Abstract
Use of long-acting progestin only contraceptives (LAPCs) offers a discrete and highly effective family planning method. Abnormal uterine bleeding (AUB) is the major side effect of, and cause for, discontinuation of LAPCs. The endometria of LAPC-treated women display abnormally enlarged, fragile blood vessels, decreased endometrial blood flow and oxidative stress. To understanding to mechanisms underlying AUB, we propose to identify LAPC-modulated unique gene cluster(s) in human endometrial stromal cells (HESCs). Protein and RNA isolated from cultured HESCs treated 7 days with estradiol (E2) or E2+ medroxyprogesterone acetate (MPA) or E2+ etonogestrel (ETO) or E2+ progesterone (P4) were analyzed by quantitative Real-time (q)-PCR and immunoblotting. HSCORES were determined for immunostained-paired endometria of pre-and 3 months post-Depot MPA (DMPA) treated women and ovariectomized guinea pigs (GPs) treated with placebo or E2 or MPA or E2+MPA for 21 days. In HESCs, whole genome analysis identified a 67 gene group regulated by all three progestins, whereas a 235 gene group was regulated by E2+ETO and E2+MPA, but not E2+P4. Ingenuity pathway analysis identified glucocorticoid receptor (GR) activation as one of upstream regulators of the 235 MPA and ETO-specific genes. Among these, microarray results demonstrated significant enhancement of FKBP51, a repressor of PR/GR transcriptional activity, by both MPA and ETO. q-PCR and immunoblot analysis confirmed the microarray results. In endometria of post-DMPA versus pre-DMPA administered women, FKBP51 expression was significantly increased in endometrial stromal and glandular cells. In GPs, E2+MPA or MPA significantly increased FKBP51 immunoreactivity in endometrial stromal and glandular cells versus placebo- and E2-administered groups. MPA or ETO administration activates GR signaling and increases endometrial FKBP51 expression, which could be one of the mechanisms causing AUB by inhibiting PR and GR-mediated transcription. The resultant PR and/or GR-mediated functional withdrawal may contribute to associated endometrial inflammation, aberrant angiogenesis, and bleeding.
Introduction
Long-acting progestin-only contraceptives (LAPCs) are safe, effective and inexpensive making them particularly ideal for use by women in underdeveloped countries with limited access to medical care [1]. Unlike estrogen-containing contraceptives, these progestins can be used safely during lactation and by women in whom estrogen use is contraindicated [2]. Currently available formulations include the depot form of medroxyprogesterone acetate (DMPA), an injectable form whose action persists for three months; intrauterine systems (IUS) s that release levonorgestrel (LNG) for either three or five years; and a single subdermal implantable rod, which releases etonogestrel (ETO) for three years [3–5].
More than a million unintended pregnancies occur in the U.S. each year due to the misuse or discontinuation of contraceptives [6]. The major reason for discontinuation of LAPC is the occurrence of abnormal uterine bleeding (AUB) [1, 7], which is a source of personal annoyance, discomfort and/or religious and social taboo in specific societies [7, 8]. Unlike menstrual bleeding originating globally from spiral arterioles in response to progesterone (P4) withdrawal, LAPC-associated AUB occurs intermittently and focally from irregularly distributed superficial, abnormally enlarged, fragile microvessels [9, 10]. Microscopic examination of endometrial biopsies from adjacent bleeding (BL) and non-bleeding (NBL) sites in LAPC users revealed that only BL sites displayed these abnormal microvessels enmeshed in a compromised extracellular matrix (ECM). These damaged microvessels are contiguous with decidualized human endometrial stromal cells (HESCs) expressing high levels of tissue factor (TF), the primary initiator of hemostasis via factor VIIa and factor Xa/Va generated thrombin [11, 12]. Thus, LAPC-induced microvascular damage should contribute to excess local thrombin generation by increasing delivery of circulating clotting factors to HESC-derived TF. While thrombin prevents bleeding by activating platelets and generating fibrin, it binds to protease activated receptors (PARs) on human endometrial endothelial cell (HEEC) to increase endothelial permeability [13]. Thrombin also binds HESC-expressed PAR–1 to promote aberrant angiogenesis and inflammation by increasing levels of vascular endothelial growth factor (VEGF) [14] and interleukin–8 (IL–8) [15]. Thrombin also induces HESC-derived matrix metalloproteinase (MMP)-1, which preferentially degrades interstitial collagens, and MMP–3, which, in turn, degrades several other ECM proteins and activates secreted MMP zymogens [13]. These proteolytic and inflammatory processes markedly contribute to LAPC-induced AUB.
The FK506-binding proteins (FKBP51 and FKBP52) belong to a family of immunophilins that mediate the actions of specific immunosuppressive drugs [16, 17]. Two members, FKBP51 and FKBP52, share 70% homology and contain a tetracopeptide repeat domain that binds to the C-terminus of heat shock protein 90 (Hsp–90). The FKBP51 promoter region and introns contain several progesterone or glucocorticoid response elements (PREs or GREs), which mediate transcriptional induction of FKBP51 by progesterone receptor (PR) and/or glucocorticoid receptor (GR) [17]. In turn, elevated levels of FKBP51 can inhibit transcriptional activity of both PR and GR [18–20]. We posit that in HESCs, FKBP51 causes functional P4 and/or glucocorticoid (GC) withdrawal by inhibiting transcriptional activity of PR and/or GR.
The contraceptive action of LAPCs results from inhibition of ovulation, thickening of the cervical mucus and impaired cyclic changes in the functional endometrium that impede implantation [21]. However, the LAPC-induced cellular and molecular changes that trigger AUB are poorly understood. This study hypothesizes that MPA or ETO modulates the expression of unique gene cluster(s) in endometrial cells, which initiate molecular changes that induce AUB. To identify these molecular modifications, we performed whole genome microarray analysis on primary cultures of HESCs treated with progestogens. Comparative analysis determined unique (individual) and common signaling pathways induced by MPA or ETO vs. P4. Whole genome analysis of the current study identified FKBP51 as one of genes significantly induced by MPA and ETO, but not P4. Therefore, subsequent experiments evaluated the regulation of FKBP51 in cultured HESCs as well as paired endometrial tissues obtained from women pre- and post-DMPA therapy. Previous studies in our laboratory demonstrated that the guinea pig (GP) is a relevant model with which to evaluate the endometrial effects of LAPCs. Like human endometrium, the GP endometrium displays closely related human features such as spontaneous estrus cycling and hemochorial placentation [22]. Thus, findings obtained from HESC cultures and human tissues were augmented by observations in the endometrium of MPA-treated GPs. In addition given the critical role that thrombin plays in mediating LAPC-induced AUB, we evaluated the effects of FKBP51 on thrombin modulation of inflammation in MPA-treated HESCs.
Materials and Methods
Antibodies and Compounds
The FKBP51 goat polyclonal antibody (Cat# AF4094) was obtained from R&D Systems, Minneapolis, MN. The β-actin (Cat# 5125), total and phosphorylated AKT (Cat#4085 and 4060, respectively) and total and phosphorylated ERK1/2 MAPK (Cat# 4695 and 4377, respectively) rabbit monoclonal antibodies were purchased from Cell Signaling Technology, Beverly, MA. The following secondary antibodies were obtained from Vector Labs, Burlingame, CA; peroxidase-conjugated anti-goat (Cat# PI–9500), biotinylated anti-goat (Cat# BA–0500). The following chemicals were obtained from Sigma-Aldrich, St. Louis, MO; estradiol (E2, Cat#E8875), progesterone (Cat#P8783) and MPA (Cat#M1629). ETO was purchased from Organon, Roseland, NJ.
Cell Cultures
Frozen HESCs was acquired from previously banked samples isolated and characterized as previously described [23] from specimens of human endometrium obtained from reproductive age undergoing hysterectomy for benign disease after obtaining informed written consent under approval of the Yale University School of Medicine Human Investigation Committee (HIC#22334) and pending approval of University of South Florida (HIC#Pro00019480). All samples used in our study were de-identified when they were obtained. Thawed HESCs (n = 6) were grown to confluence in basal medium (BM), a phenol red-free 1:1 v/v mix of Dulbecco's MEM/Ham's F–12 (Gibco, Grand Island, NY), with 100U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone complex (Gibco) supplemented with 10% charcoal-stripped calf serum (Gibco). Confluent HESCs were incubated in parallel in BM with 0.1% ethanol (vehicle control) or 10−8 M E2 or E2+10−7 M P4 (E2+P4), or 10−7 M of ETO (E2+ETO) or 10−7 M of MPA (E2+MPA). After 7 days, the cultures were washed twice with 1X phosphate-buffered saline (PBS) to remove residual serum, then placed in a serum-free defined media (DM) comprising BM plus ITS+ (insulin, transferrin, selenious and linoleic acid) premix (BD Biosciences, Bedford, MA), 5 μM of FeSO4, 50 μM of ZnSO4, 1 nM of CuSO4, 20 nM of Na2SeO3 trace elements (Sigma-Aldrich), 50 μg/ml of ascorbic acid (Sigma-Aldrich) and 50 ng/ml of recombinant epidermal growth factor (Becton-Dickinson, Bedford, MA) for 24h. The cultures were washed twice with 1X PBS to remove the residual steroids and DM containing corresponding vehicle control or steroids was added to the cultures for 6h or 24h. At the end of incubation periods, HESCs were washed with ice-cold 1X PBS and stored at −70°C until used for total RNA and protein extraction.
Microarray Analysis
Total RNA from HESCs treated with steroids for 6h was isolated using miRNeasy Mini Kit and RNeasy MinElute Cleanup Kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). The quality of isolated RNAs was confirmed using an Agilent 2100 Bioanalyzer. Extracted total RNAs were sent to the Keck Biotechnology Resource Laboratory at Yale University (New Haven, CT, USA) for microarray analysis using Illumina Human HT–12 v4 Expression BeadChip (Illumina Inc., San Diego, CA). Microarray analysis was limited to specimens whose RNA Integrity Number (RIN) value exceeded 8. Sample labeling and hybridization was performed per manufacturer’s instructions. Raw data without normalization were analyzed by GeneSpring GX12.5 software (Agilent Technologies-Silicon Genetics, Redwood City, CA). Gene readouts were normalized to the 75th percentile of the distribution of all measurements in each chip. Normalization for each gene across chips was performed using the median value of each gene throughout different chips in the same experimental condition. Normalized data were first filtered to eliminate the genes, which were absent in all experimental conditions and replicates. The resulting data were filtered on volcano plot with moderated t-test without multiple testing corrections. Genes with a fold change of > 1.25 and a p-value of < 0.05 were considered differentially expressed. Molecular functions and biological networks related to differentially expressed genes were explored using Ingenuity Pathway Analysis (IPA) software (Ingenuity Systems, Redwood City, CA) according to manufacturer’s instructions. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE72040 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72040).
Reverse Transcription and Quantitative Real Time (q)-PCR Analysis
Total RNA from cultured HESCs was isolated using miRNeasy mini kit and RNeasy MinElute cleanup kit according to the manufacturer’s instructions (Qiagen). Reverse transcription was performed using RETROscript kit (Ambion, Austin, TX) in two steps: first, 2 μg of total RNA from each sample was incubated with random decamer primers at 85°C for 3 min to eliminate any secondary structures, then a reverse transcription reaction was carried out at 42°C for 1 h consisting of 2 μl of 10X reverse transcription buffer, 4 μl of 1.25 mM dNTP mix, 1 μl of RNase inhibitor (10 units/μl) and 1 μl of MMLV-reverse transcriptase enzyme (100 units/μl). Subsequently, inactivation of the reverse transcriptase enzyme at 92°C for 10 min was performed.
The expression of FKBP51, insulin-like growth factor binding protein 2 (IGFBP2), four jointed box 1 (FJX1), thrombin receptor like–2 (F2RL2, aka proteinase-activated receptor–3; PAR3), ADAM metallopeptidase with thrombospondin type 1 motif, 1 (ADAMTS1), IL-1β, and β-actin was determined by q-PCR using TaqMan Gene Expression Assay according to the manufacturer's protocol. In brief, 10 μL of TaqMan 2× Universal PCR Master Mix was combined with 8.5 μL of nuclease-free water, 1 μL of 20× TaqMan primer/probe mix, and 0.5 μL of cDNA in the PCR tube. Amplification used 40 cycles of PCR in Applied Biosystems 7500 Real-Time PCR Detection System (Applied Biosystems, Foster City, CA), with the following program: initial denaturation at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 60 seconds. All samples were run in triplicate and the average used for each sample. A standard curve for each set of primers was first used to determine the linear dynamic range of each reaction and the PCR efficiency. Expression of the target mRNAs was normalized to β-actin levels and the 2−ΔΔCt (cycle threshold) method was used to calculate relative expression levels. Results are reported as fold change in gene expression levels among the different groups. The following TaqMan gene expression assays were used: FKBP51 (ID# Hs01561006_m1); IGFBP2; (ID# Hs01040719_m1); FJX1 (ID# Hs00534909_m1); F2RL2 (ID#Hs00765740); ADAMTS1 (ID# Hs00199608_m1); IL-1β (ID#Hs1555410_m1); and β-actin (ID# Hs99999903_m1) (Applied Biosystems).
FKBP51 overexpression
To over-expression of FKBP51 in HESCs, expression vector containing the full-length FKBP51 gene open reading frame (pCMV6-FKBP51) and empty vector (pCMV6) were purchased from Origene Inc. (Rockville, MD). Transient transfection were performed in HESC (approximately 70% confluence) using Lipofectamine LTX reagent (Invitrogen, Carlsbad, CA) in Opti-MEM I serum reduced medium (Invitrogen) according to manufacturer’s instructions. Following 44 h of transfection, HESC were incubated with E2 alone or E2+MPA±thrombin (1U/ml, American Diagnostic, Greenwich, CT) for 4h then washed with ice-cold PBS twice and stored in -80°C for subsequent experiments. Transfection efficiency for over-expression of FKBP51 was confirmed by q-PCR and immunoblotting analyses.
Immunoblot Analysis
To evaluate FKBP51 protein levels, cell lysates from HESCs cultured for 24h with E2 or E2+P4 or E2+MPA or E2+ETO were diluted in 2X sample buffer (Bio-Rad, Hercules, CA) and then boiled for 5 minutes. The cell lysates were subjected to reducing SDS-PAGE on a 10% Tris-HCl gel (Bio-Rad), with subsequent electroblotting transfer onto a 0.45-μm nitrocellulose membrane (Bio-Rad). After transfer, the membranes were blocked overnight in Tris-Buffered Saline (TBS) with 10% non-fat dry milk and then incubated with goat anti-FKBP51 polyclonal antibody at 1/1000 dilution (R&D Systems) or with rabbit anti-total (T) and phosphorylated (P)-AKT, or with rabbit anti-T and P-ERK1/2 MAPK antibodies (Cell Signaling) at 1/1000 dilution for primary antibody labeling. Membranes were rinsed in TBS-T (TBS with 0.1% Tween 20) subsequently incubated with peroxidase-conjugated anti-goat IgG at 1/5000 dilution (Vector Labs) and signals were developed using a chemiluminescence kit (Amersham; GE Healthcare, Piscataway, NJ). The membranes was sequentially stripped and re-probed with peroxidase-conjugated anti-β-actin rabbit monoclonal antibody at 1/1000 dilution (Cell Signaling Technology).
Guinea Pig Studies
Endometrial samples were obtained from oophorectomized (OVX) GPs treated in parallel with placebo or E2 or MPA or E2+MPA under the approval of Yale Institutional Animal Care and Use Committee (IACUC#2006–11002). Twelve nulliparous GPs, aged 2–6 months, were subjected to bilateral oophorectomy. Three GPs for each treatment group were given subcutaneous 50 mg MPA, cholesterol-based 21 day time-release (2.4 mg/day) pellets or 5 mg E2 cholesterol based 21 day time-release (240 μg/day) pellets or E2+MPA or placebo pellets (Innovative Research of America; Sarasota, FL). After three weeks, hysterectomy was performed and the right uterine horn was formalin-fixed for immunohistochemical studies.
Human Endometrial Tissues
Paraffin sections were derived from previously banked paired endometrial tissues obtained prior to DMPA injection (pre-DMPA) in the secretory phase and 80 to 90 days after initial DMPA injection (post-DMPA). Endometrial aspiration biopsy was used to obtain samples from woman with regular menstrual cycles during the secretory phase (n = 6), while hysteroscopic biopsy was used to obtain endometrial specimens from women following DPMA injection after receiving written informed consent at New York University under Institutional Review Board approval (#H6023) and IRB approval of University of South Florida (#Pro00019480). All samples used in our study were de-identified when they were obtained.
Immunohistochemistry
Paraffin sections of human and GP endometria were deparaffinized in xylene and rehydrated through a descending ethanol series. Antigen retrieval involved boiling the slides in citrate buffer (pH:6.0) followed by endogenous peroxidase quenching with 3% hydrogen peroxide and blocking non-specific antibody binding with 5% normal horse serum (Vector Labs). The sections were incubated overnight at 4°C with goat anti-FKBP51 polyclonal IgG at 1/200 dilution (R&D Systems). After several rinses in TBS-T, slides were incubated with biotinylated horse anti-goat IgG at 1/5000 dilution (Vector Labs) for 30 min, rinsed in TBS-T x3 and then incubated with a streptavidin-peroxidase complex (Elite ABC kit; Vector Labs). Diaminobenzidine tetrahydrochloride dehydrate (DAB; Vector Labs) was used as a chromogen (brown stain) to visualize immunoreactivity. Hematoxylin was used for background staining. Non-immune goat IgG was used at the same concentration as the primary antibody as a negative control.
The intensity of FKBP51 immunostaining was semi-quantitatively evaluated by HSCORE analysis and categorized into the following scores: 0 (no staining), 1+ (weak, but detectable, staining), 2+ (moderate staining), and 3+ (intense staining). An HSCORE value was derived for each specimen by calculating the sum of the percentage of cells stating in each category multiplied by its respective intensity score using the formula HSCORE = ∑i i*Pi, where i represents the intensity category score, and Pi is the corresponding percentage of cells [24]. For each slide, five different fields were evaluated microscopically at 200Χ magnification. HSCORE evaluation was performed independently by two investigators blinded to the source of the samples; the average score of both was then used.
Database search of Transcription factor binding sites
The MatInspector and GeneCards program (http://www.genomatix.de and http://www.genecards.org) were used to identify GR binding sites.
Statistical analysis
Immunostaining HSCOREs of FKBP51 in GP endometria, immunoblotting and q-PCR results were each normally distributed as determined by Kolmogorov-Smirnov test and each data set analyzed by One-way ANOVA followed by testing post-hoc Holm-Sidak method. p<0.05 is accepted as statistically significant. Immunostaining HSCOREs for human endometrial tissues from pre- and post-DMPA administration were compared using a t-test. Statistical calculations used SigmaStat version 3.0 software (Systat Software, San Jose, CA).
Results
MPA or ETO modulated gene expression in HESCs
The results of whole genome microarray analysis of cultured HESCs displayed in Table 1 indicate that compared with treatment with E2, E2+MPA or E2+ETO significantly altered transcription of 378 and 538 genes, respectively, whereas E2+P4 modified transcription of only 171 genes. Specifically, among: 1) 378 differentially regulated genes by E2+MPA, 222 genes (59%) were upregulated and 156 (41%) were downregulated; 2) 538 differentially regulated genes by E2+ETO, 263 (49%) were upregulated and 275 (51%) were downregulated; 3) 171 differentially regulated genes by E2+P4, 150 (88%) were upregulated and 21 (12%) were downregulated (Table 1). The 40 most highly regulated genes in HESCs in response to E2+MPA or E2+ETO or E2+P4 were given in Tables 2–4.
Table 1. Comparison of number of differentially regulated genes in cultured HESCs treated by E2+MPA, E2+ETO and E2+P4 vs. E2 according to whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit analysis (n = 3/group).
| Total number of genes altered | Number of genes upregulated | Number of genes downregulated | |
|---|---|---|---|
| E 2 +MPA (n = 3) | 378 | 222 (59%) | 156 (41%) |
| E 2 +ETO (n = 3) | 538 | 263 (49%) | 275 (51%) |
| E 2 +P4 (n = 3) | 171 | 150 (88%) | 21 (12%) |
Table 2. 40 most highly differentially regulated genes in HESCs treated by E2+MPA vs. E2 alone according to whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit.
All gene symbols were abbreviated according to GENEBANK standard nomenclature.
| Symbol | Fold Change | Location | Function |
|---|---|---|---|
| PARM1 | 3.897 | Extracellular Space | Other |
| ADAMTS1 | 2.995 | Extracellular Space | Peptidase |
| CLEC3B | 2.963 | Extracellular Space | Other |
| IGFBP2 | 2.832 | Extracellular Space | Other |
| ADH1A | 2.724 | Cytoplasm | Enzyme |
| CRISPLD2 | 2.621 | Cytoplasm | Other |
| CILP | 2.553 | Extracellular Space | Phosphatase |
| AOX1 | 2.550 | Cytoplasm | Enzyme |
| APOD | 2.541 | Extracellular Space | Transporter |
| ZBTB16 | 2.536 | Nucleus | transcription regulator |
| CFD | 2.465 | Extracellular Space | Peptidase |
| MAOA | 2.415 | Cytoplasm | Enzyme |
| MUM1L1 | 2.301 | Cytoplasm | Other |
| MEDAG | 2.139 | Cytoplasm | Other |
| FKBP5 (FKBP51) | 2.133 | Nucleus | Enzyme |
| CHST7 | 2.109 | Cytoplasm | Enzyme |
| TSC22D3 | 2.103 | Nucleus | transcription regulator |
| IMPA2 | 2.068 | Cytoplasm | Phosphatase |
| ITPR1 | 2.057 | Cytoplasm | ion channel |
| PPAP2B | 2.015 | Plasma Membrane | Phosphatase |
| FJX1 | -3.341 | Extracellular Space | Other |
| MMP11 | -3.047 | Extracellular Space | Peptidase |
| IL17RB | -2.651 | Plasma Membrane | transmembrane receptor |
| COL8A1 | -2.137 | Extracellular Space | Other |
| TENM4 | -2.104 | Other | Other |
| TFRC | -2.091 | Plasma Membrane | Transporter |
| EGR2 | -2.055 | Nucleus | transcription regulator |
| MYLIP | -1.951 | Cytoplasm | Enzyme |
| SFRP4 | -1.879 | Plasma Membrane | transmembrane receptor |
| IRS1 | -1.797 | Cytoplasm | Enzyme |
| AFAP1L2 | -1.784 | Cytoplasm | Other |
| PAMR1 | -1.765 | Extracellular Space | Peptidase |
| F2RL1 | -1.751 | Plasma Membrane | G-protein coupled receptor |
| PGR | -1.744 | Nucleus | ligand-dependent nuclear receptor |
| BMP6 | -1.726 | Extracellular Space | growth factor |
| STC2 | -1.724 | Extracellular Space | Other |
| F2RL2 | -1.701 | Plasma Membrane | G-protein coupled receptor |
| ETV5 | -1.68 | Nucleus | transcription regulator |
| KAL1 | -1.677 | Extracellular Space | Other |
| CDKN2B | -1.663 | Nucleus | transcription regulator |
Table 4. 40 most highly differentially regulated genes in HESCs treated by E2+P4 vs. E2 alone according to whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit.
All gene symbols were abbreviated according to GENEBANK standard nomenclature.
| Symbol | Fold Change | Location | Function |
|---|---|---|---|
| CLEC3B | 2.217 | Extracellular | Other |
| ADH1A | 1.973 | Cytoplasm | enzyme |
| SPARCL1 | 1.970 | Extracellular | other |
| PDGFD | 1.835 | Extracellular | growth factor |
| IGFBP2 | 1.819 | Extracellular | other |
| ADAMTS1 | 1.714 | Extracellular | peptidase |
| CILP | 1.701 | Extracellular | phosphatase |
| CRISPLD2 | 1.607 | Cytoplasm | other |
| AOX1 | 1.528 | Cytoplasm | enzyme |
| LMO3 | 1.517 | Other | other |
| TIMP3 | 1.516 | Extracellular | other |
| BCHE | 1.513 | Plasma Membrane | enzyme |
| OMD | 1.498 | Other | other |
| SULF2 | 1.477 | Plasma Membrane | enzyme |
| GAS1 | 1.466 | Plasma Membrane | other |
| LAMA2 | 1.461 | Extracellular | other |
| RPL14 | 1.435 | Other | other |
| MASP1 | 1.431 | Extracellular | peptidase |
| TMEM45A | 1.430 | Plasma Membrane | other |
| FBLN5 | 1.426 | Extracellular | other |
| SERPING1 | 1.421 | Extracellular | other |
| ANTXR1 | 1.418 | Plasma Membrane | transmembrane receptor |
| IL17RB | -1.926 | Plasma Membrane | transmembrane receptor |
| MMP11 | -1.794 | Extracellular | peptidase |
| FJX1 | -1.682 | Extracellular | other |
| COL8A1 | -1.664 | Extracellular | other |
| VGF | -1.404 | Extracellular | growth factor |
| SDC1 | -1.399 | Plasma Membrane | enzyme |
| TENM4 | -1.383 | Other | other |
| SFRP4 | -1.383 | Plasma Membrane | transmembrane receptor |
| AFAP1L2 | -1.374 | Cytoplasm | other |
| DPYSL4 | -1.358 | Cytoplasm | enzyme |
| HSPE1 | -1.354 | Cytoplasm | enzyme |
| TFRC | -1.339 | Plasma Membrane | transporter |
| KCNIP4 | -1.292 | Plasma Membrane | ion channel |
| MYLIP | -1.291 | Cytoplasm | enzyme |
| GADD45G | -1.262 | Nucleus | other |
| DBNDD1 | -1.259 | Other | other |
| RFX7 | -1.257 | Other | other |
| RALGDS | -1.251 | Cytoplasm | other |
Table 3. 40 most highly differentially regulated genes in HESCs treated by E2+ETO vs. E2 alone according to whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit.
All gene symbols were abbreviated according to GENEBANK standard nomenclature.
| Symbol | Fold Change | Location | Function |
|---|---|---|---|
| PARM1 | 4.546 | Extracellular Space | other |
| ADAMTS1 | 3.293 | Extracellular Space | peptidase |
| IGFBP2 | 3.117 | Extracellular Space | other |
| APOD | 3.068 | Extracellular Space | transporter |
| ZBTB16 | 2.972 | Nucleus | transcription regulator |
| ALDH1A3 | 2.820 | Cytoplasm | enzyme |
| ADH1A | 2.717 | Cytoplasm | enzyme |
| CLEC3B | 2.705 | Extracellular Space | other |
| MUM1L1 | 2.648 | Cytoplasm | other |
| MAOA | 2.592 | Cytoplasm | enzyme |
| CRISPLD2 | 2.588 | Cytoplasm | other |
| CHST7 | 2.580 | Cytoplasm | enzyme |
| CILP | 2.442 | Extracellular Space | phosphatase |
| FKBP5(FKBP51) | 2.357 | Nucleus | enzyme |
| AOX1 | 2.353 | Cytoplasm | enzyme |
| IMPA2 | 2.348 | Cytoplasm | phosphatase |
| RGS2 | 2.335 | Nucleus | other |
| PPAP2B | 2.186 | Plasma Membrane | phosphatase |
| MEDAG | 2.179 | Cytoplasm | other |
| CFD | 2.137 | Extracellular Space | peptidase |
| FJX1 | -3.325 | Extracellular Space | other |
| COL8A1 | -2.569 | Extracellular Space | other |
| IL17RB | -2.263 | Plasma Membrane | transmembrane receptor |
| MMP11 | -2.239 | Extracellular Space | peptidase |
| TFRC | -2.137 | Plasma Membrane | transporter |
| TENM4 | -2.100 | Other | other |
| GREM1 | -2.019 | Extracellular Space | other |
| MYLIP | -1.984 | Cytoplasm | enzyme |
| IRS1 | -1.966 | Cytoplasm | enzyme |
| ETV5 | -1.946 | Nucleus | transcription regulator |
| EGR2 | -1.939 | Nucleus | transcription regulator |
| TNFAIP6 | -1.924 | Extracellular Space | other |
| BMP6 | -1.890 | Extracellular Space | growth factor |
| F2R | -1.861 | Plasma Membrane | G-protein coupled receptor |
| SFRP4 | -1.849 | Plasma Membrane | transmembrane receptor |
| STC2 | -1.848 | Extracellular Space | other |
| F2RL1 | -1.837 | Plasma Membrane | G-protein coupled receptor |
| CDKN2B | -1.803 | Nucleus | transcription regulator |
| PGR | -1.775 | Nucleus | ligand-dependent nuclear receptor |
| CADM1 | -1.758 | Plasma Membrane | other |
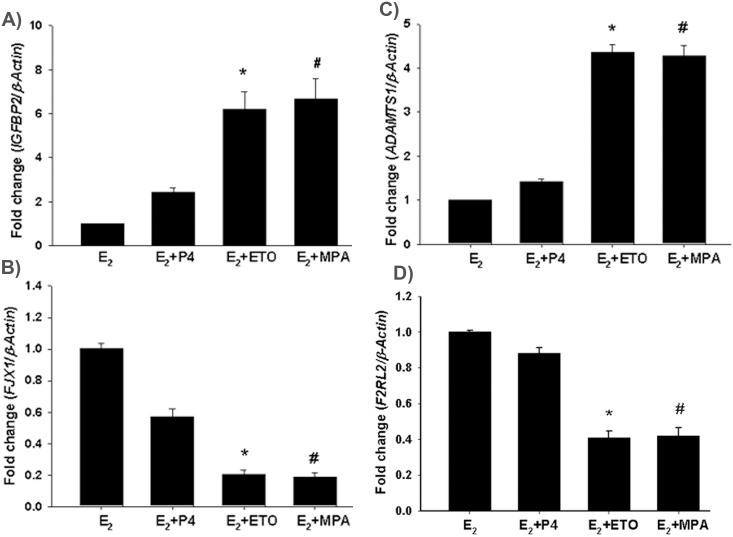
Several of the highly regulated genes (Tables 2–4) identified by microarray analysis responded to either MPA or ETO, but not to P4. This observation suggested that modulation of these genes by MPA and ETO may reflect a glucocorticoid action. This observation was supported by use of the web-based programs i.e. MatInspector and GeneCards (http://www.genomatix.de and http://www.genecards.org) which identified the presence of GREs in the promoter of the IGFBP2, ADAMTS1, F2RL2 and FKBP51 genes, but not in the FJX1 gene promoter (S1 Fig). Thus, these MPA and ETO specific regulated genes obtained from microarray analysis were confirmed by performing q-PCR on five genes (IGFBP2, FJX1, F2RL2, ADAMTS1, and FKBP51) found to be differentially regulated in HESCs following treatment with E2+MPA or E2+ETO (Fig 1A–1D). In parallel with microarray results, IGFBP2 and ADAMTS1 mRNA levels are significantly upregulated by E2+ETO or E2+MPA (Fig 1A and 1C), whereas FJX1 and F2RL2 mRNA levels are significantly downregulated by E2+MPA and E2+ETO (Fig 1B and 1D). By comparison, none of the modest responses elicited by P4 for each of the endpoints attained statistical significance (Fig 1A–1D).
Fig 1. Conformational evaluation of genes commonly regulated by all three progestins.
q-PCR for (A) IGFBP2, (B) FJX1, (C) ADAMTS1 and (D) F2RL2 (D) in HESCs. HESC treated with estradiol (E2, 10−8M) ± progesterone (P4, 10−7 M) or etonogestrel (ETO, 10−7 M) or medroxyprogesterone acetate (MPA, 10−7 M) for 6h. Bars represent mean ± SEM (n = 3). * p<0.001 in E2+ETO vs. E2; # p<0.001 in E2+MPA vs. E2.
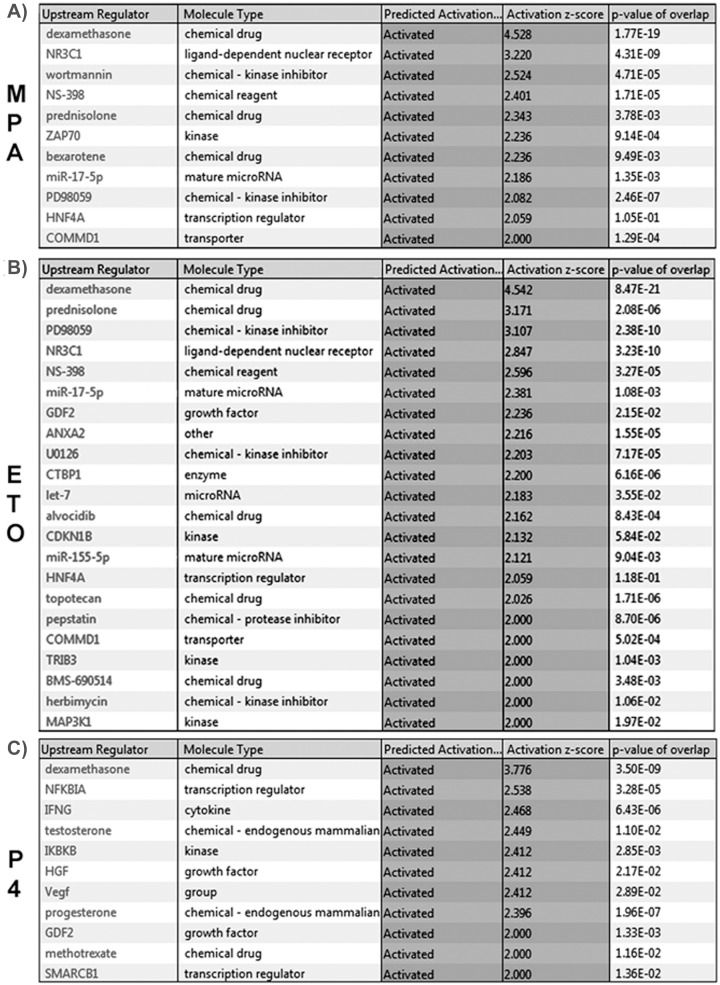
Evaluation of the microarray results using IPA revealed associations between differentially regulated gene clusters and activation of several common and unique upstream regulators in HESCs in response to either E2+MPA or E2+ETO or E2+P4 treatment (see Z-value and overlap p-value for each progestogen; Fig 2). Among the signaling pathways revealed by IPA, inspection of Fig 2 indicates that genes altered by each of the progestins are associated with activation of dexamethasone mediated signaling, whereas genes altered by treatment with E2+ETO or E2+MPA (Fig 2A and 2B), but not E2+P4 (Fig 2C), are associated with ligand-dependent activation of GR (NR3C1, see S2 Fig), PD98059 (ERK1/2 MAPK inhibitor) and miR-17-5p (a mature cytoplasmic micro-RNA), hepatocyte nuclear factor 4, alpha (HNF4A) (Fig 2A and 2B). In contrast with the modulation of common genes by either E2+ETO or E2+MPA, genes regulated by E2+P4 treatment are uniquely associated with upstream activation of nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha, aka IĸBα (NFKBIA), IFNG (interferon γ), P4, VEGF (Fig 2C).
Fig 2. Common and unique upstream regulator of differentially regulated genes by MPA, ETO and P4.
Ingenuity Pathway Analyzer (IPA) software identifies activation of ligand-dependent glucocorticoid receptor (GR,NR3C1) by (A) MPA (Z-score 3.22; overlap p value 4.31 E−9) and (B) ETO (Z-score 2.85; overlap p value 3.22 E−10), but not (C) P4 (Z score = 0 and overlap p value = 0.03). All gene symbols were abbreviated according to GENEBANK standard nomenclature. (n = 3/group).
Further analysis of the microarray data displayed by Venn diagram indicates that 65 genes are regulated solely by E2+MPA, and 222 genes are regulated solely by E2+ETO whereas 79 genes are regulated solely by E2+P4 (Fig 3A). A group of 67 genes are regulated in common by all three progestins. Strikingly, transcriptional levels of 235 genes are regulated in common by E2+ETO and E2+MPA, but not by E2+P4 (Fig 3A). Among these, the 40 most highly regulated genes by ETO and MPA are listed in Fig 3B. IPA of the microarray gene expression data revealed the activation of GR (NR3C1) as one of upstream regulators of several of these 235 genes regulated by ETO and MPA (S2 Fig).
Fig 3. MPA, ETO and P4 mediated differentially regulated genes in HESCs.
(A) Venn diagram analysis of genes regulated individually or in common by MPA or ETO or P4 (B) 40 most highly regulated genes by ETO and MPA, but not P4 according to whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit (n = 3/group). All gene symbols were abbreviated according to GENEBANK standard nomenclature.
MPA and ETO induce FKBP51 mRNA and protein expression in cultured HESCs
FKBP51 represses GR and PR transcriptional activity in several cell types [18, 20, 25] and is highlighted in bold among the genes specifically altered by either E2+ETO or E2+MPA treatment (Fig 3B). Analysis by q-PCR confirmed that incubation of HESCs with E2+ETO or E2+MPA, but not E2+P4, significantly induces steady state FKBP51 mRNA levels vs. E2 alone (Fig 4A) and, immunoblot analysis revealed that compared with E2 alone, FKBP51 protein levels are increased significantly in HESCs in response to E2+ETO or E2+MPA, but not to E2+P4 (Fig 4B). Unlike FKBP51, microarray analysis revealed no change in FKBP52 mRNA expression in response to all three progestins and immunoblotting confirmed that none of the three progestins affected FKBP52 protein expression (not shown).
Fig 4. Increased FKBP51 mRNA and protein levels by MPA or ETO but not P4.
(A) q-PCR and (B) immunoblot analysis of FKBP51 levels in HESCs treated with estradiol (E2, 10−8M) ± progesterone (P4, 10−7 M) or etonogestrel (ETO, 10−7 M) or medroxyprogesterone acetate (MPA, 10−7 M) for 6h or 24 h, respectively. Bars represent mean ± SEM (n = 3). * p<0.001 in E2+ETO vs. E2; # p<0.001 in E2+MPA vs. E2.
Endometrial FKBP51 expression in women receiving DMPA treatment
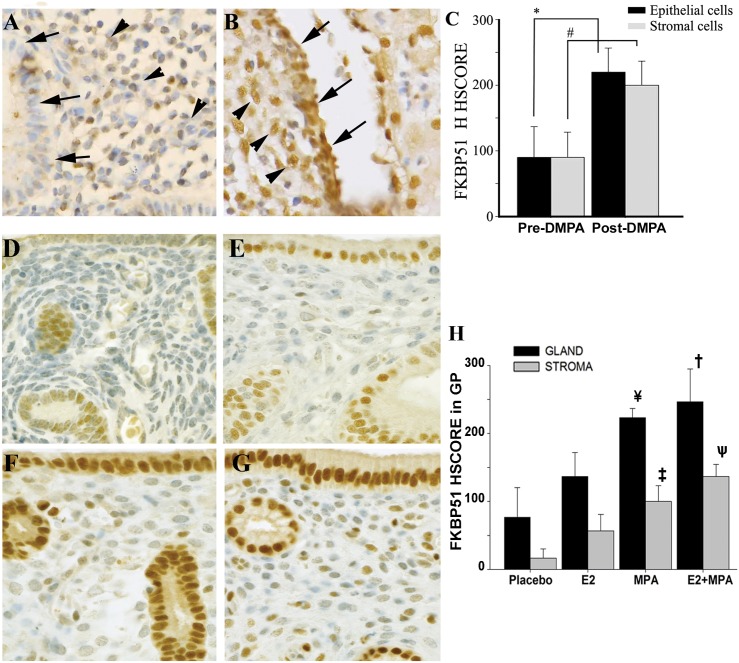
Immunohistochemical analysis was performed on paired endometrial sections obtained from women pre- and post-DMPA administration. Both endometrial stromal and glandular epithelial cells express weak to moderate FKBP51 immunoreactivity in pre-DMPA specimens (Fig 5A). Post-DMPA specimens displayed a significant increase in FKBP51 immunoreactivity in endometrial stromal cells (HSCORE mean ± SEM; 200 ± 18.3 vs. 100 ± 14.1; p<0.009 respectively) and endometrial glandular epithelial cells (220 ± 18.3 vs. 92.5 ± 22.1; p<0.006, respectively) compared with pre-DMPA administration (Fig 5B and 5C).
Fig 5. Increased FKBP51 immunoreactivity in women using DMPA and in endometria of OVX-GPs treated by MPA.
(A_C) FKBP51 expression in paired endometrium of women pre- and post- DMPA administration. Immunoreactive FKBP51 in endometrial stromal (arrowheads) and glandular (arrow) cells pre- (A) and post- (B) DMPA use. HSCORE analysis of FKBP51 expression (C) in stromal and glandular cells. Bars represent mean ± SEM (n = 6). * p< 0.01 and # p<0.01. Pre-DMPA: before DMPA use; Post-DMPA: 3 months DMPA use. (D-H) FKBP51 expression in endometria of OVX-GPs treated by MPA. Immunoreactivity for FKBP51 (brown) in stromal (arrowhead) and epithelial (arrows) cells of endometria of OVX-GPs treated with vehicle (D) or E2 (E) or MPA (F) or E2+MPA (G) for 21 days. HSCORE analysis of FKBP51 immunoreactivity (H) in stromal and glandular cells. Bars represent mean ± SEM (n = 3 per treatment group). † p<0.001 in E2+MPA vs. E2 or placebo (Pla); ѱ p<0.001 in E2+MPA vs. MPA or E2 or Pla; ¥ in p<0.05 in MPA vs. E2 or Pla; ‡ p<0.05 in MPA vs. E2 and Pla. Original Magnification: A-G x40.
MPA induces FKBP51 expression in the endometrium of OVX-GPs
FKBP51 immunoreactivity in endometrial sections obtained from OVX-GPs treated with placebo or E2 or MPA or E2+MPA is displayed in Fig 5D–5H. In endometrial stromal cells, FKBP51 expression is significantly increased by administration of MPA (HSCORE mean ± SEM; 100.0 ± 11.5) or E2+MPA (136.7 ± 8.8) compared to placebo (16.7 ± 6.7) or E2 alone (56.7 ± 12.02; p<0.001, Fig 5D–5G). Similar increases in FKBP51 expression were also observed in endometrial glandular epithelial cells of GPs treated with either MPA (223.3 ± 6.7) or E2+MPA (246.7 ± 24.1) compared to placebo (76.7 ± 21.9) or E2 alone (136.7 ± 7.6; p<0.001, Fig 5D–5G). Consistent with E2 mediated up-regulation of PR expression [26], HSCORE analysis (Fig 5H) indicates that E2+MPA significantly enhanced FKBP51 expression in endometrial stromal cells vs. MPA alone (p<0.05). No statistically significant differences between placebo and E2 alone were detected in either endometrial stromal or glandular epithelial cells (Fig 5H).
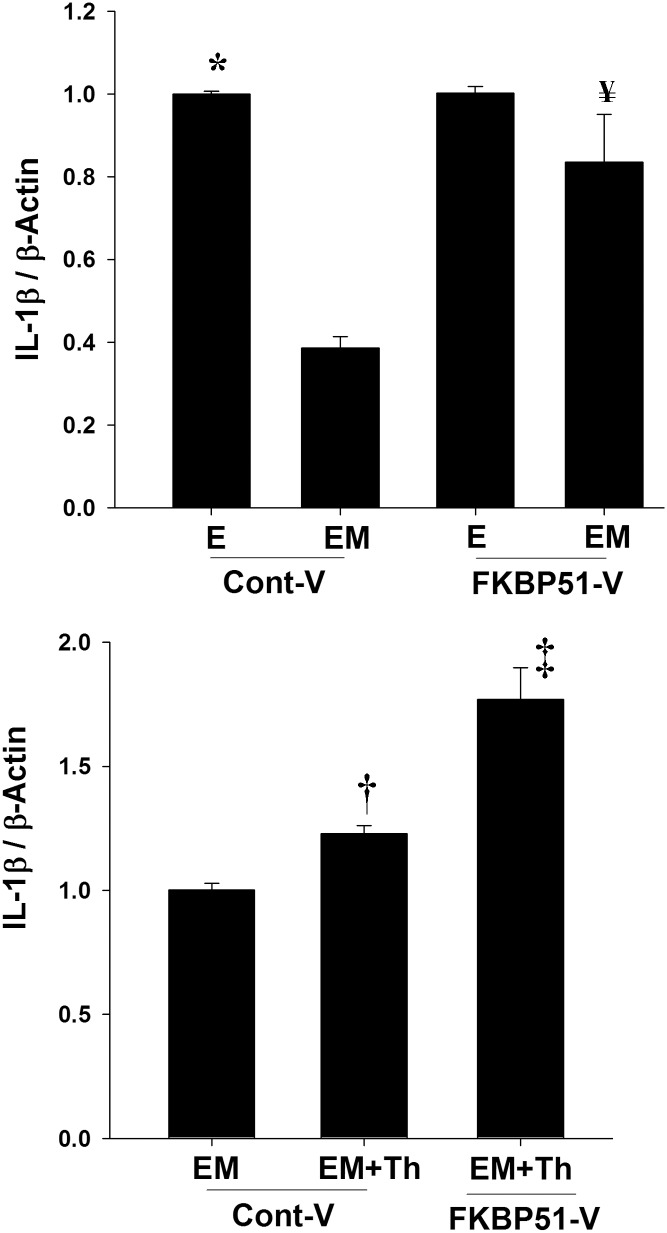
Overexpression of FKBP51 reverses MPA-mediated inhibition of endogenous IL-1β expression in HESCs
Our previous studies showed that LAPCs increase TF expression in decidualized HESCs, which generates excess thrombin [11, 12]. In cultured HESCs, thrombin enhances expression of VEGF, IL–8, and MMP–1 expression [13, 14, 23] and therefore, thrombin plays an essential role in LAPC-induced AUB by promoting a proteolytic and inflammatory milieu. To investigate the effect of FKBP51 in MPA-mediated inhibition of inflammation, IL-1β mRNA levels were measured in HESCs transfected with FKBP51 vector for overexpression. The FKBP51 expression was confirmed by q-PCR and immunoblotting in cultured HESCs (data not shown) after transfection. Compared with E2 treatment, E2+MPA treatment significantly inhibited IL-1β mRNA levels in HESC transfected with a control vector, whereas MPA failed to inhibit IL-1β mRNA levels in HESCs overexpressing FKBP51 (Fig 6A). Furthermore, thrombin treatment of HESCs that overexpressed FKBP51 exacerbates thrombin-induced IL-1β mRNA expression in the presence of MPA (Fig 6B).
Fig 6. FKBP51 overexpression reverses the anti-inflammatory action of MPA in HESCs.
(A) Basal IL-1β mRNA levels in 10−8 M estradiol (E) vs. E+ 10−7 M MPA (EM) treated HESCs at 44 hrs post-transfection with either a control (Cont-V) or FKBP51 vector (FKBP51-V). Bars represent mean ± SEM (n = 4 with 3 replicates per treatment group). (B) In parallel incubations, thrombin- (Th-) induced IL-1β mRNA levels in E2+MPA treated HESCs at 44 hrs post-transfection with control or FKBP51 vector. Bars represent mean ± SEM (n = 2 and 3 replicates per treatment group). E: E2 and EM: E2+MPA, * p<0.05 vs. EM treated in Cont-V transfected HESCs; ¥ p<0.05 vs. EM treatment in Cont-V transfected HESCs; † p<0.05 vs. EM; ‡ p<0.05 vs. EM+Th.
Discussion
LAPC methods are the most effective reversible contraceptive method [21]. For example, in a large prospective cohort study in which over 75% of teenage participants received LAPCs there was a substantial reduction in mean annual rates of pregnancy, birth, and elective abortion compared with the overall population of sexually experienced U.S. teens [27]. However, AUB occurs in the majority of LAPC users and is a major reason for non-adherence, with three year discontinuation rates as high as 47% for implants and 27% for IUS users [28]. Moreover, unlike the predictable and controlled nature of menstrual bleeding, LAPC associated AUB is unpredictable and occurs sporadically [13]. The contraceptive action of LAPCs results from inhibition of ovulation, cervical thickening and/or endometrial thinning and explains the agents greater than 99% pregnancy preventing efficacy [29]. It is unclear how the various progestins employed in LAPC induce AUB. Seeking to shed light on this question, the current study identified several gene clusters and signaling pathways that are both uniquely and commonly modulated by each progestin. To our knowledge, this is the first study to compare changes in global gene expression profiles in HESCs in response to natural vs. synthetic progestins and consequently to identify common and unique alterations in gene expression triggered by each progestin.
During the follicular phase of the human menstrual cycle, circulating P4 is maintained at low levels that increase markedly following ovulation. This increased concentration does not induce uterine bleeding. Indeed it is the physiological withdrawal of circulating P4 that triggers menstrual bleeding [13, 30]. During human pregnancy circulating P4 levels continue to rise [31] unaccompanied by AUB. These physiological observations strongly suggest that AUB accompanying LAPC administration reflects molecular changes that are distinct from those induced by circulating P4. Consistent with this hypothesis, while our microarray results reveal that 65 genes are modulated in common by P4 and by the synthetic progestins, MPA and ETO, in cultured HESCs, these commonly modulated genes match only 17% and 12% of the total genes altered by MPA or ETO, respectively, and 38% of the genes modulated by P4 alone indicating that only a portion of the action of these LAPCs reflects native progesterone effects.
The current study identified a total of 235 genes unaffected during incubation of HESCs with P4, which corresponded to 62% and 44% of genes whose expression was altered by MPA and ETO, respectively. Further analysis by IPA software indicated ligand-dependent activation of GR (NR3C1) as one of the upstream regulators of these MPA or ETO-modulated 235 genes, suggesting the involvement of GR signaling in LAPC-induced AUB. Moreover, results from IPA predicted that activation of wortmannin, an AKT signal inhibitor, and PD89059, an ERK1/2 signal inhibitor, are other upstream signaling pathways associated with these genes. However, no changes in AKT or ERK1/2 phosphorylation levels were observed in HESCs treated with either MPA or ETO (S3 Fig), ruling out involvement of both signaling cascades in MPA and ETO-mediated modulation of these genes.
Prior studies demonstrated upregulation of FKBP51 mRNA and protein levels in response to ligand-dependent activation of both the GR and PR [17]. Microarray results obtained in the current study identified FKBP51 among genes that are upregulated following incubation of HESCs with either MPA or ETO, but not by P4. Confirmation of this selective elevation of FKBP51 mRNA was confirmed by q-PCR and extended to include the FKBP51 protein by immunoblotting. Lack of changes in FKBP52 expression in HESCs in response to MPA, ETO and P4 suggests that FKBP51 acts as a dominant co-chaperone in regulating PR or GR-mediated transcriptional activity in these cells. Reports that MPA displays mixed progestin/glucocorticoid effects [32] taken together with the absence of a response to P4 suggest that regulation of FKBP51 expression in HESCs by MPA as well as ETO reflects ligand-dependent activation of the GR, but not the PR. This observation is in contrast to information describing absence of glucocorticoid action by ETO. Alternatively, P4 is more susceptible to metabolism than synthetic progestins by cultured cells [33], which may reduce the effect of P4 on FKBP51 expression. Furthermore, compared with P4, MPA and ETO may exert a stronger PR mediated transcriptional activity on the FKBP51 gene. Supporting the latter statement, several genes presented in Tables 2–4 (such as ADAMTS1, ADH1A, CLEC3B) display higher fold increased transcriptional levels in response to MPA or ETO vs. P4. In several cell systems, elevated levels of FKPB51 suppresses transcriptional activity of both the GR and PR suggesting the existence of a negative-feedback loop that induces local functional P4 and GC withdrawal in target tissue [34]. The current observations of increased FKBP51 immunoreactivity in stromal and glandular cells in human endometrial tissues obtained from women from post-DMPA versus pre-DMPA use suggests that MPA administration results in local inhibition of GR and PR transcriptional activity. This may account for increase in inflammation observed in such endometria. In support of this hypothesis, several previous studies have shown a significant reduction in expression of tissue inhibitor of matrix metalloproteinases (TIMPs) in stromal, epithelial and endothelial cells, accompanied by focal elevation of MMP–1 and -3 expression and increased influx of MMP–9 positive neutrophils, CD3+ T cells, macrophages and uterine natural killer (uNK) cells in the endometria of DMPA users [35, 36]. Moreover, LAPCs increase epithelial neutrophil-activating peptide–78 (ENA–78) [37] and IL–8 in HESCs [15], and increase numbers of CD45 positive cells [35], which may be associated with functional loss of the anti-inflammatory properties of PR and GR activated by MPA or ETO.
The endometria of women receiving LAPCs contain enlarged thin-walled fragile microvessels that are irregularly distributed across the superficial layer, and display intermittent focal bleeding. We previously observed that LAPC administration results in reduced endometrial blood flow which promotes local hypoxia (HX) and reactive oxygen species (ROS) generation damaging microvascular endothelial cells and enhancing production of pro-angiogenic factors secreted from HESCs (Fig 7) [8]. Specifically, the endometria of women receiving LAPCs display elevated levels of VEGF, the primary mediator of angiogenesis (Fig 7) [14]. Although physiological VEGF levels promote angiogenesis, over-expression of VEGF produces abnormal blood vessels with increased permeability and reduced perivascular ECM [38]. Moreover, our in situ observations and studies using primary cultures of HESCs and HEECs demonstrate that progestins inhibit HESC-expressed Ang–1, a vessel-stabilizing factor, while HX enhances HEEC-expressed Ang–2, which promotes vessel branching and enhances endothelial cell permeability [10]. Another recent study demonstrates that MPA and ETO treated HESCs secrete factors that promote HEEC apoptosis (Fig 7) [39].
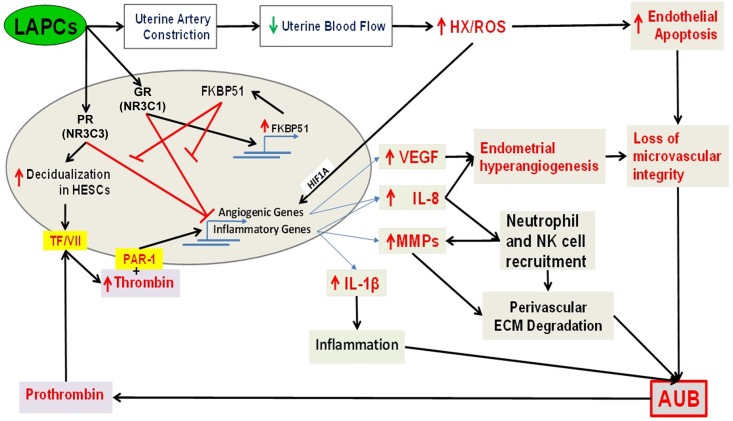
Fig 7. A schematic model for molecular interactions inducing AUB in women using LAPC.
LAPCs reduce endometrial blood flow causing hypoxia (HX) and generating reactive oxygen species (ROS), to directly damage blood vessels and enhance HEEC expression of angiopoietin (Ang)-2 and HESC expression of vascular endothelial growth factor (VEGF), interleukin (IL)-8 and matrix metalloproteinases (MMPs) while inhibiting HESC Ang–1 expression. The resultant inflammation and aberrant angiogenic stimulus leads to vessel damage causing local bleeding. The later delivers circulating factor VII to HESC membrane bound tissue factor (TF) which activates factor Xa to ultimately generate thrombin. The later exacerbates HX/ROS effects on endometrial angiogenesis and inflammation. In turn, HX and LAPCs trigger HEEC apoptosis by a paracrine manner. In addition, LAPCs also enhance FKBP51 expression in HESCs to inhibit progesterone receptor (PR; NR3C3) and glucocorticoid receptor (GR; NR3C1)-mediated transcription. In turn, the resultant PR and/or GR-mediated functional withdrawal reduces progestin and glucocorticoid mediated inhibition of endometrial inflammation, aberrant angiogenesis, thus contributing to AUB.
Like women, GPs exhibit spontaneous estrous cycling and hemochorial placentation [40–43] justifying previous use by our laboratory of GPs as a relevant animal model to study LAPC-mediated endometrial changes [44, 45]. Our previous observations that LAPC administration reduced endometrial blood flow and induced local HX-mediated ROS generation in women [10], taken together with similar observations in the LAPC-treated GP endometrium [45], prompted evaluation of FKBP51 expression in the endometrium of GPs following administration of MPA. Increased FBP51 expression in MPA treated GP endometria confirms the observations made on post-DMPA administrated human endometria and supports presence of similar MPA mediated molecular mechanism in regulation of FKBP51 expression in both species.
A recent study [46] demonstrated that GR mediates anti-inflammatory action of MPA in cervical epithelial cells. Given the predominant expression of the GR in primary endocervical epithelial cells, this GR-mediated action may be essential in discriminating between the effects on inflammation caused by different progestins and P4. In support of the existence of functional cross-talk between the GR and LAPCs, Tomasicchio et al. [47] found that induction of apoptosis in primary CD4 (+) T-cells by MPA, but not P4 and suggested that progestin component of LAPCs may promote susceptibility to HIV–1. Complementing the conclusions drawn by these studies [46, 47], the current finding that MPA reduces basal IL-1β mRNA levels in HESCs is consistent with an anti-inflammatory action of MPA. Furthermore, reversal of this anti-inflammatory action of MPA by FKBP51 overexpression in HESCs supports our hypothesis that FKBP51 promotes functional P4 and/or GC withdrawal. In view of the microarray results in Tables 2–4 indicating that both MPA and ETO downregulate thrombin receptor expression, the finding that FKBP51 overexpression exacerbates thrombin induced IL-1β expression in the presence of progestin is even more striking.
In conclusion, results obtained from the current study reveal that MPA and ETO alter the expression of significantly greater numbers of genes than P4 in endometrial cells. Distinct from P4, MPA and ETO mediated regulation of numerous genes as exemplified by FKBP51 are linked to ligand dependent activation of the GR, but not the PR. These observations suggest the need for future studies that evaluate the critical role played by FKBP51 in mediating LAPC induced functional P4 and/or glucocorticoid withdrawal leading to AUB.
Supporting Information
IGFBP1, ADAMTS1, F2RL1 and FKBP51 genes are regulated by MPA and ETO in HESCs. GREs are identified by the MatInspector program. Specifically, GR binding sites represent the glucocorticoid responsive and related elements (V$GREF; purple box) and the negative glucocorticoid response elements (V$NGRE; blue box) matrix families. Arrows represent transcription start site.
(TIF)
Upstream regulators of MPA and ETO induced differentially regulated genes in cultured HESCs using IPA analysis detected activation of NR3C1, the glucocorticoid receptor according to the gene list obtained from whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit analysis (n = 3/group). Orange squares indicate predicted increase in activity while blue squares indicate predicted decrease in activity. The circular network shows the upstream regulator in the center with its targets colored by the expression results (upregulated genes in red color, down-regulated genes in green color). Orange edge, leads to activation; yellow edge, inconsistent state; grey edge, effect not predicted. All gene symbols were abbreviated according to GENEBANK standard nomenclature.
(TIF)
Immunoblot analysis of phosphorylated (p-) and total (T-) levels of AKT and ERK1/2 MAPK in HESCs incubated with E2 (10−8 M) or E2 + P4 (10−7 M) or E2 + ETO (10−7 M) or E2 +MPA (10−7 M) for 24 hr (n = 3). β-actin was used as a loading control.
(TIF)
Acknowledgments
Authors would like to thank to Dr. J.S. Huang for his technical help to analyze and upload Microarray data to GEO webpage.
Data Availability
All relevant data including supporting data are presented in manuscript as main figures, tables or supplemental figures. Moreover, microarray data are deposited in NCBI's Gene Expression Omnibus database, that can be accessible through GEO Series accession number GSE72040 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72040).
Funding Statement
This study was funded by NIH/NICHD 2 RO1 HD 033937 to CJL.
References
- 1. Affandi B. An integrated analysis of vaginal bleeding patterns in clinical trials of Implanon. Contraception. 1998;58(6 Suppl):99S–107S. . [DOI] [PubMed] [Google Scholar]
- 2. Kaunitz AM. Injectable contraception. New and existing options. Obstetrics and gynecology clinics of North America. 2000;27(4):741–80. . [DOI] [PubMed] [Google Scholar]
- 3. Chaovisitsaree S, Piyamongkol W, Pongsatha S, Morakote N, Noium S, Soonthornlimsiri N. One year study of Implanon on the adverse events and discontinuation. J Med Assoc Thai. 2005;88(3):314–7. . [PubMed] [Google Scholar]
- 4. Mutihir JT, Daru PH. Implanon sub-dermal implants: a 10-month review of acceptability in Jos, North-Central Nigeria. Niger J Clin Pract. 2008;11(4):320–3. . [PubMed] [Google Scholar]
- 5. Varney SJ, Guest JF. Relative cost effectiveness of Depo-Provera, Implanon, and Mirena in reversible long-term hormonal contraception in the UK. Pharmacoeconomics. 2004;22(17):1141–51. . [DOI] [PubMed] [Google Scholar]
- 6. Rosenberg MJ, Waugh MS, Long S. Unintended pregnancies and use, misuse and discontinuation of oral contraceptives. The Journal of reproductive medicine. 1995;40(5):355–60. . [PubMed] [Google Scholar]
- 7. Collins J, Crosignani PG, Group ECW. Hormonal contraception without estrogens. Human reproduction update. 2003;9(4):373–86. . [DOI] [PubMed] [Google Scholar]
- 8. Krikun G, Critchley H, Schatz F, Wan L, Caze R, Baergen RN, et al. Abnormal uterine bleeding during progestin-only contraception may result from free radical-induced alterations in angiopoietin expression. Am J Pathol. 2002;161(3):979–86. 10.1016/S0002-9440(10)64258-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hickey M, Fraser IS. The structure of endometrial microvessels. Human reproduction. 2000;15 Suppl 3:57–66. . [DOI] [PubMed] [Google Scholar]
- 10. Hickey M, Krikun G, Kodaman P, Schatz F, Carati C, Lockwood CJ. Long-term progestin-only contraceptives result in reduced endometrial blood flow and oxidative stress. The Journal of clinical endocrinology and metabolism. 2006;91(9):3633–8. 10.1210/jc.2006-0724 . [DOI] [PubMed] [Google Scholar]
- 11. Lockwood CJ, Runic R, Wan L, Krikun G, Demopolous R, Schatz F. The role of tissue factor in regulating endometrial haemostasis: implications for progestin-only contraception. Human reproduction. 2000;15 Suppl 3:144–51. . [DOI] [PubMed] [Google Scholar]
- 12. Runic R, Schatz F, Wan L, Demopoulos R, Krikun G, Lockwood CJ. Effects of norplant on endometrial tissue factor expression and blood vessel structure. The Journal of clinical endocrinology and metabolism. 2000;85(10):3853–9. 10.1210/jcem.85.10.6856 . [DOI] [PubMed] [Google Scholar]
- 13. Lockwood CJ. Mechanisms of normal and abnormal endometrial bleeding. Menopause. 2011;18(4):408–11. 10.1097/GME.0b013e31820bf288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lockwood CJ, Krikun G, Koo AB, Kadner S, Schatz F. Differential effects of thrombin and hypoxia on endometrial stromal and glandular epithelial cell vascular endothelial growth factor expression. The Journal of clinical endocrinology and metabolism. 2002;87(9):4280–6. 10.1210/jc.2001-011969 . [DOI] [PubMed] [Google Scholar]
- 15. Lockwood CJ, Kumar P, Krikun G, Kadner S, Dubon P, Critchley H, et al. Effects of thrombin, hypoxia, and steroids on interleukin–8 expression in decidualized human endometrial stromal cells: implications for long-term progestin-only contraceptive-induced bleeding. The Journal of clinical endocrinology and metabolism. 2004;89(3):1467–75. 10.1210/jc.2003-030141 . [DOI] [PubMed] [Google Scholar]
- 16. Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19(6):1654–66. 10.1210/me.2005-0071 . [DOI] [PubMed] [Google Scholar]
- 17. Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell stress & chaperones. 2004;9(3):243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144(6):2380–7. 10.1210/en.2003-0092 . [DOI] [PubMed] [Google Scholar]
- 19. Sanchez ER. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochimica et biophysica acta. 2012;1823(3):722–9. 10.1016/j.bbamcr.2011.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tatro ET, Everall IP, Kaul M, Achim CL. Modulation of glucocorticoid receptor nuclear translocation in neurons by immunophilins FKBP51 and FKBP52: implications for major depressive disorder. Brain research. 2009;1286:1–12. 10.1016/j.brainres.2009.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Preventing Unintended Pregnancy: The Contraceptive CHOICE Project in Review. Journal of women's health. 2015;24(5):349–53. 10.1089/jwh.2015.5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Krikun G, Booth CJ, Buchwalder L, Caze R, Rahman M, Schatz F, et al. Long-term progestin-only contraception in humans versus animal models. Annals of the New York Academy of Sciences. 2011;1221:119–23. 10.1111/j.1749-6632.2010.05930.x . [DOI] [PubMed] [Google Scholar]
- 23. Lockwood CJ, Schatz F, Krikun G. Angiogenic factors and the endometrium following long term progestin only contraception. Histol Histopathol. 2004;19(1):167–72. . [DOI] [PubMed] [Google Scholar]
- 24. Basar M, Yen CF, Buchwalder LF, Murk W, Huang SJ, Godlewski K, et al. Preeclampsia-related increase of interleukin–11 expression in human decidual cells. Reproduction. 2010;140(4):605–12. 10.1530/REP-10-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Current opinion in pharmacology. 2011;11(4):332–7. 10.1016/j.coph.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lockwood CJ, Kayisli UA, Stocco C, Murk W, Vatandaslar E, Buchwalder LF, et al. Abruption-induced preterm delivery is associated with thrombin-mediated functional progesterone withdrawal in decidual cells. Am J Pathol. 2012;181(6):2138–48. 10.1016/j.ajpath.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Secura GM, Madden T, McNicholas C, Mullersman J, Buckel CM, Zhao Q, et al. Provision of no-cost, long-acting contraception and teenage pregnancy. The New England journal of medicine. 2014;371(14):1316–23. 10.1056/NEJMoa1400506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weisberg E, Bateson D, McGeechan K, Mohapatra L. A three-year comparative study of continuation rates, bleeding patterns and satisfaction in Australian women using a subdermal contraceptive implant or progestogen releasing-intrauterine system. The European journal of contraception & reproductive health care: the official journal of the European Society of Contraception. 2014;19(1):5–14. 10.3109/13625187.2013.853034 . [DOI] [PubMed] [Google Scholar]
- 29. Schrager S. Abnormal uterine bleeding associated with hormonal contraception. American family physician. 2002;65(10):2073–80. . [PubMed] [Google Scholar]
- 30. Papp C, Schatz F, Krikun G, Hausknecht V, Lockwood CJ. Biological mechanisms underlying the clinical effects of mifepristone (RU 486) on the endometrium. Early pregnancy. 2000;4(4):230–9. . [PubMed] [Google Scholar]
- 31. Byrns MC. Regulation of progesterone signaling during pregnancy: implications for the use of progestins for the prevention of preterm birth. The Journal of steroid biochemistry and molecular biology. 2014;139:173–81. 10.1016/j.jsbmb.2013.01.015 . [DOI] [PubMed] [Google Scholar]
- 32. Africander D, Verhoog N, Hapgood JP. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids. 2011;76(7):636–52. 10.1016/j.steroids.2011.03.001 . [DOI] [PubMed] [Google Scholar]
- 33. Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. Classification and pharmacology of progestins. Maturitas. 2003;46 Suppl 1:S7–S16. . [DOI] [PubMed] [Google Scholar]
- 34. Jaaskelainen T, Makkonen H, Palvimo JJ. Steroid up-regulation of FKBP51 and its role in hormone signaling. Current opinion in pharmacology. 2011;11(4):326–31. 10.1016/j.coph.2011.04.006 . [DOI] [PubMed] [Google Scholar]
- 35. Vincent AJ, Zhang J, Ostor A, Rogers PA, Affandi B, Kovacs G, et al. Decreased tissue inhibitor of metalloproteinase in the endometrium of women using depot medroxyprogesterone acetate: a role for altered endometrial matrix metalloproteinase/tissue inhibitor of metalloproteinase balance in the pathogenesis of abnormal uterine bleeding? Human reproduction. 2002;17(5):1189–98. . [DOI] [PubMed] [Google Scholar]
- 36. Vincent AJ, Salamonsen LA. The role of matrix metalloproteinases and leukocytes in abnormal uterine bleeding associated with progestin-only contraceptives. Human reproduction. 2000;15 Suppl 3:135–43. . [DOI] [PubMed] [Google Scholar]
- 37. Chegini N, Luo X, Pan Q, Rhoton-Vlasak A, Archer DF. Endometrial expression of epithelial neutrophil-activating peptide–78 during the menstrual cycle or in progestin-only contraceptive users with breakthrough bleeding and the influence of doxycycline therapy. Human reproduction. 2007;22(2):427–33. 10.1093/humrep/del398 . [DOI] [PubMed] [Google Scholar]
- 38. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. The American journal of pathology. 1995;146(5):1029–39. [PMC free article] [PubMed] [Google Scholar]
- 39. Guzeloglu-Kayisli O, Basar M, Shapiro JP, Semerci N, Huang JS, Schatz F, et al. Long-acting progestin-only contraceptives enhance human endometrial stromal cell expressed neuronal pentraxin–1 and reactive oxygen species to promote endothelial cell apoptosis. The Journal of clinical endocrinology and metabolism. 2014;99(10):E1957–66. 10.1210/jc.2014-1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Carter AM. Animal models of human placentation–-a review. Placenta. 2007;28 Suppl A:S41–7. 10.1016/j.placenta.2006.11.002 . [DOI] [PubMed] [Google Scholar]
- 41. Hutz RJ, Bejvan SM, Durning M, Dierschke DJ. Changes in follicular populations, in serum estrogen and progesterone, and in ovarian steroid secretion in vitro during the guinea pig estrous cycle. Biology of reproduction. 1990;42(2):266–72. . [DOI] [PubMed] [Google Scholar]
- 42. Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128(6):679–95. 10.1530/rep.1.00340 . [DOI] [PubMed] [Google Scholar]
- 43. Makker A, Bansode FW, Srivastava VM, Singh MM. Antioxidant defense system during endometrial receptivity in the guinea pig: effect of ormeloxifene, a selective estrogen receptor modulator. The Journal of endocrinology. 2006;188(1):121–34. 10.1677/joe.1.06324 . [DOI] [PubMed] [Google Scholar]
- 44. Krikun G, Booth CJ, Buchwalder L, Schatz F, Osol G, Mandala M, et al. Effects of etonogestrel treatment in the reproductive organs and uterine arteries of nonoophorectomized guinea pigs. Reproductive sciences. 2012;19(4):400–6. 10.1177/1933719111424452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krikun G, Buhimschi IA, Hickey M, Schatz F, Buchwalder L, Lockwood CJ. Long-term progestin contraceptives (LTPOC) induce aberrant angiogenesis, oxidative stress and apoptosis in the guinea pig uterus: A model for abnormal uterine bleeding in humans. Journal of angiogenesis research. 2010;2:8 10.1186/2040-2384-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Govender Y, Avenant C, Verhoog NJ, Ray RM, Grantham NJ, Africander D, et al. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PloS one. 2014;9(5):e96497 10.1371/journal.pone.0096497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tomasicchio M, Avenant C, Du Toit A, Ray RM, Hapgood JP. The progestin-only contraceptive medroxyprogesterone acetate, but not norethisterone acetate, enhances HIV–1 Vpr-mediated apoptosis in human CD4+ T cells through the glucocorticoid receptor. PloS one. 2013;8(5):e62895 10.1371/journal.pone.0062895 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IGFBP1, ADAMTS1, F2RL1 and FKBP51 genes are regulated by MPA and ETO in HESCs. GREs are identified by the MatInspector program. Specifically, GR binding sites represent the glucocorticoid responsive and related elements (V$GREF; purple box) and the negative glucocorticoid response elements (V$NGRE; blue box) matrix families. Arrows represent transcription start site.
(TIF)
Upstream regulators of MPA and ETO induced differentially regulated genes in cultured HESCs using IPA analysis detected activation of NR3C1, the glucocorticoid receptor according to the gene list obtained from whole genome microarray analysis using Illumina HumanHT–12 v4 expression BeadChip kit analysis (n = 3/group). Orange squares indicate predicted increase in activity while blue squares indicate predicted decrease in activity. The circular network shows the upstream regulator in the center with its targets colored by the expression results (upregulated genes in red color, down-regulated genes in green color). Orange edge, leads to activation; yellow edge, inconsistent state; grey edge, effect not predicted. All gene symbols were abbreviated according to GENEBANK standard nomenclature.
(TIF)
Immunoblot analysis of phosphorylated (p-) and total (T-) levels of AKT and ERK1/2 MAPK in HESCs incubated with E2 (10−8 M) or E2 + P4 (10−7 M) or E2 + ETO (10−7 M) or E2 +MPA (10−7 M) for 24 hr (n = 3). β-actin was used as a loading control.
(TIF)
Data Availability Statement
All relevant data including supporting data are presented in manuscript as main figures, tables or supplemental figures. Moreover, microarray data are deposited in NCBI's Gene Expression Omnibus database, that can be accessible through GEO Series accession number GSE72040 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72040).