Abstract
During blooms of the dinoflagellate Karenia brevis, filter-feeders such as oysters and clams bioaccumulate brevetoxins, often to levels that are toxic to humans. In controlled aquarium experiments, we exposed live oysters to bloom levels of toxic K. brevis, followed by 10 weeks of exposure to non-toxic microalgae. Oysters were harvested weekly and analyzed for brevetoxins and brevetoxin metabolites to quantify toxin bioaccumulation and depuration. All of the PbTx-2 concentrated by oysters was immediately converted to a mixture of polar metabolites that were then slowly eliminated from the oysters. However, 90% of measured PbTx-3 was eliminated within two weeks of toxic exposure but without apparent biotransformation. Extracts of oysters containing high levels of PbTx-3 were toxic to mice by intraperitoneal (IP) injection. Extracts of oysters harvested after PbTx-3 had been eliminated were non-toxic despite high concentrations of PbTx-2 metabolites. Oysters collected in Florida during and after a bloom of K. brevis contained polar metabolites of PbTx-2 as well as PbTx-3, but no PbTx-2. Again, PbTx-3 concentration was a good predictor of mouse toxicity. One hundred percent conversion of PbTx-2 to polar metabolites was also accomplished in vitro by spiking oyster or clam homogenate with PbTx-2, followed by a brief incubation at room temperature. These PbTx-2 metabolites did not kill mice, either orally or by intraperitoneal injection, even at concentrations 30 times greater than toxic PbTx-3 levels.
Introduction
Neurotoxic shellfish poisoning (NSP) is a form of food poisoning caused by ingestion of shellfish contaminated with brevetoxins. A series of brevetoxin metabolites have been identified from toxic shellfish and the urine of humans suffering from NSP (Poli et al., 2000). In this study, we used this competitive ELISA (Naar et al., 2002), as well as HPLC and mouse bioassays, to track the bioaccumulation, derivatization, and elimination of brevetoxins and metabolites in the Eastern oyster, Crassostrea virginica. Experiments were conducted on oysters from commercial shellfish beds following a natural bloom of Karenia brevis in Florida as well as on oysters exposed to controlled levels of K. brevis in an aquarium. We explore the depuration of brevetoxins by shellfish and evaluate the analytical methods currently in use. The potential consequences for human health and the fisheries industry are discussed as well as the discovery of this significant environmental sink for brevetoxins into metabolites.
Materials and Methods
Oysters from Florida
Oysters were collected from two shellfish beds in northwest Florida (east and central Choctawhatchee Bay) on December 18, 2000, and again on January 2, 2001. Four different samples of oysters were homogenized and subjected to one of two treatments:
Extraction with diethyl ether then acetone for separation of parent brevetoxins (ether extract) from polar brevetoxin metabolites (acetone post ether extract). Each 1-g aliquot was heated with 10 μL HCl/10 mg NaCl for 5 minutes. After the sample cooled to room temperature, 1–2 mL diethyl ether was added and the sample agitated for 3–5 minutes. Brief centrifugation was used to resolve the aqueous and ether layers. Three more extractions with diethyl ether followed, with all extracts being combined. These extracts are referred to as ether extracts. Residual mollusk meat was then extracted with acetone (2 × 5 mL).
Extraction with acetone to extract simultaneously parent brevetoxins and metabolites. Each 1-g aliquot was extracted twice with 5 mL acetone only (as described above).
Aquarium Oysters
Oysters were obtained from a shell-fish farm in Wilmington, NC, maintained in an aquarium, and fed Isochrysis for several months. Six additions of cultured Karenia brevis were made over three days to maintain a concentration of K. brevis in the aquarium of 5.0 × 105 cells/L. Oysters were harvested at the times indicated below.
In Vitro Production of Brevetoxin Metabolites Using Oyster and Clam Homogenate
One gram of non-toxic oyster or clam meat from a local (Wilmington, NC) fish market was homogenized and spiked with up to 100 μg of pure PbTx-2 or PbTx-3. Each sample was agitated at room temperature for three hours and extracted with acetone (2 × 5 mL) as described for Florida samples above. Conversion of brevetoxins to brevetoxin metabolites was measured by HPLC followed by ELISA analysis (below).
Samples generated by the above methods were subjected to ELISA analysis, HPLC, or mouse bioassay:
ELISA Methodology
ELISA analyses were performed according to Naar et al. (2002).
HPLC Methodology
HPLC was used to separate compounds from ether extracts, acetone post-ether extracts, and acetone-only extracts. In each case extracts were dissolved in a minimum amount of methanol and injected onto a reversed-phase HPLC column (Phenomenex Inertsil ODS-2). Following injection, materials eluting from the column were collected into separate vials at 1 or 2 min intervals and subjected to analysis by ELISA.
Mouse Toxicity Assays
Mouse bioassays were performed according to the regulatory protocol for shellfish monitoring (APHA, 1970).
Results and Discussion
Oysters harvested from two sites along the Florida gulf coast in December 2000 and January 2001 were deemed too toxic for human consumption using the mouse bioassay method, which involves extraction of toxins from shellfish with diethyl ether (Samples 2 and 3; Fig. 1)
Figure 1.
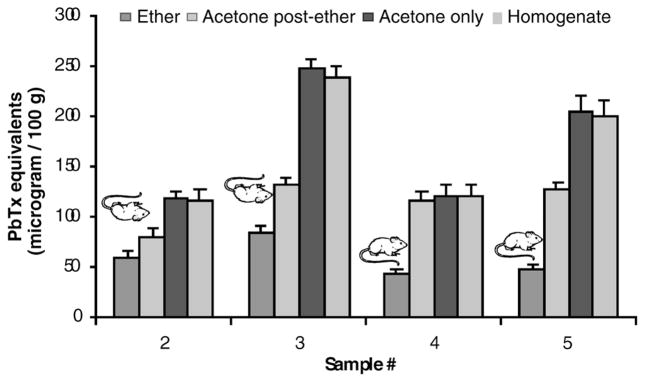
Brevetoxin analyses of four Florida oyster samples by ELISA. Extraction with ether only, ether followed by acetone, or acetone only. Samples 2 and 4 were collected from a commercial bed in central Choctawhatchee Bay two weeks apart. Samples 3 and 5 were collected from east Choctawatchee Bay at the same interval. ELISA analyses were performed on ether extracts (parent brevetoxins), acetone post-ether extracts (polar metabolites of brevetoxins), acetone extracts, and shellfish homogenates (parent brevetoxins and metabolites). Mouse symbols indicate 1) shellfish toxicity according to mouse bioassay (upside down) or 2) absence of toxicity according to mouse bioassay (upside up).
There was close agreement between the mouse bioassay results, which were run using ether extracts only, and the ether-soluble brevetoxin concentrations as measured by ELISA. ELISA results on acetone post-ether extracts indicated that not all brevetoxin-like materials were extracted with ether, consistent with the findings of Dickey et al. (1999). Extraction with acetone or direct ELISA analysis of the homogenate without extraction appears necessary to accurately account for all of the brevetoxins and brevetoxin-like material in shellfish that has been exposed to K. brevis.
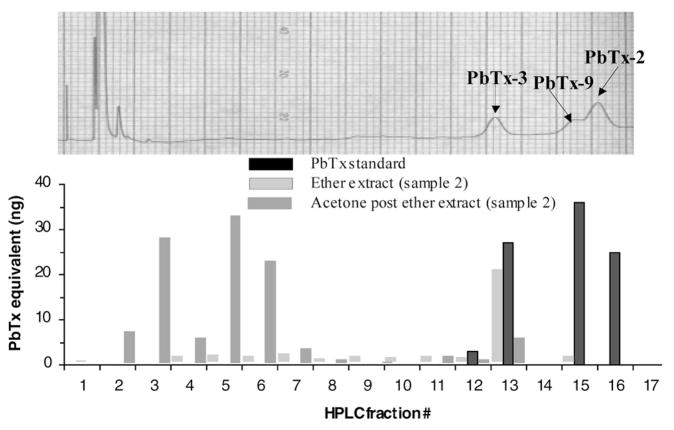
Ether-insoluble materials eluted earlier than the parent brevetoxins on reversed-phase columns (acetone post ether extract, Fig. 2) and, therefore, are likely to be closely related to polar metabolites of brevetoxins described by Murata et al. (1998) and Poli et al. (2000). Quantities of brevetoxin metabolites obtained in this study were insufficient for purification of individual compounds and spectroscopic analysis. In the current study, HPLC separation of shell-fish constituents followed by ELISA analysis of fractions indicated that the oysters from Florida contained no PbTx-2, and that the mouse toxicity of the ether-soluble components could be attributed entirely to high levels of PbTx-3 (Fig. 2). This supported the notion that PbTx-2 is rapidly converted to brevetoxin metabolites by shellfish (R. Dickey, pers. comm.).
Figure 2.
ELISA analysis of HPLC fractions.
Using an HPLC method similar to that described by Poli et al. (2000) followed by ELISA analysis of HPLC fractions, it was possible to separate parent brevetoxins (such as PbTx-2, -3, and -9) and the compounds that resisted ether extraction (see Fig. 2).
In order to directly test the hypothesis that shellfish bio-transformed PbTx-2, toxins were incubated with shellfish meat and then extracted and analyzed by HPLC, followed by ELISA (Fig. 3). PbTx-3 was not biotransformed by the oyster meat, but after three hours of incubation, all PbTx-2 was biotransformed into polar metabolites. Toxicity analysis of the polar metabolites indicated that they had no acute toxicity to mice after IP injection (90 μg/mouse = 20 times LD50 of PbTx-2) or oral administration (200 mg/mouse, 20 times LD50 for PbTx-3).
Figure 3.
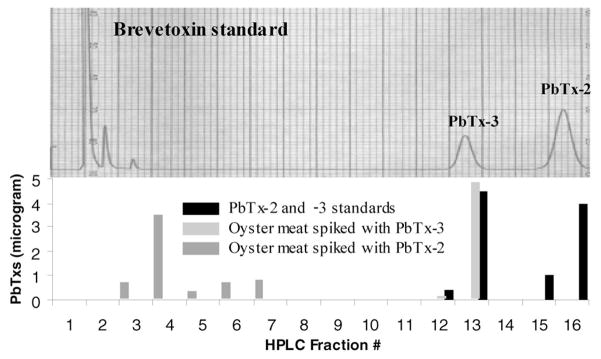
In vitro derivatization of brevetoxins by shellfish meat. HPLC fractionation of PbTx standard (5 micrograms of PbTx-2 and PbTx-3, top) and ELISA analysis of HPLC fractions. Standards (solid bars, represent analysis of fraction from HPLC chromatogram presented), oyster meat spiked with PbTx-3 (gray bars), and oyster meat spiked with PbTx-2 (striped bars) versus PbTx equivalents.
Since the discovery of brevetoxin “metabolites” in biological fluids of people suffering from NSP (Poli et al., 2000), detection and characterization of these compounds has been a high priority for the protection of human health. In this study, the presence of polar metabolites of brevetoxins has been demonstrated in both field oysters exposed to a bloom of K. brevis as well as in oysters exposed in an aquarium to bloom levels of K. brevis (data not shown). From the peak of a bloom to several months afterwards, these metabolites represent 60–90% of the total amount of brevetoxin-reactive compounds present in exposed shellfish. Being more polar, they are resistant to ether extraction and so are not taken into account by the regulatory agencies involved in shellfish monitoring. In vitro derivatization of brevetoxins by shellfish meat clearly identified PbTx-2 as a precursor of polar metabolites. This biotransformation process is rapid and efficient, with 100% transformation observed within 3 hours of incubation. Interestingly, these polar metabolites appear to be non-toxic to mice injected or fed with high doses of these compounds (200 μg/mouse). Even though these metabolites do not induce acute toxicity in mice, potential long-term effects linked to chronic exposure are possible and need to be investigated.
Acknowledgments
This work was supported by funds from Seagrant and the MERHAB program and NIEHS P01 ES10594.
References
- Greenberg AE, Hunt DA, editors. APHA. Recommended Procedures for the Examination of Sea Water and Shellfish. 4. American Public Health Association; Washington, DC: 1970. pp. 61–66. [Google Scholar]
- Dickey R, Jester E, Granade R, Mowdy D, Moncreiff C, Rebarchik D, Robl M, Musser S. Nat Toxins. 1999;7:157–165. doi: 10.1002/(sici)1522-7189(199907/08)7:4<157::aid-nt52>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Murata K, Satake M, Naoki H, Kaspar HF, Yasumoto T. Tetrahedron. 1998;54:735–742. [Google Scholar]
- Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney PL, Flewelling L, Steidinger K, Lancaster J, Baden DG. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli MA, Musser SM, Dickey RW, Eilers PP, Hall S. Toxicon. 2000;38:981–993. doi: 10.1016/s0041-0101(99)00191-9. [DOI] [PubMed] [Google Scholar]
- Van Dolah FM, Finley EL, Haynes BL, Doucette GJ, Moeller PD, Ramsdell JS. Nat Toxins. 1994;2:189–19. doi: 10.1002/nt.2620020407. [DOI] [PubMed] [Google Scholar]