Abstract
While differences in the rate of virus fusion and budding from the host cell membrane have been correlated with pathogenicity, no systematic study of the contribution of differences in viral envelope composition has previously been attempted. Using rigorous virus purification, marked differences between virions and host were observed. Over 125 phospholipid species have been quantitated for three strains of influenza (HKx31- H3N2, PR8- H1N1, and VN1203- H5N1) grown in eggs. The glycerophospholipid composition of purified virions differs from that of the host or that of typical mammalian cells. Phosphatidylcholine is the major component in most mammalian cell membranes, while in purified virions phosphatidylethanolamine dominates. Due to its effects on membrane curvature, it is likely that the variations in its content are important to viral processing during infection. This integrated method of virion isolation with systematic analysis of glycerophospholipids provides a tool for the assessment of species specific biomarkers of viral pathogenicity.
Keywords: lipidomics, virion, influenza, phospholipids, plasmalogens, membrane lipid composition, infectious disease
Viruses hijack the enzymatic machinery of host cells in order to generate the necessary building blocks for new virus replication. While the glycerophospholipids (GPL) in mammalian cells are highly organized and their composition tightly regulated, pathogenic organisms and viruses can tolerate large variations in the GPL composition of their envelopes.1 Previous work from our group has shown that GPL composition of the infected systems may be only subtly modified from that of non-infected cells and the purified virion lipid composition is drastically different from that of either.2 Influenza viruses of different pathogenicity have been associated with high mortality and development of severe complications.3 Influenza A virus is an enveloped, negative-sense, single-stranded RNA virus which can acquire mutations and some can be resistant to the currently available antiviral drugs.4 Viral envelopes possess relatively strong positive curvature owing to the inert extracellular lipid bilayers of their membrane. Variations in lipid composition of the viral envelope can be correlated to virus infectivity as they modulate viral fusion.5 Lipids such as free fatty acids or lysophosphatidic acid (inverted cone lipids) are known to promote positive curvature by introducing inverted cone shape into the membrane, while cone-shaped lipids like phosphatidylethanolamine, lysolipids or phosphatidic acid tend to adopt negative curvature required for fusion.6,7
As a basis for evaluating differences in the lipid composition that may affect viral pathogenicity, three influenza viruses were studied here, PR8/H1N18 – a highly pathogenic strain in laboratory mice, which for the past 30 years has been used to produce inactivated influenza vaccine, X31/H3N29,10- a low pathogenicity strain, and a modified VN1203/H5N111- lethal to domestic chickens and highly pathogenic in ferret and mouse models. All viruses in this study were grown in eggs. The three viruses used were all generated by reverse genetics on the PR8 backbone in order to compare differences in virion composition associated with differences in the surface hemagglutinin (HA) and neuraminidase (NA) proteins. The choice for a growing medium was based on this well characterized system used by CDC for vaccine development and production. Over 125 phospholipid species have been quantitated in each strain and in the host – non-infected egg allantoic fluid. The quantitative analysis of low abundance lipid species led to the identification of subtle differences between strains, which may lead to a potential correlation between phospholipid composition and influenza severity.
Alteration of the cellular membrane plays an important role in viral replication and infectivity.12 The viral lipid membrane is somewhat similar to that of the host cells as they rely on the host for membrane structural fragments but it also involves de novo lipid synthesis.13 Evaluation of the structural lipid composition of the viral envelope is essential when comparing strains of different lethality or morbidity. One of the major prerequisites for an accurate determination of the viral lipid composition is the virion purification and normalization between samples. This allows assurance that the amount of lipid recovered from an individual preparation reflects similar infection/growth trajectories and degree of purification as compared to another replicate experiment. The procedure utilized in this study reliably produced pure virion particles suitable for further analysis (Fig. 1). Interest in lipid composition of viral envelope and its relation/comparison to that of its host dates several decades back and provides an excellent foundation for this study.14-16 The advancement in lipidomics technology took this exploration further and allowed a more detailed interrogation of that system at the molecular species level. Profiling of the purified virions and non-infected allantoic fluid (NAF) allowed a comprehensive characterization and comparison of the major phospholipid classes’ composition at a molecular species level between all preparations. Absolute quantitation of over 125 molecular species in each strain and host was achieved by the use of LC-MS analysis and computational platforms developed in our laboratory.17 In all more than 200 molecular species (including isobaric species) were identified in the seven major classes – phosphatidic acid (PA), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI), phosphatidylserine (PS) and sphingomyelin (SM) and their respective lysolipids in each strain and NAF (Supplementary Tables S1-S4.). This is an illustration of the large number of lipid species present in virions. While some of the classes are remarkably similar in number of species present and their composition between strains (e.g. PG, PA, SM), others show a marked difference in diversity and quantity (PC, PE, PS).
Figure 1.
Representative electron micrograph (EM) of purified PR8 virus, grown in egg.
The glycerophospholipid makeup of the purified virions is different from that of the typical mammalian cells.2 Phosphatidylcholine (PC) is the major structural component of the mammalian cell membrane comprising about 50% of the total lipids and second largest fraction belonging to phosphatidylethanolamine (PE). In purified virions PE is the major class of the phospholipids. The ratio of PE: PC in NAF is close to 1 while in purified virions it is between 5 and 9 (Fig. 2a). In addition to the large differences between the lipidome of the virions as compared to the NAF, there were also differences in the lipid composition between strains. The diversity in the GPL composition between the three strains and NAF are presented in Fig. 2a and Fig. 2b for major diacyl phospholipid classes and SM and in Table 1a, b.
Figure 2.
Glycerophospholipid profiles of egg-grown influenza virus differ from each other and that of non-infected allantoic fluid (NAF) (a). Representation of molecular species within each lipid class (b).
Table 1.
a Comparison of major GPL classes distribution between three influenza strains and host (NAF)
b Lysolipids in each influenza strain and NAF.
| HKx31 (n=8) |
PR8 (n=8) |
VN1203 (n=8) |
ANOVA p-value |
NAF (n=9) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| headgroup | mean | sem | mean | sem | mean | sem | across virions | mean | sem |
| %PA | 2.5% | 0.3% | 2.0% | 0.3% | 1.4% | 0.1% | 0.014 | 7.8% | 0.5% |
| %PC | 9.0% | 0.7% | 7.5% | 0.9% | 6.3% | 0.5% | 0.042 | 27.2% | 2.19% |
| %PE | 48.3% | 2.1% | 47.9% | 2.4% | 54.5% | 1.0% | 0.042 | 31.7% | 1.0% |
| %PG | 0.3% | 0.0% | 0.2% | 0.0% | 0.1% | 0.0% | 0.002 | 1.0% | 0.1% |
| %PI | 0.6% | 0.1% | 0.3% | 0.1% | 0.2% | 0.0% | 0.007 | 2.0% | 0.2% |
| %PS | 24.4% | 0.8% | 24.5% | 0.4% | 24.7% | 0.4% | 0.929 | 17.2% | 0.9% |
| %SM | 14.9% | 1.4% | 17.6% | 1.2% | 12.6% | 1.0% | 0.027 | 13.0% | 0.6% |
| %PEp of tot GPL | 19.5% | 1.0% | 18.9% | 0.7% | 22.4% | 1.0% | 0.031 | 17.2% | 1.0% |
| PE/PC ratio | 5.3 | 0.6 | 7.0 | 1.2 | 9.0 | 0.8 | 0.035 | 1.2 | 0.1 |
| HKx31 (n=8) |
PR8 (n=8) |
VN1203 (n=8) |
ANOVA p-value |
NAF (n=9) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| lysolipid class | mean | sem | mean | sem | mean | sem | across virions | mean | sem |
| %LPA | 0.03% | 0.01% | 0.12% | 0.05% | 0.03% | 0.02% | 0.042 | 3.8% | 0.4% |
| %LPC | 0.87% | 0.12% | 0.80% | 0.10% | 1.08% | 0.07% | 0.140 | ||
| %LPE | 7.5% | 0.6% | 8.7% | 1.3% | 10.3% | 1.0% | 0.162 | ||
| %LPG | 0.02% | 0.00% | 0.02% | 0.00% | 0.03% | 0.01% | 0.553 | ||
| %LPI | 0.04% | 0.01% | 0.01% | 0.01% | 0.22% | 0.03% | 0.190 | ||
| %LPS | 1.0% | 0.2% | 1.0% | 0.1% | 0.9% | 0.1% | 0.321 | ||
| %lyso of tot GPL | 9.5% | 0.8% | 10.5% | 1.3% | 12.3% | 1.0% | 0.172 | 3.8% | 0.4% |
As shown in Table 1a, NAF exhibits a GPL profile similar to most mammalian systems and corresponding well with published data.18 At the same time virions grown in egg reveal much lower amounts of the structural phospholipid PC and the anionic phospholipids PA, PG and PI, while PE and PS are present to a higher extent. Considering the role of the spatial phospholipid presentation and charge, and their role in membrane dynamics, this class distribution clearly demonstrates that virions require a membrane configuration, favorable for fusion which is dependent on lipid composition.19 Influenza viruses also contain higher sphingomyelin content than their host cells, and this phenomenon is associated with the ability of the virus to infect cells.20 It is very likely that this rise is an illustration of the expand in lipid rafts in the virion lipid membrane compared to that of NAF. Influenza viruses have been shown to use raft platforms for budding.21,22 Thus enrichment in SM provides lipid raft organization, which not only influences membrane fluidity but also facilitates virus budding.23 The three viral strains studied here also differ in their GPL composition. HKx31 displays most structural lipids (cylindrical steric presentation) (PC, PI, PG) compared to other strains, while VN1203 has the least. At the same time PE content of VN1203 is the largest and this strain also shows the highest PE: PC ratio of 9. Cone shaped lipids like PE with suitable molecular geometry facilitate appropriate membrane fluidity and dynamics necessary for virus infection.7,24 Modification in the PE content can probably be exploited as a means to decrease its content and thus render viruses unable to acquire the necessary membrane conformation by affecting the de novo PE biosynthesis via ethanolamine kinase inhibition.25 Another phospholipid class which just like PE is predominantly found in the inner membrane leaflet, PS, appears to enhance virus entry through interaction with annexin proteins26,27 and has also been implicated in augmented infectivity and viral transmission of enteroviruses.28 And all three strains show similar PS content, much higher than in the NAF host. Apart from lysophosphatidic acid (LPA), lysolipids were essentially not detected in the NAF, but there was considerably more LPA detected in the NAF than the virions (Table 1b). Additionally, the molecular fraction of this class of lipid species was significantly elevated in PR8 relative to the other two virus strains (p=0.04). Lysophosphatidylethanolamine (LPE) was the largest lysolipid class represented in the virions. The VN1203 strain had the highest molecular fraction (10.3%) of this class. A difference in the overall lysolipids as a proportion of the total GPL pool was also observed, with VN1203 displaying the highest at 12.3%, followed by PR8 with 10.5% and HKx31 with 9.5%, while there was only 3.8% of lysolipid quantitated in NAF (Table 1b). Incorporation of lysolipids in the membrane leads to increased permeability29 which can be beneficial for cytosolic constituents exchange during viral replication and assembly. The differences in lysolipid distribution lead to different membrane profiles and can probably be implicated in different viral infectivity.7,29
The fatty acid composition of each class of glycerophospholipids was examined. HKx31 was the most different from the other two virions, with noticeably more representation by polyunsaturated (PUFA) PS and PI species and less PUFA PG (Fig. 3a, b). The NAF contains very little PUFA outside of PC, PI, and PS. NAF displayed a different profile of fatty acid unsaturation across classes compared to the virions, particularly for PA and PG, where no PUFA species were above the limit of quantitation in NAF. Similar relationships exist when the data is viewed by the percentage of long chain (>36 carbons) in each class (Supplementary Fig. S1). Comparison of the fatty acid (FA) composition between the viral strains and that of the NAF demonstrated some differences in the saturated fatty acids-containing phospholipids. As an illustration, extracts of VN 1203 and PR8 virions had higher fractions of saturated FA-containing LPE, PE and LPC compared to HKx31 and NAF (Table 2 and Supplementary Table S5). The elevated presence of phospholipids containing the “initiators” of the lipid biosynthesis (palmitic, C16:0 and stearic acids, C18:0) is an indication of lipogenesis. Similar upregulation of de novo lipid biosynthesis has been previously reported during DENV infection.30
Figure 3.
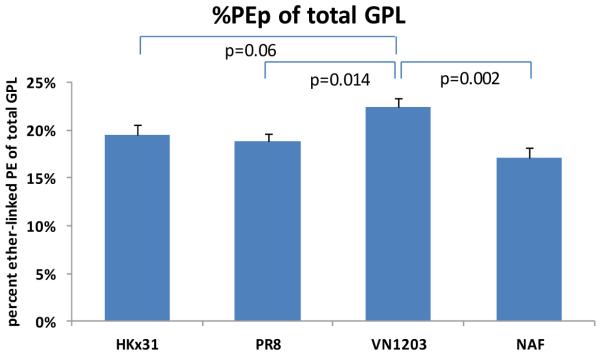
Fatty acid distribution between phospholipid classes in purified virions and NAF. Representation of fractions of polyunsaturated (red) versus saturated, monounsaturated and diunsaturated FA-containing glycerophospholipids (blue) across virions ( vertical) and glycerophospholipids classes (horizontal (a). PUFA content by class in virions and non-infected allantoic fluid (NAF) (b). Long chain fatty acids ( >36 carbon atoms) content by class in virions and NAF (c). Plasmalogen ethanolamines as a fraction of total GPL for all viral strains and host (NAF) (d).
Table 2.
Saturated FA-containing glycerophospholipids distribution comparison between influenza strains and host (NAF)(mol.%).
| PA | LPA | PS | LPS | PG | LPG | PI | LPI | PC | LPC | PE | LPE | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAF | 7 | 5.8 | ||||||||||
| VN1203 | 4.6 | 39.5 | 1.9 | 44.2 | 0 | 63.6 | 44.4 | 7.94 | 96.4 | 8.4 | 72.4 | |
| PR8 | 2.8 | 64 | 14.1 | 42 | 3.1 | 50 | 50 | 8.6 | 93 | 7.7 | 66.5 | |
| HKx31 | 4 | 100 | 0.9 | 37.4 | 1.7 | 60 | 55.6 | 11 | 91.7 | 5.1 | 64.6 |
Viral budding and entry depend on the disruption of the original membrane bilayers and generation of a spontaneous membrane curvature, which would allow membrane rupture and complete fission or fusion actions. Accumulation of curvature-forming lipids (cone or inverted cone) and decrease of cylindrical lipids would result in a highly curved membrane.24 Viral envelopes of HCMV2 or influenza virus18 showed augmented PE content. Recent lipidomics analysis of HCMV and HIV demonstrated strong enrichment of the virion in plasmalogen ethanolamines (PEp)2,31 a highly fusogenic lipid32, as a fraction of total GPL. Plasmalogen species have different physical properties than their diacyl counterparts and are well known to affect the membrane fluidity.32 Several studies suggest viruses bud from lipid raft formations, requiring viral lipid membrane modification.21,23,33 Increased SM content in purified virions compared to that of NAF is consistent with a rise in lipid raft domains, and their utilization for viral replication, membrane fusion, intracellular transport, etc. Viruses benefit from sphingolipid metabolism and alter cellular signaling, as with the influenza A virus, which activates sphingosine kinase 1 and the transcription factor NF-κB.22
METHODS
Materials
All glycerophospholipids used as standards in the MS analysis were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). HPLC grade solvents were supplied by VWR (West Chester, PA) and used without further purification.
Virion Cultivation and Purification
The H1N1 virus studied was a reverse genetics-generated A/Puerto Rico/8/1934 (PR8). The H3N2 and H5N1 viruses were recombinant strains made on the PR8 backbone: A/Aichi/2/1968xPR8 (HKx31) and A/Vietnam/1203/2004 (VN1203). The VN1203 strain has a modification in the HA protein to remove the polybasic cleavage site, allowing it to be used under BL2 conditions.34,35
Viruses were grown in 10-day old embryonated chicken eggs. Stock strains generated by reverse genetics were diluted to 105.5 egg infectious dose50 and injected onto the allantoic membrane. Each individual preparation of virus was grown and purified separately.
After 48 hours of incubation, eggs were chilled for 24 hours at 4°C and allantoic fluid collected. The subsequent fluid was cleared by centrifugation at 500×g. Next, the material was concentrated by pumping through a Pellicon 2 ultrafiltration cassette (P2B100v01, Millipore). The concentrate was run across a discrete 25-70% sucrose gradient by ultracentrifugation at 27,000 rpm for one hour. The virus collects in a band at the interface and is removed by pipetting. A second continuous 25-70% sucrose gradient was run at the same speed. The harvested virus band was diluted in a sterile Tris-EDTA buffer (STE) and pelleted in the same buffer at 10,000 rpm for one hour.
Characterization of virion purity
Ten percent of the viral prep was removed at the final spin stage after resuspension in STE. Plaque assays were performed on the resuspended virus. Viruses were added for one hour to confluent monolayers of Madin-Darby Canine Kidney (MDCK) cells in 10-fold dilutions (10-5 to 10-10). The fluid was then discarded and replaced with agar containing 1μg/mL L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK) -treated trypsin (Sigma-Aldrich). After incubation for 72 hours at 37°C, the agar overlays were removed, the cells were stained with crystal violet, and plaques were counted for virus titer calculation as PFU (plaque forming units) per mg of protein. Protein concentrations were tested by BCA Assay (Pierce) according to the manufacturer’s instructions. In order to generate a measure of virion purity, a ratio of pfu/protein was calculated and used for normalization between samples. Purity was also assessed by electron microscopy of the resuspended virus.36
Glycerophospholipid Extraction
Glycerophospholipids from purified virion pellets of different influenza strains were extracted using a modified Bligh and Dyer procedure.37 Briefly, each pellet was homogenized in 800 μL of ice-cold 0.1 N HCl: CH3OH (1:1) by vortexing for one minute at 4°C. Suspension was then vortexed with 400 μL of cold CHCl3 for one minute at 4°C and the extraction proceeded with centrifugation (5 min, 4°C, 18,000 × g) to separate the two phases. Lower organic layer was collected and solvent evaporated.
Extraction of phospholipids from non-infected allantoic fluid (NAF) proceeded with addition of 1 mL of ice-cold 0.1 N methanolic HCl and 1 mL of ice-cold CHCl3 to 1 mL of NAF. Following one minute of vortexing at 4°C, layers were separated by centrifugation (5 min, 4°C, 18,000 × g) and the solvents from the lower layer evaporated. All procedures were performed quickly and at low temperature to prevent plasmalogen phospholipids degradation. The resulting lipid film was dissolved in 100 μL of isopropanol: hexane:100 mM NH4COOH (aq) 58:40:2 (mobile phase A).
Mass Spectrometric Analysis
Quantification of glycerophospholipids was achieved by the use of an LC-MS technique employing synthetic odd-carbon diacyl and lysophospholipid standards and was based on standard curves, constructed from even-carbon species with spanning acyl chain length and degree of unsaturation. Typically, 200 ng of each odd-carbon standard was added per sample. Glycerophospholipids were analyzed on an Applied Biosystems/MDS SCIEX 4000 Q TRAP hybrid triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems, Foster City, CA, USA) and a Shimadzu high pressure liquid chromatography system with a Phenomenex Luna Silica column (2 × 250 mm, 5-μm particle size) using a gradient elution as previously described.17,38 The identification of the individual species, achieved by LC-MS/MS, was based on their chromatographic and mass spectral characteristics compared to synthetic standards. This analysis allows identification of the two fatty acid moieties but does not determine the relative position on the glycerol backbone (sn-1 versus sn-2). All analyses were performed from multiple preparations of purified virions and NAF (n=8) and mean data is reported in the figures and tables.
As a result of a comprehensive phospholipid profiling of purified virions from three different influenza virus strains with a range of pathogenicity in humans and NAF, we have identified differences in membrane lipid compositions. Biophysical properties of the virion are highly affected by its lipid composition and are reacting to the environmental conditions.39 Literature indicates that virions with lower interferon expression (H5N1 is lower than for H1N1 or H3N2) have higher replication rates and vice versa.40,41 Host cells may be less able to defend against virions that evoke interferon responses. Related early lipid changes may be critical to membrane dynamics through factors such as changes in plasmalogen ethanolamines, generation of lysolipids, and increased SM synthesis. Thus membrane organization and architecture is highly dependent on the lipid environment during viral infection and suggests that viral replication can be influenced by modifying its structure. For instance, inhibitors of lipid biosynthesis (such as fatty acid synthase, ethanolamine kinase, sphingosine kinase) can be perceived as pharmacological targets for developing effective antivirals.
In addition, GPL themselves can serve as potential therapeutics. Volker and colleagues42 showed that two strains of influenza A bind to palmitoyl-oleoyl-phosphatidylglycerol, which is a minor component of pulmonary surfactant. These findings suggest that addition of this molecular species as part of a surfactant supplement might be an effective treatment approach against viral infections.43 Similarly, it was recently demonstrated that changes in host cell lipid composition play key roles in influenza infectivity. Our group recently demonstrated that phospholipase D (PLD) is activated in the host cells following presentation with influenza virus and the PLD2 isoenzyme plays a major role in assisting the virus escape from immune defenses.44 Allosteric modulators of PLD greatly decelerate the kinetics of entry and data suggests that PA may be a novel target for development of new antiviral therapeutics.
Supplementary Material
Acknowledgments
This work was partially supported by funds from NIAID Grant HHSN 272200800058C. HAB holds the Bixler Johnson Mayes Endowed Chair in Pharmacology and received support from the Vanderbilt Institute of Chemical Biology. PGT received support from The Hartwell Foundation as an Individual Biomedical Research Fellow and from ALSAC.
Abbreviations
- ESI-MS
electrospray ionization mass spectrometry
- FA
fatty acid
- GPL
glycerophospholipids
- LC-MS
liquid chromatography mass spectrometry
- MS/MS
tandem mass spectrometry
- NAF
non-infected allantoic fluid
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PEp
plasmalogen ethanolamine
- PG
phosphatidylglycerol
- PI
phosphatidylinositol
- PS
phosphatidylserine
- SM
sphingomyelin
Footnotes
Supporting Information
Tables with identified phospholipid species in three strains of influenza and NAF,
Table with quantitated lipid species in the influenza strains and NAF,
Figure comparing long chain fatty acid compositions.
This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Hermansson M, Hokynar K, Somerharju P. Mechanisms of glycerophospholipid homeostasis in mammalian cell. Progr. Lipid Res. 2011;50:240–257. doi: 10.1016/j.plipres.2011.02.004. DOI:10.1016/j.plipres.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Liu STH, Friling-Sharon R, Ivanova PT, Milne SB, Myers DS, Rabinowitz JD, Brown HA, Shenk T. Synaptic vesicle-like lipidome of human cytomegalovirus virions reveals a role for SNARE machinery in virion egress. Proc. Natl. Acad. Sci. U.S.A. 2011;108:12869–12874. doi: 10.1073/pnas.1109796108. DOI:10.1073/pnas.1109796108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark NM, Lynch JP. Influenza: epidemiology, clinical features, therapy, and prevention. Semin. Respir. Crit. Care Med. (3rd) 2011;32:373–392. doi: 10.1055/s-0031-1283278. DOI:10.1055/S-0031-1283278. [DOI] [PubMed] [Google Scholar]
- 4.Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337:199–204. doi: 10.1126/science.1222213. DOI:10.1126/science.1222213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teissier E, Pecheur E-I. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur. Biophys. J. 2007;36:887–899. doi: 10.1007/s00249-007-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kooijman EE, Chupin V, de Kruijff B, Burger KN, Rand PR. Spontaneous curvature of phosphatidic acid and lysophosphatidic acid. Biochemistry. 2005;44:2097–2102. doi: 10.1021/bi0478502. [DOI] [PubMed] [Google Scholar]
- 7.Chernomordik LV, Leikina E, Kozlov MM, Frolov VA, Zimmerberg J. Structural intermediates in influenza haemaglutinin-mediated fusion. Mol. Membr. Biol. 1999;16:33–42. doi: 10.1080/096876899294733. [DOI] [PubMed] [Google Scholar]
- 8.Winter G, Fields S, Brownlee GG. Nucleotide sequence of the haemagglutinin gene of a human influenza virus H1 subtype. Nature. 1981;292:72–75. doi: 10.1038/292072a0. [DOI] [PubMed] [Google Scholar]
- 9.Hartley CA, Reading PC, Ward AC, Anders EM. Changes in the haemagglutinin molecule of influenza A (H3N2) virus associated with increased virulence for mice. Arch. Virol. 1997;142:75–88. doi: 10.1007/s007050050060. [DOI] [PubMed] [Google Scholar]
- 10.Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral Immunol. 2011;24:77–88. doi: 10.1089/vim.2010.0118. DOI:10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- 11.Yen H-L, Aldridge JR, Boon ACM, Ilyushina NA, Salomon R, Hulse-Post DJ, Marjuki H, Franks J, Boltz DA, Bush D, Lipatov AS, Webby RJ, Rehg JE, Webster RG. Changes in H5N1 influenza virus hemagglutinin receptor binding domain affects systemic spread. Proc.Natl. Acad.Sci. U. S. A. 2009;106:286–291. doi: 10.1073/pnas.0811052106. DOI:10.1073/pnas.0811052106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chukkapalli V, Heaton NS, Randall G. Lipids at the interface of virus-host interactions. Curr. Opin. Microbiol. 2012;15:512–518. doi: 10.1016/j.mib.2012.05.013. DOI:10.1016/j.mib.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munger J, Bennett BD, Parikh A, Feng X-J, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. DOI:10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klenk H-D, Choppin PW. Lipids of plasma membranes of monkey and hamster kidney cells and of parainfluenza virions grown in these cells. Virology. 1969;38:255–268. doi: 10.1016/0042-6822(69)90367-5. [DOI] [PubMed] [Google Scholar]
- 15.Klenk H-D, Rott R, Becht H. On the structure of the influenza virus envelope. Virology. 1972;47:579–591. doi: 10.1016/0042-6822(72)90547-8. [DOI] [PubMed] [Google Scholar]
- 16.Blough HA. Newly synthesized lipids incorporated into influenza virus membranes. Nature. 1974;251:333–335. doi: 10.1038/251333a0. [DOI] [PubMed] [Google Scholar]
- 17.Myers DS, Ivanova PT, Milne SB, Brown HA. Quantitative analysis of glycerophospholipids by LC-MS: Acquisition, data handling and interpretation. Biochim. Biophys. Acta. 2011;1811:748–757. doi: 10.1016/j.bbalip.2011.05.015. DOI:10.1016/j.bbalip.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkin HM, Makino S, Townsend D, Riera MC, Barron AL. Lipid composition of the hemagglutinating active fraction obtained from chick embryos infected with Chlamydia psittaci 6BC. Infection and Immunity. 1970;2:316–319. doi: 10.1128/iai.2.3.316-319.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorizate M, Kräusslich H-G. Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 2011;3:a004820. doi: 10.1101/cshperspect.a004820. DOI:10.1101/cshperspect.a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tafesse FG, Sanyal S, Ashour J, Guimaraes CP, Hermansson M, Somerharju P, Ploegh HL. Intact sphingomyelin biosynthetic pathway is essential for intracellular transport of influenza virus glycoproteins. Proc. Natl. Acad. Sci. U.S.A. 2013;110:6406–6411. doi: 10.1073/pnas.1219909110. DOI:10.1073/pnas.1219909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerl M-J, Sampaio JL, Urban S, Kalvodova L, Verbavatz J-M, Binmington B, Lindemann D, Lingwood CA, Shevchenko A, Schroeder C, Simons K. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J. Cell Biol. 2012;196:213–221. doi: 10.1083/jcb.201108175. DOI:10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider-Schaulies J, Schneider-Schaulies S. Sphingolipids in viral infection. Biol. Chem. 2015;396:585–595. doi: 10.1515/hsz-2014-0273. DOI:10.1515/hsz-2014-0273. [DOI] [PubMed] [Google Scholar]
- 23.Rawat SS, Viard M, Gallo SA, Rein A, Blumenthal R, Puri A. Modulation of entry of enveloped viruses by cholesterol and sphingolipids. Mol. Membr. Biol. 2003;20:243–254. doi: 10.1080/0968768031000104944. DOI:10.1080/0968768031000104944. [DOI] [PubMed] [Google Scholar]
- 24.Chan RB, Tanner L, Wenk MR. Implications for lipids during replication of enveloped viruses. Chem. Phys. Lipids. 2010;163:449–459. doi: 10.1016/j.chemphyslip.2010.03.002. DOI:10.1016/j.chemphyslip.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberge B, Gannoun-Zaki L, Bascunana C, TranVan Ba C, Vial H, Cerdan R. Comparison of the cellular and biochemical properties of Plasmodium falciparum choline and ethanolamine kinases. Biochem. J. 2010;425:149–158. doi: 10.1042/BJ20091119. DOI:10.1042/BJ20091119. [DOI] [PubMed] [Google Scholar]
- 26.Huang RT, Lichtenberg B, Rick O. Involvement of annexin V in the entry of influenza viruses and the role of phospholipids in infection. FEBS Lett. 1996;392:59–62. doi: 10.1016/0014-5793(96)00783-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Pekosz A, Lamb RA. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y-H, Du WL, Hagemeijer MC, Takvoryan PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma H-C, Jiang P, Wimmer E, Altan-Bonnet G, Altan-Bonnet N. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. DOI: 10.1016/j.cell.2015.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davidsen J, Mouritsen OG, Jørgensen K. Synergistic permeability enhancing effect of lysophospholipids and fatty acids on lipid membranes. Biochim. Biophys. Acta. 2002;1564:256–262. doi: 10.1016/s0005-2736(02)00461-3. [DOI] [PubMed] [Google Scholar]
- 30.Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, et al. Dengue virus non-structural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. DOI:10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brügger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. The HIV lipidome: a raft with an unusual composition. Proc. Natl. Acad. Sci. U.S.A. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glaser PE, Gross RW. Plasmenylethanolamine facilitates rapid membrane fusion: a stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry. 1994;33:5805–5812. doi: 10.1021/bi00185a019. [DOI] [PubMed] [Google Scholar]
- 33.Simons K, Ehehalt R. Cholesterol, lipid rafts, and disease. J. Clin. Invest. 2002;110:597–603. doi: 10.1172/JCI16390. DOI:10.1172/JCI200216390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann E, Lipatov AS, Webby RJ, Govorkova EA, Webster RG. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proc. Natl. Acad. Sci. USA. 2005;102:12915–12920. doi: 10.1073/pnas.0506416102. DOI:10.1073/pnas.0506416102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutigliano JA, Morris MY, Yue W, Keating R, Webby RJ, Thomas PG, Doherty PC. Protective memory responses are modulated by priming events prior to challenge. J. Virol. 2010;84:1047–1056. doi: 10.1128/JVI.01535-09. DOI:10.1128/JVI.01535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noda T. Native morphology of influenza virions. Front. Microbiol. 2012;2:269. doi: 10.3389/fmicb.2011.00269. article. DOI:10.3389/fmicb.2011.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 38.Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Meth. Enzymol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- 39.Reddy T, Shorthouse D, Parton DL, Jefferys E, Fowler PW, Chavent M, Baaden M, Sansom MSP. Nothing to sneeze at: A dynamic and integrative computational model of an influenza A virion. Structure. 2015;23:1–14. doi: 10.1016/j.str.2014.12.019. DOI:10.1016/j.str.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan RW, Yuen KM, Yu WC, Ho CC, Nicholls JM, Peiris JS, Chan MC. Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS ONE. 2010;5:e8713. doi: 10.1371/journal.pone.0008713. DOI:10.1371/journal.pone.0008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, Tumpey TM, Katz JM. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J. Virol. 2007;81:12439–12449. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Numata M, Kandasamy P, Nagashima Y, Posey J, Hartshorn K, Woodland D, Voelker DR. Phosphatidylglycerol suppresses influenza A virus infection. Am. J. Respir. Cell. Mol. Biol. 2012;46:479–487. doi: 10.1165/rcmb.2011-0194OC. DOI:10.1165/rcmb.2011-0194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Numata M, Nagashima Y, Moore ML, Berry KZ, Chan M, Kandasamu P, Stokes Peebles R, Jr., Murphy RC, Voelker DR. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. J. Lipid Res. 2013;54:2133–2143. doi: 10.1194/jlr.M037077. DOI:10.1194/jlr.M037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oguin TH, Sharma S, Stuart AD, Duan S, Scott SA, Jones CK, Daniels JS, Lindsley CW, Thomas PG, Brown HA. Phospholipase D facilitates efficient entry of influenza virus, allowing escape from innate immune inhibition. J. Biol. Chem. 2014;289(37):25405–25417. doi: 10.1074/jbc.M114.558817. DOI:10.1074/jbc.M114.558817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







