Indoor tanning exposes users to intense UV radiation, which is a known carcinogen.1 However, little is known about the more immediate adverse outcomes of indoor tanning. To our knowledge, this study provides the first national estimates of indoor tanning–related injuries treated in US hospital emergency departments (EDs).
Methods
Data
Nonfatal indoor tanning–related injury data from 2003 to 2012 were obtained from the National Electronic Injury Surveillance System–All Injury Program (NEISS-AIP), a nationally representative sample of 66 NEISS hospital EDs, on approximately 500 000 nonfatal injury-related ED visits annually.2 Trained coders review ED medical records to extract data, including age, sex, diagnosis, body region affected, consumer products involved, disposition at discharge, location where injury occurred, and a case narrative describing the cause of injury. Deidentified nonfatal injury surveillance data for this study were obtained by the Centers for Disease Control and Prevention through an interagency agreement with the US Consumer Product Safety Commission, which operates the NEISS-AIP. Use of these deidentified NEISS data did not require Centers for Disease Control and Prevention institutional review board approval.
Case Definition
Cases were initially selected if they were classified as unintentional injuries, involved the use of an indoor tanning device, and the narrative contained one of the following keywords: indoor tanning, tanning, tanning salon, tanning booth, tanning bed, sun lamp, ultraviolet, or UV. Cases were reviewed and classified by 3 study researchers (G.P.G., M.W., and J.L.A.) to confirm they met the case definition; classification differences were resolved by consensus. Injuries were classified into 5 types: skin burns, eye injuries, lacerations and muscle and bone injuries, syncope, and other injuries (Table).
Table.
National Estimates of Indoor Tanning–Related Injuries Treated in Hospital Emergency Departments, United States, 2003-2012
| Characteristic | Sample Cases, No. |
Average Annual No. of Injuries (95% CI)a |
Average Annual % of Injuries (95% CI)a |
|---|---|---|---|
| Total | 405 | 3234 (2344-4123) | 100 |
|
| |||
| Sex | |||
|
| |||
| Male | 77 | 574 (387-762) | 17.8 (12.0-23.6) |
|
| |||
| Female | 328 | 2659 (1866-3452) | 82.2 (57.7-106.8) |
|
| |||
| Race/ethnicity | |||
|
| |||
| Non-Hispanic white | 313 | 2517 (1649-3386) | 77.8 (51.0-104.7) |
|
| |||
| Other/unknown | 92 | 716 (360-1073) | 22.2 (11.1-33.2) |
|
| |||
| Age, y | |||
|
| |||
| <18 | 54 | 412b | 12.7b |
|
| |||
| 18-24 | 145 | 1150 (752-1547) | 35.5 (23.3-47.8) |
|
| |||
| 25-34 | 103 | 870 (598-1143) | 26.9 (18.5-35.3) |
|
| |||
| 35-44 | 69 | 579 (262-895) | 17.9 (8.1-27.7) |
|
| |||
| ≥45 | 34 | 223 (121-326) | 6.9 (3.7-10.1) |
|
| |||
| Location where injury occurred | |||
|
| |||
| Home | 23 | 168 (95-241) | 5.2 (3.0-7.4) |
|
| |||
| Public property/place | 248 | 2084 (1628-2540) | 64.4 (50.3-78.5) |
|
| |||
| Unknown | 134 | 982 (461-1503) | 30.4 (14.2-46.5) |
|
| |||
| Type of injuryc | |||
|
| |||
| Skin burn | 319 | 2572 (1690-3455) | 79.5 (52.3-106.8) |
|
| |||
| Eyed | 22 | 187 (94-281) | 5.8 (2.9-8.7) |
|
| |||
| Laceration/muscle/bonee | 28 | 180 (88-272) | 5.6 (2.7-8.4) |
|
| |||
| Syncopef | 37 | 308 (203-412) | 9.5 (6.3-12.8) |
|
| |||
| Otherg | 14 | 111b | 3.4b |
|
| |||
| Visit disposition | |||
|
| |||
| Treated and released | 389 | 3107 (2233-3980) | 96.1 (69.1-123.1) |
|
| |||
| Otherh | 16 | 126b | 3.9b |
Numbers may not sum to totals and percentages may not sum to 100 due to rounding.
Estimate may be unstable because the number of sample cases is fewer than 20 or the coefficient of variation is greater than 30%.
Percentages do not total 100 because categories are not mutually exclusive.
Includes eye burns, keratosis, and foreign bodies in the eye.
Includes lacerations, cuts, strains, sprains, spasms, contusions, fractures, and dislocations.
Includes syncope, fainting, dizziness, falls, and passing out.
Includes allergy, rash, conjunctivitis, urticaria, nausea, vomiting, and other.
Includes transferred, hospitalized, observation, and left without being seen or against medical advice.
Statistical Analysis
Researchers identified 405 nonfatal indoor tanning–related cases from the NEISS-AIP. Sample weights were applied to provide annualized national estimates of indoor tanning–related injuries. Trends in indoor tanning–related injuries from 2003 to 2012 were examined with negative binomial regression. Data were analyzed using SAS, version 9.3 (SAS Institute, Inc), and Joinpoint, version 4.1.0 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute; http://surveillance.cancer.gov/joinpoint/), software.
Results
On average, an estimated 3234 indoor tanning– related injuries were treated each year in US hospital EDs from 2003 to 2012 (Table). Most injuries occurred among females (82.2%), non-Hispanic whites (77.8%), persons aged 18 to 24 years (35.5%), and in public settings (such as tanning salons) (64.4%). Most injuries were skin burns (79.5%), followed by syncope (9.5%) and eye injuries (5.8%). Indoor tanning– related injuries have decreased significantly from 6487 in 2003 to 1957 in 2012 (P < .001) (Figure).
Figure.
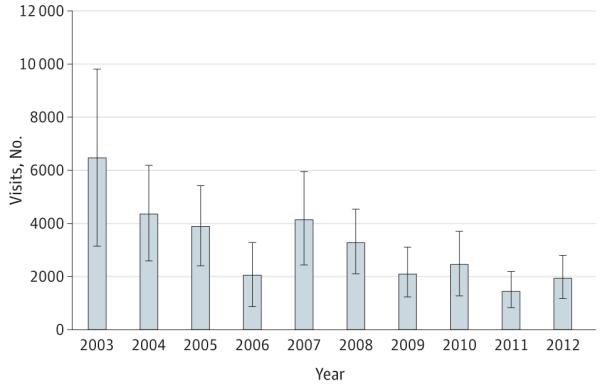
National Estimates of Indoor Tanning–Related Injuries Treated in Hospital Emergency Departments, United States, 2003-2012
The number of indoor tanning–related injuries decreased significantly from 2003 to 2012 (P < .001). Bars represent 95% CIs.
Discussion
Indoor tanning is associated with a substantial number of injuries treated in US hospital EDs. The majority of injuries were skin burns, and injuries occurred at the highest rates among younger adults and non-Hispanic white females, the population with the highest rates of indoor tanning.3 From 2003 to 2012, indoor tanning–related injuries treated in hospital EDs declined, likely due to reductions in indoor tanning.4
Most patients were treated in the ED and released, not requiring hospitalization. However, burns severe enough to warrant an ED visit clearly indicate overexposure to UV radiation and increase skin cancer risk.
Serious injuries occur despite US Food and Drug Administration standards and guidelines on indoor tanning devices.5 Although the Food and Drug Administration requires manufacturers of tanning devices to install timers to limit exposure,5 several case narratives in our study described patients falling asleep while tanning, raising concerns about timers either malfunctioning or being intentionally overridden. A study of tanning salons in North Carolina found that only 5% complied with Food and Drug Administration–recommended exposure schedules.6 The Food and Drug Administration reclassified indoor tanning devices in 2014, requiring new standards and labeling.5
Limitations of this study include not being able to capture injuries left untreated or treated in other settings. In addition, NEISS-AIP case narratives may not provide enough details to characterize injury circumstances. Lastly, location of injury was unknown for 30.4% of cases, and small sample sizes resulted in some unstable estimates. Despite these limitations, this study provides the first nationally representative estimates of indoor tanning–related injuries, allowing for continued monitoring of such injuries. Compliance with current federal and state regulations could be monitored to identify opportunities to decrease harm from indoor tanning. A decrease in indoor tanning could reduce associated injuries and future cases of skin cancer.
Footnotes
Author Contributions: Dr Guy and Mr Haileyesus had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Guy, Watson, Annest. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Guy, Watson, Annest.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Guy, Haileyesus, Annest.
Administrative, technical, or material support: Guy, Watson, Annest.
Conflict of Interest Disclosures: None reported.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Additional Contributions: Tom Schroeder, MS, and other staff of the Division of Hazard and Injury Data Systems, US Consumer Product Safety Commission, Bethesda, Maryland, collected the nonfatal injury data. They were not compensated for their contributions.
References
- 1.US Department of Health and Human Services . The Surgeon General’s Call to Action to Prevent Skin Cancer. US Dept of Health and Human Services, Office of the Surgeon General; Washington, DC: 2014. [Google Scholar]
- 2.Schroeder T, Ault K. The NEISS Sample (Design and Implementation) 1997 to Present. US Consumer Product Safety Commission; Washington, DC: 2001. pp. 11–12. http://www.cpsc.gov/en/media/documents/research--statistics/neiss-injury-data/neiss-sample-design-1997-present/. Accessed May 13, 2014. [Google Scholar]
- 3.Guy GP, Jr, Berkowitz Z, Watson M, Holman DM, Richardson LC. Indoor tanning among young non-Hispanic white females. JAMA Intern Med. 2013;173(20):1920–1922. doi: 10.1001/jamainternmed.2013.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy GP, Jr, Berkowitz Z, Everett Jones S, Holman DM, Garnett E, Watson M. Trends in indoor tanning among US high school students, 2009-2013. JAMA Dermatol. doi: 10.1001/jamadermatol.2014.4677. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration CFR—Code of Federal Regulations Title 21. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=1040.20. Accessed May 30, 2014.
- 6.Hornung RL, Magee KH, Lee WJ, Hansen LA, Hsieh YC. Tanning facility use: are we exceeding Food and Drug Administration limits? J Am Acad Dermatol. 2003;49(4):655–661. doi: 10.1067/s0190-9622(03)01586-x. [DOI] [PubMed] [Google Scholar]


