SUMMARY
SETTING
Primary care clinic serving a high tuberculosis (TB) and human immunodeficiency virus (HIV) prevalence community in South Africa.
OBJECTIVE
To evaluate a program combining TB and HIV contact investigation with tracing of individuals lost to TB or HIV care.
DESIGN
Contacts were offered home-based HIV testing, TB symptom screening, sputum collection and referral for isoniazid preventive therapy (IPT). Effectiveness was assessed by the number needed to trace (NNT).
RESULTS
Only 419/1197 (35.0%) households were successfully traced. Among 267 contacts, we diagnosed 27 new HIV cases (10 linked to care) and two TB cases (both initiated treatment) and three started IPT. Of 630 patients lost to care, 132 (21.0%) were successfully traced and 81 (61.4%) re-engaged in care. The NNT to locate one individual lost to care was 4.8 (95%CI 4.1–5.6), to re-engage one person in care 7.8 (95%CI 6.4–9.7), to diagnose one contact with HIV 44.3 (95%CI 30.6–67.0), to link one newly diagnosed contact to HIV care 120 (95%CI 65.3–249.2) and to find one contact with active TB and initiate treatment 599 (95%CI 166.0–4940.7).
CONCLUSION
The effectiveness of this contact tracing approach in identifying new TB and HIV cases was low. Methods to optimize contact investigation should be explored and their cost-effectiveness assessed.
Keywords: tuberculosis, human immunodeficiency virus, intensified case finding, South Africa
THE DUAL epidemics of tuberculosis (TB) and human immunodeficiency virus (HIV) continue to pose a public health problem in resource-poor countries, particularly sub-Saharan Africa.1 Un-awareness of TB and HIV status prevents individuals from receiving care and results in preventable morbidity, mortality and ongoing transmission.2-4 While facility-based active TB case finding in people living with HIV (PLHIV) and provider-initiated HIV counseling and testing (HCT) for people with TB have become routine, TB contact investigation remains a low priority in high-burden settings. Household contacts are at high risk of infection and disease,5,6 and contact investigation may result in earlier diagnosis.7,8 Home-based TB screening and HCT could also reduce the socio-economic barriers and stigma posed by screening at health care facilities.9 The growing evidence of a large pool of undetected TB in the community7,10-12 has increased interest in contact investigations in these settings.
In 2012, the World Health Organization (WHO) published recommendations for investigating contacts of TB cases in low- and middle-income countries.13 The guidelines recommend contact investigations for index cases with smear-positive TB, multidrug-resistant TB, PLHIV or those aged <5 years. They also recommend prioritization of contacts with symptoms suggestive of TB, age <5 years or PLHIV. The guidelines state, in addition, that HCT should be offered as part of TB contact investigation to all contacts in high HIV burden settings, contacts of an HIV-infected index case living with HIV, and contacts with TB symptoms. As the guidelines do not advise on implementation, the optimal strategy remains unclear.14
We aimed to evaluate a pragmatic approach to TB and HIV contact investigation by combining these activities with tracing individuals lost to TB and HIV care. We assessed the program’s yield in identifying cases of undiagnosed TB and HIV, its effectiveness in linking newly diagnosed individuals to care and its success in re-engaging individuals lost to care.
METHODS
Study population
We performed a prospective study of index cases and household contacts. Five types of index cases were included: newly diagnosed TB cases, newly diagnosed HIV cases, individuals lost to anti-tuberculosis treatment and individuals lost to pre-antiretroviral therapy (pre-ART) care or ART. People lost to anti-tuberculosis treatment were those who failed to pick up their TB drugs within 2 weeks of the scheduled visit. PLHIV who failed to return for CD4 count results, and ART-eligible patients (CD4 count <350 cells/mm3) who missed their ART initiation appointment by ≥1 month, were classified as lost to pre-ART care. Those who did not pick up their ART drugs within 1 month of the scheduled refill date were classified as lost to ART.
Study procedures
The outreach team at the Witkoppen Health and Welfare Center, a busy primary care clinic in Johannesburg, South Africa, consisted of eight lay health workers certified in HCT and trained in TB symptom screening, sputum collection and isoniazid preventive therapy (IPT). Pairs performed household visits from Monday to Saturday during working hours. Two local drivers used their cars for outreach team transportation, and visits were geographically clustered to maximize efficiency.
Index cases were identified through chart review and the clinic’s electronic data management system. The index case name, address, telephone number, most recent CD4 count and other relevant information were entered into a mobile phone database. Index cases were called to confirm addresses and determine availability during the day. A single household visit was attempted if anyone was expected to be home or when a household could not be reached by phone.
During household visits, a mobile phone-based questionnaire prompted when to offer onsite HCT using two rapid HIV tests, perform TB symptom screening, collect sputum samples, provide adherence counseling and attempt to re-engage index cases who were lost to follow-up into care and refer for IPT. Symptomatic contacts of new HIV cases and contacts of cases lost to pre-ART or ART care were asked to produce one spontaneous sputum sample. Contacts of new TB patients or index cases lost to anti-tuberculosis treatment were asked to provide sputum samples regardless of TB symptoms. Specimens were transported by the team to the clinic for testing using Xpertw MTB/RIF (Cepheid, Sunnyvale, CA, USA). Individuals were only re-contacted if TB was diagnosed.
Definitions and statistical analysis
A successfully traced household was defined as a household for which the house was located and at least one adult was home. Linkage or re-engagement into care was defined as care within 1 month of the household visit.
Standard descriptive statistics were used to describe the study population. Program effectiveness was assessed by calculating the number of household visits needed to locate one person lost to care, reengage one person in care, diagnose a contact with TB or HIV, or link one new case of HIV or TB to care. Analyses were conducted using Stata 11 (StataCorp, College Station, TX, USA).
Ethics approval
The study was approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill, NC, USA, and the University of the Witwatersrand, Johannesburg, South Africa. All study participants gave written informed consent.
RESULTS
Index case identification and study population characteristics
Among 1850 index cases identified from June to December 2012, a household visit was not attempted in 653 (35.3%), the majority because they reported during the initial phone call that they had moved or that nobody would be home during working hours. Among the 1197 index cases for whom tracing was attempted, 172 were new TB diagnoses, 395 new HIV diagnoses, 38 were patients lost to anti-tuberculosis treatment, 135 lost to pre-ART care and 457 lost to ART.
Participants primarily resided in informal settlements and were poor, with 68.5% unemployed, 81.0% lacking money to buy food regularly and 48.3% of households living on ≤$6 per day. One fourth (28.1%) of contacts were immigrants from other African countries. Access to care was good, with the nearest clinic about 25 min from home (Table).
Table.
Characteristics of 419 successfully traced households and 267 household contacts stratified by type of index case
| Newly diagnosed TB index case n (%) |
Newly diagnosed HIV index case n (%) |
Index case lost to TB or HIV care and treatment n (%) |
|
|---|---|---|---|
| Household characteristics | |||
| Total households, n | 81 | 139 | 199 |
| Number of people living in household, median [IQR] | 3 [2–4] | 2 [2–4] | 3 [2–4] |
| Sleeping rooms in the house, median [IQR] | 1 [1–2] | 1 [1–1] | 1 [1–2] |
| Monthly household income, median [IQR]* | 150 [194–313] | 188 [184–313] | 188 [168–350] |
| Time to nearest clinic, min, median [IQR] | 20 [15–30] | 25 [18–30] | 25 [20–30] |
| Cost to reach the nearest clinic, $, median [IQR] | 2.25 [2.25–2.50] | 2.25 [2.25–2.25] | 2.25 [2.25–2.50] |
| Material of the walls of the house | |||
| Corrugated iron | 39 (48.1) | 78 (56.1) | 91 (45.7) |
| Brick/cement blocks | 39 (48.1) | 56 (40.3) | 101 (50.8) |
| Other | 3 (3.7) | 5 (3.6) | 7 (3.5) |
| Household lacks money to buy food | |||
| Never | 11 (13.6) | 12 (8.6) | 18 (9.0) |
| Seldom | 7 (8.6) | 12 (8.6) | 24 (12.1) |
| Sometimes | 57 (70.4) | 110 (79.1) | 133 (66.8) |
| Often | 6 (7.4) | 5 (3.6) | 24 (12.1) |
| First place household seeks care | |||
| Health clinic or hospital | 78 (96.3) | 134 (96.4) | 196 (98.5) |
| Traditional/faith-based | 3 (3.7) | 4 (2.9) | 3 (1.5) |
| Other | 0 (0) | 1 (0.7) | 0 (0) |
| Contact characteristics | |||
| Total number of individual household contacts enrolled | 67 | 75 | 125 |
| Female | 47 (70.1) | 52 (69.3) | 78 (62.4) |
| Educational level | |||
| None | 16 (23.9) | 7 (9.3) | 10 (8.0) |
| Primary school | 10 (14.9) | 16 (21.3) | 25 (20.0) |
| Secondary or higher | 41 (61.2) | 52 (69.4) | 90 (72.0) |
| Unemployed | 51 (76.1) | 48 (64.0) | 84 (67.2) |
| Foreign national | 20 (29.9) | 26 (34.7) | 29 (23.2) |
| Lives in house with poor ventilation† | 26 (38.8) | 28 (37.3) | 43 (34.4) |
| Current smoker | 5 (7.5) | 5 (6.7) | 13 (10.4) |
| ≥1 other household member smokes | 14 (20.9) | 20 (26.7) | 39 (31.2) |
| Ever tested for HIV | 51 (76.1) | 55 (73.3) | 104 (83.2) |
| Known HIV-positive | 19 (28.4) | 26 (34.7) | 40 (32.0) |
| Knows partner’s HIV status | 25 (37.3) | 37 (49.3) | 50 (40.0) |
| History of anti-tuberculosis treatment | 7 (10.4) | 8 (10.7) | 12 (9.6) |
| History of IPT | 0 (0.0) | 3 (4.0) | 1 (0.8) |
| ≥1 TB symptom | 27 (40.3) | 22 (29.3) | 36 (28.8) |
| TB symptoms | |||
| Cough | 18 (26.9) | 8 (10.7) | 22 (17.6) |
| Weight loss | 7 (10.4) | 10 (13.3) | 15 (12.0) |
| Fever | 5 (7.5) | 3 (4.0) | 13 (10.4) |
| Night sweats | 10 (14.9) | 8 (10.7) | 10 (8.0) |
Missing for 1 household of a newly diagnosed TB index case, 3 households of newly diagnosed HIV index cases, and 1 household of index cases lost to care; US$1 = 8ZAR.
Defined as no windows present or windows that are never opened.
TB = tuberculosis; IQR = interquartile range; HIV = human immunodeficiency virus; IPT = isoniazid preventive therapy; ZAR = South African Rand
Tracing contacts and individuals lost to care
Only 419 (35.0%) of 1197 households were successfully traced. Tracing was unsuccessful in 778 cases due to failure to locate the address (30.7%) or no-one being home (65.3%). Among the 508 households where no-one was home, neighbors provided information in 238 cases, reporting that the index case had moved (n = 99, 19.5%) or that all household members were at work (n = 139, 27.4%).
During household visits, 70 new TB index cases, 110 new HIV index cases, 5 individuals lost to TB care, 31 individuals lost to pre-ART care, 96 individuals lost to ART care and 267 contacts were assessed. Tracing households of newly diagnosed TB or HIV patients was more successful than tracing people lost to TB or HIV care (38.8% vs. 31.6%, P = 0.078). While the median household size was 3 (interquartile range [IQR] 2–4), the median number of contacts evaluated per household successfully traced was 0.64.
Linkage to care of patients lost to TB or HIV care
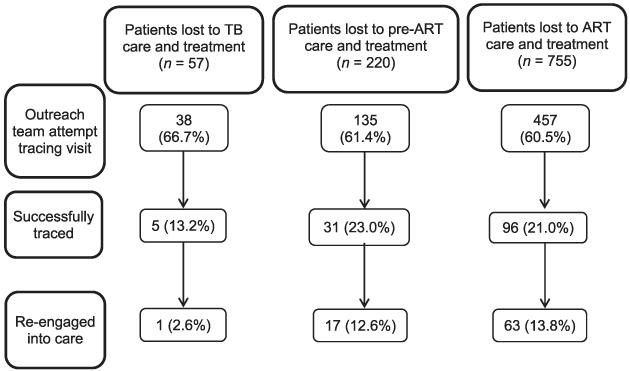
A household visit was attempted in 630 (61.0%) of 1032 patients lost to TB or HIV care. Of these, 132 (21.0%) were successfully traced and 81 re-engaged in care after a median of 14 days (IQR 3–21). This represents 7.9% (81/1032) of all index cases lost to care, 12.9% (81/630) where tracing was attempted and 61.4% (81/132) of those successfully traced (Figure 1).
Figure 1.
Linkage to care of 1032 patients lost to TB or HIV care and treatment. TB=tuberculosis; ART=antiretroviral therapy; HIV = human immunodeficiency virus.
Yield of case finding for TB and HIV among household contacts
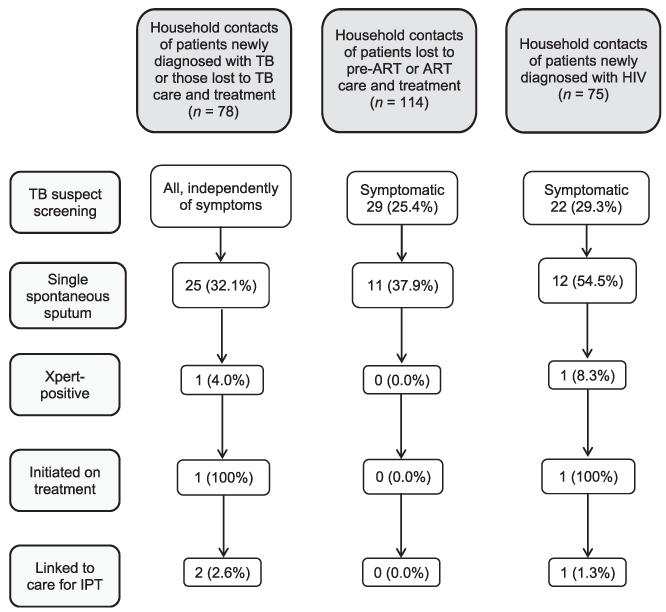
Overall, 267 contacts of index cases were assessed; 10% had a history of anti-tuberculosis treatment and 1.5% reported a history of IPT. Many contacts lived in a house where the windows were never opened (36.3%) or they were exposed to smoking (27.3%), but few (8.6%) reported smoking themselves (Table). One in four contacts of HIV-infected index cases reported TB symptoms, mostly cough (10.7%) and weight loss (13.3%) (Table). Only 45.1% of symptomatic contacts and 32.1% of contacts of TB index cases were able to produce a spontaneous sputum sample (Figure 2). Two contacts were diagnosed with active TB, representing 0.7% (95% confidence interval [CI] 0.09–2.7) of all household contacts and 4.2% (95%CI 0.51–14.3) of those assessed by sputum, corresponding to a TB prevalence of 749/100 000 (95%CI 91–2679) among household contacts.
Figure 2.
TB screening of 267 successfully traced household contacts by type of index case. TB = tuberculosis; ART = antiretroviral therapy; HIV = human immunodeficiency virus; IPT = isoniazid preventive therapy.
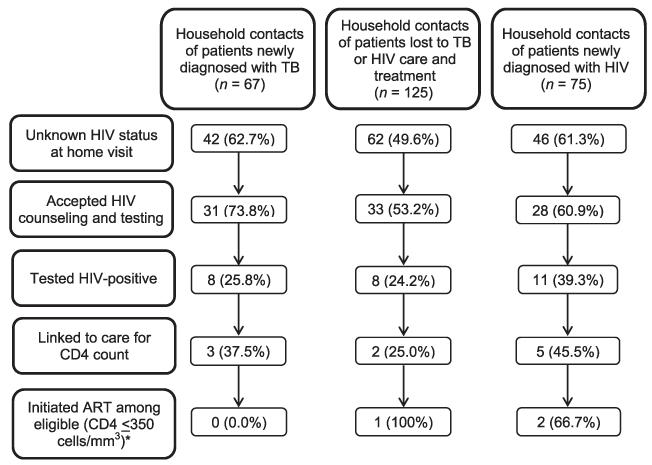
Knowledge of HIV status among contacts was high, with 78.7% ever undergoing an HIV test, 43.8% knowing their recent HIV status and 31.8% being HIV-positive. Among 150 contacts who were unaware of their HIV status, 61.3% accepted HCT and 27 (29.3%, 95%CI 18.4–37.3%) tested positive (Figure 3). The proportion of contacts testing HIV-positive did not differ by type of index case (P = 0.380). Female contacts and those of South African nationality were more likely to test positive (P = 0.158 and P = 0.025, respectively).
Figure 3.
HIV screening of 267 successfully traced household contacts by type of index case. *In addition, two contacts with CD4 counts of 380–500 cells/mm3 were initiated on ART. TB = tuberculosis; ART = antiretroviral therapy; HIV = human immunodeficiency virus.
Household contact linkage to care
Both contacts newly diagnosed with TB initiated anti-tuberculosis treatment, but only one in three (10/27) contacts newly diagnosed with HIV presented for ART eligibility assessment. Median CD4 count for these contacts was 389 cells/mm3 (IQR 254–487), higher than the median CD4 count of 268 cells/mm3 (IQR 137–416, P = 0.258) among new HIV diagnoses at the clinic. Among the four individuals eligible for ART, three initiated it. Three of 15 asymptomatic HIV-positive household contacts presented for IPT.
Effectiveness of targeted household TB and HIV case finding
Among successfully traced households, the number of households needed to trace (NNT) to locate one individual lost to TB or HIV care was 4.8 (95%CI 4.1–5.6), and the NNT to re-engage one person in TB or HIV care was 7.8 (95%CI 6.4–9.7). The NNT to newly diagnose one household contact with HIV was 44.3 (95%CI 30.6–67.0). The NNT to link one newly diagnosed contact to HIV care was 120 (95%CI 65.3–249.2). The NNT to diagnose one contact with TB and to initiate one contact newly diagnosed with TB on anti-tuberculosis treatment was 599 (95%CI 166.0–4940.7).
DISCUSSION
The outreach program combining tracing of individuals lost to TB or HIV care with household TB-HIV contact investigation faced many challenges, resulting in relatively low effectiveness when considering the entire cascade from home visit to initiation of care. Over a 7-month period, a team of eight lay health workers successfully traced 419 households and found 2 new TB cases and 27 new HIV cases, of whom only 10 presented for ART eligibility assessment and 3 initiated ART.
The yield of active TB among contacts assessed by sputum microscopy was 4.2%, similar to the 3.1% (95%CI 2.2–4.4) prevalence estimate of TB in household contacts reported in a recent meta-analysis.15 This corresponds to a TB prevalence of 749/100 000 (95%CI 91–2679), substantially lower than the 6075/100 000 (95%CI 5789–6360) estimate from another South African household contact study.6 The effectiveness of contact investigation for TB was low, with 599 households needed to trace to diagnose one contact with TB. This may not be surprising, as Shapiro et al. found that 94% of newly diagnosed TB cases in contacts were smear-negative, culture-positive and 89% were asymptomatic.6 The low proportion of contacts from whom we were able to collect a sputum sample and our deliberately pragmatic design may, in part, explain the lower yield in our study compared to two other South African studies. We used lay health workers instead of nurses,6 made only one household visit attempt instead of up to three,6,16 collected a single spontaneous sputum rather than one spot and two early morning sputum samples16 or induced sputum,6 and did not perform culture.6,16
The program was effective in providing HCT, with 61.3% of contacts with unknown HIV status consenting to home-based HCT, which falls within the 58.1–99.8% range reported in a meta-analysis of home-based HCT in sub-Saharan Africa.17 The yield of new HIV diagnoses among those with unknown HIV status was 29.3% (95%CI 20.3–39.8), similar to the 30.4% HIV prevalence estimate for Gauteng Province.18 This corresponded to relatively high program effectiveness, with an NNT of 44.3 (95%CI 30.6–67.0) to newly diagnose one contact with HIV. Median CD4 count of these new HIV diagnoses was relatively high (389 cells/mm3), similar to other home-based HCT programs.19-22 Unfortunately, linkage to care was low, with 120 households needed to trace to engage one newly diagnosed contact in HIV care and 239 visits to initiate one person on ART. While information on linkage to care from home-based HIV case finding is limited, the effectiveness of our program was lower than that reported by others. In a South African study using point-of-care CD4 count testing and follow-up visits after home-based HCT, 86% of those eligible initiated ART within 3 months and 96% visited an HIV clinic within 6 months.23 In a Kenyan home-based HCT program, 44–54% had visited a primary health clinic within 1 month of diagnosis.19 We may have underestimated linkage to care, as some may have sought care at health care facilities other than one the outreach team was linked to.
In addition to contact investigations for TB and HIV, the program aimed to re-engage those lost to TB or HIV care. While many individuals lost to care could not be traced, two thirds (61.4%) of those successfully traced re-engaged in care, resulting in the need to trace 7.8 households (95%CI 6.4–9.7) to re-engage one individual into TB or HIV care. Our results are consistent with other studies targeting individuals lost to care. In Nairobi, Kenya, nearly 60% of patients reached were re-engaged in care through a TB-HIV tracing program.24 In Malawi, 51% of individuals lost to ART care re-engaged in care after household tracing.25
Our study highlights challenges with community contact investigation for TB and HIV and tracing individuals lost to care. Many addresses could not be located, and the population often moved within the community or returned temporarily to their home countries. The observed low effectiveness of case finding may therefore, at least in part, be due to the fact that study participants were living in informal settlements and were of low socio-economic status, which may not be representative of other populations in sub-Saharan countries.
We attempted to optimize program effectiveness by using lay health workers from the community, as well as confirming addresses and obtaining landmark-based directions before visits. Use of community health workers (CHWs) instead of clinic-based outreach teams travelling to the community may improve efficiency, as it could allow for repeat visits or visits outside of working hours. Use of CHWs is a key component of the new South African Department of Health (SA DOH) strategic vision for primary health care services,26 which plans to deploy one million CHWs by 2020.27 Other improvements could include diligent collection of locator information on all patients at the time of clinic visit and follow-up of contacts newly diagnosed with HIV who do not link to care.
We set out to develop a sustainable intervention in response to WHO recommendations for investigating contacts of TB cases, and the SA DOH strategy to deliver health services directly to communities. We demonstrated that home-based tracing of individuals lost to TB care, a standard component of TB control programs, can be expanded to include tracing of individuals lost to HIV care, home-based HCT, and investigation of contacts with TB. The effectiveness of our pragmatic approach was lower than hoped, raising the question of whether such a program provides sufficient benefit at a reasonable cost. Because of competing demands for resources, methods to optimize contact tracing need to be explored and cost-effectiveness addressed before high burden, low-income countries integrate these approaches into their public health programs.
Acknowledgements
The authors wish to sincerely thank the contact tracing team: L Aphane, M Dlamini, G Mashiane, J Mogwasi, E Mokoena, G Mopereo, L Musekwa, E Paledi, N Phidane and N Ximba. We are also thankful to J Faber with GeoMed for their help in developing the mobile phone system. We are deeply grateful to the patients and staff at Witkoppen Health and Welfare Center.
This work was supported by the President’s Emergency Plan For AIDS Relief, Washington DC, USA, and the National Institutes of Health (Bethesda, MD, USA) grant UM1 AI069463, and by the United States Agency for International Development (Washington DC, USA) grants to Right to Care: 674-A-00-08-00007 and 674-A-12-00020, and to Witkoppen Health and Welfare Center (Johannesburg, South Africa): 674-A-12-00033.
Footnotes
Conflict of interest: none declared.
References
- 1.World Health Organization . Global tuberculosis control, 2011. WHO/HTM/TB/2011.16. WHO; Geneva, Switzerland: [Accessed January 2014]. 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [Google Scholar]
- 2.Howard AA, El-Sadr WM. Integration of tuberculosis and HIV services in sub-Saharan Africa: lessons learned. Clin Infect Dis. 2010;50(Suppl 3):S238–S244. doi: 10.1086/651497. [DOI] [PubMed] [Google Scholar]
- 3.Kranzer K, Zeinecker J, Ginsberg P, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLOS ONE. 2010;5:e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44:1500–1502. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 5.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AE, Variava E, Rakgokong MH, et al. Community-based targeted case finding for tuberculosis and HIV in household contacts of patients with tuberculosis in South Africa. Am J Respir Crit Care Med. 2012;185:1110–1116. doi: 10.1164/rccm.201111-1941OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah S, Demissie M, Lambert L, et al. Intensified tuberculosis case finding among HIV-infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr. 2009;50:537–545. doi: 10.1097/QAI.0b013e318196761c. [DOI] [PubMed] [Google Scholar]
- 9.Helleringer S, Kohler HP, Frimpong JA, Mkandawire J. Increasing uptake of HIV testing and counseling among the poorest in sub-Saharan countries through home-based service provision. J Acquir Immune Defic Syndr. 2009;51:185–193. doi: 10.1097/QAI.0b013e31819c1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbett EL, Bandason T, Duong T, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010;376:1244–1253. doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eang MT, Satha P, Yadav RP, et al. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health. 2012;12:469. doi: 10.1186/1471-2458-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayles H, Schaap A, Nota A, et al. Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLOS ONE. 2009;4:e5602. doi: 10.1371/journal.pone.0005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . Recommendations for the investigation of contacts of persons with infectious tuberculosis in low- and middle-income countries. WHO/HTM/TB/2012.9. WHO; Geneva, Switzerland: [Accessed January 2014]. 2012. http://apps.who.int/iris/bitstream/10665/77741/1/9789241504492_eng.pdf. [PubMed] [Google Scholar]
- 14.Lönnroth K, Corbett E, Golub J, et al. Systematic screening for active tuberculosis: rationale, definitions and key considerations [State of the art series. Active case finding/screening. Number 1 in the series] Int J Tuberc Lung Dis. 2013;17:289–298. doi: 10.5588/ijtld.12.0797. [DOI] [PubMed] [Google Scholar]
- 15.Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41:140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thind D, Charalambous S, Tongman A, Churchyard G, Grant AD. An evaluation of ‘Ribolola’: a household tuberculosis contact tracing programme in North West Province, South Africa. Int J Tuberc Lung Dis. 2012;16:1643–1648. doi: 10.5588/ijtld.12.0074. [DOI] [PubMed] [Google Scholar]
- 17.Sabapathy K, Van den Bergh R, Fidler S, Hayes R, Ford N. Uptake of home-based voluntary HIV testing in sub-Saharan Africa: a systematic review and meta-analysis. PLOS Med. 2012;9:e1001351. doi: 10.1371/journal.pmed.1001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Republic of. [Accessed January 2014];South Africa, National Department of Health. The 2010 National Antenatal Sentinel HIV and Syphilis Prevalence Survey in South Africa. Pretoria, South Africa: National Department of Health. 2011 http://www.doh.gov.za/docs/reports/2011/hiv_aids_survey.pdf.
- 19.Dalal W, Feikin DR, Amolloh M, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62:e47–e54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]
- 20.Wachira J, Kimaiyo S, Ndege S, Mamlin J, Braitstein P. What is the impact of home-based HIV counseling and testing on the clinical status of newly enrolled adults in a large HIV care program in Western Kenya? Clin Infect Dis. 2012;54:275–281. doi: 10.1093/cid/cir789. [DOI] [PubMed] [Google Scholar]
- 21.Tumwesigye E, Wana G, Kasasa S, Muganzi E, Nuwaha F. High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care STDS. 2010;24:735–741. doi: 10.1089/apc.2010.0096. [DOI] [PubMed] [Google Scholar]
- 22.Menzies N, Abang B, Wanyenze R, et al. The costs and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 23.van Rooyen H, Barnabas RV, Baeten JM, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64:e1–e8. doi: 10.1097/QAI.0b013e31829b567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson KA, Cheti EO, Reid T. Implementation and outcomes of an active defaulter tracing system for HIV, prevention of mother to child transmission of HIV (PMTCT), and TB patients in Kibera, Nairobi, Kenya. Trans R Soc Trop Med Hyg. 2011;105:320–326. doi: 10.1016/j.trstmh.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the ‘Back-to-Care’ project in Lilongwe, Malawi. Trop Med Int Health. 2010;15(Suppl 1):S82–S89. doi: 10.1111/j.1365-3156.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 26.Pillay Y, Baron P. The implementation of PHC re-engineering in South Africa. Public Health Association of South Africa; Johannesburg, South Africa: [Accessed January 2014]. 2010. http://www.sarrahsouthafrica.org/LinkClick.aspx?fileticketWcC6br4QaHk%3D&tabid 2067. [Google Scholar]
- 27.World Health Organization . Community health workers: what do we know about them? The state of the evidence on programmes, activities, costs and impact on health outcomes of using community health workers. Evidence and Information for Policy, Department of Human Resources for Health. WHO; Geneva, Switzerland: [Accessed January 2014]. 2007. http://www.who.int/hrh/documents/community_health_workers_brief.pdf. [Google Scholar]