Abstract
Background
Yersinia enterocolitica causes an estimated 116 716 illnesses annually in the United States. Black children have historically had the highest rates of infection, with incidence peaking in the winter.
Methods
The Foodborne Diseases Active Surveillance Network (FoodNet) conducts active surveillance for laboratory-confirmed Y. enterocolitica infections, defined as the isolation of Y. enterocolitica or unspeciated Yersinia from a human clinical specimen. We calculated the average annual crude incidence rate per 100 000 persons from 1996 through 2009 and described rates by age, race, and geographic site. To account for changes in the FoodNet catchment area, we used a negative binomial model to estimate statistical changes in incidence using the average annual incidence in 1996–1998 as the baseline.
Results
From 1996 through 2009, 2085 Y. enterocolitica infections were reported to FoodNet. The average annual crude incidence was 0.5 per 100 000 persons and was highest in blacks (0.9 per 100 000 persons). Over time, the rate in blacks declined from 3.9 to 0.4 per 100 000 persons. Declines among other racial groups were not as pronounced. The largest decline occurred in black children <5 years old (from 41.5 per 100 000 persons in 1996 to 3.5 per 100 000 persons in 2009). From 2007 through 2009, the highest rate of infection was in Asian children (5.1 per 100 000 persons). Compared with 1996–1998, the incidence in 2009 was 66% (95% confidence interval, 51%–77%) lower among children <5 years old.
Conclusions
Y. enterocolitica infections in FoodNet sites have significantly declined since 1996. These declines were greatest in young black children, the group that initially had the highest incidence, possibly as the result of educational efforts in Georgia.
Yersinia enterocolitica causes an estimated 116 716 infections annually in the United States [1]. Infection causes acute febrile diarrhea, often accompanied by severe abdominal pain that can mimic appendicitis. Y. enterocolitica is a pharyngeal commensal organism in pigs, the major animal reservoir [2]. In the United States, only a few outbreaks have been reported; most have been associated with consumption of pork, particularly chitterlings (prepared pig intestine, a traditional winter holiday dish prepared most frequently in African-American households in the South) [3, 4]. Transmission to young children is thought to occur through contact with adult caregivers preparing chitterlings [5–7]. Young black children have historically had by far the highest rates of infection, with incidence peaking in the winter [8].
A previous population-based study of the incidence of and trends in Y. enterocolitica infections in the United States from 1996 to 1999 reported the highest rates of infection among black and Asian children <5 years old and a decline in incidence in black children aged <5 years over the study period [8]. We extend these initial observations through 2009.
METHODS
The Foodborne Diseases Active Surveillance Network (FoodNet) is a collaborative project of the Centers for Disease Control and Prevention (CDC), 10 state departments, the US Department of Agriculture’s Food Safety and Inspection Service (USDA-FSIS), and the Food and Drug Administration (FDA). FoodNet conducts active, population-based surveillance for laboratory-confirmed infections caused by 9 pathogens, including Yersinia species (except Yersinia pestis). FoodNet began in 1996 with sites in 5 states—Connecticut, Georgia, Minnesota, Oregon, and selected counties in California—and expanded over time to include Maryland, New Mexico, and Tennessee, and selected counties in Colorado and New York [9]. The FoodNet catchment area has been stable since 2004. In 2009, the FoodNet catchment area had a population of 45.2 million persons, approximately 15% of the US population.
FoodNet personnel routinely contact all clinical laboratories (approximately 650) serving the catchment area to ascertain all laboratory-confirmed Yersinia infections in residents of the area. For this analysis, we defined a Y. enterocolitica infection as isolation of Y. enterocolitica or unspeciated Yersinia (92% of speciated Yersinia were identified as Y. enterocolitica) from a clinical human specimen. Regular audits of laboratory records from clinical laboratories were completed to ensure that all Yersinia isolations were identified and reported.
For each infection, surveillance officers collected demographic (patient’s age, sex, race, and state of residence), laboratory (date of specimen collection, specimen source, species), and outcome (hospitalization or death within 7 days of specimen collection) information, using a standard case report form.
Age was categorized as <1, 1–4, 5–59, or ≥60 years. Race was reported as Asian/Pacific Islander, black, white, multiracial, Native American/Alaskan, other, and unknown. Only persons reported as Asian/Pacific Islander, black, or white were included in analyses by race. The date of specimen collection was used to assign the month in which an infection occurred. We grouped reports into 4 seasons (summer [June, July, August], fall [September, October, November], winter [December, January, February], and spring [March, April, May]) and 3 periods (1996–1999, 2000–2004, and 2005–2009).
We calculated crude incidence rates per 100 000 persons, using population estimates from the US Census Bureau [10], and described annual rates by age, race, and site. We examined and compared changes in reported infections, hospitalizations, and seasonality by period and by race. To account for the changing catchment area, we used a negative binomial model to estimate statistical changes in incidence in children <5 years old [9].
This surveillance data review was determined not to be research, so the project did not undergo institutional review board review.
RESULTS
Demographics and Clinical Information
From 1996 to 2009, 2085 laboratory-confirmed Y. enterocolitica infections were reported in FoodNet sites. Fifty-one percent of cases were in females. Most infections occurred in children; 47% were in children <5 years old and 32% were in infants <1 year old. The proportion of cases in children <5 years old was higher in southeastern sites (Georgia and Tennessee, 75% and 68%, respectively) than in other sites (31%; P < .001). Of the 1637 Y. enterocolitica infections (79%) in persons for whom race was reported, 49% were in whites, 40% were in blacks, 10% were in Asian/Pacific Islanders, and 2% were in persons of another race. Blacks and Asian/Pacific Islanders with Y. enterocolitica infection were significantly younger than whites with infection (median ages, 7 months among blacks, 3 years among Asian/Pacific Islanders, and 35 years among whites; P < .001) (Table 1).
Table 1.
Demographic Characteristics of Persons With Yersinia enterocolitica Infection, Foodborne Diseases Active Surveillance Network, 1996–2009
| Characteristics | No. (%)of Cases | Cases per 100 000a |
|---|---|---|
| Overall | 2085 (100) | 0.5 |
| Sex (n = 2080) | ||
| Female | 1056 (51) | 0.5 |
| Male | 1024 (49) | 0.5 |
| Median age, years | 7 | |
| Age (years) | ||
| <1 | 677 (32) | 12.3 |
| 1 to <5 | 311 (15) | 1.4 |
| 5 to <60 | 788 (38) | 0.2 |
| ≥60 | 309 (15) | 0.4 |
| Site | ||
| California | 272 (13) | 0.7 |
| Colorado | 52 (2) | 0.2 |
| Connecticut | 216 (10) | 0.5 |
| Georgia | 624 (30) | 0.7 |
| Maryland | 120 (6) | 0.2 |
| Minnesota | 270 (13) | 0.4 |
| New Mexico | 15 (1) | 0.1 |
| New York | 146 (7) | 0.4 |
| Oregon | 179 (9) | 0.4 |
| Tennessee | 191 (9) | 0.4 |
| Race (n = 1637) | ||
| Asian/Pacific Islander | 164 (10) | 0.7 |
| Black | 649 (40) | 0.9 |
| White | 795 (49) | 0.2 |
| Other | 29 (2) | 0.3 |
| Year | ||
| 1996 | 147 (7) | 1.0 |
| 1997 | 132 (6) | 0.8 |
| 1998 | 174 (8) | 0.8 |
| 1999 | 150 (7) | 0.6 |
| 2000 | 124 (6) | 0.4 |
| 2001 | 137 (7) | 0.4 |
| 2002 | 163 (8) | 0.4 |
| 2003 | 150 (7) | 0.4 |
| 2004 | 160 (8) | 0.4 |
| 2005 | 148 (7) | 0.3 |
| 2006 | 152 (7) | 0.3 |
| 2007 | 150 (7) | 0.3 |
| 2008 | 150 (7) | 0.3 |
| 2009 | 148 (7) | 0.3 |
Denominators are 2085 infections, unless otherwise indicated.
Average annual crude rate per 100 000 persons calculated using population estimates from the US Census Bureau.
Overall, the average annual crude incidence of Y. enterocolitica infection in FoodNet sites was 0.5 per 100 000 persons, with the highest site-specific crude incidence in California and Georgia (both 0.7). Among racial groups, the average annual crude incidence was highest in blacks (0.9 per 100 000 persons), followed by Asian/Pacific Islanders (0.7 per 100 000 persons), and whites (0.2 per 100 000 persons) (Table 1). Among age groups, the highest average annual crude incidence was in infants aged <1 year (12.3 per 100 000 persons) (Table 1), with a racial pattern consistent with the overall pattern (black, 49.3 per 100 000 persons; Asian/Pacific Islander, 19.7 per 100 000 persons; and white, 2.2 per 100 000 persons) (Table 2).
Table 2.
Characteristics of Persons With Yersinia enterocolitica Infection, by Race, Foodborne Diseases Active Surveillance Network, 1996–2009
| Black
|
White
|
Asian/Pacific Islander
|
Other
|
Totala
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 649
|
n = 795
|
n = 164
|
n = 29
|
n = 2085
|
|||||||||||
| No. | % | Rateb | No. | % | Rateb | No. | % | Rateb | No. | % | Rateb | No. | % | Rateb | |
| Sex | |||||||||||||||
|
| |||||||||||||||
| Female | 331 | 51 | 0.9 | 407 | 51 | 0.2 | 69 | 42 | 0.7 | 19 | 66 | 0.3 | 1056 | 51 | 0.5 |
|
| |||||||||||||||
| Male | 317 | 49 | 0.9 | 388 | 49 | 0.2 | 95 | 58 | 1.0 | 10 | 34 | 0.2 | 1024 | 49 | 0.5 |
|
| |||||||||||||||
| Missing | 1 | <1 | … | 0 | 0 | … | 0 | 0 | … | 0 | 0 | … | 5 | <1 | … |
|
| |||||||||||||||
| Median age | 0.7 | 35 | 3 | 2.6 | 7 | ||||||||||
|
| |||||||||||||||
| Age (y) | |||||||||||||||
|
| |||||||||||||||
| <1 | 426 | 66 | 49.3 | 96 | 12 | 2.2 | 60 | 37 | 19.7 | 10 | 34 | 5.5 | 677 | 32 | 12.3 |
|
| |||||||||||||||
| 1 to <5 | 100 | 15 | 3.1 | 87 | 11 | 0.5 | 37 | 23 | 3.0 | 8 | 28 | 1.2 | 311 | 15 | 1.4 |
|
| |||||||||||||||
| 5 to <60 | 89 | 14 | 0.2 | 418 | 53 | 0.2 | 54 | 33 | 0.3 | 9 | 31 | 0.1 | 788 | 38 | 0.2 |
|
| |||||||||||||||
| ≥60 | 34 | 5 | 0.4 | 194 | 24 | 0.3 | 13 | 8 | 0.4 | 2 | 7 | 0.2 | 309 | 15 | 0.4 |
Includes 448 cases with unknown race.
Average annual crude rate per 100 000.
Most isolates were obtained from stool (1761 [84%]), followed by blood (123 [6%]). The proportion of isolates from stool decreased with increasing age: 90% of isolates from children <1 year old were from stool, compared with 57% from stool in adults ≥60 years old (P < .001), a pattern seen in every racial group. The proportion of isolates obtained from stool decreased from 92% in 1996–1999 to 78% in 2005–2009 (P < .0001). This decrease corresponded with an increase in the proportion of isolates obtained from urine (from <1% in 1996–1999 to 5% in 2005–2009; P < .0001) and blood (from 4% in 1996–1999 to 7% in 2005–2009; P = .06). This pattern also occurred in every racial and age group.
Overall, 590 persons (28%) were hospitalized for a median of 4 days (range, 3–5 days). The proportion hospitalized was highest (48%) among persons ≥60 years old. The proportion of black patients hospitalized (40%) was higher than the proportions of white or Asian/Pacific Islander patients (30% each). This pattern occurred in every age group. There were 19 deaths (1%), nearly all (15 [79%]) of which were in adults ≥60 years old. The remaining 4 deaths were in an infant aged 1 year old and in adults aged 37, 45, and 51 years old.
There was a winter peak of Y. enterocolitica infections among black children <5 years old; 363 black children (70%) with a reported date had onset of illness during winter. Over time, however, the winter peak in this group became much less pronounced (P < .0001), although winter remained the season with the highest incidence in black children (Figure 1). There was no seasonal peak in cases among other races.
Figure 1.
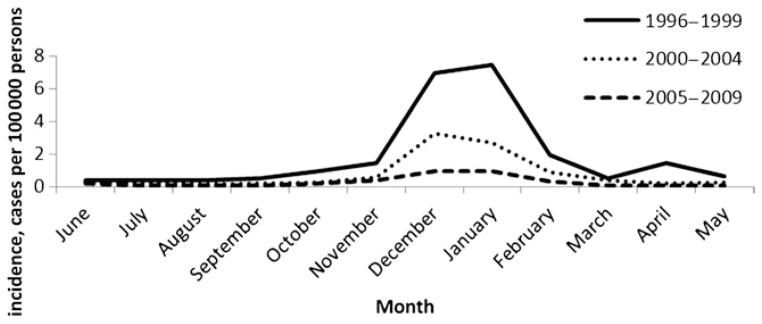
Seasonal distribution of Yersinia enterocolitica infection in black children <5 years old, by period, Foodborne Diseases Active Surveillance Network, 1996–2009.
Trends
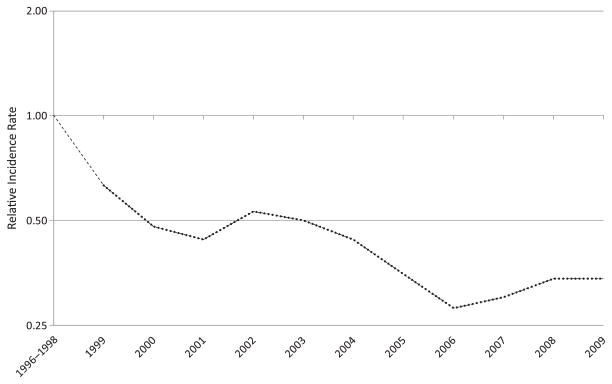
Overall, the crude incidence rate of Y. enterocolitica infection declined from 1.0 per 100 000 persons in 1996 to 0.3 per 100 000 persons in 2009 (Table 1). The decline was most pronounced in children <5 years old, from 9.2 per 100 000 persons in 1996 to 1.9 per 100 000 persons in 2009. In persons ≥5 years old, the rate initially declined from 0.4 per 100 000 persons in 1996 to 0.2 per 100 000 persons in 2000, and then remained unchanged through 2009. The largest declines occurred in Georgia, where the incidence dropped sharply from a high of 2.3 per 100 000 persons in 1996 to 0.8 per 100 000 persons in 1999, with continued declines to a low of 0.3 per 100 000 persons in 2005 before leveling off from 2006 to 2009 (average rate, 0.4 per 100 000 persons). The rate of Y. enterocolitica infections declined in all racial groups in all sites. The largest decline occurred in black children <5 years old, from a high of 41.5 per 100 000 persons in 1996, followed by a steep decline to 10.2 per 100 000 persons in 2000, and 3.5 per 100 000 persons in 2009 (Figure 2). Beginning in 2007, the highest rate of infection was in Asian children <5 years old (5.1 per 100 000 persons for 2007–2009; Figure 2). Use of the negative binomial model estimated that the incidence in children <5 years old was 66% (95% confidence interval [CI], 51%–77%) lower in 2009 than in 1996–1998 (Figure 3). We were not able to use the negative binomial model to evaluate changes in incidence among children aged <5 years by race because of sparse data.
Figure 2.
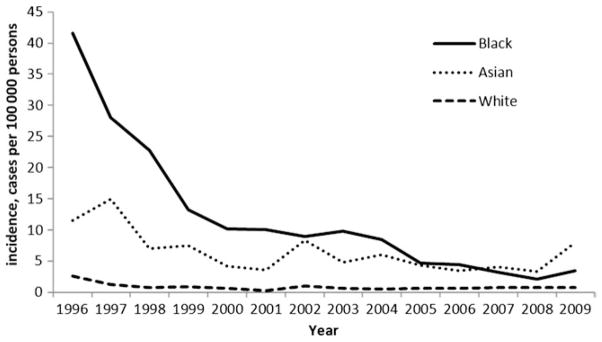
Crude annual incidence rate of Yersinia enterocolitica infection in children <5 years old, by year and race, Foodborne Diseases Active Surveillance Network, 1996–2009.
Figure 3.
Change in the incidence rate of laboratory-confirmed Yersinia enterocolitica infection in children <5 years old during 2009, relative to the average incidence rate during 1996–1998, Foodborne Diseases Active Surveillance Network, 1996–2009. Data were calculated using the negative-binomial model. The position of the line indicates the relative change in the incidence compared with 1996–1998. The actual incidence of infection cannot be determined from this graph.
DISCUSSION
We report large declines in rates of laboratory-confirmed Y. enterocolitica infections in FoodNet over the past 14 years, particularly in children <5 years old. These declines were driven mainly by reductions that occurred in Georgia, particularly among black children during winter months, almost completely eliminating what had been a notable racial disparity [8].
Georgia experienced an 83% reduction in incidence, more than any other FoodNet site. The reasons for this decline are not known, but, beginning in 1998, Georgia implemented an educational campaign about Y. enterocolitica targeted at high-risk groups, particularly blacks [4], including pamphlets on safe preparation and handling of chitterlings, particularly during the winter months and holidays (eg, http://www.health.state.ga.us/archives/pdfs/Chitlins_flyer.pdf). Although other factors could also be important, this campaign may have played a role in the declines. However, the continued presence of somewhat higher incidence in winter among black children <5 years old suggests that chitterlings might still be a source of infection in this group. Similarly, while rates of Y. enterocolitica infection initially declined in persons ≥5 years old, rates in this group remain unchanged for nearly a decade, suggesting continued exposures to the bacterium via this or other routes of infection.
Beginning in 2007, Asian children <5 years old have been the group with the highest incidence rates. Pork products, including intestines, are common food items in the Asian community [11]. These food items are often prepared at home, and young children may be exposed to Y. enterocolitica because of their proximity to food preparation or food preparers handling contaminated product. Although FoodNet sites are largely demographically representative of the US population as a whole [9], the Asian population included in FoodNet surveillance is small; thus, the rates among Asian children might be less stable than in larger groups. Changes to FoodNet to include a site with a larger Asian population would help to further define the burden of yersiniosis in this racial group.
Several other possible reasons for the observed declines should be considered. First, although there is no systematic testing of swine for Y. enterocolitica, an analysis by the Agricultural Research Service in 2008 identified correlations between the presence of Y. enterocolitica infections in swine on farms in central states and swine deaths attributed to scours. Both of these factors have declined in recent surveys conducted by the USDA [12, 13]. This suggests that fewer pigs may be colonized with Y. enterocolitica as they enter the slaughter process. Second, although data on household practices in preparation and handling of pork products are not available, the general emphasis on safe handling of meats could have led to a decrease in risky practices that contributed to the decline in rates we observe. Finally, several laboratory diagnostic issues may have contributed to a decrease in reported Y. enterocolitica isolations. Specifically, to improve cost-effectiveness of stool cultures, some practice guidelines allow for Yersinia culture only in winter months and in other situations indicated by clinical and epidemiologic evidence [14]. Although this practice might have had some impact on the number of Yersinia infections reported, it cannot explain the decline we observed in the winter peak among young black children. Laboratories might not always routinely speciate Yersinia isolates; we included unspeciated Yersinia in the analysis because most Yersinia isolations are Y. enterocolitica; however, we could not be certain this small number of isolates were in fact other less common Yersinia species.
The negative-binomial model we used to describe changes in incidence among persons with Y. enterocolitica infection is designed to account for the increase in size of the FoodNet surveillance area and site-to-site variation in incidence. The model requires a minimum set of observations per subgroup examined to produce a reliable estimate of change. As a result, we were not able to use the model to quantify changes in incidence by racial group. Instead, we report the crude incidence rates by race and year. Despite changes in catchment in Georgia during the study period—specifically, the addition of counties outside of metropolitan Atlanta to FoodNet surveillance—the crude rates presented likely provide an accurate description of the changes in incidence in each racial group.
In summary, we describe significant sustained declines in Y. enterocolitica infections in young children in FoodNet sites. Although a notable racial disparity has largely improved, there continue to be higher rates of disease among Asians, blacks, and children <5 years old. Yersiniosis can be a serious disease, causing serious illness, hospitalization, and death; public health efforts should continue to focus on these groups. In addition, further exploration into the causes of the declines may prove useful in identifying potential interventions that could be used to address other diseases affecting vulnerable populations.
Footnotes
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Supplement sponsorship. This article was published as part of a supplement entitled “Studies From the Foodborne Diseases Active Surveillance Network,” sponsored by the Division of Foodborne, Waterborne, and Environmental Diseases of the National Center for Emerging and Zoonotic Infectious Diseases from the Centers for Disease Control and Prevention, and the Association of Public Health Laboratories.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhaegen J, Charlier J, Lemmens P, et al. Surveillance of human Yersinia enterocolitica infections in Belgium: 1967–1996. Clin Infect Dis. 1998;27:59–64. doi: 10.1086/514636. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Yersinia enterocolitica gastroenteritis among infants exposed to chitterlings—Chicago, Illinois, 2002. MMWR Morb Mortal Wkly Rep. 2003;52:956–8. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Topics in minority health Yersinia enterocolitica infections during the holidays in black families—Georgia. MMWR Morb Mortal Wkly Rep. 1990;39:819–20. [PubMed] [Google Scholar]
- 5.Lee LA, Gerber AR, Lonsway DR, et al. Yersinia enterocolitica O:3 infections in infants and children, associated with the household preparation of chitterlings. N Engl J Med. 1990;322:984–7. doi: 10.1056/NEJM199004053221407. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Haq NM, Asmar BI, Abuhammour WM, Brown WJ. Yersinia enterocolitica infection in children. Pediatr Infect Dis J. 2000;19:954–8. doi: 10.1097/00006454-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 7.McDonald A, Segler S, McNeil M, et al. Population-based surveillance for Yersinia enterocolitica infection in metropolitan Atlanta. J Invest Med. 1998;46:62a-a. [Google Scholar]
- 8.Ray SM, Ahuja SD, Blake PA, et al. Population-based surveillance for Yersinia enterocolitica infections in FoodNet sites, 1996–1999: higher risk of disease in infants and minority populations. Clin Infect Dis. 2004;38(Suppl 3):S181–9. doi: 10.1086/381585. [DOI] [PubMed] [Google Scholar]
- 9.Henao OL, Scallan E, Mahon B, Hoekstra RM. Methods for monitoring trends in the incidence of foodborne diseases: Foodborne diseases active surveillance Network 1996–2008. Foodborne Pathog Dis. 2010;7:1421–6. doi: 10.1089/fpd.2010.0629. [DOI] [PubMed] [Google Scholar]
- 10.US Census Bureau. Population projections 2007. US Census Bureau; 2007. [Accessed February 2012]. Available at: http://www.census.gov. [Google Scholar]
- 11.Solomon C. Encyclopedia of Asian food. Melbourne: William Heinemann Australia; 1996. [Google Scholar]
- 12.US Department of Agriculture. Part IV: changes in the U.S. Pork industry, 1990–2006. Fort Collins, CO: USDA-APHIS-VS; 2008. Report No. N520.1108. [Google Scholar]
- 13.Wesley IV, Bhaduri S, Bush E. Prevalence of Yersinia enterocolitica in market weight hogs in the United States. J Food Prot. 2008;71:1162–8. doi: 10.4315/0362-028x-71.6.1162. [DOI] [PubMed] [Google Scholar]
- 14.Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–51. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]