010). Missing from previous work among children and adolescents have been assessments of the multidimensional nature of symptoms that tap not only prevalence but also severity and distress (Erickson et al, 2012), findings that would empower healthcare providers to develop interventions that both diminish symptoms and also improve quality of life (Reid, McKenna, Fitzsimons, & McCance, 2009).
Biomarkers
Given the symptom profile described above, patients are at risk of impaired nutritional status. Nutritional well-being of post-HSCT patients has been measured relatively simply as body mass index (BMI), using height and weight, as muscle mass and fat tissue using mid-arm circumference and skinfold triceps measurements (Muscaritoli, Grieco, Capria, Iori, & Fanelli, 2002), or with bioelectrical impedance (Jaime-Pérez et al, 2013) or whole body dual-energy x-ray absorptiometry (Kyle et al, 2005). Among adults in two studies, significant weight loss of 12 kilograms occurred between transplant and engraftment (Jaime-Pérez et al, 2013), and significant lean BMI loss of 1.0 kg/m2 and a body fat mass loss of 1.2 kg/m2 occurred over 6 months that was not regained by one-year post HCST (Kyle et al, 2005), respectively. Children and adolescents experienced significant declines in weight, skinfold triceps, and mid-arm circumference measurements from baseline to 4 months post-HSCT, illustrating a significant loss of muscle mass and fat tissue (Rodgers et al, 2008).
Laboratory measurements such as serum protein markers (albumin and prealbumin) have also been used to assess patients’ nutritional status following HSCT, with mixed findings. Jaime-Pérez and colleagues (2013) studied albumin levels between HSCT and engraftment in 77 adult patients and found no significant change. Uderzo and colleagues (1991) studied albumin and prealbumin levels during total parenteral nutrition (TPN) supplementation after HSCT in 25 pediatric patients. Although albumin levels did not fluctuate, prealbumin level showed a statistically significant rise approximately one week after starting TPN. Protein markers have not been studied longitudinally among adolescents during HSCT recovery.
Given the dearth of research on the symptom and biological marker profiles of adolescent patients and the feasibility of the EAT! app as a symptom management intervention during recovery from HSCT, this study used Symptom Management Theory (SMT) (Dodd et al, 2001) as a framework for testing two research questions. (1) What are the symptom prevalence, symptom-related distress, and biomarker profiles of adolescent patients at hospital discharge and 20, 40 and 60 days post-discharge following HSCT? And, (2) is the use of the EAT! app related to better or worse symptom experiences or nutritional status (weight, BMI, serum albumin, or serum prealbumin) in this sample? The SMT states that with a full understanding of symptom experiences (research question 1), symptom management strategies can be used to create either positive or negative outcomes (research question 2) (Dodd et al, 2001).
Methods
Design, Setting, & Sample
This study used a repeated measures design, as previously reported (Rodgers, Krance, Street, & Hockenberry, 2013). Briefly, data were collected at four time points (initial HSCT hospital discharge, 20, 40, and 60 days post hospital discharge) between September 2011 and September 2012 from patients within the HSCT service at Texas Children’s Hospital. Sixteen consecutively discharged adolescents who were 11–18 years of age, English-speaking, and discharged within 50 days of a first time allogeneic HSCT participated. Adolescents who received an autologous HSCT or a repeat allogeneic HSCT or had neurological or developmental delays were excluded. The study was approved by the institutional review board at Baylor College of Medicine in Houston, Texas. Informed consent and patient assent were obtained if the patient was younger than 18 years of age, and patient consent was obtained if the patient was 18 years or older.
Intervention
Development and feasibility of the EAT! intervention have been described previously (Rodgers, Krance, Street, & Hockenberry, 2013). Briefly, EAT! is a touch screen mobile phone application that includes descriptions and strategies regarding appetite, choosing foods, nausea and vomiting, taste changes, dry mouth, control of eating, and returning to normal following HSCT. The app allowed patients to access the information at any time between its introduction prior to HSCT hospital discharge and the conclusion of the 60-day study period.
Research Variables
Symptoms were assessed using the Memorial Symptom Assessment Scale 10–18 (MSAS 10–18) (Portenoy et al, 1994), a 30-item patient-rated instrument adapted from the original MSAS questionnaire to accommodate a reading and comprehension age of 10 years (Collins et al, 2000). Items refer to presence/absence of a specific symptom in the past week and, if present, assessed frequency and severity on a 4-point Likert scale and distress on a 5-point Likert scale. Symptom frequencies were totaled to measure symptom prevalence, and symptom distress scores were averaged for an overall symptom distress score. Higher scores signify more frequent symptoms and more distress from symptoms. Validity and reliability of the MSAS 10–18 has been assessed by Collins and colleagues (2000). Cronbach’s alpha coefficient of the MSAS 10–18 ranged from 0.83 for psychological symptoms and 0.87 for physical symptoms. Test-retest reliability has been mixed: correlation was significant for 26 of the 30 symptoms (p < 0.005), but 4 symptoms (pain, nervousness, drowsiness and constipation) lacked correlation possibly due to instability of symptoms over time. Convergent and discriminate validity of the MSAS 10–18 has shown significant correlations to the pediatric Memorial Pain Assessment Card and nausea visual analog scales (p < 0.01).
Physiologic markers included BMI calculated from weight and height, prealbumin, and albumin. Weight in kilograms and height in centimeters were obtained from the patient’s medical record on each of the four days that the symptom questionnaire was completed; BMI was calculated as height in meters squared divided by weight in kilograms. Prealbumin levels in milligrams per deciliter were obtained from the patient’s medical record, based on serum blood drawn within two days of symptom assessments at each data collection time point, due to prealbumin’s approximate 2-day half-life (Rzepecki, Barzal, Sarosiek, & Szczylik, 2007). Albumin levels in grams per deciliter were also based on serum blood data from the patient’s medical record, drawn within 7 days following symptom assessments over time, given albumin’s half-life of 20 days (Rzepecki et al, 2007). TPN and appetite stimulant medications may affect the physiological markers due to fluid volume variations and/or oral intake; therefore, use of either of these variables was monitored from the patient’s medical record and recorded as either receiving or not receiving.
Use of the EAT! application was measured in seconds and recorded individually for each patient. To avoid any potential influence on the use of the app, patients were unaware of the monitoring, and the principal investigator (PI) downloaded the information remotely from a secure website.
Procedure
After consent/assent, adolescents were introduced and oriented to the EAT! intervention via a cell phone provided to them for the duration of the study (n=14) or downloaded onto their own smart phones (n=2). All adolescents were recruited to the study prior to hospital discharge, which occurred between 18–28 days post HSCT. Next, patients completed the MSAS 10–18 questionnaire, and the PI documented the patient’s physiologic parameters and TPN/appetite stimulant medication use from their medical record. If pre-discharge prealbumin results were not available within the past 2 days or albumin results were not available within the past 7 days, the PI ordered the testing to be done with the next blood draw (later that day or the following morning). At 20, 40, and 60 days post HSCT hospital discharge, patients again completed the MSAS 10–18 questionnaire during a routine clinic visit, and the PI recorded the patient’s time using the EAT! app and obtained the patient’s physiologic parameters and TPN/appetite stimulant medication use from the patient’s medical record. Upon completion of the study, patients returned the cell phone or discarded the application from their cell phone, and were given a small monetary incentive for their participation in the study.
Statistical Analysis
Descriptive statistics, including measures of central tendency and variability, were used to evaluate symptom reports from the MSAS10–18 and physiologic markers. Friedman repeated measures analyses of variance were used to examine the differences in symptom scores and physiologic markers across time. If differences were noted, pairwise comparison testing was conducted using a Wilcoxon test that controlled for Type I errors, using 5% significance level. Correlation between use of EAT! and symptom prevalence and distress was evaluated with Spearman rank testing while correlation between the use of EAT! and physiological markers was evaluated with Pearson correlation coefficient testing.
Results
As previously reported (Rodgers, Krance, Street, and Hockenberry, 2013), patients completed data at all collection times except for one patient who relapsed prior to completion of the 60-day post-HSCT assessment. The majority of patients were male, Hispanic, had a diagnosis of leukemia, and received chemotherapy and radiation as part of their HSCT conditioning regiment (Table 1). All patients accessed the EAT! app initially, and its use declined significantly over time (Rodgers, Krance, Street, & Hockenberry, 2013).
Table 1.
Sample Characteristics
| Characteristic | Number | Percent |
|---|---|---|
|
| ||
| Age | ||
| 11–12 years | 5 | 31 |
| 13–14 years | 3 | 19 |
| 15–16 years | 5 | 31 |
| 17–18 years | 3 | 19 |
|
| ||
| Gender | ||
| Male | 9 | 56 |
| Female | 7 | 44 |
|
| ||
| Ethnicity | ||
| Hispanic | 9 | 56 |
| Caucasian | 5 | 31 |
| African American | 1 | 6.5 |
| Asian | 1 | 6.5 |
|
| ||
| Diagnosis | ||
| Leukemia | 11 | 69 |
| Lymphoma | 2 | 12.5 |
| Immunological disease | 2 | 12.5 |
| Myelodysplastic syndrome | 1 | 6 |
|
| ||
| HSCT Conditioning Regimen | ||
| Chemotherapy alone | 4 | 25 |
| Chemotherapy and radiation | 12 | 75 |
Symptoms
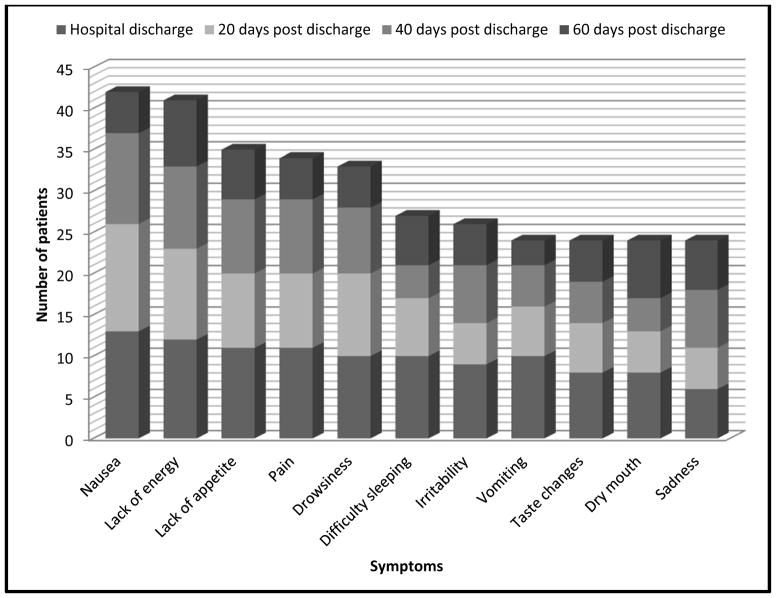
All 30 symptoms in the MSAS were reported by at least one participant at each time point. The total prevalence of all symptoms significantly decreased over time (p=0.006): at hospital discharge (n=217), at 20 days post hospital discharge (n=153), at 40 days post hospital discharge (n=147), at 60 days post hospital discharge (n=121). The most prevalent symptoms (n=11) are illustrated in Figure 1.
Figure 1.
Most Prevalent Symptoms
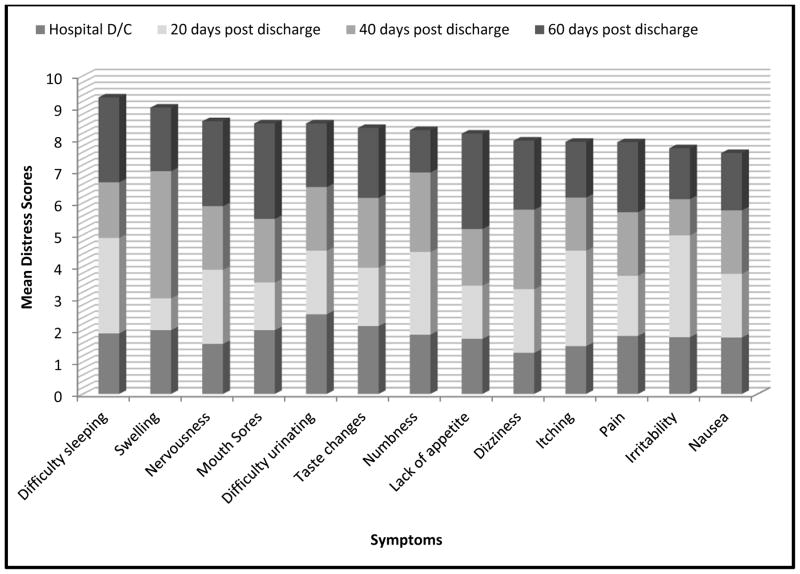
At least one patient reported some degree of distress with all 30 symptoms at all four time points, with one exception: two patients reported no distress with “not looking like myself” at 60 days post hospital discharge. Mean distress scores of the most prevalent 11 symptoms are illustrated in Figure 2. Four of the most prevalent symptoms (difficulty sleeping, taste changes, lack of appetite, and nausea) were among the symptoms reported as most distressing. Symptom distress scores did not show a linear decline over time, and no statistically significant difference was noted over time (p=0.22): overall mean symptom distress scores were lowest at hospital discharge (mean=1.5), increased at 20 days post hospital discharge (mean=1.8), then decreased at 40 days post hospital discharge (mean=1.7) and 60 days post hospital discharge (mean=1.6). Use of the EAT! intervention was not correlated with symptom prevalence or symptom distress at any time during HSCT recovery (Table 2).
Figure 2.
Highest Mean Distress Symptoms
Table 2.
Correlation of EAT! with Symptoms and Physiological Markers
| EAT! Use | |||
|---|---|---|---|
| 20 days post discharge | 40 days post discharge | 60 days post discharge | |
| Symptom Prevalence | rs=.369, p=.159 | rs=−.442, p=.086 | rs=.372, p=.172 |
| Symptom Distress | rs=.289, p=.278 | rs=−.304, p=.253 | rs=.366, p=.180 |
| Weight | r=.331, p=.211 | r=−.199, p=.459 | r=.201, p=.472 |
| Body Mass Index | r=.380, p=.147 | r=.120, p=.659 | r=.196, p=.484 |
Physiologic Markers
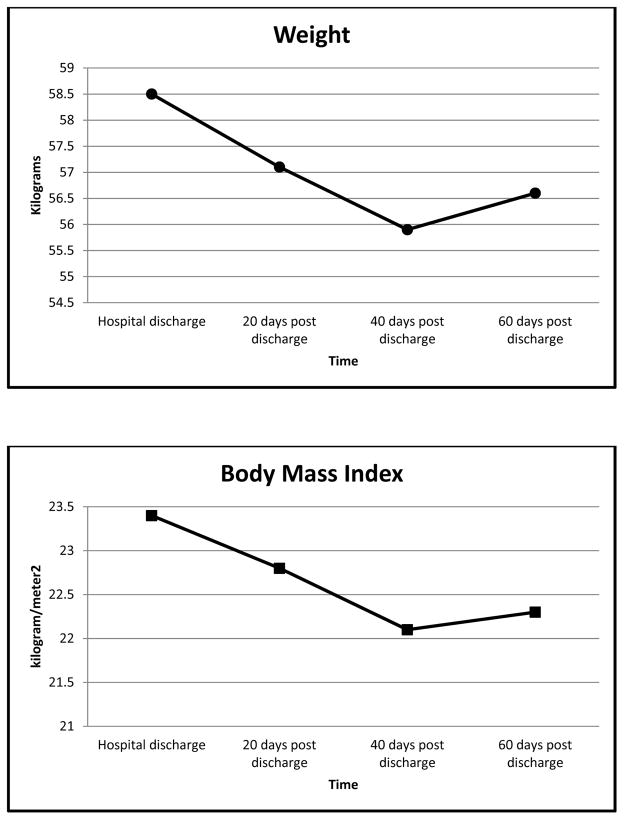
There was a statistically significant decline in the overall mean weight and BMI from the start to the end of the study (p=0.029 and 0.002, respectively), despite the slight increase in weight and BMI noted at the end of the study (Figure 3). Use of the EAT! intervention was not correlated with weight or BMI changes at any time during HSCT recovery (Table 2).
Figure 3.
Mean Weight and Body Mass Index Changes over Time
Mean albumin and prealbumin levels remained in the normal range throughout the course of the study (Table 3). There were no statistically significant changes for either of the serum proteins over time. The majority of patients (n=12) were receiving TPN upon HSCT hospital discharge, four of whom were receiving an additional oral medication to stimulate their appetite. The number of patients receiving TPN decreased over time, but the use of appetite stimulants remained the same (Table 3).
Table 3.
Mean Serum Protein Markers and Frequency of Nutritional Supplements
| Time | Albumin Mean (SD) | Prealbumin Mean (SD) | TPN/Appetite Stimulant Frequency |
|---|---|---|---|
| Hospital discharge | 3.6 (0.6) | 26.4 (6.9) | 12/4 |
| 20 days post hospital discharge | 3.8 (0.5) | 24.6 (10.5) | 5/4 |
| 40 days post hospital discharge | 3.7(0.6) | 27.0 (10.8) | 2/5 |
| 60 days post hospital discharge | 3.8 (0.5) | 25.1 (10.7) | 0/3 |
Discussion
As expected, symptom prevalence was highest at hospital discharge and steadily declined over time, likely corresponding to overall HSCT recovery. This study reported similar symptom prevalence during the first 100 days post HSCT as did adult HSCT studies, including reports of nausea, fatigue, and lack of appetite, despite the mean age in this study being only 14 years. Age may not be a significant factor in symptom prevalence during HSCT recovery but should be evaluated further with various age groups, including school-age, adolescents, and young adults. Furthermore, reports of symptom prevalence in this study overlapped with many of the symptoms reported by adolescents receiving cancer treatment, according to a recent literature review (Erickson et al, 2012). Fatigue, sleep disturbances, nausea/eating problems, pain, mood disturbances, and appearance changes were common symptoms found in 12 studies evaluating symptoms in adolescents during cancer treatment. This comparable symptom prevalence may be due to the fact that many HSCT patients receive similar chemotherapy agents as patients undergoing cancer treatment. Further evaluations should be performed to identify symptom trajectories related to specific chemotherapy medications.
The most frequent symptoms were not always the most distressing symptoms reported in this study. Nausea was the most frequently reported symptom yet ranked the eleventh most distressing score. Difficulty sleeping was the most distressing symptom yet the sixth most frequently reported. Symptom-related distress is not routinely reported in symptom assessment studies but should be evaluated as results may differ from symptom prevalence. A thorough evaluation of symptoms should be performed in order to understand fully the experience of and appropriate support for patients recovering from HSCT.
Patients with high symptom prevalence and distress might be expected to use the EAT! intervention more frequently than patients with lower symptom prevalence and distress; however, this was not supported. All patients used the intervention frequently immediately after HSCT hospital discharge and less frequently over time. This may be due to the fact that adolescents were interested in using a novel intervention to receive information (Rodgers, Krance, Street, & Hockenberry, 2013). Expanded options on this phone application could provide a more effective mechanism for patient education and engagement of adolescents in their healthcare and well-being.
Weight and BMI declined significantly during the study. These changes correspond to the frequency patterns of anorexia and nausea, which likely results in limited oral intake during early HSCT recovery. Adolescents typically need 45 days post HSCT to regain 50% of their appetite (Cunningham et al, 1983). Despite weight loss, mean albumin and prealbumin levels remained essentially the same throughout the study. The stable albumin levels are similar to findings in studies of adults (Jaime-Pérez et al, 2013; Uderzo et al, 1991). This may be due to the use of supplemental nutrition, such as TPN, commonly used during HSCT recovery, which maintains adequate serum protein levels. Seventy-five percent of the patients in this study (n=12) were discharged with TPN. Further studies should evaluate the influence of nutritional supplements on the nutritional well-being of patients during HSCT recovery.
Limitations of the study include a small sample size from a single institution. A larger and more diverse sample would provide more generalizable conclusions concerning symptom trajectories and physiologic markers throughout HSCT recovery. Adolescents’ symptom reports may have been influenced by the receipt of a mobile phone to use during the duration of the study. Although patients were told that their symptom reports had no influence over availability of the phone or application, some adolescents may still have altered their symptom reports.
Nursing Implications
Patients in this study reported a high prevalence of symptoms at hospital discharge, with symptom frequency and distress decreasing but not diminishing by 100 days post HSCT. Awareness of symptom trajectories throughout HSCT recovery illustrates the importance of performing a thorough symptom assessment with patients including separate queries of incidence and distress of symptoms known to occur during HSCT recovery. These assessments can increase awareness of symptoms that might otherwise be overlooked and not addressed.
A thorough understanding of symptom experiences during HSCT recovery allows healthcare providers to educate patients about techniques to assist in relieving frequent and distressing symptoms that may decrease the need for further medical treatment and ultimately improve well-being (Baggott, Dodd, Kennedy, Marina, & Miaskowski, 2009). Patients may be unaware of self-initiated strategies that can be used to minimize symptoms. Sharing strategies such as deep breathing and avoidance of noxious smells to relieve nausea may allow patients to increase their ability to eat that will minimize the need for nutritional supplements. Sharing strategies such as relaxation techniques and avoidance of daytime naps to minimize difficulty sleeping at night may allow patients to be more active during the day that will reduce the potential for further medical complications.
Only through a comprehensive understanding of symptom experiences will caregivers be able to develop robust interventions to educate patients on effective symptom interventions. Use of a mobile phone application should be considered for future interventions, especially with symptom management, as it has the ability to guide a number of individuals through the ease of accessibility. Adolescent patients are especially comfortable using mobile phone technology to obtain information and communicate with others. This valuable tool can be used as a means to educate and empower patients to use effective strategies to minimize symptoms and create a more positive experience during HSCT recovery that will promote well-being.
Acknowledgments
This study was funded by a Dan L. Duncan Cancer Center Grant, P30 CA125123
Contributor Information
Cheryl C. Rodgers, Assistant Professor, Duke University School of Nursing.
Robert Krance, Professor and Director of Pediatric Stem Cell Transplant Program, Baylor College of Medicine.
Richard L. Street, Jr., Assistant Professor, Baylor College of Medicine, Professor and Chair of Department of Communication, Texas A&M.
Marilyn J. Hockenberry, Professor, Duke University School of Nursing.
References
- Baggott C, Dodd M, Kennedy C, Marina N, Miaskowski C. Multiple symptoms in pediatric oncology patients: A systematic review. Journal of Pediatric Oncology Nursing. 2009;26(2):325–339. doi: 10.1177/1043454209340324. [DOI] [PubMed] [Google Scholar]
- Barker CC, Anderson RA, Sauve RS, Butzner JD. GI complications in pediatric patients post-BMT. Bone Marrow Transplantation. 2005;36:51–58. doi: 10.1038/sj.bmt.1705004. [DOI] [PubMed] [Google Scholar]
- Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation. Supportive Care Cancer. 2008;16:1243–1254. doi: 10.1007/s00520-008-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MZ, Rozmus CL, Mendoza TR, Padhye NS, Neumann J, Gning I, Cleeland CS. Symptoms and quality of life in diverse patients undergoing hematopoietic stem cell transplantation. Journal of Pain and Symptom Management. 2012;44(2):168–180. doi: 10.1016/j.jpainsymman.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, Byrnes ME, Dunkel IJ, Lapin J, Nadel T, Thaler T, Portenoy RK. The measurement of symptoms in children with cancer. Journal of Pain and Symptom Management. 2000;19(5):363–377. doi: 10.1016/s0885-3924(00)00127-5. [DOI] [PubMed] [Google Scholar]
- Cunningham B, Lenssen P, Aker S, Gittere K, Cheney C, Hutchison M. Nutritional considerations during marrow transplantation. Nursing Clinics of North America. 1983;18:585–593. [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher E, Humphreys J, Taylor D. Advancing the science of symptoms management. Journal of Advanced Nursing. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Enskar K, Carlsson M, Golsater M, Hamrin E. Symptom distress and life situation in adolescents with cancer. Cancer Nursing. 1997;20(1):23–33. doi: 10.1097/00002820-199702000-00004. [DOI] [PubMed] [Google Scholar]
- Erickson JM, MacPherson CF, Ameringer S, Baggott C, Linder L, Stegenga K. Symptoms and symptom clusters in adolescents receiving cancer treatment: A review of the literature. International Journal of Nursing Studies. 2012 doi: 10.1016/j.ijnurstu.2012.10.011. in press. Epub ahead of print retrieved January 28, 2013 from http://www.journalofnursingstudies.com/article/S0020-7489(12)00360-4. [DOI] [PubMed]
- Grant M, Cooke L, Bhatia S, Forman S. Discharge and unscheduled readmissions of adult patients undergoing hematopoietic stem cell transplantation: Implications for developing nursing interventions. Oncology Nursing Forum. 2005;32(1):E1–E8. doi: 10.1188/05.onf.e1-e8. [DOI] [PubMed] [Google Scholar]
- Hacker E, Ferrans C. Quality of life immediately after peripheral blood stem cell transplantation. Cancer Nursing. 2003;26(4):312–322. doi: 10.1097/00002820-200308000-00010. [DOI] [PubMed] [Google Scholar]
- Iestra JA, Fibbe WE, Zwinderman AH, van Staveren WA, Kromhout D. Body weight recovery, eating difficulties and compliance with dietary advice in the first year after stem cell transplantation: A prospective study. Bone Marrow Transplantation. 2002;29:417–424. doi: 10.1038/sj.bmt.1703375. [DOI] [PubMed] [Google Scholar]
- Jaime-Pérez JC, Colunga-Pedraza PR, Gutiérrez-Gurrola B, Brito-Ramírez AS, Gutiérrez-Aguirre H, Cantú-Rodríguez OG, Gómez-Almaguer D. Obesity is associated with higher overall survival in patients undergoing an outpatient reduced-intensity conditioning hematopoietic stem cell transplant. [Accessed February 27, 2013];Blood Cells, Molecules and Diseases. 2013 doi: 10.1016/j.bcmd.2013.01.010. in press. at http://dx.doi.org/10.1016/j.bcmd.2013.01.010. [DOI] [PubMed]
- Kyle U, Chalandon Y, Miralbell R, Karsegard V, Hans D, Trombetti A, Pichard C. Longitudinal follow-up of body composition in hematopoietic stem cell transplant patients. Bone Marrow Transplantation. 2005;35:1171–1177. doi: 10.1038/sj.bmt.1704996. [DOI] [PubMed] [Google Scholar]
- Larson P. Perception of needs of hospitalized patients undergoing bone marrow transplant. Cancer Practice. 1995;17:1173–179. [PubMed] [Google Scholar]
- Muscaritoli M, Grieco G, Capria S, Iori A, Fanelli F. Nutritional and metabolic support in patients undergoing bone marrow transplantation. American Journal of Clinical Nutrition. 2002;75:183–190. doi: 10.1093/ajcn/75.2.183. [DOI] [PubMed] [Google Scholar]
- National Marrow Donor Program. Transplants by cell source. 2012 Retrieved from: http://marrow.org/Physicians/Unrelated_Search_and_Transplant/Trends_in_Allo_Transplants.aspx.
- Portenoy RK, Thaler HT, Kornblith AB, Lepore JM, Friedlander-Klar H, Kiyasu E, Scher H. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer. 1994;30(9):1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Reid J, McKenna H, Fitzsimons D, McCance T. Fight over food: Patient and family understanding of cancer cachexia. Oncology Nursing Forum. 2009;36(4):439–445. doi: 10.1188/09.ONF.439-445. [DOI] [PubMed] [Google Scholar]
- Rodgers C, Krance R, Street RL, Hockenberry MJ. Feasibility of a symptom management intervention for adolescents recovering from a hematopoietic stem cell transplant. Cancer Nursing. 2013 doi: 10.1097/NCC.0b013e31829629b5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers C, Wills-Alcoser P, Monroe R, McDonald L, Trevino M, Hockenberry M. Growth patterns and gastrointestinal symptoms in pediatric patients after hematopoietic stem cell transplantation. Oncology Nursing Forum. 2008;35(3):443–448. doi: 10.1188/08.ONF.443-448. [DOI] [PubMed] [Google Scholar]
- Rodgers C, Young A, Hockenberry M, Binder B, Symes L. The meaning of adolescents’ eating experiences during bone marrow transplant recovery. Journal of Pediatric Oncology Nursing. 2010;27(2):65–72. doi: 10.1177/1043454209355984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepecki R, Barzal J, Sarosiek T, Szczylik C. Biochemical indices for the assessment of nutritional status during hematopoietic stem cell transplantation. Bone Marrow Transplantation. 2007;40:567–572. doi: 10.1038/sj.bmt.1705767. [DOI] [PubMed] [Google Scholar]
- Uderzo C, Rovelli A, Bonomi M, Fomia L, Pirovano L, Masera G. Total parenteral nutrition and nutritional assessment in leukaemic children undergoing bone marrow transplantation. European Journal of Cancer. 1991;27(6):758–762. doi: 10.1016/0277-5379(91)90183-e. [DOI] [PubMed] [Google Scholar]
- Woodgate R, Degner L. Expectations and beliefs about children’s cancer symptoms: Perspective of children with cancer and their families. Oncology Nursing Forum. 2003;30:479–491. doi: 10.1188/03.ONF.479-491. [DOI] [PubMed] [Google Scholar]