Abstract
Interleukin–23 (IL–23) is a conventional proinflammatory cytokine that plays a role in tumor progression by inducing inflammation in the tumor microenvironment. However, the role of IL–23 in thyroid cancer migration and invasion remains unclear. In the present study, we observed that the treatment with IL–23, induced migration and invasion in human thyroid cancer cells. Additional data demonstrate that SOCS4 negatively regulates IL-23-mediated migration and invasion. On investigating the mechanisms involved in IL–23 mediated migration and invasion, we observed that miR–25 promotes the migration and invasion of thyroid cancer cells by directly binding to the 3′-UTR of SOCS4 that leads to the inhibition of SOCS4. In addition, we also demonstrated that IL–23 increases miR–25 expression levels, and overexpressed miR–25 is involved in IL-23-associated SOCS4 inhibition and cell migration and invasion. Together, our data suggest that IL–23 induces migration and invasion in thyroid cancer cells by mediating the miR–25/SOCS4 signaling pathway.
Introduction
Thyroid cancer is a common type of endocrine malignancy that has shown a rapid increase in worldwide incidence during the past few decades [1]. Despite improvements in therapeutic strategies, some patients are difficult to treat and develop invasion and metastasis [2]. Therefore, it is essential to identify the molecular mechanisms underlying thyroid cancer invasion and metastasis. Recent reports demonstrated that inflammation is a strong promoter of carcinogenesis and malignancy in many forms of cancer [3,4]. Inflammation seems to be an important mediator for the development of cancer and provides the cancer cells a hospitable microenvironment [5]. Interleukin–23 (IL–23), a heterodimeric type 1 cytokine composed of the IL–12/p40 subunit and the specific p19 subunit, belongs to the interleukin–6 superfamily [6]. Previous studies have shown that IL–23 is associated with carcinogenesis as well as inflammation. High levels of IL–23 were found in human hepatocellular carcinoma, colorectal carcinoma, squamous carcinoma, and esophageal carcinoma [7–10]. Evidence suggests that IL–23 overexpression can induce metastasis in colorectal, lung, and oral cancer [7–10].
The suppressors of cytokine signaling (SOCS) are important negative feedback regulators of cytokine signaling [11]. The SOCS proteins are a family of 8 proteins (SOCS1-7 and a cytokine-inducible SH2-containing protein or CIS). Each SOCS protein contains a central SH2 domain that interacts with phosphorylated tyrosines [12]. SOCS proteins have been recently investigated for their role in the development of different cancers [13–18]. However, little is known about the role of SOCS4 in carcinoma, and their possible influence on tumor growth and malignancy.
MicroRNAs (miRNAs) are a species of small noncoding single stranded RNAs that play an important role in the development of different cancers by binding the 3′-untranslated region (3′-UTR) of targeted genes [19]. Aberrant miRNA expression has also been frequently reported in numerous tumors [20]. In recent years, multiple evidence point to a role for miRNAs in tumor cell biological processes, including cell proliferation, differentiation, migration, and invasion [21]. MicroRNA–25 belongs to the miR-106b-25 cluster that includes miR-106b, miR–93, and miR–25. MicroRNA–25 has been reported to be aberrantly overexpressed in several tumors, such as ovarian cancer, lung cancer, gastric cancer, and colorectal cancer [22–26]. Although the expression of miR–25 in different tumors has been described, a clear role for miR–25 in thyroid carcinoma remains unclear.
In this study, we demonstrate that IL–23 promotes thyroid cancer cell migration and invasion. We further demonstrate that IL–23 regulates the migration and invasion of thyroid cancer cells via a miR–25/SOCS4 signaling pathway.
Materials and Methods
Ethics statement
All participants gave written informed consent to participate in the study. The study was conducted according to the principles of the Declaration of Helsinki and approved by the Institutional Review Board of the Remin Hospital of Wuhan University, in accordance with its guidelines for the protection of human subjects.
Samples and cases
Thyroid tissues were collected at the Remin Hospital of Wuhan University from February 2010 to February 2014. Tissue samples were cut into two parts, one was reviewed by two expert pathologists to verify the histologic diagnosis, the other immediately snap-frozen in liquid nitrogen, and stored in liquid nitrogen until RNA extraction. None of the patients had received any preoperative treatment. Tumors were staged according to the American Joint Committee on Cancer (AJCC) pathologic tumor-node-metastasis (TNM) classiication. The characteristics of patients are described in S1 and S2 Tables.
Cell culture
Human thyroid cancer cell lines K1 (papillary) and WRO (follicular) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco BRL), 100 units/ml penicillin, and 100 μg/ml streptomycin sulfate. Cells were maintained at 37°C in a 5% CO2 incubator. All these cell lines were original purchased from cell bank of the Chinese Academy of Science, Shanghai.
Reagents
Recombinant human IL–23 (rhIL–23) was purchased from R&D Systems (Minneapolis, MN). Monoclonal antibodies (Abs) against human SOCS4 and GAPDH were purchased from Sigma (St Louis, MO). Chemically synthesized miRNA mimics and miRNA inhibitors were purchased from Ambion (Austin, TX, USA). TRIzol, Lipfectamine–2000, Enzyme MIX were purchased from Invitrogen (Basel, Switzerland).
Quantitative real time PCR
Quantitative real time PCR analysis was performed to determine mature miRNA and mRNA levels. For quantitative mature microRNAs detection, Total miRNAs were isolated using a mirVana miRNA isolation kit (Ambion), according to the manufacturer’s instructions. Total RNA (2 μg) was reversetranscribed with Bulge-Loop mature miRNA-specific reverse transcription primers (Ambion) and Moloney murine leukemia virus reverse transcriptase (Promega). Quantitative Real-time PCR-based quantification of miRNAs was performed using the miRNA analysis kits (Ambion), according to the manufacturer’s instructions. The levels of miRNAs were normalized to those of the internal control U6 snRNA. To detect cellular mRNAs, total RNA was isolated using TRIzol (Invitrogen, Basel, Switzerland). Cellular RNA samples were reverse-transcribed using random primers. Real-time PCR was performed using a LightCycler 480 (Roche) and the SYBR Green system (Applied Biosystems). GAPDH was amplified as an internal control. Primers used this study are listed in S3 Table and the SYBR green products verified by sequencing.
Transwell assay
Cell migration and invasion assay were performed as described previously [27]. Briefly, cells were treated either with 50 ng/mL rhIL–23 or BSA buffer control for described time and dose and observed accordingly. The mean number of migrating and invading cells was expressed as a percentage relative to the control, which was designated as 100%.
Wound closure assay
A wound was introduced on the confluent monolayer cells using a micropipette tip. Photographs were taken at 40 X magnification using phase-contrast microscopy immediately after wound incision and at selected timepoints. Wound closure was measured by calculating pixel densities in the wound area by Cella software (Olympus Biosystem Gmb, Hamburg, Germany) and expressed as percentage of wound closure of triplicate areas ± SD.
MTT assay
The 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used in the evaluation of cells proliferation. Cells were seeded into 96-well plates at 5×103 cells/well. Twenty-four hours later, MTT assay was conducted. Finally, the optical density was determined at 570nm using the ELISA plate reader (Model 550; Bio-Rad). At least three independent experiments were ensured.
Transfection and luciferase reporter assay
Cells were seeded on 24-well or 6-well dishes, depending on the experiment, and were grown to the confluence reaching approximately 80–90% at the time of transfection. Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the protocol provided by the manufacturer. A Renilla luciferase reporter vector pRL-TK was used as internal control. Luciferase assays were performed with a dual-specific luciferse assay kit (Promega, Madison, WI). Firefly luciferase activities were normalized on the basis of Renilla luciferase activities. All reporter assays were repeated for at least three times. Data shown were average values ± SD from one representative experiment.
RNA interference
SOCS4 shRNA and irrelevant shRNA control (shRNA-control) were purchased from GenePharma (GenePharma, Shanghai) and prepared by ligation of the corresponding pairs of oligonucleotides to PGPU6/GFP/Neo. The target sequence can be found in S4 Table.
Western blot analysis
Whole-cell lysates were prepared by lysing cells with PBS pH 7.4 containing 0.01% Triton X–100, 0.01% EDTA, and 10% protease inhibitor cocktail (Roche, Applied Science). Protein concentration was determined by the Bradford assay (Bio-Rad). The polypeptides from cell lysates were separated on SDS 12% polyacrylamide gels cross-linked with N,N -methylenebisacylamide, and transferred electrically to nitrocellulose membranes (Millipore, Billerica, MA). Nonspecific binding was blocked with 5% milk in PBST before adding primary antibodies used in this study. Horseradish peroxidase–linked anti-rabbit and anti-mouse antibodies (Sigma, St Louis, MO) were used as secondary antibodies. Protein levels were quantified by scanning blots on a Gel Doc EZ imager (Bio-Rad) and analysis with Quantity One 1D image analysis software 4.4.0 (Bio-Rad)
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). Parametric and nonparametric data were analyzed using a two-tailed t-test and the Mann–Whitney U test respectively. A value of P < 0.05 was considered statistically significant. Data are presented as mean±S.D. or mean±S.E.M.
Results
IL–23 promotes the migration and invasion of thyroid cancer cells
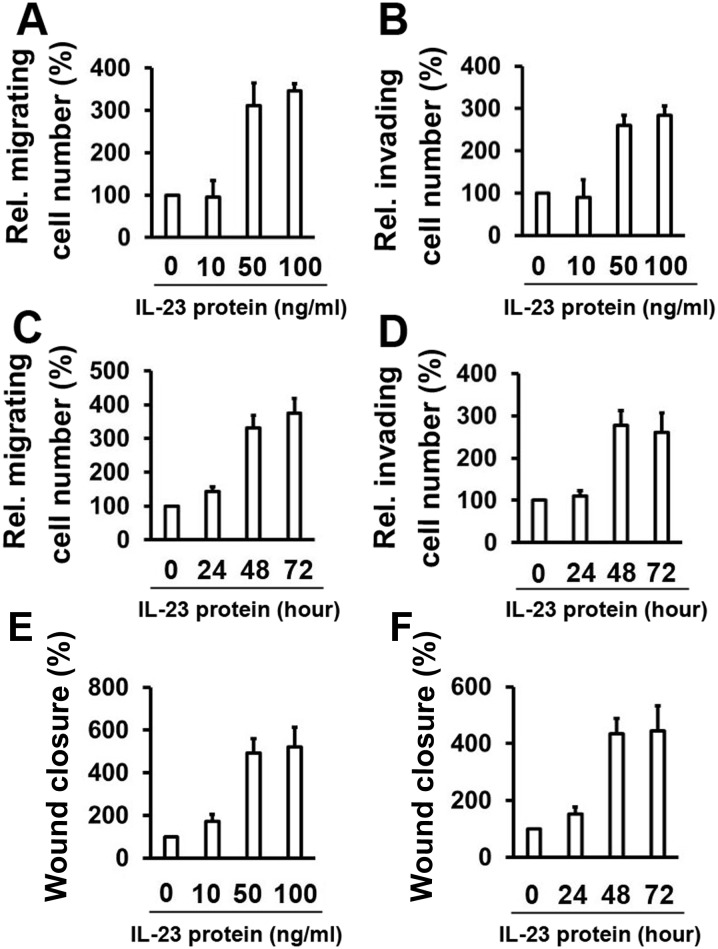
We wanted to test the effect of IL–23 on the cell proliferation of thyroid cancer cells in order to observe if cell proliferation disturbs the migration and invasion capacity of the cells. The MTT assay showed that the proliferation of K1 cells and WRO cells were hardly affected by any dose of IL–23 (S1 Fig). We next investigated whether IL–23 could affect the migration and the invasion of thyroid cancer cells. Enhanced movement and invasion of K1 cells were detected in the presence of 50 and 100 ng/ml of IL–23 (Fig 1A and 1B). Similarly, increased migration and invasion were also detected as early as 48 hours (h) following treatment with 50 ng/ml IL–23 protein (Fig 1C and 1D). We also demonstrated that IL–23 increased the migration and invasion of WRO cells in a dose- and time-dependent manner (S2 Fig). In addition, wound healing assay also show that IL–23 increased the migration of K1 cells (Fig 1E and 1F). Together, these results confirm the dose- and time-dependent promigratory and proinvasive effect of IL–23.
Fig 1. IL–23 promotes K1 cell migration and invasion.
(A) K1 cells were treated with rhIL–23 for 48 h at the indicated concentrations, followed by culturing in a transwell system for 24 hours. (B) Cell invasion assay experiments were performed with K1 cells that were treated as in (A). (C) K1 cells were treated with a concentration of 50 ng/ml rhIL–23 for the indicated time points, followed by culturing in a transwell system for 24 h. (D) The experiments were performed as in (C) for the cell invasion assay. (E) K1 cells were treated with rhIL–23 for 48 h at the indicated concentrations, followed by introducing a wound. Cell migration into the wound was monitored at 24 hours. (F) K1 cells were treated with a concentration of 50 ng/ml rhIL–23 for the indicated time points, followed by introducing a wound. Cell migration into the wound was monitored at 24 hours. Wound closure was measured by calculating pixel densities in the wound area and expressed as percentage of wound closure of triplicate areas ± standard deviations In the transwell migration, invasion and wound healing assay, the data are expressed as a percentage of the control. Data represent mean ± SD, n = 3 (**P < 0.01; *P < 0.05).
IL–23 induces the migration and invasion of thyroid cancer cells through SOCS4
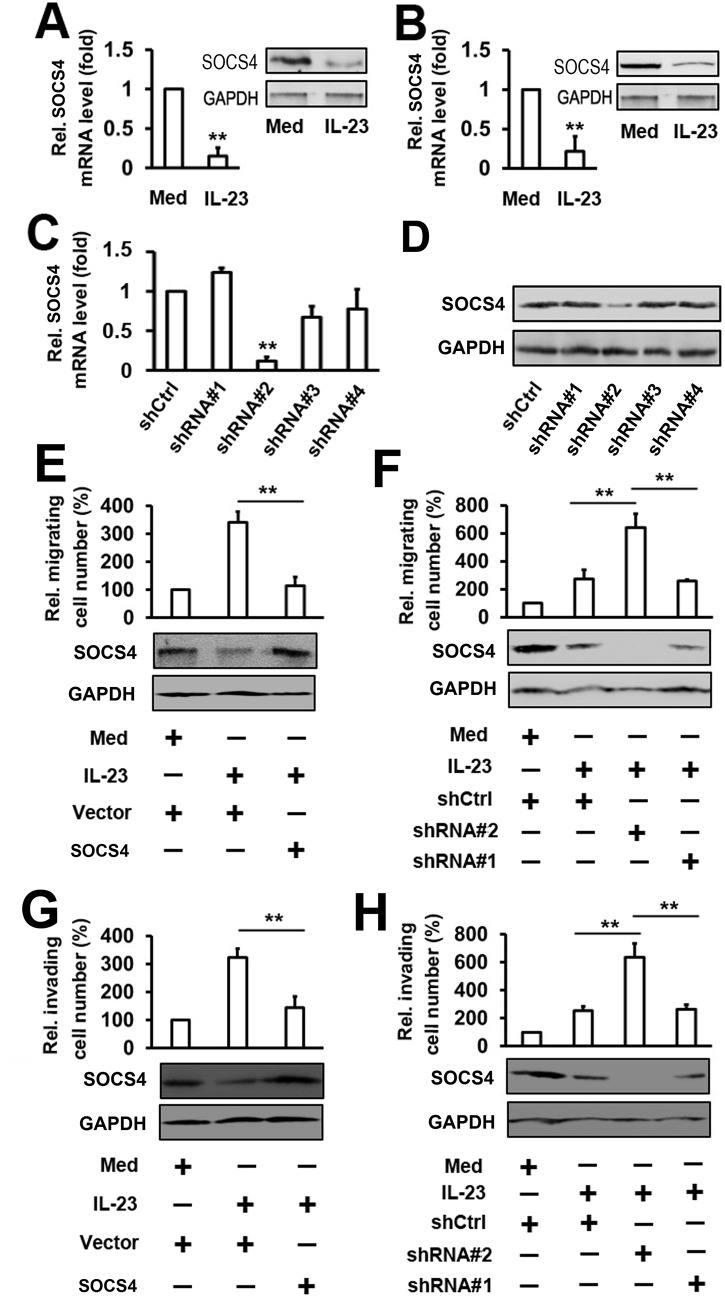
To identify the role of IL–23 in the expression of SOCS4, K1 cells were stimulated with IL–23 protein for 24 h at the concentration of 50 ng/ml. The suppressors of cytokine signaling (SOCS4) mRNA and protein were detected by real-time PCR and western blot, respectively. Results showed that IL–23 suppressed SOCS4 mRNA and protein expression in K1 cells (Fig 2A). Similar results were also obtained in the WRO cell line (Fig 2B). To determine whether SOCS4 participates in the IL-23-mediated migration and invasion of thyroid cancer cells, we constructed 4 human SOCS4-specific short hairpin RNA (shRNA). Real-time PCR results showed that the #2 shRNA plasmids could markedly inhibit the expression of SOCS4 in K1 cells, whereas the other shRNA plasmids had little effect on the expression of SOCS4 (Fig 2C). SOCS4 protein levels showed a similar trend as determined by Western blot (Fig 2D). In cell migration experiments, the overexpression of SOCS4 inhibited IL-23-induced migration in K1 cells (Fig 2E). Conversely, the knockdown of SOCS4 expression enhanced IL-23-induced migration in K1 cells (Fig 2F). The degrees of induction were correlated with the efficiencies of SOCS4 knockdown by each shRNA plasmid (Fig 2F). Similar results were obtained with transwell invasion experiments (Fig 2G and 2H). Since SOCS4 negatively regulates migration, we next analyzed the role of SOCS4 in the cell proliferation of thyroid cancer cells. Results from MTT assay indicated that the proliferation of K1 cells were hardly affected by the expression of SOCS4. In addition, overexpression or Knockdown of SOCS4 and treatment with IL–23 could not affect the cell proliferation of thyroid cancer cells (S3 Fig). These results suggest that the activation of IL-23-regulated migration and invasion may require SOCS4.
Fig 2. Analysis of the role of So4 in the regulation of thyroid cancer cell migration and invasion mediated by IL–23.
(A) K1 cells were treated with 50 ng/ml rhIL–23 for 48 h. SOCS4 RNA levels were quantified by qRT-PCR (left panel) and the protein levels of SOCS4 were detected by western blot (right panel). (B) Experiments with WRO cells were performed as in A. (C, D) K1 cells were transfected with different SOCS4 shRNA (shRNA#1, shRNA#2, shRNA#3 and shRNA#4) or shCtrl as mock. After 48 h, SOCS4 mRNA levels were measured by qRT-PCR (C) and western blot (D). (E) K1 cells were transfected with the indicated plasmid, then treated with rhIL–23 (50 ng/ml) for 48 h and allowed to migrate towards serum for 24 h (upper panel). Protein levels of SOCS4 were detected by western blot (lower panel). (F) The cells were transfected with indicated shRNA-SOCS4 and the experiments were performed as in (E). (G, H) The cell invasion assay experiments were performed under similar conditions as described in (E) and (F). In the real-time RT-PCR experiments, the control was designated as 1. All the experiments were repeated at least 3 times with similar results. Bar graphs represent mean ± SD, n = 3 (**P < 0.01; *P < 0.05).
MicroRNA–25 downregulates SOCS4 expression by directly targeting its 3′-UTR
To analyze the miRNAs that may target the 3′-UTR of SOCS4, we used 3 online databases, TargetScanHuman, miRDB, and miRWalk2.0, to search for potential candidates. Fourteen of the common potential miRNAs were found in the 3 databases. To determine the effect of the predicted miRNAs on the expression of SOCS4, the SOCS4 3′-UTR was cloned into a firefly luciferase reporter plasmid. The luciferase activity assays indicated that 6 miRNAs inhibited the SOCS4 luciferase activity below 50% as compared with the miR-Ctrl (S4A Fig). We next investigated which miRNAs were activated by IL-23-induced migration and invasion. K1 cells were treated with IL–23 protein at 50 ng/ml for 48 h. Results from real-time PCR demonstrated that among the miRNAs that could attenuate SOCS4 luciferase activity, the expression of miR–25 was significantly induced (S4B Fig).
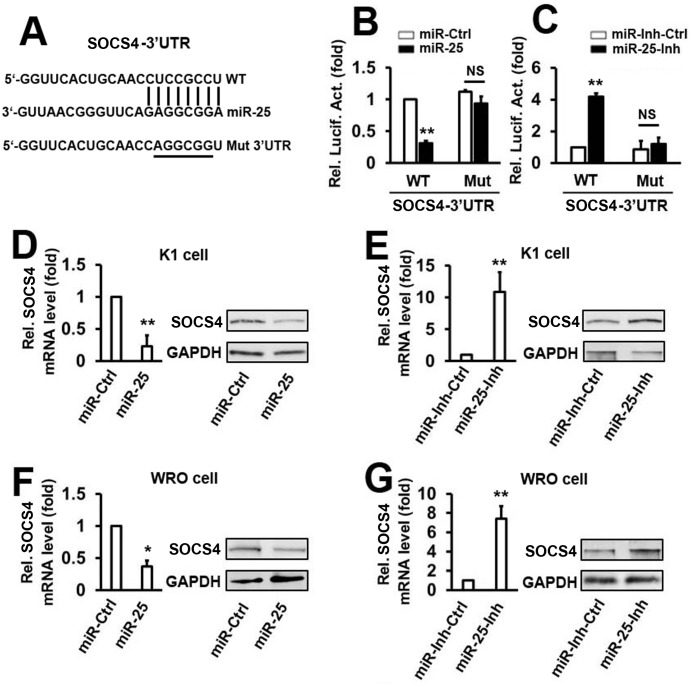
From our data we hypothesized that miR–25 may play a role in the IL–23/SOCS4 signaling pathway. We then investigated whether miR–25 regulates SOCS4 expression by post-transcriptional targeting of its 3′-UTR. A distinct mutation was generated in the 3′-UTR of SOCS4 at predicted seed-matching sites to test the interaction between miR–25 and SOCS4 3′-UTR (Fig 3A). Transient transfection of K1 cells with wild type (WT) SOCS4 3′-UTR, and the miR–25 mimic, leads to a significant decrease in the reporter activity compared with that of the miR-control (Fig 3B). This phenomenon was disrupted when the same cell lines were transfected with SOCS4 3′-UTR mutants (Fig 3B). The miR–25 inhibitor (miR-25-Inh) significantly stimulated the luciferase activity of the WT SOCS4 3′-UTR, without any effect on Mut SOCS4 3′-UTR in K1 cells (Fig 3C). MicroRNA–25 overexpression in K1 cells significantly suppressed both the mRNA and protein levels of SOCS4 (Fig 3D). Conversely, miR–25 inhibitor-mediated knockdown of endogenous miR–25 increased SOCS4 mRNA and protein expression in K1 cells (Fig 3E). To determine whether the miR-25-mediated downregulation of SOCS4 expression is a common feature in thyroid cancer cells, similar experiments were performed in WRO cells (Fig 3F and 3G). Together, these results suggest that SOCS4 is a target of miR–25 in K1 cells and WRO cells.
Fig 3. MicroRNA–25 downregulates SOCS4 expression by directly targeting its 3′UTR.
(A) The sequences of miR–25 binding sites within the human SOCS4 3′UTRs and the schematic reporter constructs. In this panel, WT represents the reporter constructs containing the entire 3′UTR sequences of SOCS4. SOCS4-MUT represents the reporter constructs containing mutated nucleotides. (B, C) K1 cells were co-transfected with either WT or MUT SOCS4 3′UTR reporter plasmids and either miR–25 mimic (B) or miR–25 inhibitor (C). Luciferase activities were measured after 48 h using a dual-luciferase assay kit and normalized to Renilla luciferase. (D) K1 cells were transfected with miR–25 mimic or controls for 48 h. SOCS4 mRNA (left panel) and protein (right panel) were detected by qRT-PCR and western blot, respectively. (E) Cells were transfected with miR–25 inhibitor or control and the experiments were performed as in D. (F, G) WRO cells were utilized and experiments were performed as in D and E. All the experiments were repeated at least 3 times with similar results. Bar graphs represent mean ± SD, n = 3 (**P < 0.01; *P < 0.05).
MicroRNA–25 promotes the motility of thyroid cancer cells by targeting SOCS4
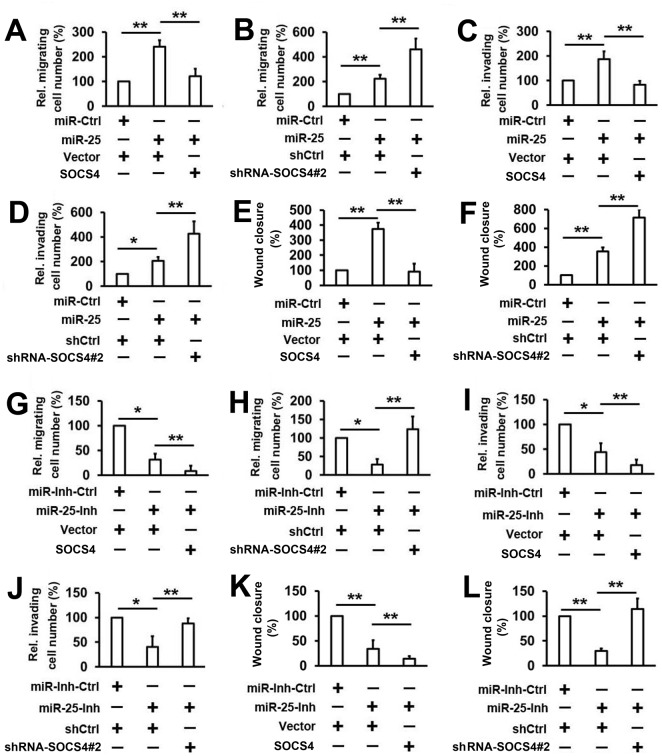
Since miR–25 can suppress SOCS4 expression by sequence-specific binding to its 3′-UTR, we hypothesized that miR–25 may affect the migration and invasion of thyroid cancer via SOCS4. To test the hypothesis, K1 cells were co-transfected with the SOCS4 expression vector (or empty vector) and the miR–25 mimics (or miR-Ctrl). Transwell migration assays demonstrate that miR–25 promotes the migration of K1 cells and cell migration induced by miR–25 is reversed by SOCS4 overexpression (Fig 4A). In addition, we observed that the knockdown of SOCS4 may significantly enhance the effect of miR–25 on cell migration (Fig 4B). Similar results were obtained in the invasion assays and wound healing assay (Fig 4C-4F). The effect of the miR–25/SOCS4 signaling pathway on the migration of thyroid cancer cells was further evaluated using the miR–25 inhibitor. As shown in Fig 4G, miR–25 inhibitor significantly inhibits the migration of K1 cells. In addition, the miR–25 inhibitor and the SOCS4 expression vector synergistically inhibit the migration of K1 cells (Fig 4G). Conversely, high levels of migration were present in K1 cells when SOCS4 was knocked down by shRNA-SOCS4#2 (Fig 4H). Similar results were also obtained in invasion assays and wound healing assay (Fig 4I-4L). These results suggest that the inhibition of SOCS4 expression is responsible for the ability of miR–25 to promote cell invasion and migration.
Fig 4. Determination of the effect of miR–25 on the regulation of SOCS4-suppressed thyroid cancer cell migration and invasion.
(A, B) K1 cells were co-transfected with indicated miR-RNA mimic and plasmid (A) or shRNA (B) for 48 h and allowed to migrate towards serum for 24 h. (C, D) Cell invasion assay experiments were performed with the same conditions as in A and B. (E, F) Wound healing assay experiments were performed with the same conditions as in A and B. (G, H) Cells were transfected with miR–25 inhibitor or control and the experiments were performed as in A and B. (I, J) Cell invasion assay experiments were performed with the same conditions as in G and H. (K, L) Wound healing assay experiments were performed with the same conditions as in G and H. Bar graphs represent mean ± SD, n = 3 (**P < 0.01; *P < 0.05).
IL–23 stimulates miR–25 expression in thyroid cancer cells
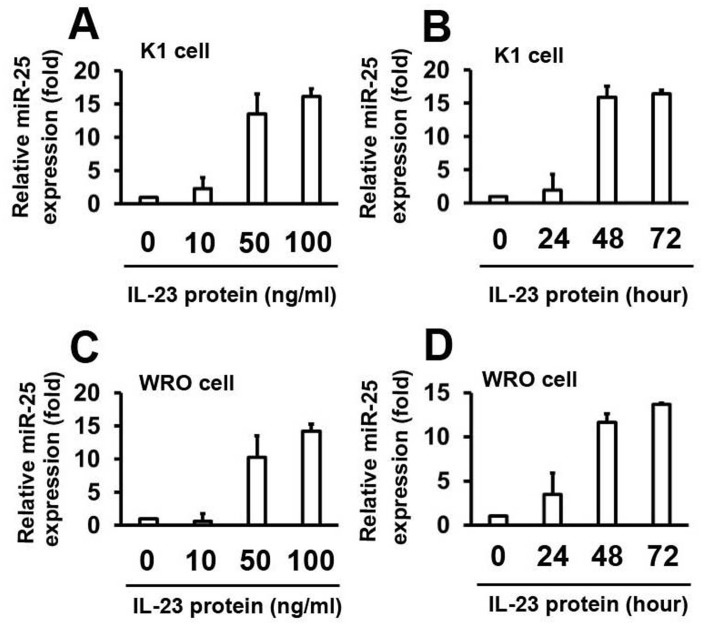
To identify the role of IL–23 in the expression of miR–25, K1 cells were stimulated with human IL–23 protein at different concentrations for 48 h. MicroRNA–25 levels were detected by real-time PCR. Results show that miR–25 levels are upregulated by the IL–23 protein in a dose-dependent manner (Fig 5A). Consistently, K1 cells were treated with IL–23 protein at different time points, at a concentration of 50 ng/ml. Results from real-time PCR analyses demonstrate that miR–25 levels increase as the time increases (Fig 5B). The role of IL–23 on miR–25 expression was confirmed by repeating the experiments using WRO cells (Fig 5C and 5D). These results suggest that IL–23 activates miR–25 expression.
Fig 5. IL–23 stimulates miR–25 expression.
MicroRNA–25 levels were quantified by real-time PCR and the experiments were performed as described in Fig 1. The expression levels were normalized to U6 snRNA. Data represent mean ± SD, n = 3 (**P < 0.01; *P < 0.05).
MicroRNA–25 plays an important role in IL-23-mediated SOCS4 inhibition and cell migration and invasion
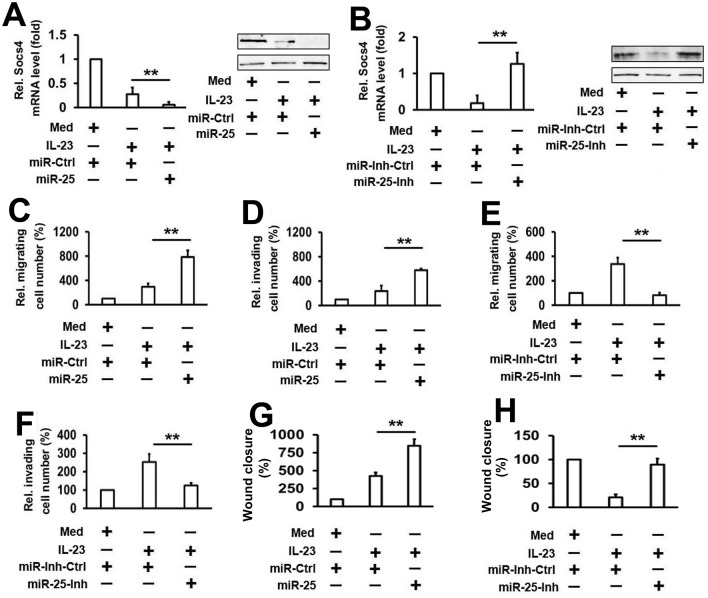
To define the role of miR–25 in the downregulation of IL-23-mediated SOCS4 expression, K1 cells were transfected with either miR–25 mimics or miR-Ctrl and treated with or without IL–23 protein for 48 h. Results from real-time PCR and western blot analyses show that transfection with miR–25 mimics increases IL-23-mediated inhibition of SOCS4 mRNA and protein expression (Fig 6A). In contrast, high levels of SOCS4 mRNA and protein are present in K1 cells, when the expression of miR–25 is inhibited by the miR–25 inhibitor (Fig 6B). We also examined whether miR–25 is involved in IL-23-mediated thyroid cancer cell line motility. Using transwell migration and invasion assays, we showed that miR–25 overexpression stimulates IL-23-mediated activation of migration and invasion (Fig 6C and 6D), and knockdown of miR–25 expression inhibits IL-23-mediated activation of migration and invasion (Fig 6E and 6F). Similar results were also obtained in wound healing assay (Fig 6G and 6H).Taken together, these data suggest that miR–25 is the key component involved in IL-23-mediated thyroid cancer cell migration and invasion through the inhibition of SOCS4 expression.
Fig 6. Analysis of the role of miR–25 in the regulation of SOCS4 expression and IL-23-mediated thyroid cancer cell migration and invasion.
(A) K1 cells were transfected with the indicated plasmid, then treated with rhIL–23 (50 ng/ml) for 48 h. SOCS4 mRNA (left panel) and protein (right panel) were detected by qRT-PCR and western blot, respectively. (B) Cells were transfected with miR–25 inhibitor or control, and the experiments were performed as in A. (C, D) K1 cells were transfected with miR–25 mimic (C) or inhibitor (D) and controls, then treated with rhIL–23 (50 ng/ml) for 48 h and allowed to migrate towards serum for 24 h. (E, F) Cell invasion assay experiments were performed with the same conditions as in C and D. (G, H) Wound healing assay experiments were performed with the same conditions as in C and D. All the experiments were repeated at least 3 times with similar results. Bar graphs represent mean ± SD, n = 3 (**P < 0.01; *P < 0.05).
The expression of IL–23, miR–25 and SOCS4 in thyroid cancer tissues
To validate the role of IL–23, miR–25 and SOCS4 in thyroid cancer, the expression of IL–23, miR–25 and SOCS4 were analyzed in 35 pairs of clinical PTC, 26 pairs of clinical FTC, and 22 normal thyroid samples. As shown in S5A-S5C Fig, the expression of SOCS4 was lower and the expression level of IL–23 and miR–25 was higher in PTC and FTC specimens than normal thyroid samples. In addition, high levels of IL–23 were correlated with high levels of miR–25 (S5D Fig). Interestingly, low levels of SOCS4 expression were correlated with high levels of IL–23 and miR–25 expression in PTC and FTC specimens (S5E and S5F Fig). These observations strongly suggest that alterations of IL–23, miR–25 and SOCS4 expression could be involved in thyroid cancer progression.
Discussion
In this study, we defined a novel signaling pathway implicated in the control of thyroid cancer cell migration and invasion. First, we demonstrated that IL–23 upregulated miR–25 expression as well as downregulated SOCS4 expression in thyroid cancer cell migration and invasion. Further, we also showed that miR–25 promotes thyroid cancer cell migration and invasion by targeting SOCS4. Finally, our data suggest that miR–25 is involved in IL-23-associated SOCS4 expression and cell migration and invasion.
Tumor cell migration and invasion is a very complicated process in which cancer cells spread from the primary tumor, survive in the circulation, and grow in distant locations in the body [28]. Each process is determined by the migration and invasion ability of the tumor cells and the local tumor microenvironment that provide a favorable environment for tumor cells to survive and metastasize [29]. Recent investigations have demonstrated that high expression of levels of IL–23, which can be detected in the microenvironment, could help to facilitate tumor metastasis. For example, IL–23 promotes hepatocellular carcinoma metastasis by NF-κB-upregulated MMP9 expression [9]. IL–23 is highly expressed in metastases-associated astrocytes, and IL–23 induces the progression of melanoma brain metastasis [8]. IL–23 plays a pivotal role in the development of esophageal cancer via an epithelial-mesenchymal transition [7]. IL–23 can enhance the proliferation and invasion of colorectal carcinoma cells [10]. However, the role of IL–23 in thyroid cancer cell migration and invasion is still unknown. To our knowledge, this is the first study to demonstrate the direct effects of IL–23 on the migration and invasion of thyroid cancer cells. Interestingly, a recent study demonstrated that IL–23 regulates the proliferation of lung cancer cells [30]. Nonetheless, unlike lung cancer cells, we did not find evidence to support that IL–23 induces the proliferation of thyroid cancer cells (S1 Fig). We speculate that there may be some intrinsic differences between thyroid cancer and lung cancer. On the other hand, IL–23 promotes the migration and invasion of thyroid cancer cells.
Currently, two reports have implied the potential role of SOCS4 in cancer. One study used a double combination array analysis to prove that SOCS4 is a novel gastric cancer suppressor gene [31]. The other study compared the expression differences of SOCS1-7 between breast cancer tissue and background breast tissue [32]. High expression of SOCS4 is significantly associated with an earlier tumor stage and a better clinical outcome in human breast cancer [32]. In this study, we observed that the levels of SOCS4 are decreased in IL-23-induced migration and invasion of thyroid cancer cells (Fig 2). Treatment with IL–23 resulted in reduced expression levels of SOCS4 in thyroid cancer cells (Fig 2). Through overexpression and knockdown experiments, we demonstrate that SOCS4 negatively regulates IL-23-induced migration and invasion (Fig 2). Recently, several studies have shown evidence that the SOCS family has a strong tumor suppressing role in several types of solid and hematological tumors [32,33]. When considering the next step, exploring the relationship between the SOCS family and IL–23 could be of great help in further clarifying the role that is played by the SOCS family in the development of thyroid tumors.
The role of miR–25 in cancer is not consistent and is occasionally controversial. In some studies, miR–25 may suppress the proliferation and the migration of colon cancer cells as a tumor suppressor gene in vitro and in vivo [24,26], whereas in others, miR–25 could remarkably promote cell proliferation and suppress apoptosis in gastric cancer [26]. In this study, we found that miR–25 promotes the migration and invasion of thyroid cancer cells by targeting SOCS4 (Figs 3 and 4). Additional data reveal that miR–25 is involved in IL-23-regulated cell migration and invasion (Fig 6). These results are consistent with the above studies showing that miR–25 promotes tumor cell migration and invasion. Besides, we also analyzed the role of miR–25 in proliferation, apoptosis, and cell cycle in thyroid cancer cell lines. However, there was no association between miR–25 and cell proliferation, apoptosis, and cell cycle in thyroid cancer cells (data not shown). These results indicate that deregulated miR–25 expression plays different roles in different types of cancers.
In summary, our present study provides a novel evidence indicating that miR–25 and SOCS4 play a functional role in regulating IL-23-mediated migration and invasion in thyroid cancer cells. Although additional studies are required to understand the intricate regulatory mechanisms of thyroid cancer cell migration and invasion, the results obtained may shed new light in understanding cancer resistance to therapy.
Supporting Information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (No.81372880); the Independent research project of Wuhan University (No. 2042014kf0184; NO. 2042014kf0119); the doctoral program of Higher Education Research Fund (No. 20130141120093; No. 20110141110062); the Natural Science Foundation of Hubei province (No.2012FFA045). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Chen AY, Jemal A, Ward EM (2009) Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer 115: 3801–3807. 10.1002/cncr.24416 [DOI] [PubMed] [Google Scholar]
- 2. Davies L, Welch HG (2006) Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 295: 2164–2167. [DOI] [PubMed] [Google Scholar]
- 3. Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331: 1565–1570. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 4. Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7: 41–51. [DOI] [PubMed] [Google Scholar]
- 5. Chen D, Jiang R, Mao C, Shi L, Wang S, Yu L, et al. (2012) Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum Immunol 73: 1068–1072. 10.1016/j.humimm.2012.07.333 [DOI] [PubMed] [Google Scholar]
- 6. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL–23, with biological activities similar as well as distinct from IL–12. Immunity 13: 715–725. [DOI] [PubMed] [Google Scholar]
- 7. Chen D, Li W, Liu S, Su Y, Han G, Xu C, et al. (2015) Interleukin–23 promotes the epithelial-mesenchymal transition of oesophageal carcinoma cells via the Wnt/beta-catenin pathway. Sci Rep 5: 8604 10.1038/srep08604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein A, Schwartz H, Sagi-Assif O, Meshel T, Izraely S, Ben Menachem S, et al. (2015) Astrocytes facilitate melanoma brain metastasis via secretion of IL–23. J Pathol. [DOI] [PubMed] [Google Scholar]
- 9. Li J, Lau G, Chen L, Yuan YF, Huang J, Luk JM, et al. (2012) Interleukin 23 promotes hepatocellular carcinoma metastasis via NF-kappa B induced matrix metalloproteinase 9 expression. PLoS One 7: e46264 10.1371/journal.pone.0046264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suzuki H, Ogawa H, Miura K, Haneda S, Watanabe K, Ohnuma S, et al. (2012) IL–23 directly enhances the proliferative and invasive activities of colorectal carcinoma. Oncol Lett 4: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hilton DJ (1999) Negative regulators of cytokine signal transduction. Cell Mol Life Sci 55: 1568–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ram PA, Waxman DJ (1999) SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem 274: 35553–35561. [DOI] [PubMed] [Google Scholar]
- 13. Fujitake S, Hibi K, Okochi O, Kodera Y, Ito K, Akiyama S, et al. (2004) Aberrant methylation of SOCS–1 was observed in younger colorectal cancer patients. J Gastroenterol 39: 120–124. [DOI] [PubMed] [Google Scholar]
- 14. To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai AH, et al. (2004) Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS–1 in human gastric cancer cell line. Br J Cancer 91: 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, et al. (2003) SOCS–3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci U S A 100: 14133–14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, et al. (2005) SOCS–3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene 24: 6699–6708. [DOI] [PubMed] [Google Scholar]
- 17. Lai RH, Hsiao YW, Wang MJ, Lin HY, Wu CW, Chi CW, et al. (2010) SOCS6, down-regulated in gastric cancer, inhibits cell proliferation and colony formation. Cancer Lett 288: 75–85. 10.1016/j.canlet.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 18. Storojeva I, Boulay JL, Ballabeni P, Buess M, Terracciano L, Laffer U, et al. (2005) Prognostic and predictive relevance of DNAM–1, SOCS6 and CADH–7 genes on chromosome 18q in colorectal cancer. Oncology 68: 246–255. [DOI] [PubMed] [Google Scholar]
- 19. Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20. [DOI] [PubMed] [Google Scholar]
- 20. Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233. 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang B, Pan X, Cobb GP, Anderson TA (2007) microRNAs as oncogenes and tumor suppressors. Dev Biol 302: 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Petrocca F, Vecchione A, Croce CM (2008) Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor beta signaling. Cancer Res 68: 8191–8194. 10.1158/0008-5472.CAN-08-1768 [DOI] [PubMed] [Google Scholar]
- 23. Feng S, Pan W, Jin Y, Zheng J (2014) MiR–25 promotes ovarian cancer proliferation and motility by targeting LATS2. Tumour Biol 35: 12339–12344. 10.1007/s13277-014-2546-0 [DOI] [PubMed] [Google Scholar]
- 24. Li Q, Zou C, Zou C, Han Z, Xiao H, Wei H, et al. (2013) MicroRNA–25 functions as a potential tumor suppressor in colon cancer by targeting Smad7. Cancer Lett 335: 168–174. 10.1016/j.canlet.2013.02.029 [DOI] [PubMed] [Google Scholar]
- 25. Yang T, Chen T, Li Y, Gao L, Zhang S, Wang T, et al. (2015) Downregulation of miR–25 modulates non-small cell lung cancer cells by targeting CDC42. Tumour Biol 36: 1903–1911. 10.1007/s13277-014-2793-0 [DOI] [PubMed] [Google Scholar]
- 26. Zhao H, Wang Y, Yang L, Jiang R, Li W (2014) MiR–25 promotes gastric cancer cells growth and motility by targeting RECK. Mol Cell Biochem 385: 207–213. [DOI] [PubMed] [Google Scholar]
- 27. Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, et al. (2011) Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One 6: e21816 10.1371/journal.pone.0021816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiong T, Li Y, Li Z, Xie X, Lu L (2013) In vivo monitoring the process of tumor growth, metastasis and bacterial infection expressing GFP via real-time optical imaging. J Biomed Nanotechnol 9: 274–280. [DOI] [PubMed] [Google Scholar]
- 29. Spano D, Zollo M (2012) Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis 29: 381–395. 10.1007/s10585-012-9457-5 [DOI] [PubMed] [Google Scholar]
- 30. Li J, Zhang L, Zhang J, Wei Y, Li K, Huang L, et al. (2013) Interleukin 23 regulates proliferation of lung cancer cells in a concentration-dependent way in association with the interleukin–23 receptor. Carcinogenesis 34: 658–666. 10.1093/carcin/bgs384 [DOI] [PubMed] [Google Scholar]
- 31. Kobayashi D, Nomoto S, Kodera Y, Fujiwara M, Koike M, Nakayama G, et al. (2012) Suppressor of cytokine signaling 4 detected as a novel gastric cancer suppressor gene using double combination array analysis. World J Surg 36: 362–372. 10.1007/s00268-011-1358-2 [DOI] [PubMed] [Google Scholar]
- 32. Sasi W, Jiang WG, Sharma A, Mokbel K (2010) Higher expression levels of SOCS 1,3,4,7 are associated with earlier tumour stage and better clinical outcome in human breast cancer. BMC Cancer 10: 178 10.1186/1471-2407-10-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sutherland KD, Lindeman GJ, Choong DY, Wittlin S, Brentzell L, Phillips W, et al. (2004) Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene 23: 7726–7733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.