Abstract
Over the past quarter century it has become clear that adult onset chronic diseases like heart disease and type 2 diabetes have their roots in early development. The report by David Barker and colleagues showing an inverse relationship between birthweight and mortality from ischemic heart disease was the first clear-cut demonstration of fetal programming. Because fetal growth depends upon the placental capacity to transport nutrients from maternal blood, it has been a suspected causative agent since the original Barker reports. Epidemiological studies have shown that placental size and shape have powerful associations with offspring disease. More recent studies have shown that maternal phenotypic characteristics, such as body mass index and height, interact with placental size and shape to predict disease with much more precision than does birthweight alone. For example, among people in the Helsinki Birth Cohort, who were born during 1924–1944, the risk for acquiring colorectal cancer increased as the placental surface became longer and more oval. Among people in whom the difference between the length and breadth of the surface exceeded 6 cm, the hazard ratio for the cancer was 2.3 (95% CI 1.2–4.7, p=0.003) compared with those in whom there was no difference. Among Finnish men, the hazard ratio for coronary heart disease was 1.07 (1.02–1.13, P =0.01) per 1% increase in the placental weight/birthweight ratio. Thus, it appears that the ratio of birthweight to placental weight, known as placental efficiency, predicts cardiovascular risk as well. Babies born with placentas at the extremes of efficiency are more vulnerable for adult onset chronic diseases. Recent evidence suggests that placental growth patterns are sex specific. Boys’ placentas are, in general, more efficient than those made by girls. Another recent discovery is that the size, shape and efficiencies of the placenta can change over years of time with very narrow confidence limits. This suggests that the growth of the placenta within a population of women is strongly affected by their nutritional environment. Even though it is known that an individual placenta can expand to improve its nutrient acquisition capacity in the first 2/3rd of gestation, the mechanisms by which placentas grow in response to a specific nutritional environment are not known. Discovering those mechanisms is the task of the current generation of scientists. While it may seem obvious that good nutrition is highly important for women who are pregnant because it supports optimal placentation and fetal development, more research is needed to determine the mechanisms by which maternal nutrition, placenta growth and fetal health are related.
Keywords: Chronic Disease, Fetus, Maternal Nutrition, Placenta, Programming
Introduction Fetal Programming
Over the past quarter century the scientific community has gained a new perspective on the origins of chronic disease which now includes a central role for the placenta. It began when Professor David Barker’s team reported an inverse relationship between death risk from cardiovascular disease and birthweight among English men and women.1 They found that term babies born at the 5 lb end of the birthweight scale had a 3–5 times greater risk for cardiovascular disease compared to babies born at the 9 lb pound end. Later, it was discovered that there is an increasing risk for disease in term babies as their weights exceed the 9 lb birthweight at the high end of the birthweight scale.2,3,4 The discovery of the relationship between birthweight and later chronic disease stimulated extensive research among scientists worldwide and brought to light a new level of understanding regarding lifelong health in offspring.5,6–10 The mechanism by which compromised development leads to adult onset disease is called “programming.”
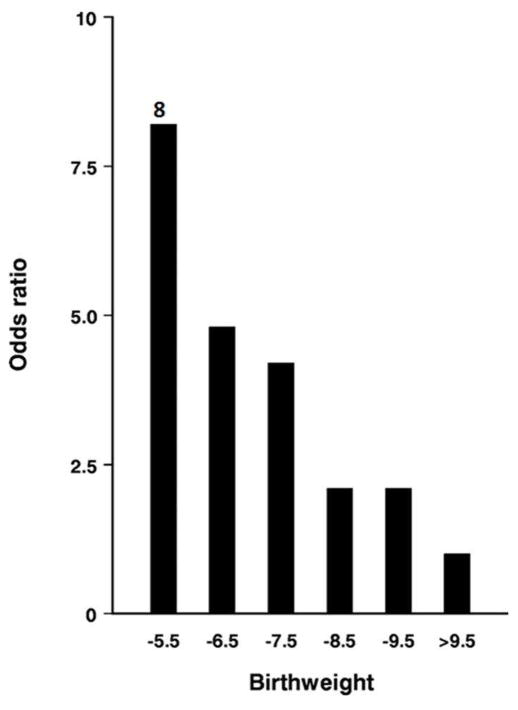
It was the unexpected trends in public health that brought a new urgency to the concept of programming in the USA. Beginning in the mid ‘90s the prevalence of obesity and type 2 diabetes began to rise in the western world in a dramatic way (http://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm). The upsurge in these two interrelated conditions, plus the ever increasing numbers of people who have uncontrolled blood pressure,11 led medical scientists to predict that the current generation of young people in the USA are likely to live shorter lives than will their parents.12 The links between increasing prevalence of diseases like diabetes and heart disease and early life development are powerful and exist across mammalian species. Figure 1 shows the relationship between type 2 diabetes or insulin resistance and birth weight where there is an 8 fold risk for diabetes across the birthweight scale. The relationship between birthweight and disease risk was so clear cut that Barker and colleagues estimated that diabetes would be reduced by some 60% in one generation if babies were born at the lowest risk birthweight and did not cross BMI centiles in childhood.13
Figure 1. Relationship between type II diabetes and birthweight.
Figure 1 shows the relationship between the risk for impaired glucose tolerance or type 2 diabetes and birthweight in 370 men aged approximately 64 years in Hertfordshire UK (adjusted for body mass index).80 (With permission.)
Much of what we understand about placental function has been discovered in animal models. The field of developmental origins of disease has gained enormous insight into the biological mechanisms that underlie developmental plasticity from animal studies. Langley-Evans et al.14 were among the first to demonstrate that rat pups born to dams eating a low protein diet during pregnancy had high blood pressure as adults. Dozens of additional studies using different animal models have revealed the central role of the placenta.9,15,5,16–18
Under adverse conditions, like poor maternal nutrition or periods of chronic hypoxia, or high levels of thyroid hormone, or glucocorticoids, the fetus suffers alterations in fetal organ structures including reduced coronary arterial dimensions,19 low arterial elastin,20,21 reduced endowment of beta cells in the pancreas,22 decreased numbers of nephrons in the kidney23,24 and changes in brain structure and function.25 The result is increased appetite, decreased cognitive function, endothelial dysfunction, compromised anti-oxidant protection systems as well as dyslipidemias.26 The sum of these effects leads to increased vulnerability for heart disease, diabetes, stroke and obesity for the remainder of an individual’s life.
The Placenta as Culprit
Thus, it is now clear that patterns of growth and accommodations to maternal stress before birth are a major driver of disease risk in offspring. The relationship between maternal dietary and tissue sources of nutrients, placental function and eventual embryonic and fetal growth is complex but not well studied. Nevertheless, because the placenta is the source of nutrients for the fetus, the provision of nutrients by the mother gives a central place to the placenta as a driver of adult onset disease.
The role of the placenta can be either active or passive. Low rates of fetal growth are generally associated with reduced nutrient fluxes across the placenta. Furthermore, a long list of chronic diseases are associated with specific placental phenotypes.27 The transport of required nutrients from mother to fetus requires optimal function of a myriad of separate transport mechanisms including 1) diffusional permeability to blood gases,28–31 2) transporters facilitating diffusion of glucose and fatty acids,32,33,34 3) active transporters for amino acids and some ions,34–36 4) vesicular transport systems that regulate the transport of iron and immunoglobulins and many others.37,38 Thus, each of these processes are known to be, or thought to be, associated with compromised fetal growth.39
Maternal Stress and Placental Function
Fetal glucocorticoid levels increase toward the end of gestation in a number of mammalian species including humans.40 Glucocorticoids are important for the maturation of several organs before birth including the lungs and heart, which underlies the rationale for administration of corticosteroids to women at risk for preterm delivery (reviewed by Challis et al., 200141). However, when maternal levels exceed those found under normal physiological conditions, as during high levels of social stress, glucocorticoids cross the placenta and cause reduced fetal growth rates.42 An exception to the suppression of growth is found in the heart in which cell proliferation and growth is stimulated by the actions of glucocorticoids.43 In most cases of human intrauterine growth retardation both maternal and fetal concentrations of circulating cortisol are elevated.44,45 Ordinarily active cortisol in the human (and corticosterone in small mammals) is inactivated in the placenta by 11β-HSD2 which catalyzes the rapid metabolism of active cortisol and corticosterone to inert, inactive, 11-keto forms. Unfortunately, when maternal levels exceed the rate of inactivation in the placenta, active cortisol will cross the placenta and exert programming effects on the fetus. Thus, either elevated levels of maternal cortisol or reduced levels of placental 11β-HSD2 will lead to programming in offspring. Expression levels of 11β-HSD2 are down regulated by a number of factors including sex steroids and hypoxia and upregulated by glucocorticoids themselves and cyclin AMP (reviewed by Seckl and Holmes, 20076). Such offspring will have higher resting levels of cortisol as adults46 and will have higher cortisol peaks during periods of stress.47,48 Babies who were born small and had high glucocorticoid levels have elevated risks for chronic diseases later in life including hypertension, hyperinsulinemia, hyperglycemia and hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis.6
Placental Inflammation
There is increasing evidence that in addition to characteristic inflammatory responses to infectious agents, placental inflammation that derives from maternal conditions such as diabetes and obesity leads to fetal programming. Acute and chronic inflammation conditions in the placenta are associated with fetal morbidity and mortality including preterm birth.49,50 In addition to these nicely described categories, there may be milder forms of inflammation that do not fit easily under current definitions. O’Tierney et al. showed that women who lack muscle have placentas characterized by elevated expression of pro-inflammatory genes.51 In this study, expression of interferon- gamma in the placentas of women who had low muscle mass was elevated, as were a host of placental target genes. However, there was no sign of classical inflammation in the tissue. For example, neither T cells bearing CD3 markers, nor B cells (CD20), nor macrophages (CD68) nor neutrophils (CD64) were elevated in these placentas. The lack of cellular response contrasts placentas from obese mothers where CD68 and CD14 positive cells more than doubled.52 However, the placental response to the low muscle mass condition and the augmentation of inflammatory signals associated with obesity53 suggest the need for more precise definitions of placental inflammation which are not characterized by a full blown immune response. This need has been suggested in cases of systemic inflammatory changes in cancer that stimulate known signaling cascades but lack the full response seen in local “hot” inflammation where granulocytes accumulate.54 We hypothesize that the known stressors that lead to fetal programming, including poor nutrition, toxic social stress and hypoxia, can alter immune function, reduce the actions of protectants of oxidative stress and lead to a “cold” form of inflammation.54 Many of the same signaling pathways, including activation of AP1, NF-kappa B and IRFs, mediate tissue responses in both hot and cold conditions. We speculate that the cold type of inflammation is often present in the human placenta and that it mediates a persisting “smoldering inflammation” in the fetus that makes it vulnerable for chronic disease over years in the future.
Plasticity of the Placenta
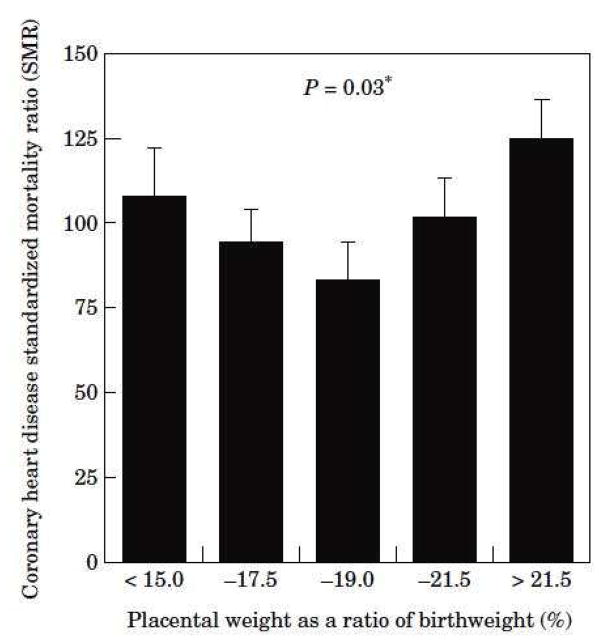
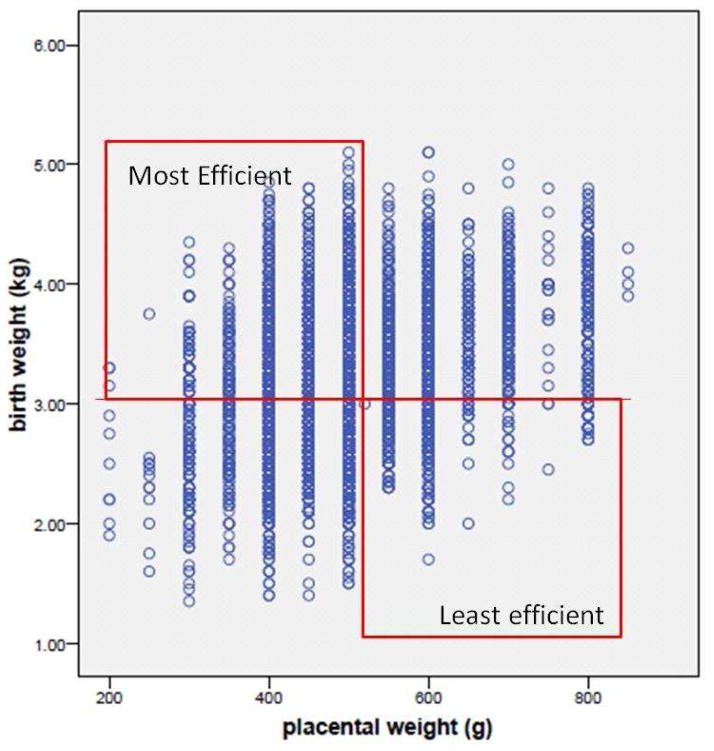
David Barker’s team showed a “U” shaped relationship between cardiovascular death risk and the ratio of placental weight to birthweight (Fig 2).55 Small placentas bearing large babies are defined as highly efficient and large placentas bearing small babies are deemed inefficient. Fig 3 shows the birthweight of some 17,000 placentas from babies born in Saudi Arabia according to their placental weight. In the upper left hand quadrant are large babies born with small efficient placentas; the lower right hand quadrant shows small babies born with large inefficient placentas. Based on the placental ratios in the UK study,8 we can predict that the efficiency extremes of this population will carry elevated risks for chronic disease. We also know that boys tend to make placentas that are more efficient than do girls.56,57 This may explain some of the differences in disease patterns between the sexes during adulthood.
Figure 2. Risk of coronary heart disease based upon the ratio of placental weight to birthweight.
Figure 2 shows the risk of coronary heart disease based upon the ratio of placental weight to birthweight in an English population81 Low placental weight to birthweight is defined as “efficient” and represented by the left hand bars. This “U” shaped curve suggests that placental efficiencies are related to risk for disease. 82 This relationship has been found in other studies as well.79 (With permission.)
Figure 3. High and low efficiency placentas.
Figure 3 showing 17,000 live births in Unizah, Saudi Arabia. Boxes show quadrants of high and low efficiency placentas. Efficiency is the weight of a newborn baby per unit weight of placenta. Figure 3 suggests that efficiencies at the extremes are associated with chronic disease. Modified from.83
The regulation of growth of the placenta is not well understood. It is well known that to grow heavier lambs, farmers placed previously well fed pregnant ewes on poor pasture early in pregnancy to stimulate growth of the placenta and later returned the ewes to good pasture.58 Human placentas may also respond to inadequate nutrient delivery by expanding their tissue mass. Data from non-human primates illustrate the loss of plasticity of the placenta as a function of gestational age. When the bridge vessels between the two lobes of the rhesus placenta are ligated, the non-ligated primary lobe is able to compensate by enlargement.59 But this is only true at mid-gestation and not if the vessels are ligated at or after 67% of the gestational period. Thus, there seems to be a period of time when the growth of the placenta responds to the demands placed upon it. When that period is passed, it can no longer accommodate increasing demands for nutrients. This change in placental plasticity will influence the fetal response to maternal insults.
Placental Lessons from the Helsinki Birth Cohort
Epidemiological studies demonstrate that a woman’s body composition, including her relative fat, muscle and pelvic bone masses, are important regulators of placental function and fetal outcomes.27,60 These epidemiological associations and others suggest that maternal body composition affects placentation.61 High BMI is associated with adverse pregnancy outcomes including preeclampsia, thromboembolism, and gestational diabetes mellitus,62,63 and has detrimental effects on the fetus including macrosomia and preeclampsia.64,65 Furthermore, babies born to obese mothers have a compromised immune system.66
The Helsinki Birth Cohort comprises 13,345 men and women born during 1934–1944,67,68 and an older cohort comprising 7086 people born during 1924–1933.69 The Helsinki Birth Cohort is a gold mine for placentologists because at the time of birth, midwives and nurses measured the weights, widths and lengths of all births in Helsinki hospitals during those periods of time. Among ~6,000 placentas, the lengths exceeded their widths by an average of 2.6 cm with the difference ranging from 0–21 cm. From these data and others, it is now possible to link poor fetal growth and/or placental phenotype with70 metabolic disease and obesity,71 coronary heart disease,72 heart failure,73 sudden cardiac death,55 asthma,74 osteoporosis,75 as well as cancers including Hodgkin’s lymphoma,76 lung cancer,77 and colorectal cancer.78 Many examples have not been published.
Placental Thickness is Associated with Sudden Cardiac Death
Among 187 men and 47 women, sudden cardiac death outside the hospital55 was associated with a thin placenta and had a hazard ratio of 1.47 ( C.I. 1.11–1.93) for every g/cm2 decrease in placental thickness. Sudden cardiac death is thought to be associated with excess sympathetic tone and subsequent ventricular fibrillation. Thus, one can speculate that an inadequate placenta, caused perhaps by inadequate trophoblast invasion, compromised nutrient exchange and the development of the autonomic nervous system.
Chronic Heart Failure is Associated with a Small Placenta
Among 187 people in the Helsinki Birth Cohort of 1934–1944, chronic heart failure was associated with a small surface area of the delivered placenta.73 In people who were born with a placenta of less than 225 cm2 the odds ratio was 1.7 (C.I. 1.1–2.5) compared to people with larger placentas having a surface area of >295 cm2. Short placental width but not length predicted the disease also but only in short mothers. Other factors were associated with heart failure, too. A rapid gain in body mass index between 2 and 11 years of age was also associated with chronic heart failure, a path of growth that has been associated with insulin resistance. It appears that the combination of a small placenta and rapid childhood weight gain leads to poor glucose control which predisposes to heart failure later in life.
Coronary Heart Disease is Associated with 3 Different Maternal/Placental Phenotypes
Among 7000 men born in the Helsinki Birth Cohort during 1934–44,72 those who developed coronary heart disease were thin at birth and their disease was associated with three different placental/maternal phenotypes. 1) In short primiparous mothers, the hazard ratio for coronary disease was related to the difference between the length and width of the placental surface. 2) In tall mothers whose body mass index was above the median, a small placental surface predicted the disease (See Table 1). 3) In tall mothers who were thin, coronary heart disease was related to placental efficiency. The hazard ratio was elevated with an increase in the placental weight/birthweight ratio. Thus, there was a profound interaction between maternal phenotype, placental deviation from roundness, placental surface area at delivery and placental efficiency. These complex relationships suggest a profound interaction between maternal body composition and placental form and function as suggested by others.61
Table 1.
Coronary heart disease in men born to tall mothers (>160 cm) according to her body mass index (BMI)
| Mother’s BMI ≤ 26 kg/m2 | Mother’s BMI > 26 kg/m2 | |
|---|---|---|
| Placental weight (g) | ||
| HR (95% CI) | HR (95% CI) | |
| ≤550 | 0.8 (0.4 to 1.3) | 2.2 (1.3 to 4.0) |
| −650 | 0.9 (o.6 to 1.5) | 1.9 (1.2 to 3.2) |
| −750 | 0.8 (0.5 to 1.4) | 1.0 (baseline) |
| p for trend | 0.5 | 0.002 |
| Placental Area (cm2) | ||
| ≤225 | 1.0 (0.6 to 1.7) | 2.2 (1.4 to 3.7) |
| −255 | 1.0 (0.6 to 1.6) | 1.3 (0.8 to 2.2) |
| −295 | 1.1 (0.7 to 1.9) | 1.7 (1.0 to 2.7) |
| >295 | 1.0 (baseline) | 1.0 (baseline) |
| p for trend | 0.5 | <0.001 |
Table 1 shows that risks for acquiring coronary heart disease in men depends on maternal stature and body mass index. Among men born in Helsinki to taller mothers with a high body mass index, low placental weight and surface area were associated with coronary heart disease.79
Conclusions
The placenta is at the center of the programming universe because fetal growth determines the degree of vulnerability that a neonate carries for adult onset disease. Stress, poor diet and hypoxia are major stressors that are mediated by the placenta. When it comes to understanding the mechanisms which regulate the growth and function of the placenta, we are stifled by ignorance. We can say with confidence, however, that unless we ensure that women and their offspring are able to eat healthy diets and generate healthy placentas, we cannot expect much improvement in the health of US population. Thus, in order to optimize fetal growth and improve life-long health, it now falls on scientists to study placental nutrient transport to provide nutritional guidance for future generations.
Acknowledgments
This work was supported by NIH grants R01 AG032339, R01 HL102763 and P01 HD034430. KLT was supported by the M. Lowell Edwards Endowment and NM by 1K23HD069520.
Johan Eriksson led the Helsinki Birth Cohort team of which David Barker and KLT were a part. Office support was provided by Lisa Rhuman, Kim Rogers, Susan McGinn and Mae Culbertson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989 Mar 4;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plagemann A, Harder T, Dudenhausen JW. The diabetic pregnancy, macrosomia, and perinatal nutritional programming. Nestle Nutrition workshop series. Paediatric programme. 2008;61:91–102. doi: 10.1159/000113179. [DOI] [PubMed] [Google Scholar]
- 3.Oken E, Gillman MW. Fetal origins of obesity. Obesity research. 2003 Apr;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 4.Wei JN, Sung FC, Li CY, et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in taiwan. Diabetes care. 2003 Feb;26(2):343–348. doi: 10.2337/diacare.26.2.343. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. The New England journal of medicine. 2008 Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nature clinical practice. Endocrinology & metabolism. 2007 Jun;3(6):479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 7.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006 Apr 1;572(Pt 1):25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002 Apr;23( Suppl A):S20–27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 9.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological reviews. 2005 Apr;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 10.de Rooij SR, van Pelt AM, Ozanne SE, et al. Prenatal undernutrition and leukocyte telomere length in late adulthood: the Dutch famine birth cohort study. Am J Clin Nutr. 2015 Jul 15; doi: 10.3945/ajcn.115.112326. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV. Shattuck Lecture. The hypertension paradox--more uncontrolled disease despite improved therapy. The New England journal of medicine. 2009 Aug 27;361(9):878–887. doi: 10.1056/NEJMsa0903829. [DOI] [PubMed] [Google Scholar]
- 12.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. The New England journal of medicine. 2005 Mar 17;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 13.Barker DJP, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 14.Langley-Evans SC, Phillips GJ, Jackson AA. In utero exposure to maternal low protein diets induces hypertension in weanling rats, independently of maternal blood pressure changes. Clinical nutrition. 1994 Oct;13(5):319–324. doi: 10.1016/0261-5614(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 15.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clin Obstet Gynecol. 2013 Sep;56(3):591–601. doi: 10.1097/GRF.0b013e3182993a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Zhang L. Epigenetic mechanisms in developmental programming of adult disease. Drug discovery today. 2011 Dec;16(23–24):1007–1018. doi: 10.1016/j.drudis.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynolds LP, Borowicz PP, Vonnahme KA, et al. Placental angiogenesis in sheep models of compromised pregnancy. J Physiol. 2005 May 15;565(Pt 1):43–58. doi: 10.1113/jphysiol.2004.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Long NM, Hein SM, Ma Y, Nathanielsz PW, Ford SP. Maternal obesity in ewes results in reduced fetal pancreatic beta-cell numbers in late gestation and decreased circulating insulin concentration at term. Domestic animal endocrinology. 2011 Jan;40(1):30–39. doi: 10.1016/j.domaniend.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang B, Godfrey KM, Martyn CN, Gale CR. Birth weight and cardiac structure in children. Pediatrics. 2006 Feb;117(2):e257–261. doi: 10.1542/peds.2005-1325. [DOI] [PubMed] [Google Scholar]
- 20.Martyn CN, Greenwald SE. A hypothesis about a mechanism for the programming of blood pressure and vascular disease in early life. Clinical and experimental pharmacology & physiology. 2001 Nov;28(11):948–951. doi: 10.1046/j.1440-1681.2001.03555.x. [DOI] [PubMed] [Google Scholar]
- 21.Dodson RB, Rozance PJ, Fleenor BS, et al. Increased arterial stiffness and extracellular matrix reorganization in intrauterine growth-restricted fetal sheep. Pediatric research. 2013 Feb;73(2):147–154. doi: 10.1038/pr.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumortier O, Blondeau B, Duvillie B, Reusens B, Breant B, Remacle C. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia. 2007 Dec;50(12):2495–2503. doi: 10.1007/s00125-007-0811-0. [DOI] [PubMed] [Google Scholar]
- 23.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney international. 2004 Apr;65(4):1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 24.Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated outcomes-a global concern. Nature reviews. Nephrology. 2015 Mar;11(3):135–149. doi: 10.1038/nrneph.2014.251. [DOI] [PubMed] [Google Scholar]
- 25.Buss C, Entringer S, Wadhwa PD. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Science signaling. 2012 Oct 9;5(245):pt7. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornburg KL. The programming of cardiovascular disease. Journal of developmental origins of health and disease. 2015 doi: 10.1017/S2040174415001300. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013 Oct;34(10):841–845. doi: 10.1016/j.placenta.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 28.Sibley CP, Coan PM, Ferguson-Smith AC, et al. Placental-specific insulin-like growth factor 2 (Igf2) regulates the diffusional exchange characteristics of the mouse placenta. Proceedings of the National Academy of Sciences of the United States of America. 2004 May 25;101(21):8204–8208. doi: 10.1073/pnas.0402508101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansson T, Powell TL, Illsley NP. Non-electrolyte solute permeabilities of human placental microvillous and basal membranes. J Physiol. 1993 Aug;468:261–274. doi: 10.1113/jphysiol.1993.sp019770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis DM, O'Grady JP, Faber JJ, Thornburg KL. Diffusion permeability of cyanocobalamin in human placenta. Am J Physiol. 1986 Mar;250(3 Pt 2):R459–464. doi: 10.1152/ajpregu.1986.250.3.R459. [DOI] [PubMed] [Google Scholar]
- 31.Bain MD, Copas DK, Taylor A, Landon MJ, Stacey TE. Permeability of the human placenta in vivo to four non-metabolized hydrophilic molecules. J Physiol. 1990 Dec;431:505–513. doi: 10.1113/jphysiol.1990.sp018343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illsley NP. Glucose transporters in the human placenta. Placenta. 2000 Jan;21(1):14–22. doi: 10.1053/plac.1999.0448. [DOI] [PubMed] [Google Scholar]
- 33.Hay WW., Jr Placental-fetal glucose exchange and fetal glucose metabolism. Transactions of the American Clinical and Climatological Association. 2006;117:321–339. discussion 339–340. [PMC free article] [PubMed] [Google Scholar]
- 34.Lager S, Powell TL. Regulation of nutrient transport across the placenta. Journal of pregnancy. 2012;2012:179827. doi: 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jansson T. Amino acid transporters in the human placenta. Pediatric research. 2001 Feb;49(2):141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Avagliano L, Garo C, Marconi AM. Placental amino acids transport in intrauterine growth restriction. Journal of pregnancy. 2012;2012:972562. doi: 10.1155/2012/972562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faber J, Thornburg KL. Placental Physiology. Raven Press; 1983. [Google Scholar]
- 38.Dilworth MR, Sibley CP. Review: Transport across the placenta of mice and women. Placenta. 2013 Mar;34( Suppl):S34–39. doi: 10.1016/j.placenta.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clinical science. 2007 Jul;113(1):1–13. doi: 10.1042/CS20060339. [DOI] [PubMed] [Google Scholar]
- 40.Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? The Proceedings of the Nutrition Society. 1998 Feb;57(1):113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- 41.Challis JR, Sloboda D, Matthews SG, et al. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Molecular and cellular endocrinology. 2001 Dec 20;185(1–2):135–144. doi: 10.1016/s0303-7207(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 42.Fowden AL, Szemere J, Hughes P, Gilmour RS, Forhead AJ. The effects of cortisol on the growth rate of the sheep fetus during late gestation. The Journal of endocrinology. 1996 Oct;151(1):97–105. doi: 10.1677/joe.0.1510097. [DOI] [PubMed] [Google Scholar]
- 43.Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology. 2006 Aug;147(8):3643–3649. doi: 10.1210/en.2006-0061. [DOI] [PubMed] [Google Scholar]
- 44.Goland RS, Tropper PJ, Warren WB, Stark RI, Jozak SM, Conwell IM. Concentrations of corticotrophin-releasing hormone in the umbilical-cord blood of pregnancies complicated by pre-eclampsia. Reproduction, fertility, and development. 1995;7(5):1227–1230. doi: 10.1071/rd9951227. [DOI] [PubMed] [Google Scholar]
- 45.McTernan CL, Draper N, Nicholson H, et al. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. The Journal of clinical endocrinology and metabolism. 2001 Oct;86(10):4979–4983. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- 46.Phillips DI, Jones A. Fetal programming of autonomic and HPA function: do people who were small babies have enhanced stress responses? J Physiol. 2006 Apr 1;572(Pt 1):45–50. doi: 10.1113/jphysiol.2005.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006 Apr 1;572(Pt 1):31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikaelsson MA, Constancia M, Dent CL, Wilkinson LS, Humby T. Placental programming of anxiety in adulthood revealed by Igf2-null models. Nature communications. 2013;4:2311. doi: 10.1038/ncomms3311. [DOI] [PubMed] [Google Scholar]
- 49.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Seminars in fetal & neonatal medicine. 2006 Oct;11(5):317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salafia C, Popek E. Women's med. DOI. 2008;10(3843/GLOWM.10150) [Google Scholar]
- 51.O'Tierney PF, Lewis RM, McWeeney SK, et al. Immune response gene profiles in the term placenta depend upon maternal muscle mass. Reproductive sciences. 2012 Oct;19(10):1041–1056. doi: 10.1177/1933719112440051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008 Mar;29(3):274–281. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aye IL, Lager S, Ramirez VI, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biology of reproduction. 2014 Jun;90(6):129. doi: 10.1095/biolreprod.113.116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calay ES, Hotamisligil GS. Turning off the inflammatory, but not the metabolic, flames. Nature medicine. 2013 Mar;19(3):265–267. doi: 10.1038/nm.3114. [DOI] [PubMed] [Google Scholar]
- 55.Barker DJ, Larsen G, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The placental origins of sudden cardiac death. Int J Epidemiol. 2012 Oct;41(5):1394–1399. doi: 10.1093/ije/dys116. [DOI] [PubMed] [Google Scholar]
- 56.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. American journal of human biology : the official journal of the Human Biology Council. 2010 May-Jun;22(3):330–335. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roseboom TJ, Painter RC, de Rooij SR, et al. Effects of famine on placental size and efficiency. Placenta. 2011 May;32(5):395–399. doi: 10.1016/j.placenta.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 58.McCrabb GJEA, Hasking BJ. Maternal undernutrition during med-pregnancy in sheep; variable effects on placental growth. J Agricult Sci. 1992;118:127–132. [Google Scholar]
- 59.Roberts VH, Rasanen JP, Novy MJ, et al. Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta. 2012 Jan;33(1):73–76. doi: 10.1016/j.placenta.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clinical obstetrics and gynecology. 2013 Sep;56(3):511–519. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- 61.Roland MC, Friis CM, Godang K, Bollerslev J, Haugen G, Henriksen T. Maternal factors associated with fetal growth and birthweight are independent determinants of placental weight and exhibit differential effects by fetal sex. PloS one. 2014;9(2):e87303. doi: 10.1371/journal.pone.0087303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sebire NJ, Jolly M, Harris JP, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001 Aug;25(8):1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 63.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health. 2001 Mar;91(3):436–440. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006 Oct;113(10):1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 65.Marshall NE, Guild C, Cheng YW, Caughey AB, Halloran DR. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol. 2012 May;206(5):417.e411–416. doi: 10.1016/j.ajog.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson RM, Marshall NE, Jeske DR, Purnell JQ, Thornburg K, Messaoudi I. Maternal obesity alters immune cell frequencies and responses in umbilical cord blood samples. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 2015 Jun;26(4):344–351. doi: 10.1111/pai.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sandboge S, Fellman J, Nilsson PM, Eriksson AW, Osmond C, Eriksson JG. Regional differences in birth size: a comparison between the Helsinki Birth Cohort Study and contemporaneous births on the Aland Islands. Journal of developmental origins of health and disease. 2015 Aug;6(4):263–267. doi: 10.1017/S2040174415000136. [DOI] [PubMed] [Google Scholar]
- 68.Osmond C, Kajantie E, Forsen TJ, Eriksson JG, Barker DJ. Infant growth and stroke in adult life: the Helsinki birth cohort study. Stroke; a journal of cerebral circulation. 2007 Feb;38(2):264–270. doi: 10.1161/01.STR.0000254471.72186.03. [DOI] [PubMed] [Google Scholar]
- 69.Forsen T, Eriksson JG, Tuomilehto J, Teramo K, Osmond C, Barker DJ. Mother's weight in pregnancy and coronary heart disease in a cohort of Finnish men: follow up study. BMJ. 1997 Oct 4;315(7112):837–840. doi: 10.1136/bmj.315.7112.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. The International journal of developmental biology. 2010;54(2–3):525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. American journal of physiology. Endocrinology and metabolism. 2000 Jul;279(1):E83–87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 72.Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother's body size and placental size predict coronary heart disease in men. European heart journal. 2011 Sep;32(18):2297–2303. doi: 10.1093/eurheartj/ehr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barker DJ, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. European journal of heart failure. 2010 Aug;12(8):819–825. doi: 10.1093/eurjhf/hfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barker DJ, Osmond C, Forsen TJ, Thornburg KL, Kajantie E, Eriksson JG. Foetal and childhood growth and asthma in adult life. Acta paediatrica. 2013 Jul;102(7):732–738. doi: 10.1111/apa.12257. [DOI] [PubMed] [Google Scholar]
- 75.Goodfellow LR, Cooper C, Harvey NC. Regulation of placental calcium transport and offspring bone health. Frontiers in endocrinology. 2011;2:3. doi: 10.3389/fendo.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barker DJ, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The intrauterine origins of Hodgkin's lymphoma. Cancer epidemiology. 2013 Jun;37(3):321–323. doi: 10.1016/j.canep.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 77.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The prenatal origins of lung cancer. II. The placenta. American journal of human biology : the official journal of the Human Biology Council. 2010 Jul-Aug;22(4):512–516. doi: 10.1002/ajhb.21041. [DOI] [PubMed] [Google Scholar]
- 78.Barker DJ, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The shape of the placental surface at birth and colorectal cancer in later life. American journal of human biology : the official journal of the Human Biology Council. 2013 Jul-Aug;25(4):566–568. doi: 10.1002/ajhb.22409. [DOI] [PubMed] [Google Scholar]
- 79.Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother's body size and placental size predict coronary heart disease in men. Eur Heart J. 2011 Sep;32(18):2297–2303. doi: 10.1093/eurheartj/ehr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 81.Martyn CN, Barker DJ, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996 Nov 9;348(9037):1264–1268. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- 82.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002 Apr;23( Suppl A):S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 83.Alwasel SH, Abotalib Z, Aljarallah JS, et al. Secular increase in placental weight in Saudi Arabia. Placenta. 2011 May;32(5):391–394. doi: 10.1016/j.placenta.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]