Abstract
Background
Adolescents undergoing a hematopoietic stem cell transplant (HSCT) experience a variety of side effects and eating difficulties. Few interventions exist to assist patients with self-care after HSCT hospitalization. The Eating After Transplant (EAT!) program is a mobile phone application developed to assist adolescents with self-management of common eating related issues during HSCT recovery.
Objective
This study examined the acceptability and usability of the EAT! program among adolescents and assessed the competency of the participants using the program after hospital discharge through the first 100 days post HSCT.
Methods
A repeated measures design was used to evaluate the EAT! application with 16 adolescent patients recovering from an allogeneic HSCT. Participants provided verbal feedback and used a Likert-scale to rate acceptability and usability of the application. Additionally, a tracking device monitored use of the application. Competency was measured with orientation time and independent demonstration of use of the application.
Results
Acceptability remained high throughout the study, but use significantly decreased over time. Patients reported familiarity with the program’s content as the reason for the declining use. Competency was excellent with a short orientation period and independent demonstration throughout the study.
Conclusions
A mobile phone application is a feasible intervention to educate adolescents with symptom management strategies. Future research needs to examine factors affecting sustainability of use over time.
Implications for Practice
Healthcare providers need to continue to develop and evaluate innovative methods to educate adolescents on effective self-care strategies throughout HSCT recovery.
Introduction
Hematopoietic stem cell transplantation (HSCT) is a common form of treatment for a variety of pediatric malignant and nonmalignant diseases. This treatment requires the use of high dose chemotherapy and total body irradiation that cause side effects including nausea, vomiting, anorexia, diarrhea, dry mouth, altered taste, and decreased oral intake, which can persist through the first 100 days post HSCT, known as the acute recovery phase.1,2 Gastrointestinal (GI) symptoms and poor eating can increase the need for medical care and negatively affect adolescents’ development, compliance to treatment, and quality of life.3 While hospitalized, the side effects and nutritional needs are managed by healthcare providers but once discharged, patients and family caregivers assume responsibility of care. Nutritional deficits during the acute recovery phase can be significant and adolescents typically resume eating only 50% of their caloric needs at 45 days post HSCT.4 Effective self-care interventions are not available to adolescent outpatients who are attempting to self-manage their treatment related side effects and resume eating.5 The current study addressed a dearth of self-care interventions for adolescents following discharge after HSCT by testing the feasibility of a mobile phone application that provided adolescents with descriptive information and self-care strategies related to common GI symptoms and eating issues.
Background
Self-assessment and management mobile phone application have been developed for various health conditions. This technology has been effective in supporting patients with asthma,6 diabetes,7 and sickle cell anemia.8 Only two studies have involved adult oncology patients using mobile phones for symptom assessment. In one study, patient responses to a phone-based self-assessment of nausea, vomiting, diarrhea, mucositis, hand-foot syndrome, and temperature were sent remotely to a nurse who reviewed the information and called the patient for any moderate or severe symptoms.9 In a second study, a mobile phone application was designed to evaluate ten symptoms (pain, fatigue, worry, weight loss, cough, difficulty sleeping, shortness of breath, problems urinating, anorexia, difficulty concentrating) among adult oncology patients during a single hospitalization.10 Patients’ responses were sent to a secured website where the healthcare team reviewed the information and determined best management for the hospitalized patient. Of 97 patients approached for that study, 41 (42%) refused participation because of unfamiliarity with a mobile phone; the remaining 56 patients completed the symptom questionnaire without difficulty and spontaneously commented on the ease of use. Age was thought to be a factor in the refusal rate; patients who refused the study had a mean age of 61 years while patients who completed the study had a mean age of 45 years.
Mobile phone applications for oncology patients that assist not only with assessment but also with the management of symptoms are limited.11 One such application is the advanced symptom management system (ASyMS), developed to monitor and manage six symptoms (nausea, vomiting, mucositis, hand-foot syndrome, diarrhea, and fatigue) during treatment for lung, breast, or colorectal cancer among adult patients in the United Kingdom.12 Software determined whether patient-reported symptoms required an immediate call from a healthcare professional or a self-care message tailored to the severity of their symptom. Patients using the ASyMS reported positive experiences with the program and requested it be expanded to other symptoms.12 The ASyMS is now being developed for use among young adults undergoing cancer treatment in the United Kingdom. ASyMS will contain information on five common symptoms (mouth sores, nausea, vomiting, weight loss, and diarrhea) and has potential to promote self-care among the young adults.13
Patients recovering from HSCT express a desire to participate in self-care activities to manage their symptoms but want strategies presented in an organized and practical method.14 The Eating After Transplant (EAT!) intervention is delivered as a mobile phone application with information and self-care strategies regarding seven common GI symptoms and eating issues described in an organized manner. EAT! was created to provide adolescents with information on demand after HSCT hospitalization and is readily available to patients when needed. Use of the EAT! application was intended to increase knowledge regarding symptom experiences and management strategies, which would enable adolescents to make informed decisions about self-care independently, thus assisting with their developmental goal of achieving autonomy.15 Mobile phones have significant potential for supporting health care because they are portable, can deliver self-care resources at the time of need, and are a socially accepted means of communication.16 Smartphones are used for more than talking and texting among adolescents, who use their phones to access the Internet and share videos, music, and information with peers.17 Initial acquisition of a mobile phone typically occurs during the adolescent years with mobile phone ownership occurring in 58% of 12 year olds, 73% of 13 year olds, and 83% of 17 year olds.17
In the absence of research that evaluated the feasibility of a mobile phone intervention among adolescents following HSCT, this pilot study examined the acceptance and use of the EAT! intervention among adolescents during an HSCT acute recovery phase and the competency of the participants using the intervention. Determining feasibility of a clinical intervention is important for ascertaining information leading to a successful and sustainable intervention.18,19
Methodology
Design
A repeated measures design was used to evaluate the feasibility of the EAT! phone application during HSCT recovery. Adolescents completed assessments at three time points following their initial HSCT hospital discharge to evaluate the EAT! application over time. This study was approved by the Institutional Review Board at Baylor College of Medicine.
Setting and Sample
The study was performed at a large pediatric teaching hospital in the southern part of the United States. The hospital is a tertiary pediatric center performing at least 80 autologous and allogeneic HSCTs annually. The HSCT facility consists of a 15 bed inpatient unit with an adjoining outpatient clinic.
Inclusion criteria included patients aged 11–18 years, with any disease requiring an allogeneic HSCT, were able to read and speak English by self-report (Spanish speaking parents were approached using a translator), and were discharged before day 51 from a first time allogeneic HSCT (due to the need for the adolescents to have time for self-directed care during the acute recovery phase). Exclusion criteria included failure to engraft (due to the likelihood of prolonged hospitalization and treatment), receipt of an autologous HSCT or a repeat allogeneic HSCT, or presence of a neurological or developmental delay (to minimize potential confounding factors). A convenience sample of 16 patients was used for this repeated measures study. For pilot studies, a sample size in the range of 10–20 subjects is sufficient for feasibility assessments.19
Intervention
The EAT! phone application was based on work by Rodgers et al.,20 in which adolescents described seven key eating issues during their HSCT acute recovery: appetite recovery, choosing foods, control of eating, nausea and vomiting, taste changes, dry mouth, and returning to normal. The initial screen displayed seven topics with the instructions “Select the topic for more information” (Figure 1). When touched, each topic opened to a touch screen with the selection options of “Descriptions” or “What to do”. Descriptions included quotes from patients about their experiences related to the topic while what to do included effective, non-pharmacological strategies that patients discussed in the Rodgers et al. study.21 Examples of the content are listed in table 1. Patients had the option to press a back button to move to the previous page, or the home button to return to the initial screen of topic listings.
Figure 1.
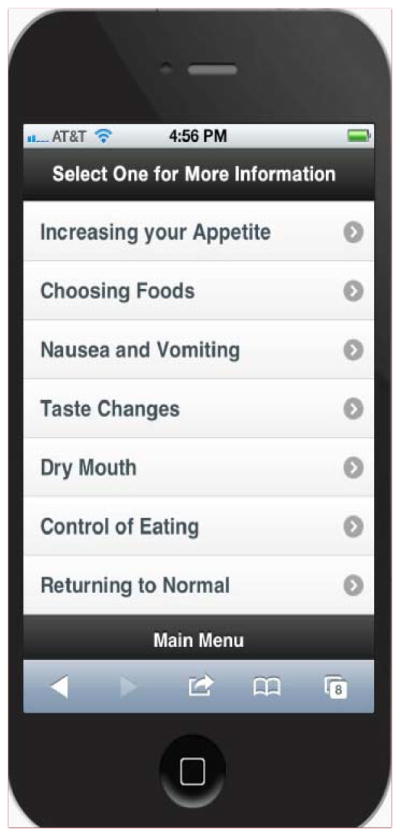
EAT! Sample Screen
Table 1.
EAT! Content Examples
| Topic | Options | Examples |
|---|---|---|
| Increasing your Appetite | Description | Gradually you start eating more and more as each day goes on. After awhile, you wake up and you’re hungry. |
| What to do | My stomach didn’t want too much volume at once; so I’d eat little amounts throughout the day. | |
| Choosing Foods | Description | If I’m not hungry I don’t really want to talk about food. If I can talk about food, then I would know I could eat it. |
| What to do | I made a list of all the foods that I really like and then try them whenever I’m hungry. | |
| Nausea and Vomiting | Description | It’s just easier to eat when I start to get hungry… but if I wait awhile after I get hungry then I start to feel nauseous and I can’t eat. |
| What to do | Relax and concentrate on your breathing by taking slow deep breaths. I take a couple deep breaths and like, lay my head back, and close my eyes and that usually helps. | |
| Taste Changes | Description | Foods need to have flavor. If you eat bland foods, you won’t want to eat anymore because it takes away your appetite. |
| What to do | Use ketchup, barbeque sauce, steak sauce, or anything to add flavor | |
| Dry Mouth | Description | All of a sudden, it’s like my mouth will just get really dry and it feels gross and sticky. |
| What to do | I always drink water before I put food in my mouth; it cleans out my mouth and moistens it. | |
| Control of Eating | Description | My dad gives me too much food, so I eat and eat and makes it looks like I didn’t eat anything but really there’s so much there. |
| What to do | Take only some food on your own plate because if you see a big portion, you’re like, where am I gonna start? | |
| Returning to Normal | Description | Eating is not the easiest road but it’s the best road to eventual health and getting back to your normal life. |
Study Instruments
Feasibility was assessed as acceptability, usability, and competency. Acceptability of the application was measured using six questions rated on a 5-point Likert scale: ease of use, readability, quickness of use, enjoyment, helpfulness, and program recommendation. Usability of the application was measured using three questions: time needed to locate desired information, estimated time of use, and number of topics reviewed within the program over the past 20 days or since the last data collection. Higher scores indicated better acceptability and usability. In addition, adolescents were asked to provide verbal feedback to the researcher about what they liked and what they would change with the application. Usability was also measured by the amount of time recorded in seconds that adolescents spent reading each topic within the program, based on a tracking device in the phone application of which participants were unaware. Competency was measured as both the amount of time in minutes to initially orient the patient to the phone application and by asking the adolescent to locate an intervention for a specific topic within the EAT! application. This verification ensured that, if the program was not used, it was the adolescent’s choice and was not due to a lack of understanding on the use of the program.
Procedure
Eligible participants were identified by the principle investigator (PI) from a list of hospitalized patients that was updated weekly by the HSCT team. Every patient that met inclusion criteria was introduced to the study by the PI prior to hospital discharge. For patients aged 11–17, parent or legal guardian consent and adolescent assent were obtained; for adolescents aged 18 years, consent from the adolescent was obtained.
Initial training to use EAT! was completed by the PI prior to the patient’s hospital discharge and participants were encouraged to use the EAT! program whenever needed after training. If the participant did not have a smart phone, a phone was provided to them for the duration of the study. If the participant had a smart phone, the application was loaded onto the home screen. The time required to orient the patient to the EAT! program was measured and documented by the PI. After orientation to the phone, the participant was asked to locate an intervention within a specific topic to confirm independent use of the program, reinforce information if needed, and answer any further questions. Participants were encouraged to contact the PI if they experienced any problems or questions with the phone or the application. Contact information for the PI was provided verbally and in writing.
At approximately 20 days, 40 days, and 60 days post hospital discharge, the adolescent was approached during a clinic visit or if re-hospitalized, in their hospital room, and asked to locate specific information within the application to confirm retained knowledge on use of the program. Adolescents were also asked to complete the EAT! acceptability and usability scale and to verbally provide suggestions or comments about the program. The PI downloaded the tracking information via a website and recorded the length of time that the participant looked at each topic. At the end of the study, adolescents returned the mobile phone, if applicable, and all participants were given a small monetary incentive for participation.
Statistical Analysis
Descriptive statistics were used to report the acceptability and usability scores. Differences of acceptability and usability scores across the measurement periods were tested using Friedman repeated measure analysis of variance, with follow-up pairwise comparison testing conducted using a Wilcoxon test, controlling for Type I errors across these comparisons at the 5% significance level. Usability measured as tracking time was evaluated with descriptive statistics, including measures of central tendency and variability. Competence was documented using descriptive statistics.
Results
Sixteen adolescents were recruited to the study with no adolescent or legal guardian refusals. One participant relapsed prior to study completion and did not complete the last data collection at 60 days post HSCT hospital discharge. All other participants completed data collection at all time points. Characteristics of the sample are presented in table 2. A majority of the sample was Hispanic, patients with a leukemia diagnosis, and patients receiving a matched or mismatched related HSCT. Fourteen of the participants used the cell phone provided by the PI, and two adolescents used their own smart phone for the study. None of the participants reported problems with the EAT! program during the study. All study phones were returned to the PI upon completion of the study without damage.
Table 2.
Sample Characteristics
| Characteristics | N | Percent |
|---|---|---|
|
| ||
| Gender | ||
| Male | 9 | 56 |
| Female | 7 | 44 |
|
| ||
| Ethnicity | ||
| Caucasian | 5 | 31 |
| Hispanic | 9 | 56 |
| African American | 1 | 6.5 |
| Asian | 1 | 6.5 |
|
| ||
| Age | ||
| 11–14 years | 8 | 50 |
| 15–18 years | 8 | 50 |
|
| ||
| Diagnosis | ||
| Leukemia | 11 | 69 |
| Lymphoma | 2 | 12.5 |
| Myelodysplastic Syndrome | 1 | 6 |
| Immunological Disease | 2 | 12.5 |
|
| ||
| Transplant Source | ||
| Related Matched | 5 | 31 |
| Related Mismatched | 6 | 38 |
| Unrelated Matched | 4 | 25 |
| Unrelated Mismatched | 1 | 6 |
Acceptability
All adolescents reported high acceptability of the EAT! program throughout the study. Of a potential maximum score of 30, participants rated the application’s acceptability an average of 28.5–28.9 throughout the study (Table 3). In addition, participants verbalized positive comments throughout the study. One boy reported, “I knew some of the things in there but I didn’t know that adding lemon would help with my dry mouth, so I tried it, and it helped”. Participants reported that they liked reading information from other patients. One girl reported, “I was afraid to start eating again, but I read that another patient was scared but started eating, so I tried it, and it was okay”. All participants would recommend the program to other patients; however, they wanted the program expanded to include more information on more topics. One boy stated, “Make the app have the capability for us to add tips and ask questions”.
Table 3.
Acceptability and Usability Scores
| Days post HSCT Hospital Discharge | Acceptability Scoresa Mean (SD) Range |
Usability Scoresb Mean (SD) Range |
|---|---|---|
| 20 | 28.5 (1.3) 26–30 |
11.2 (1.5) 9–14 |
| 40 | 28.9 (1.1) 27–30 |
10.1 (1.9) 7–12 |
| 60 | 28.8 (1.1) 27–30 |
10.3 (2.7) 6–15 |
Maximum score of 30
Maximum score of 15
Usability
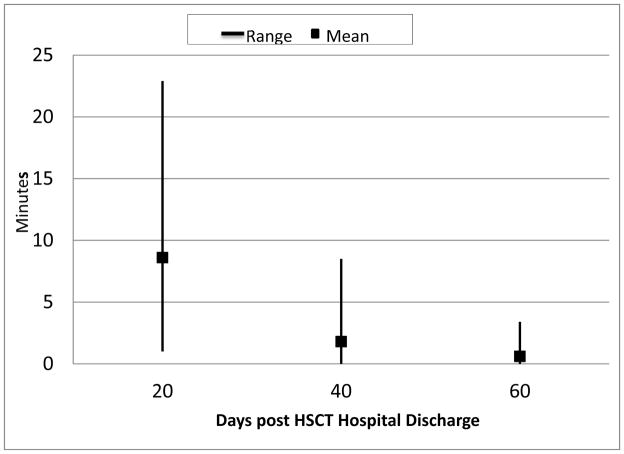
Participants reported moderate usability of the program. Of a potential maximum score of 15, patients reported an average usability score of 10.1–11.2 throughout the study (Table 3). Use of the program significantly decreased over time (p<0.001). On average, participants used the program 8.6 minutes within the first 20 days post HSCT hospital discharge, which decreased to 1.8 minutes of use during the next 20 days, and declined even further to 0.6 minutes during the last 20 days of the study (figure 2). Adolescents reported that the program had no new information added to it, so once they read it they were familiar with all of the content. One boy stated, “It’s good information, just not enough to keep coming back” and another boy stated, “It is a really helpful app, [it] just needs constant updating of information to keep it fresh”.
Figure 2.
EAT! Application Usage
All adolescents liked having the information on a cell phone because it was readily available. One boy said, “I like it on the cell phone because I have it with me whenever I need it”. None of the participants wanted the information on the computer, and one participant stated, “I hardly ever use the computer, only for my homework”.
Competency
All adolescents were able to use the program within 1–5 minutes after orientation (mean 3.3 minutes). In addition, all of the adolescents were able to locate information independently, as requested by the PI, at all points during the study. No participant required re-orientation to the program, and no participant contacted the PI for issues or problems with the program.
Discussion
The EAT! program is a feasible intervention for symptom management education among adolescents recovering from HSCT. Participants were eager to enroll in the study and reported the program was easy to read, quick to use, and helpful. All adolescents viewed the application initially after HSCT hospital discharge but use declined over time. Orientation to the application was quick, less than 5 minutes, and adolescents were able to retain the knowledge to use the program independently without difficulties.
The EAT! application is different from other smartphone application as patients have access to all of the content in EAT! whenever requested, whereas other mobile phone interventions, such as ASyMS, only provide tailored information depending on the prevalence and severity of the participants’ symptoms.14 The unrestricted information in EAT! allows participants to use the application at any time regardless of their current symptom status.
All adolescents reported they would recommend EAT! to other patients; however, participants requested updated and more information within the program. The application could be enhanced by allowing participants to add their own comments into the program during their HSCT recovery. This method of experiential sharing could provide a sense of engagement and peer support for the participant, while providing a continual update of information for other users. Further enhancements of the application could include an exchange of topics as the patient progresses through recovery. Eating issues, which are more common at HSCT hospital discharge, could be replaced later with information on fatigue and sleeping difficulties, which are symptoms more common beyond the acute recovery phase.19
Generalizability of these study findings is limited because data was collected from a single institution with a narrow age range of patients. There may have been the possibility of bias due to participants receiving a mobile phone to use during the study. Fourteen of the adolescents were given a cell phone to use during the study, which may have influenced their attitudes about the program; however, adolescents were informed that their opinion of the program had no effect on accessibility to the phone.
Future studies should evaluate the intervention from different institutions to determine any variation in the use of the program among various HSCT centers. In addition, future studies should include a larger sample size with a wider age range of participants to determine usability of the application with younger patients and acceptability of the application with older patients.
Nursing Implications
Healthcare providers need to develop innovative methods to educate patients on effective symptom management interventions throughout their lengthy HSCT recovery. Educating patients on self-care activities can allow patients to become more active in their care and increase autonomy. Patients who are better informed about side effects of their treatment may initiate more strategies to manage their symptoms and/or eating and may experience increased confidence and independence.23
Children and adolescents use electronic equipment, such as cell phones, on a daily basis and are eager to use new programs to learn and share information.24 Because cell phones are a socially acceptable means of delivering and receiving information, phone applications should be developed to enhance healthcare education and encourage self-care activities. However, formal evaluation of the interventions must be performed to confirm its impact and effectiveness.9 Nurses are ideal candidates to perform these evaluations and customize interventions based on feedback to create effective and innovative interventions for patients.
Acknowledgments
This study was funded by a Dan L. Duncan Cancer Center Grant, P30 CA125123
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Rodgers C, Wills-Alcoser P, Monroe R, McDonald L, Trevino M, Hockenberry M. Growth patterns and gastrointestinal symptoms in pediatric patients after hematopoietic stem cell transplantation. Oncology Nursing Forum. 2008;35(3):443–448. doi: 10.1188/08.ONF.443-448. [DOI] [PubMed] [Google Scholar]
- 2.Barker CC, Anderson RA, Sauve RS, Butzner JD. GI complications in pediatric patients post-BMT. Bone Marrow Transplantation. 2005;36:51–58. doi: 10.1038/sj.bmt.1705004. [DOI] [PubMed] [Google Scholar]
- 3.Enskar K, Carlsson M, Golsater M, Hamrin E. Symptom distress and life situation in adolescents with cancer. Cancer Nursing. 1997;20(1):23–33. doi: 10.1097/00002820-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Reid J, McKenna H, Fitzsimons D, McCance T. Fight over food: Patient and family understanding of cancer cachexia. Oncology Nursing Forum. 2009;36(4):439–445. doi: 10.1188/09.ONF.439-445. [DOI] [PubMed] [Google Scholar]
- 5.Dodd M, Miaskowski C. The PRO-SELF Program: A self-care intervention program for patients receiving cancer treatment. Seminars in Oncology Nursing. 2000;16(4):300–307. doi: 10.1053/sonu.2000.16586. [DOI] [PubMed] [Google Scholar]
- 6.Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Professional and patient attitudes to using mobile phone technology to monitor asthma: Questionnaire survey. Primary Care Resp Journal. 2006;15:237–245. doi: 10.1016/j.pcrj.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll AE, Marrero DG, Downs SM. The HealthPia GlucoPack diabetes phone: A usability study. Diabetes Technology & Therapeutics. 2007;9(2):158–164. doi: 10.1089/dia.2006.0002. [DOI] [PubMed] [Google Scholar]
- 8.Stinson J. Improving the assessment of pediatric chronic pain: Harnessing the potential of electronic diaries. Pain Research Management. 2009;14(1):59–64. doi: 10.1155/2009/915302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver A, Young AM, Rowntree J, Townsend N, Pearson S, Smith J, Gibson O, Cobern W, Larsen M, Tarassenko L. Application of mobile phone technology for managing chemotherapy-associated side-effects. Annals of Oncology. 2007;18(11):1887–1892. doi: 10.1093/annonc/mdm354. [DOI] [PubMed] [Google Scholar]
- 10.Bielli E, Carminati F, La Capra S, Lina M, Brunelli C, Tamburini M. A wireless health outcomes monitoring system (WHOMS): development and field testing with cancer patients using mobile phones. BMC Medical informatics and Decision Making. 2004;4:7. doi: 10.1186/1472-6947-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClellan CB, Schatz JC, Puffer E, Sanchez CE, Stancil MT, Roberts CW. Use of handheld wireless technology for a home-based sickle cell pain management protocol. Journal of Pediatric Psychology. 2009;34(5):564–573. doi: 10.1093/jpepsy/jsn121. [DOI] [PubMed] [Google Scholar]
- 12.McCann L, Maguire R, Miller M, Kearney N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. European Journal of Cancer Care. 2009;18:156–164. doi: 10.1111/j.1365-2354.2008.00938.x. [DOI] [PubMed] [Google Scholar]
- 13.Gibson F, Aldiss S, Taylor R, Maguire R, Kearney N. Involving health professionals in the development of an advanced symptom management system for young people. European Journal of Oncology Nursing. 2009;13:187–192. doi: 10.1016/j.ejon.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Larson P. Perception of needs of hospitalized patients undergoing bone marrow transplant. Cancer Practice. 1995;17:1173–1179. [PubMed] [Google Scholar]
- 15.Saewyc EM. Health promotion of the adolescent and family. In: Hockenberry MJ, Wilson D, editors. Wong’s Nursing Care of Infants and Children. St. Louis, MO: Elsevier; Mosby: 2011. pp. 738–804. [Google Scholar]
- 16.Blake H. Mobile phone technology in chronic disease management. Nursing Standard. 2008;23(12):43–46. doi: 10.7748/ns2008.11.23.12.43.c6728. [DOI] [PubMed] [Google Scholar]
- 17.Lenhart A, Ling R, Campbell S, Purcell K. Teens and mobile phones. [Accessed February 10, 2013];Pew Internet Website. http://pewinternet.org/Reports/2010/Teens-and-Mobile-Phones.aspx.
- 18.Bruckenthal P, Broderick J. Assessing treatment fidelity in pilot studies assist in designing clinical trials. Advances in Nursing Science. 2007;30(1):E72–E84. doi: 10.1097/00012272-200701000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Breitenstein S, Gross D, Garvey C, Hill C, Fogg L, Resnick B. Implementation fidelity in community-based interventions. Research in Nursing & Health. 2010;33:164–173. doi: 10.1002/nur.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertzog M. Considerations in determining sample size for pilot studies. Research in Nursing & Health. 2008;31:180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 21.Rodgers C, Young A, Hockenberry M, Binder B, Symes L. The meaning of adolescents’ eating experiences during bone marrow transplant recovery. Journal of Pediatric Oncology Nursing. 2010;27(2):65–72. doi: 10.1177/1043454209355984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT) Supportive Care Cancer. 2008;16(11):1243–1254. doi: 10.1007/s00520-008-0420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCorkle R, Ercolano E, Lazenby M, Schulman-Green D, Schilling L, Lorig K, Wagner E. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA: A Cancer Journal for Clinicians. 2011;61(1):50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy C, Charlesworth A, Chen J. Interactive data collection: Benefits of integrating New Media into Pediatric Research. Computers, Informatics, Nursing. 2003;21(3):120–127. doi: 10.1097/00024665-200305000-00007. [DOI] [PubMed] [Google Scholar]