Abstract
The teleost fishes represent over half of all extant vertebrates; they occupy nearly every body of water and in doing so, occupy a diverse array of environmental conditions. We propose that their success is related to a unique oxygen (O2) transport system involving their extremely pH-sensitive haemoglobin (Hb). A reduction in pH reduces both Hb-O2 affinity (Bohr effect) and carrying capacity (Root effect). This, combined with a large arterial-venous pH change (ΔpHa-v) relative to other vertebrates, may greatly enhance tissue oxygen delivery in teleosts (e.g., rainbow trout) during stress, beyond that in mammals (e.g., human). We generated oxygen equilibrium curves (OECs) at five different CO2 tensions for rainbow trout and determined that, when Hb-O2 saturation is 50% or greater, the change in oxygen partial pressure (ΔPO2) associated with ΔpHa-v can exceed that of the mammalian Bohr effect by at least 3-fold, but as much as 21-fold. Using known ΔpHa-v and assuming a constant arterial-venous PO2 difference (Pa-vO2), Root effect Hbs can enhance O2 release to the tissues by 73.5% in trout; whereas, the Bohr effect alone is responsible for enhancing O2 release by only 1.3% in humans. Disequilibrium states are likely operational in teleosts in vivo, and therefore the ΔpHa-v, and thus enhancement of O2 delivery, could be even larger. Modeling with known Pa-vO2 in fish during exercise and hypoxia indicates that O2 release from the Hb and therefore potentially tissue O2 delivery may double during exercise and triple during some levels of hypoxia. These characteristics may be central to performance of athletic fish species such as salmonids, but may indicate that general tissue oxygen delivery may have been the incipient function of Root effect Hbs in fish, a trait strongly associated with the adaptive radiation of teleosts.
Introduction
Haemoglobin (Hb) is one of the most well studied proteins to date and is key to blood oxygen (O2) transport in nearly all vertebrates and some invertebrates, as it increases the total O2 that can be transported in the blood and optimizes tissue O2 delivery. The Bohr effect describes the reduction in Hb-O2 affinity when blood pH decreases and has been studied for over a century to understand how metabolic CO2 production can elevate the partial pressure of O2 (PO2) in the blood to enhance tissue O2 delivery [1–3]. The effect of pH on Hb-O2 can be graphically represented using an oxygen equilibrium curve (OEC), where Hb-O2 saturation decreases in a sigmoidal pattern depending on the PO2 of the system. With a given reduction in pH, as occurs between arterial and venous blood (ΔpHa-v) due to metabolic CO2 production, the OEC shifts to the right. Thus, at a given Hb-O2 saturation, such as P50 (PO2 at which Hb is 50% saturated), the shift in the OEC increases the PO2 at P50, which increases the driving force for tissue O2 delivery. The new P50 can be calculated using the following equation (Eq 1):
| (1) |
where P50i refers to the initial P50 of the organism (e.g., under resting conditions) or system, Φ refers to the Bohr coefficient (Δlog P50/ΔpH), and ΔpHa-v is the arterial-venous pH change. For example, a human with a P50i of 27 mmHg [4], a Bohr coefficient of -0.35 [5], and ΔpHa-v of -0.035 (a typical change that can occur in vivo [6]) will therefore exhibit a new P50 of 27.77 mmHg. While this is a ΔPO2 (ΔPO2 = P50—P50i) of less than 1 mmHg, it represents the increase in the driving force for tissue O2 delivery. End capillary PO2 values in mammals have been measured at 40 mmHg [7–10], and thus the ΔPO2 resulting from the Bohr effect would represent a modest benefit (~2%) to the driving force for tissue O2 delivery in a human.
Among vertebrates, teleosts possess Hbs whereby an acidosis not only greatly decreases Hb-O2 affinity, as in the Bohr effect, but also reduces the carrying capacity of Hb for O2, termed the Root effect [11–13]. The Root effect has long been discussed in terms of its role in enhancing O2 delivery to the eye and swimbladder of fishes. The eye and swimbladder are equipped with retia–dense capillary networks that can serve to localize and magnify an acidosis–that, in conjunction with the Root effect, greatly elevate arterial PO2 (PaO2) [14–16]. In the eye, the high PaO2 serves to overcome great diffusion distances to oxygenate the metabolically active, yet poorly vascularized retinal tissue [16–18]. At the swimbladder, a gas gland also aids in producing acid, and the resulting high PaO2 serves to inflate the swimbladder against large pressure gradients (>50 atm) associated with depth, permitting precise buoyancy regulation [14]. Teleosts that possess Root effect Hbs generally possess a large Bohr coefficient, much larger than occurs in air-breathing vertebrates with a Bohr effect alone. Consequently, it has been proposed that Root effect Hbs may also be in place to enhance O2 delivery to tissues in general, beyond the eye and swimbladder [2,19,20]. The focus of this study was to quantify the degree to which this may occur.
Indeed, the Root effect in teleosts is generally associated with a large Bohr coefficient, which is one step toward generating a large ΔPO2 during blood capillary transit; however, the other prerequisite is a large pHa-v, which under steady state conditions is thought not to occur [5]. This is because a Hb with a large Bohr coefficient also has a large Haldane coefficient, which minimizes or even prevents a pHa-v difference due to binding of H+ upon Hb deoxygenation. However, recently it has been demonstrated that under some conditions teleosts may exhibit a large pHa-v that may greatly exceed that of air-breathing vertebrates. If this is coupled with a large Bohr coefficient, O2 delivery could be greatly enhanced [21,22].
A large ΔpHa-v may occur in teleosts during a generalized acidosis through the short-circuiting of red blood cell Na+/H+ exchange (NHE). During a generalized acidosis, most teleosts secure gill O2 uptake by protecting RBC pH via β-adrenergically stimulated NHE (βNHE), which upon activation, removes protons (H+) from the RBCs in exchange for Na+. However, Rummer and Brauner [23] determined that, in vitro, the βNHE and a general ‘housekeeping’ NHE could both be selectively short-circuited via plasma-accessible carbonic anhydrase (CA). This short-circuiting would create the large ΔpHa-v needed to greatly enhance O2 delivery to select tissues. Short-circuiting was further validated in vivo in rainbow trout exposed to a mild acidosis (elevated water CO2) upon which muscle PO2 –determined using fast-responding fibre optic O2 sensors implanted directly into the fish’s muscle tissue–increased by 65% [21]. The increase in red muscle PO2 was completely abolished following arterial injection of a membrane-impermeant CA inhibitor, thus linking the increase in tissue PO2 to plasma-accessible CA [21]. Therefore, qualitative support exists for a large ΔpHa-v and the associated effect on O2 delivery both in vitro and in vivo, which may only require a mild acidosis. However, the degree to which this may enhance O2 delivery during periods of severe environmental challenges, such as hypoxia or exercise, is not known.
At a given pHa-v and Hb-O2 saturation (e.g., P50 or otherwise), a ΔPO2 (P50—P50i, where the respective level of saturation is used instead of 50%) can be calculated using Eq. 1 assuming that the Bohr coefficient is constant at the different Hb-O2 saturations. This is more or less the case in human blood, where under relevant in vivo conditions the shift in the OEC due to the Bohr effect is relatively constant between 20 and 80% Hb-O2 saturation [24]. However, Root effect Hbs exhibit a strongly non-linear release of Bohr protons with oxygenation [24], and consequently, the greatest Bohr shift exists between 60 and 100% Hb-O2 saturation [25–28]. Thus, a ΔPO2 in Root effect Hbs cannot simply be calculated as described above. Rather, ΔPO2 must be interpolated directly from OECs generated at constant pH values that span the in vivo range for that organism. Surprisingly, no such data set currently exists. Furthermore, there is tremendous variability in the literature regarding even the magnitude of the Bohr coefficient at P50, let alone at other Hb-O2 saturations, in rainbow trout (Oncorhynchus mykiss), despite it being one of the most comprehensively investigated teleost species to date. These inconsistencies may be in part due to differences in methodologies (Table A in S1 File).
We chose to thoroughly characterize the Root effect Hb system in rainbow trout, and our specific objectives were as follows: i) generate complete OECs in whole blood at constant pH values that span the in vivo range for rainbow trout; ii) calculate the ΔPO2 for a given proposed pHa-v at set Hb-O2 saturations; iii) determine Bohr coefficients, Hill coefficients, and P50 values validated against other sampling and incubation techniques from our previous studies [29] to address the historical inconsistences that preclude many of these analyses otherwise; iv) compare this with the ΔPO2 calculated from models developed for a mammal (e.g., human) possessing a Bohr effect alone; v) estimate the increase in the O2 release from Hb and potential enhancement in tissue O2 delivery associated with a Root effect Hb that rainbow trout may experience during exercise or hypoxia, when oxygen delivery is most critical for performance. The latter may be particularly important in athletic species to speed up post-exercise recovery, following a predator-prey encounter, or during long, upstream migrations as exhibited in Pacific salmon [30], or to enhance O2 delivery in other species during exposure to environmental stress.
Results
Series 1: Influence of pH on the oxygen equilibrium curves of rainbow trout blood
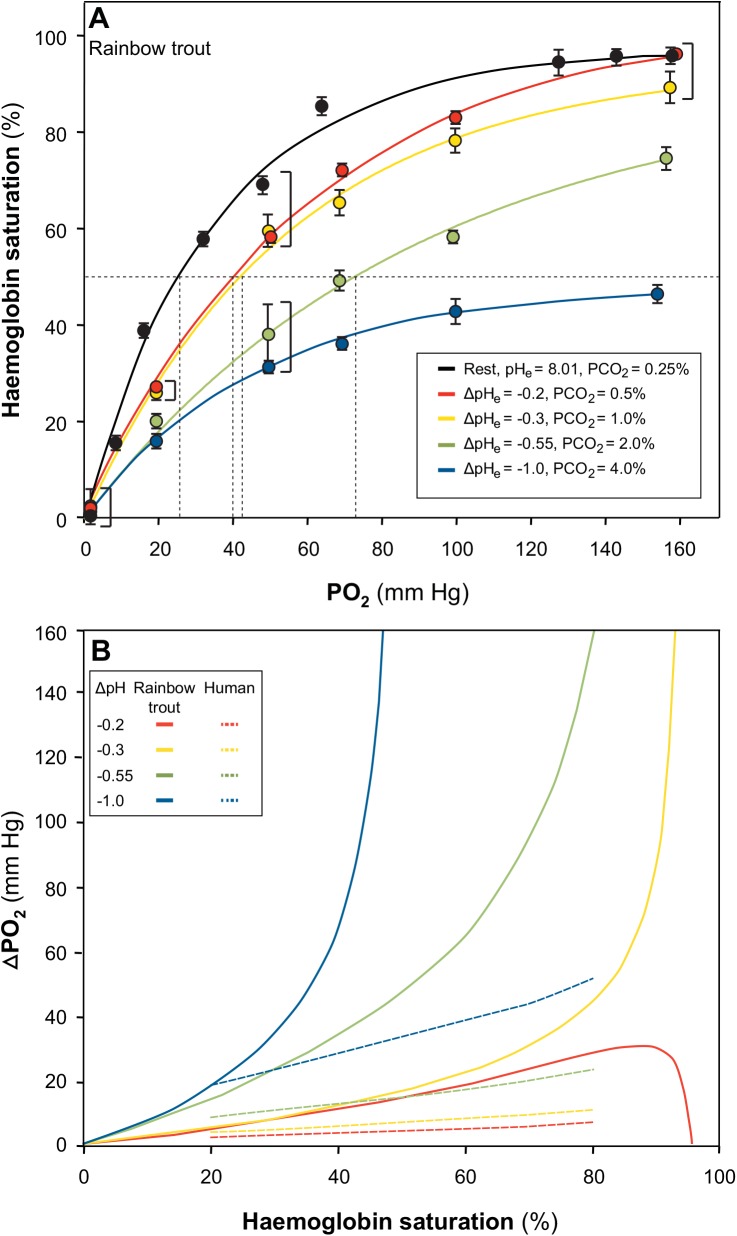
Blood was incubated at 0.25, 0.5, 1, 2, or 4% CO2 to simulate different pHa-v over the entire range of Hb-O2 saturations. At each CO2 level and therefore pH, PO2 was also decreased stepwise, and mean Hb-O2 saturation was calculated at each step so that OECs could be generated. Mean Hb-O2 saturation significantly decreased with PO2 and with each increase in CO2 tension, resulting in the characteristic rightward (Bohr effect) and downward (Root effect) shifts in the OEC (Fig 1A). The exceptions were points generated from samples incubated at 0.25, 0.5, and 1% CO2 for a PO2 of 156 to 158 mmHg, which were not statistically different from each other. This pattern was evident again when samples were incubated at a PO2 of 47 to 49 mmHg. At air-saturated oxygen tensions (~160 mmHg) there was a reduction in Hb-O2 saturation from 95 and 96% at 0.25 and 0.5% CO2, to 89% at 1% CO2, 75% at 2% CO2, and 47% at 4% CO2 (Fig 1A), a reduction in carrying capacity that is characteristic of Root effect Hbs.
Fig 1. Oxygen equilibrium curves (OECs) generated at 0.25, 0.5, 1.0, 2.0, and 4.0% CO2 with stepwise decreases in PO2 (mmHg) from 160 to 0 mmHg (balance N2) for rainbow trout rinsed RBCs (panel A).
The ΔpHe relative to the 0.25% CO2 curve (black, pHe = 8.01) is also noted for each treatment. Data are means ±S.E.M. Brackets indicate no statistically significant effect of CO2 at the respective PO2. Dashed lines extend from 50% Hb-O2 saturation to indicate the P50 for each OEC. In panel B, the magnitude of the right-shift of the OEC for a given pH change (ΔpH represented as different colours) is represented as ΔPO2 over each Hb-O2 saturation for a Bohr effect Hb system (human, dashed lines between 20 and 80% Hb-O2 saturation only) or a Root effect Hb system (rainbow trout, solid lines).
Blood samples were taken at each step of the incubation process for each curve generated. Both intracellular and extracellular pH (pHi and pHe) were measured at each CO2 tension, and both decreased with higher levels of CO2 (P<0.001) (Table 1). Changes in pHi were significantly and linearly correlated with changes in pHe (pHi = 2.747 + (0.574 * pHe), R2 = 0.930), similar to previous determinations with whole blood in vitro (pHi = 2.708 + (0.595 * pHe), R2 = 0.950) [31]. Haematocrit (Hct) was measured in whole blood samples following each CO2 incubation and was found to slightly but significantly increase at 1, 2, and 4% CO2 relative to 0.25 and 0.5% CO2 incubations.
Table 1. Effects of carbon dioxide (% CO2) on haematological parameters, pH and oxygen transport-related variables for blood of rainbow trout.
| CO 2 (%) | 0.25 | 0.5 | 1 | 2 | 4 | ||||
| PCO 2 (mm Hg) | 1.9 | 3.8 | 7.6 | 15.2 | 30.4 | ||||
| [Hb] (mM) | 0.9 ±0.0abc | 0.9 ±0.0abc | 1.0 ±0.0b | 1.0 ±0.0b | 0.8 ±0.1c | ||||
| Hct (%) | 23.2 ±0.0abd | 22.5 ±0.3b | 25.3 ±0.8c | 26.1 ±0.4c | 24.5 ±0.2cd | ||||
| MCHC | 3.7 ±0.2ab | 4.2 ±0.1a | 4.1 ±0.1a | 4.0 ±0.1a | 3.1 ±0.4b | ||||
| pH i | 7.40 ±0.00a | 7.24 ±0.05b | 7.12 ±0.03c | 6.96 ±0.05d | 6.79 ±0.02e | ||||
| pH e | 8.01 ±0.00a | 7.81 ±0.04b | 7.69 ±0.02c | 7.46 ±0.03d | 6.96 ±0.01e | ||||
| P 50 (mm Hg) | 24.8 ±0.3a | 37.7 ±0.3b | 40.6 ±2.2b | 75.0 ±4.8c | >160.0 | ||||
| n H | 1.3 ±0.1a | 1.5 ±0.0a | 1.4 ±0.0a | 1.0 ±0.0b | 0.6 ±0.1c | ||||
| Φ | -0.91 ±0.18 | -0.67 ±0.04 | -0.87 ±0.06 | -0.77 ±0.05 |
Haemoglobin concentration [Hb], haematocrit (Hct), mean corpuscular haemoglobin concentration (MCHC), intracellular pH (pHi), extracellular or plasma pH (pHe), the Hill coefficient (nH), and the Bohr coefficient (Φ). All data were collected according to Series 1 protocols on rinsed RBCs. Letters that differ within rows indicate statistically significant effects of CO2.
Both 3-parameter logistic equations as well as Hill plots were used to calculate P50 values for each OEC, which were approximately 25, 38, 41, 75, and >160 mmHg (generated from 0.25 to 0.5, 1, 2, and 4% CO2 mixtures, respectively). Each were significantly different from one another (P<0.001) except for 0.5 and 1% CO2 samples (P = 0.05) (Table 1, S1 Fig, S1 File). Statistical analyses did not include the >160 mmHg P50 from samples; while the Hill plots resulted in a value of 162 mmHg, a nominal value could not be interpolated from the OECs. The Hill coefficient (nH) was also calculated for each curve, illustrating low cooperativity (1.34, 1.48, 1.37, 1.03, and 0.55 at 0.25, 0.5, 1, 2, and 4% CO2, respectively) that is characteristic of Root effect Hbs. The nH using 2 and 4% CO2 were the only significantly different values (Table 1, S1 Fig, S1 File). Bohr coefficients for each CO2 incubation treatment were calculated at P50 and were -0.91, -0.67, -0.87, and -0.77 (at 0.25, 0.5, 1, 2, and 4% CO2, respectively) (Table 1).
Series 2: Differences in ΔPO2 in a Bohr effect Hb system (human) and a Root effect Hb system (rainbow trout)
For the human model, the ΔPO2 was calculated as described above (Eq. 1), assuming a resting P50 of 27 mmHg [4], Φ of -0.35 [5] and assuming Φ was constant at all pH values between 20 and 80% Hb-O2 saturation, as this is where the shift in the OEC due to the Bohr effect is relatively constant [24]. The PO2 values at P20, P30, P40, P60, P70, and P80 were extrapolated from a Hill plot generated using nH = 2.8 and P50 = 27 mmHg. A ΔpH of -0.2, -0.3, -0.55 and -1.0 pH units were chosen to be consistent with those values determined for rainbow trout. While the latter two values far exceed what might be seen in vivo, they serve to illustrate the dramatic differences between the two model systems investigated. It was assumed that Hb always reached 100% saturation at atmospheric O2 tensions for the human model because they do not possess Root effect Hbs. Therefore, the model was restricted to Hb-O2 saturations between 20 and 80%, as a ΔPO2 would not be expected at 0 and 100% SO2. Rainbow trout blood ΔPO2 values were obtained by direct interpolation from the OECs generated in Series 1 (Fig 1A). The SO2 values from 0 to100% for a ΔpHe of 0.1 (by comparing the 0.25 and 0.5% CO2 OECs), ΔpHe of 0.2, (by comparing the 0.25 and 1% OECs), ΔpHe of 0.5 (by comparing the 0.25 and 2% CO2 OECs), and ΔpHe of 1.0 (by comparing 0.25 and 4% CO2 OECs). The ΔPO2 depending on Hb-O2 saturation for human and rainbow trout determined at the same ΔpH values are shown superimposed in Fig 1.
For human blood between 20 and 80% Hb-O2 saturation, the ΔPO2 values for a ΔpH of -0.2, -0.3, -0.55, and –1.0 were relatively constant (because the Bohr coefficient was assumed constant over this range) and were 4.7, 7.4, 15.1, and 33.4 mmHg, respectively at P50. For rainbow trout blood, the ΔPO2 values directly interpolated from the OECs for ΔpH values of -0.2, -0.3, -0.55, and –1.0 ranged from 14.6 to 295.1 mmHg at 50% Hb-O2 saturation. Thus, at a given ΔpH, the ΔPO2 depended greatly on the Hb-O2 saturation. The ΔPO2 for each ΔpH was on average 2.5-times greater in rainbow trout blood than human blood at 40% Hb-O2, and this difference increased at higher saturations. For Hb-O2 saturations up to 80% and a ΔpH close to what might be expected in vivo in trout (0.2 pH units), the ΔPO2 for rainbow trout was, at minimum, 4-fold that of the human ΔPO2 and over 21-times greater at greater ΔpH values (Fig 1B).
Series 3: Modeling changes in O2 release from Hb in a Bohr effect system in comparison to a Root effect system.
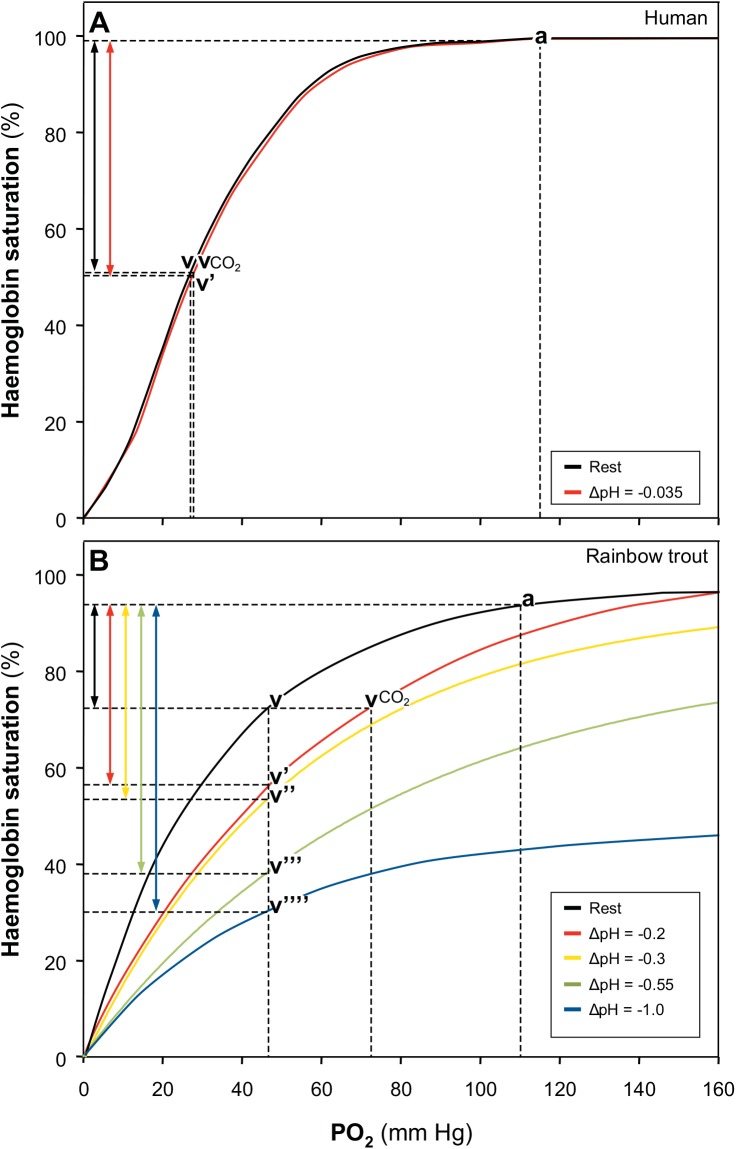
The OECs generated for human and trout blood were used to model O2 release from Hb and tissue O2 delivery. For the human model, a PaO2 of 115 mmHg (labeled “a” on Fig 2A), a PvO2 of 27 mmHg (labeled “v” on Fig 2A), and a physiologically relevant ∆pHa-v of 0.035 were used. All above values correspond to values obtained from previous studies [6,32–35]. The corresponding right-shifted OEC was plotted assuming a constant Bohr coefficient, Φ = -0.35, between 20 and 80% Hb-O2 saturation [4]. For the rainbow trout model, a PaO2 of 110 mmHg was used (labeled “a” on Fig 2B), and PvO2 (labeled “v” on Fig 2B) was estimated from muscle O2 values of 45–47 mmHg, both of which correspond to values obtained in vivo [21]. The OEC curves generated from the current study were used to simulate the various ∆pHa-v for the model and also to accommodate the non-linear Bohr shift known to occur at the different Hb-O2 saturations in teleosts.
Fig 2. The degree to which O2 release from Hb is enhanced for a given ΔpH for a Bohr effect Hb system (human, panel A) and a Root effect Hb system (rainbow trout, panel B).
The increase in O2 release with respective right-shifts in the OECs is represented by vertical double arrows here and as a percent increase over what is possible without the right shift in the text (see above section “Series 2: Differences in ΔPO2 …” for details).
Oxygen extraction over a given Pa-vO2 and without a right-shift in the OEC (“a” to “v”) is represented by a black vertical double arrow for both human and trout models (Fig 2). The increase in O2 released from Hb that is associated with a given ∆pHa-v and resulting right shift in the OEC can then be estimated by tracing “v” horizontally to the first right-shifted OEC to the point labeled “vCO2” and then moving down this new OEC to the PvO2 estimate corresponding with v’ on the model. For the human, the increase in O2 released from Hb as a result of the right-shift in the OEC (a to v’) would be a 1.3% increase from what would occur from “a to v” (i.e., without a right-shift, compare black and red vertical arrows; Fig 2A). For rainbow trout (Fig 2B), the increased O2 extraction as a result of the right-shift in the OEC (a to v’) would be 73.5% (compare black and red vertical arrows; Fig 2B). Thus, under these conditions, there is over a 50-fold increase in the additional O2 released from the Hb associated with the right shift in the OEC in trout relative to the human model. Under a more severe acidosis, the OEC shifts further to the right corresponding to v”, v”‘, or v”“and a respective 88, 160, or 197% increase or a doubling to tripling of O2 released from Hb (compare black to yellow, green, or blue vertical arrows; Fig 2B) and therefore potentially tissue O2 delivery.
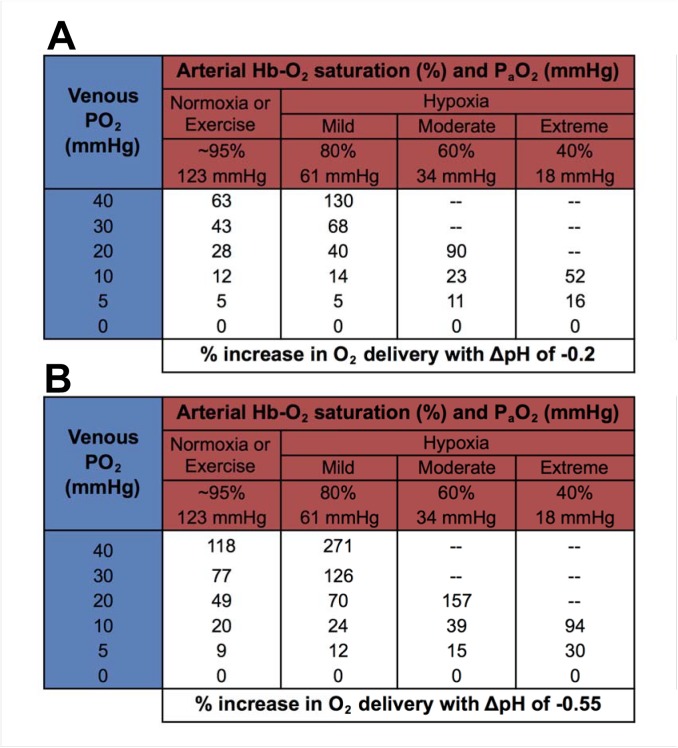
An additional model was generated for rainbow trout to predict the degree to which O2 release from Hb could be enhanced under normoxic conditions, at various levels of sustained exercise, or during exposure to hypoxia (Fig 3). We used PaO2 values corresponding to ~95% Hb-O2 saturation as well as various levels of hypoxia (e.g., 80, 60, and 40% Hb-O2 saturation) and a range of PvO2 levels (40, 30, 20, 10, 5, and 0 mmHg). Both ∆pH = -0.2 and ∆pH = -0.55 OEC curves were used to represent two potential ∆pHa-v scenarios. The first could be experienced in vivo at the tissues in the presence of a mild acidosis [21,23], and the second would represent a more severe acidosis–both cases representing involvement of selective short-circuiting of RBC NHE via plasma-accessible CA. The % increase in O2 release with Root effect Hbs, depending on the scenario, is most often at least 40% or greater and in five scenarios exceeds a 100% increase in O2 release (Fig 3). Furthermore, the increase in O2 release from Hb is almost doubled when the ∆pH is -0.55 when compared to ∆pH of -0.2 (Fig 3).
Fig 3. Model estimates for enhanced O2 release from Hb (as a %) in rainbow trout under normoxic conditions, various levels of sustained exercise, and various levels of hypoxia at two different simulated ∆pHa-v (panels A and B) compared to when there is no right shift in the oxygen equilibrium curve (OEC).
The PaO2 values corresponding to arterial Hb-O2 saturations (indicated by red cells) were derived from the resting curve of Fig 1A. Calculated values at each combination of arterial Hb-O2 saturation (red cells) and venous PO2 (PvO2) values (blue cells) represent the % increase in O2 delivery (white cells) over what is possible without a right-shift in the OEC. Curves for ∆pH = -0.2 (panel A) and ∆pH = -0.55 (panel B) were used for this model. For scenarios where the Pa-vO2 difference was less than 5 mmHg (deemed unrealistic), no data are shown (—).
Discussion
The present study quantifies the potential benefit to tissue O2 delivery associated with a ∆pHa-v in the presence of Root effect Hbs (rainbow trout) compared to that for a mammalian Bohr effect Hb system (human). Overall, it was determined that the ΔPO2 occurring in rainbow trout blood at 40% Hb-O2 saturation was 2.5-fold greater than that of a system with only a Bohr effect, using the same ∆pHa-v (Fig 1B). At Hb-O2 saturations greater than 50%, the difference was even more pronounced, 21-times that of a system possessing only a Bohr effect (Fig 1B). Upon modeling O2 release from Hb, it was determined that a ΔpHa-v of 0.2 in rainbow trout blood could increase O2 extraction by 73.5% at constant PvO2 (Fig 2B). Based on ΔPO2 values calculated from OECs associated with larger changes in pH, it follows that the enhancement in O2 release from Hb in rainbow trout could be even greater. In the human, however, a physiologically relevant pH change under otherwise similar assumptions may only increase O2 release from Hb by 1.3% (Fig 2A). This is the first time this difference has been quantified to this extent, despite qualitative predictions [19,26,36]. It follows that, if a relatively large ΔpHa-v can be generated in the general circulation of fish, which has been recently demonstrated in vitro and in vivo in rainbow trout [21,23], Root effect Hbs could be responsible for greatly enhancing general O2 delivery. Modeling with known Pa-vO2 in fish during exercise and hypoxia indicates that O2 release from Hb and thus tissue O2 delivery may double during exercise and even triple during some levels of hypoxia.
One of the assumptions associated with the model we present is that fish exhibit larger pHa-v differences than air-breathing vertebrates. To be conservative, many of the estimates presented here are based upon measured pHa-v differences in fish; although, recent data suggest that the pHa-v difference realized at the tissues under periods of stress are likely to be even larger [21,22]. This is due to the presence and then elimination of disequilibrium states [22], which are almost impossible to measure directly. It is also important to note that intracellular pH (pHi) cannot yet be reliably measured in real-time at the tissue/cellular level in vivo, and disequilibrium states in the blood preclude accurate representations of pHi once blood is collected and RBCs are lysed for measurement. So, even though many past studies have drawn arterial and venous blood samples for pH measurements, the values determined are equilibrium values that may differ greatly from pH values realized at the tissues. Currently, the fastest and most sensitive way to infer changes in RBC pHi is via changes in PO2 that we know occur as a result of a decrease in pHi, and indeed, the technology is already available for real-time measurements of PO2 at the tissues.
The magnitude of the ΔpHa-v that is possible in teleosts has recently been described and elaborated upon by Randall et al., and the sequence of events contrast the model for air-breathing vertebrates in several ways [22]. In teleosts, there is a loss of CA at the respiratory surface and in the venous circulation, an uncoupling of RBC and plasma pH, and a decrease in the role of Hb as a buffer. This collectively results in nearly all plasma bicarbonate dehydration occurring inside the RBC, which results in disequilibrium states that elevate arterial and venous bicarbonate levels [22]. With the presence of plasma-accessible CA near some tissues, disequilibrium states can be eliminated to form CO2 that re-acidifies the RBCs. All of this can be greatly magnified during acidotic stress and has been supported both in vitro [23] and in vivo [21]. In rainbow trout exposed to a mild acidosis (0.2 pH unit reduction in blood pH), for example, red muscle PO2 increased to an extent nearly identical to the right shift in the OECs derived from this study with the same pH change (0.2 pH unit). The ΔPO2 was abolished when plasma accessible CA in the red muscle was selectively inhibited [21]. These findings suggest that–at least during a mild acidosis–an entire acid load can be transferred from the plasma into the RBC, resulting in the equivalent of a ΔpHa-v of 0.2 pH units in situ. This is a much larger difference than would be inferred from arterial and venous blood drawn and then measured at equilibrium, which represents most of the literature values. Whether the same applies under more severe acidoses in vivo, as it does in vitro [23], needs to be investigated. However, the predictions generated here can now be tested experimentally. Clearly there is a tremendous potential for enhanced O2 release from the Hb associated with a large ∆pHa-v and potentially tissue O2 delivery.
Past information on haemoglobin-oxygen relationships in rainbow trout
Rainbow trout is arguably one of the most universally investigated teleost species with respect to O2 transport and respiratory physiology. The absence of a comprehensive data set sufficient to model ΔPO2 over a range of ΔpH (Table A in S1 File) was therefore surprising. An extensive review of the available literature reveals that reported O2 transport-related variables in rainbow trout blood are highly variable. For example, great variability in Bohr coefficients are observed ranging from –0.15 to –1.97 (Table A in S1 File, S2 Fig, S1 File) and P50 values for control or resting animals as low as 11 but as high as 40 mmHg (Table A in S1 File). This variability is likely due to the range of animal holding conditions, sampling techniques, blood preparation and protocols (Table A in S1 File), but it is unknown how these differences may affect the values those authors obtained. Consistent OECs and calculated Bohr and Hill coefficients and P50 values were generated using two different blood sampling protocols (rinsed RBCs or whole blood drawn from an indwelling dorsal aortic cannula), and two methods of equilibrating blood with gases (tonometry and the Tucker method vs. a modified version of the microdiffusion chamber and spectrophotometer, i.e. the Pwee50) [29] to further validate the data used for the models in the present study.
Modeling increased O2 release from Hb and tissue O2 delivery with a Bohr effect Hb (human) and a Root effect Hb (rainbow trout)
Christiansen and colleagues calculated a maximal P50 shift (ΔP50) in human blood associated with the Bohr effect in vivo of 3 mmHg, and so the predicted benefit to O2 delivery associated with the Bohr effect alone is quite modest. In modeling ΔPO2 associated with a pH change in human blood, a Bohr coefficient of –0.35 was assumed, a value deemed optimal for O2 delivery [5]. This value also falls within the middle of the range of fixed-acid and CO2 Bohr coefficients calculated from whole blood of healthy humans [37]. It was also assumed that the Bohr coefficient was constant between 20 and 80% SO2 [24]. A P50 value of 27 mmHg was chosen, which is midway between resting values of 24 and 29 mmHg, which have previously been determined at a pH of 7.4 [4,38–41]. The ΔpH values chosen were consistent with those determined in Series 1 for rainbow trout OECs to allow the two systems to be compared in terms of the contribution of a ΔpHa-v to enhancing O2 release from Hb. Our models may actually underestimate the difference between the two systems, however, because pH is a log scale, and starting pH values are lower in humans (7.4) than in rainbow trout (8.0). As such, more H+ would be added to the human system, which has a lower H+ sensitivity, than the trout system, despite using the same ΔpH. Therefore, if the same number of H+ were added to the trout system, the result may be even greater than we predict here. The measured ΔpHa-v in humans, 0.035 pH units, is quite small relative to even the lowest ΔpH value of 0.2 units used in this study, which between 20 and 80% Hb-O2 saturation, results in a ΔPO2 of less than 8 mmHg. A ΔpH of 0.035 would result in a ΔPO2 of even less than 1 mmHg, which would increase O2 release from Hb by 1.3%. However, it should also be noted that mammals can have nearly double the blood Hb concentrations of teleosts, which must be taken into account in determining the increase in tissue O2 delivery that may be realized with this modest Bohr effect. On the other hand, teleosts may be able to exploit this phenomenon that we describe here to enhance tissue O2 delivery while maintaining lower blood Hb concentrations.
In comparison, O2 release associated with a ΔpHa-v in teleost blood with Root effect Hbs is much greater than that in an air-breathing vertebrate with a Bohr effect Hb only, provided a sufficient pH change in the blood is observed. This varies with blood Hb-O2 saturation for a number of reasons. First, the Root effect results in incomplete Hb-O2 saturation at low pH, despite atmospheric PO2 levels [11–13]. This was evident in the OECs generated for rainbow trout, as larger pH changes resulted in curves that approached apparent upper asymptotic maximum Hb-O2 saturations that were distinctly and significantly lower than 100% (Fig 1A). This translated to very high ΔPO2 values upon interpolation (Fig 1B). Second, Root effect Hbs typically exhibit large Bohr coefficients [19,26,36]. As a result, the ΔPO2 in rainbow trout blood exceeds that of human blood at all comparable ΔpH values between 20 and 80% Hb-O2 saturation (Fig 1B). A 0.2 ΔpHa-v difference in human blood is unlikely, but a difference of this magnitude has been measured in rainbow trout during exercise [42,43]. This is also a value that can be realized at the level of the RBC when disequilibrium states are eliminated in the presence of plasma-accessible CA at the tissues, as described above. Even at a ΔpHa-v of 0.035, the ΔPO2 in rainbow trout blood still exceeds that of the human blood by as much as 3-fold (data not shown). Third, Root effect Hbs exhibit a non-linear release of Bohr protons with Hb-O2 saturation, and therefore the magnitude of the Bohr shift varies with Hb-O2 saturations [26,44]. This is apparent in Fig 1B, where ΔPO2 for a given ΔpHa-v is much greater in rainbow trout blood than human blood above P50, but this difference decreases below P50 (Fig 1B).
To date, only two studies have monitored real-time muscle PO2 in a teleost, and both of these studies provided evidence that tissue O2 delivery may be enhanced relative to other vertebrates [20,21]. Whether during normoxia, mild hypoxia, mild hypercarbia, or sustained or exhaustive exercise, red muscle PO2 in rainbow trout (between 45 and 60 mmHg; [20,21]) is consistently and substantially higher than in mammals (25–35 mmHg [7–10]), and this difference has been proposed to be due to the presence of Root effect Hbs. Furthermore, the fact that muscle PO2 still remains elevated during stress (hypoxia, hypercarbia, or exercise [20,21]) and above PvO2 (approximately 20 mmHg; [45]), despite decreases in PaO2, also suggests the importance of Root effect Hbs to enhancing O2 delivery. Here, the comparative model for human and trout with relevant ΔpHa-v demonstrated at least a 50-fold difference in the impact that Root effect Hbs have on enhancing O2 release from Hb when compared to a system with only a Bohr effect.
The potential for enhanced O2 delivery in rainbow trout was calculated using arterial Hb-O2 saturation, PaO2, and PvO2 values that represent normoxia, sustained exercise, and two levels of hypoxia associated with the exploitation of two different ΔpHa-v (Fig 3). The associated increase in O2 release from Hb over a given Pa-vO2 difference is remarkable, resulting in an increase of at least 40% or greater and in five scenarios exceeds a 100% increase (Fig 3). Assuming that, under these conditions, all other aspects of the oxygen cascade remain constant, and in particular there is no change in tissue metabolism or perfusion, this increase in O2 release would be directly proportional to the increase in tissue O2 delivery. Ultimately, this model serves to illustrate the remarkable potential for enhanced O2 delivery under various scenarios in a system unique to teleosts. Enhanced O2 delivery may be particularly important during exposure to environmental stress or prolonged exercise–for example–during the long, upstream migrations that the Pacific salmon undertake [30] or even to speed up post-exercise recovery, such as following a predator-prey interaction.
Conclusions
Quantitative results confirmed theoretical predictions that–for a given pH change–Root effect Hbs in rainbow trout convey an enormous benefit to blood O2 release and thus delivery when compared with human blood having a Bohr effect alone. Teleost fish evolved an extraordinary O2 delivery system associated with the extremely pH-sensitive Root effect Hbs. This system has been understood for decades as key to O2 delivery to the eye and swimbladder, which may have been important factors responsible for the extensive adaptive radiation of teleosts. Provided here is empirical evidence to suggest that Root effect Hbs, in conjunction with a mechanism to increase pHa-v (via the presence of plasma accessible CA in the tissues and absence at the gills), can also enhance general O2 delivery, which is consistent with recent in vitro and in vivo studies [20,21,23]. It may be that Root effect Hbs, which evolved prior to the appearance of the anatomical structures (retia) at the eye and swimbladder typically associated with this exceptional O2 delivery system, were initially selected for enhancing general O2 delivery through the associated large Bohr coefficient and large pHa-v difference. Studies on a model species, such as the rainbow trout, that is neither basal nor the most derived of the teleosts and exhibits a moderate level of activity and tolerance to environmental conditions provides a foundation on which to build further studies to understand how evolutionary history, activity, and habitat may play a role in the functional significance of this system.
Materials and Methods
Series 1: Influence of pH on the oxygen equilibrium curves of rainbow trout blood
1.1 Experimental animals and holding conditions
Rainbow trout, (O. mykiss, 300–600g wet body mass), were obtained from Spring Valley Trout Farm (Langley, British Columbia, Canada) and maintained at the University of British Columbia (UBC) Aquatic Facilities. Fish were held under a natural photoperiod at densities no greater than 10kg/m3 [46] in 4,000-l tanks supplied with flow-through 10°C Vancouver dechlorinated municipal tap water. Fish were fed every other day to satiation using commercial trout pellets (Skretting, Orient 4–0). Experiments were completed within the spring months over two separate years. All procedures complied with the guidelines approved by the Canadian Council on Animal Care and were approved by UBC’s animal ethics care and use committee (UBC protocol approval # A07-0080).
1.2 Caudal puncture sampling protocol
Fish were quickly removed from holding tanks and placed into a 20 l bucket of clean, well-aerated water containing benzocaine (0.2 mM final concentration, p-aminobenzoate, Sigma-Aldrich cat. no. E1501; St. Louis, MO, USA) to anaesthetize fish. Blood was drawn from the caudal vein and collected in heparinized syringes, and RBCs were rinsed twice and resuspended in ice-cold Cortland's saline [47] according to Caldwell et al. [48]. Haematocrit (Hct) of the rinsed RBCs was measured in duplicate by centrifuging 60μl whole blood in heparinized micro-capillary tubes for 3 min at 17,000 g and was standardized to 25% by removing either saline or RBCs. Blood was stored at 4°C overnight until experiments commenced, ensuring that any catecholamines present within the sample had degraded [49].
1.3 Oxygen equilibrium curves derived from tonometry
The Hct of rinsed RBCs stored at 4°C overnight was readjusted to 25% as needed. Then, 4 ml was added to each of four Eschweiler tonometers, which were incubated at 12°C, and equilibrated for one hour with a humidified gas mixture to one of the following CO2 proportions: 0.25, 0.5, 1, 2, or 4% balanced with air (21% O2). To generate an OEC at each of the above % CO2 values (n = 8), each tonometer was subjected to a step-wise decrease in O2 (21, 20, 19, 13, 9, 6.5, 4, 2.5, 1.5, or 0%) balanced with N2 using a DIGAMIX Wösthoff gas-mixing pump (DIGAMIX 275 6KM 422 Wösthoff, Bochum, Germany). Following a 20-min incubation period at each O2 tension, two 25 μl aliquots of rinsed RBCs were withdrawn into a pre-gassed Eppendorf™ tube or Hamilton™ syringe for measurement of total O2 content (TO2), and up to a further 500μl was withdrawn to measure haemoglobin concentration ([Hb]), Hct, extracellular pH (pHe), and intracellular pH (pHi). TO2 was measured according to Tucker [50], [Hb] (mM per tetramer) was measured after adding rinsed RBCs to Drabkin’s solution (Sigma-Aldrich cat. no. D5941; St. Louis, MO, USA), measuring absorbance at 540 nm, and applying a millimolar extinction coefficient of 11. The freeze-thaw technique [51] was used to measure pHi, where both pHe and pHi were measured using a BMS 3 Mk2 Blood Microsystem in conjunction with a PHM 84 meter (Radiometer, Copenhagen). A larger blood volume (500μl) was drawn when pH was measured, which was done at the highest, middle, and lowest O2 incubation tensions. All assays were performed in duplicate.
1.4 Calculations and statistical analyses
Data are presented as means ±S.E.M. Mean corpuscular haemoglobin concentration (MCHC) was calculated as Hb/(Hct/100). Haemoglobin % saturation (SO2) was calculated by dividing TO2 (after subtracting physically dissolved O2 according to [52]) by the theoretical maximum carrying capacity of the rinsed RBCs based upon the tetrameric Hb concentration obtained spectrophotometrically according to [50]. The SO2 values were plotted as a function of incubation PO2 (mmHg) for each % CO2 (0.25, 0.5, 1.0, 2.0, and 4.0%), and a curve was fit to the data using the Dynamic Fit Wizard function in SigmaPlot for Windows 13.0 (Systat Software Inc.) to generate the OECs. The P50 and Hill coefficients (nH) were calculated from Hill plots. Bohr coefficients (Φ) were calculated as ∆logP50/∆pHe for pH values corresponding to each CO2 incubation condition relative to 0.25% CO2. Statistical differences among CO2 treatments were detected via ANOVA and, when necessary, a post-hoc Holm-Sidak multiple comparisons test. All statistical analyses were conducted using SigmaPlot for Windows 13.0 (Systat Software, Inc.), using α < 0.05 to determine statistical significance.
Series 2: Differences in ΔPO2 in a Bohr effect Hb system (human) and a Root effect Hb system (rainbow trout)
2.1 Human model with a Bohr effect Hb system
The ΔPO2 was calculated (using Eq. 1), assuming a P50 of 27 mmHg [4], Φ of -0.35 [5], and assuming Φ was constant at all pH values between 20 and 80% SO2. A ΔpH of -0.2, -0.3, -0.55 and -1.0 pH units were chosen to be consistent with those values determined for rainbow trout below, and while the latter two values far exceed what might be seen in vivo, they serve to illustrate the dramatic differences between the two model systems investigated. It was assumed that Hb always reached 100% saturation at atmospheric O2 tensions. Therefore, the ΔPO2 at 0 and 100% SO2 always equalled zero.
2.2 Rainbow trout model with a Root effect Hb system
Rainbow trout blood ΔPO2 values were obtained by direct interpolation from the OECs generated in Series 1. The SO2 values from 0 to100% for a ΔpHe of 0.1 (by comparing the 0.25 and 0.5% CO2 OECs), ΔpHe of 0.2, (by comparing the 0.25 and 1% OECs), ΔpHe of 0.5 (by comparing the 0.25 and 2% CO2 OECs), and ΔpHe of 1.0 (by comparing 0.25 and 4% CO2 OECs). The same four pH shifts (ΔpH) simulated in rainbow trout blood were used for the human blood calculations as described above.
Series 3: Modeling changes in O2 release from Hb in a Bohr effect system in comparison to a Root effect system.
The OECs generated at different CO2 levels from this study were used to calculate the increased O2 release from Hb associated with a given ∆pHa-v and a constant ∆Pa-vO2. Then, O2 release can be used to estimate enhanced tissue O2 delivery associated with the respective ∆pHa-v, assuming that all other aspects of the O2 transport cascade were unaffected (e.g., including tissue metabolic rate and blood flow).
For the human model, a PaO2 of 115 mmHg, PvO2 of 27 mmHg, and a physiologically relevant ∆pHa-v of 0.035 were used because they correspond to values obtained from previous studies [6,32–35]. The corresponding right-shifted OEC was plotted assuming a constant Φ = -0.35 between 20 and 80% Hb-O2 saturation [4]. For the rainbow trout model, a PaO2 of 110 mmHg was used, which corresponds to values obtained in vivo in rainbow trout [21]. PvO2 was assumed constant and estimated from RMPO2 values (45–47 mmHg, [21]), which closely resemble PvO2. The OEC curves generated from the current study were used to simulate the various ∆pHa-v for the model and because PO2 values at each Hb-O2 could not be calculated using Eq. 1 because of the non-linear Bohr effect precluding a constant Φ at different Hb-O2 saturations.
An additional model was generated for rainbow trout to predict the degree to which tissue O2 delivery could be enhanced under normoxic conditions, hypoxic conditions, or at various levels of sustained exercise. We used PaO2 values corresponding to ~95% Hb-O2 saturation as well as various levels of hypoxia (e.g. 80, 60, and 40% Hb-O2 saturation) and a range of PvO2 levels (40, 30, 20, 10, 5, and 0 mmHg). Both ∆pH = -0.2 and ∆pH = -0.55 OEC curves were used to represent two potential ∆pHa-v that could be experienced in vivo at the tissues [21,23].
Supporting Information
(EPS)
(EPS)
(DOCX)
Acknowledgments
This project was funded by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery grant and Accelerator Supplement to C.J.B. and a UBC Graduate Fellowship to J.L.R. During the modelling and writing portion of this study, J.L.R. was funded by the Australian Research Council (ARC) Super Science Fellowship program, the ARC Centre of Excellence for Coral Reef Studies, and an ARC Discovery Early Career Fellowship. The authors wish to thank D.J. Randall, P. Morrison, C. Verhille, D.W. Baker, C. Ciuhandu, A.P. Farrell, P. Allen, N. Farrell, C. Fu, L. Hanson, K. Kamo, M. Roshan-Moniri, K. Suvadzjic, and the UBC Zoology workshop for interesting discussions and editorial and technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files. Metadata have been archived on Dryad via doi:10.5061/dryad.d0325.
Funding Statement
This project was funded by a Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery grant and Accelerator Supplement to C.J.B. and a UBC Graduate Fellowship to J.L.R. During the modelling and writing portion of this study, J.L.R. was funded by the Australian Research Council (ARC) Super Science Fellowship program, the ARC Centre of Excellence for Coral Reef Studies, and an ARC Discovery Early Career Fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bohr C, Hasselbalch K, Krogh A (1904) Über einen in biologischer Beziehung wichtigen Einfluss, den Kohlen- säurespannung des Blutes auf dessen Sauerstoffbindung übt. Skand Arch Physiol 16: 402–412. [Google Scholar]
- 2. Nikinmaa M (1997) Oxygen and carbon dioxide transport in vertebrate erythrocytes: an evolutionary change in the role of membrane transport. J Exp Biol 200: 369–380. [DOI] [PubMed] [Google Scholar]
- 3. Nikinmaa M, Soivio A (1979) Oxygen dissociation curves and oxygen capacities of blood of a freshwater fish, Salmo gairdneri . Ann Zool Fenn 16: 217–221. [Google Scholar]
- 4. Wells RMG, Weber RE (1989) The measurement of oxygen affinity in blood and haemoglobin solutions Cambridge: Cambridge University Press; pp. 279–302. [Google Scholar]
- 5. Lapennas GN (1983) The magnitude if the Bohr coefficient: Optimal for oxygen delivery. Respiration Physiology 54: 161–172. [DOI] [PubMed] [Google Scholar]
- 6. Rang LCF, Murray HE, Wells GA, Macgougan CK (2002) Can peripheral venous blood gases replace arterial blood gases in emergency department patients? CJEM 4: 7–15. [DOI] [PubMed] [Google Scholar]
- 7. Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC (2001) Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63. [DOI] [PubMed] [Google Scholar]
- 8. Hutter J, Habler O, Kleen M, Tiede M, Podtschaske A, Kemming G, et al. (1999) Effect of acute normovolemic hemodilution on distribution of blood flow and tissue oxygenation in dog skeletal muscle. J Appl Physiol 86: 860–866. [DOI] [PubMed] [Google Scholar]
- 9. Jung F, Keßler H, Pindur G, Sternitzky R, Franke RP (1999) Intramuscular oxygen partial pressure in the healthy during exercise. Clin Hemorheol Microcirc 21: 25–33. [PubMed] [Google Scholar]
- 10. Suttner SW, Lang K, Boldt J, Kumle B, Maleck WH, Piper SN (2002) The influence of hyperoxic ventilation during sodium nitroprusside-induced hypotension on skeletal muscle tissue oxygen tension. Anesthesiology 96: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 11. Root RW (1931) The respiratory function of the blood of marine fishes. Biol Bull 61: 427–456. [Google Scholar]
- 12. Root RW, Irving L (1943) The effect of carbon dioxide and lactic acid on the oxygen-combining power of whole and hemolyzed blood of the marine fish tautoga onitis (Linn.). Biol Bull 84: 207–242. [Google Scholar]
- 13. Pelster B, Weber RE (1991) The physiology of the Root effect Advances in Comparative and Environmental Physiology. Berlin: Springer-Verlag; pp. 51–77. [Google Scholar]
- 14. Scholander PF, Van Dam L (1954) Secretion of gases against high pressures in the swimbladder of deep sea fishes I. oxygen dissociation in blood. Biol Bull 107: 247–259. [Google Scholar]
- 15. Wittenberg JB, Haedrich RL (1974) The choroid rete mirabile of the fish eye. II. distribution and relation to the pseudobranch and to the swimbladder rete mirabile. Biol Bull 146: 137–156. [DOI] [PubMed] [Google Scholar]
- 16. Wittenberg JB, Wittenberg BA (1974) The choroid rete mirabile of the fish eye. I. Oxygen secretion and structure: comparison with the swimbladder rete mirabile. Biol Bull 146: 116–136. [DOI] [PubMed] [Google Scholar]
- 17. Nikinmaa M, Huestis WH (1984) Shape changes in goose erythrocytes. Biomembranes 773: 317–320. [DOI] [PubMed] [Google Scholar]
- 18. Dafré AL, Wilhelm D (1989) Root effect hemoglobins in marine fish. Comp Biochem Physiol 92: 467–471. [Google Scholar]
- 19. Brauner CJ, Randall D (1998) The linkage between the oxygen and carbon dioxide transport. Fish Physiol 17: 283–319. [Google Scholar]
- 20. McKenzie DJ, Wong S, Randall DJ, Egginton S, Taylor EW, Farrell AP (2004) The Effects of Sustained Exercise and hypoxia upon oxygen tensions in the red muscle of rainbow trout. J Exp Biol 207: 3629–3637. [DOI] [PubMed] [Google Scholar]
- 21. Rummer JL, McKenzie DJ, Innocenti A, Supuran CT, Brauner CJ (2013) Enhanced muscle oxygen delivery may represent the incipient function of the Root effect in ray-finned fishes. Science 340: 1327–1329. 10.1126/science.1233692 [DOI] [PubMed] [Google Scholar]
- 22. Randall DJ, Rummer JL, Wilson JM, Wang S, Brauner CJ (2014) A unique mode of tissue oxygenation and the adaptive radiation of teleost fishes. J Exp Biol 217: 1205–1214. 10.1242/jeb.093526 [DOI] [PubMed] [Google Scholar]
- 23. Rummer JL, Brauner CJ (2011) Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: in vitro evidence in rainbow trout, Oncorhynchus mykiss . J Exp Biol 214: 2319–2328. 10.1242/jeb.054049 [DOI] [PubMed] [Google Scholar]
- 24. Tyuma I, Ueda Y (1975) Non-linear relationship between oxygen saturation and proton release, and equivalence of the Bohr and Haldane coefficients in human hemoglobin. Biochem Biophys Res Commun 65: 1278–1283. [DOI] [PubMed] [Google Scholar]
- 25. Brauner CJ, Gilmour KM, Perry SF (1996) Effect of haemoglobin oxygenation on Bohr proton release and CO2 excretion in the rainbow trout. Respir Physiol 106: 65–70. [DOI] [PubMed] [Google Scholar]
- 26. Brauner CJ, Wang T, Val AL, Jensen FB (2001) Non-linear release of Bohr protons with haemoglobin-oxygenation in the blood of two teleost fishes; carp (Cyprinus carpio) and tambaqui (Colossoma macropomum). Fish Physiol Biochem 24: 97–104. [Google Scholar]
- 27. Jensen FB (1986) Pronounced influence of Hb-O2 saturation on red cell pH in tench blood in vivo and in vitro . J Exp Zool 238: 119–124. [DOI] [PubMed] [Google Scholar]
- 28. Brauner, Thorarensen H, Gallaugher P, Farrell A, Randall D (2000) The interaction between O2 and CO2 exchange in rainbow trout during graded sustained exercise. Respir Physiol 119: 83–96. [DOI] [PubMed] [Google Scholar]
- 29.Rummer JL (2010) A novel mechanism for enhancing tissue oxygen delivery in teleost fishes [Ph.D. thesis]. Vancouver: University of British Columbia. 171 p.
- 30. Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, et al. (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332: 109–112. 10.1126/science.1199158 [DOI] [PubMed] [Google Scholar]
- 31. Boutilier R, Iwama G, Randall D (1986) The promotion of catecholamine release in rainbow trout, Salmo gairdneri, by acute acidosis: interactions between red cell pH and haemoglobin oxygen-carrying capacity. J Exp Biol 123: 145–157. [DOI] [PubMed] [Google Scholar]
- 32. Adrogue HJ, Rashad MN, Gorin AB, Yacoub J, Madias NE (1989) Assessing acid-base status in circulatory failure—differences between arterial and central venous-blood. N Engl J Med 320: 1312–1316. [DOI] [PubMed] [Google Scholar]
- 33. Kelly AM, McAlpine R, Kyle E (2001) Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J 18: 340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malatesha G, Singh NK, Bharija A, Rehani B, Goel A (2007) Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J 24: 569–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Middleton P, Kelly AM, Brown J, Robertson M (2006) Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J 23: 622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brauner C, Weber R (1998) Hydrogen ion titrations of the anodic and cathodic haemoglobin components of the European eel Anguilla anguilla . J Exp Biol 201: 2507–2514. [DOI] [PubMed] [Google Scholar]
- 37. Meier U, Böning D, Rubenstein HJ (1974) Oxygenation dependent variations of the Bohr coefficient related to whole blood and erythrocyte pH. Pflügers Archiv 349: 203–213. [DOI] [PubMed] [Google Scholar]
- 38. Winslow R (2005) Oxygen transport agents: A new approach to red blood cell alternatives Vancouver: University of British Columbia Centre for Blood Research. [Google Scholar]
- 39. Winslow RM, Swenberg ML, Berger RL, Shrager RI, Luzzana M, Samaja M, et al. (1977) Oxygen equilibrium curve of normal human blood and its evaluation by Adair's equation. J Biol Chem 252: 2331–2337. [PubMed] [Google Scholar]
- 40. Roughton FJ, Severinghaus JW (1973) Accurate determination of O2 dissociation curve of human blood above 98.7 percent saturation with data on O2 solubility in unmodified human blood from 0 degrees to 37 degrees C. J Appl Physiol 35: 861–869. [DOI] [PubMed] [Google Scholar]
- 41. Vorger P (1985) The Bohr effect of the blood in rainbow trout (Salmo gairdneri). A comparative study with human blood, using precise oxygen equilibrium curves and the Adair model. Comp Biochem Physiol 82A: 915–924. [DOI] [PubMed] [Google Scholar]
- 42. Nikinmaa M, Vihersaari L (1993) Pre- and postbranchial carbon dioxide content of rainbow trout (Oncorhynchus mykiss) blood after catecholamine injection. J Exp Biol 180: 315–321. [Google Scholar]
- 43. Kiceniuk JW, Jones DR (1977) The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J Exp Biol 69: 247–260. [Google Scholar]
- 44. Brauner CJ, Jensen FB (1999) O2 and CO2 Exchange in Fish: The Nonlinear release of Bohr/Haldane protons with oxygenation In: Val AL, Almeida-Val V, editors. Biology of Tropical Fishes. Manaus, Brazil: INPA; pp. 393–400. [Google Scholar]
- 45. Farrell AP, Clutterham SM (2003) On-line venous oxygen tensions in rainbow trout during graded exercise at two acclimation temperatures J Exp Biol 206: 487–496. [DOI] [PubMed] [Google Scholar]
- 46. North BP, Turnbull JF, Ellis T, Porter MJ, Miguad H, Bron J, et al. (2006) The impact of stocking density on the welfare of rainbow trout (Oncorhynchus mykiss). Aquaculture 5: 466–479. [Google Scholar]
- 47. Wolf K (1963) Physiological salines for freshwater teleosts. N Am J Aquac 25: 135–140.` [Google Scholar]
- 48. Caldwell S, Rummer JL, Brauner CJ (2006) Blood sampling techniques and storage duration: Effects on the presence and magnitude of the red blood cell ß -adrenergic response in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A 144: 188–195. [DOI] [PubMed] [Google Scholar]
- 49. Tetens V, Lykkeboe G, Christensen NJ (1988) Potency of adrenaline and noradrenaline for ß-adrenergic proton extrusion from red cells of rainbow trout, Salmo gairdneri . J Exp Biol 134: 267–280. [DOI] [PubMed] [Google Scholar]
- 50. Tucker VA (1967) Method for oxygen content and dissociation curves on microliter blood samples. J Appl Physiol 23: 410–414. [DOI] [PubMed] [Google Scholar]
- 51. Zeidler R, Kim DH (1977) Preferential hemolysis of postnatal calf red cells induced by internal alkalinization. J Gen Physiol 70: 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boutilier RG, Heming TA, Iwama GK (1984) Appendix—Physicochemical parameters for use in fish respiratory physiology. Fish Physiol 10: 403–430. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)
(EPS)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Metadata have been archived on Dryad via doi:10.5061/dryad.d0325.