Abstract
Behavioral measures of spatial selectivity in cochlear implants are important both for guiding the programing of individual users’ implants and for the evaluation of different stimulation methods. However, the methods used are subject to a number of confounding factors that can contaminate estimates of spatial selectivity. These factors include off-site listening, charge interactions between masker and probe pulses in interleaved masking paradigms, and confusion effects in forward masking. We review the effects of these confounds and discuss methods for minimizing them. We describe one such method in which the level of a 125-pps masker is adjusted so as to mask a 125-pps probe, and where the masker and probe pulses are temporally interleaved. Five experiments describe the method and evaluate the potential roles of the different potential confounding factors. No evidence was obtained for off-site listening of the type observed in acoustic hearing. The choice of the masking paradigm was shown to alter the measured spatial selectivity. For short gaps between masker and probe pulses, both facilitation and refractory mechanisms had an effect on masking; this finding should inform the choice of stimulation rate in interleaved masking experiments. No evidence for confusion effects in forward masking was revealed. It is concluded that the proposed method avoids many potential confounds but that the choice of method should depend on the research question under investigation.
Keywords: cochlear implant, spatial selectivity, psychophysical tuning curve, masking
Introduction
Cochlear implant (CI) users rely on a finite number of electrodes, each of which typically conveys acoustic information from a restricted spectral region. Ideally, each channel should excite a discrete set of auditory nerve fibres that are spatially distributed along a narrow region of the basilar membrane. In practice, however, a number of factors can prevent this ideal spatial selectivity. These limiting factors include increased electrode-to-neuron distance caused by positioning of the electrode far from the modiolus (e.g., on the lateral wall) and incomplete neural survival such as the presence of “dead regions” along the auditory nerve. Being able to identify electrodes with poor spatial selectivity has clinical relevance, as it could guide patient-specific (“bespoke”) frequency-to-electrode maps that maximize individual performance. At present, however, approaches that estimate spatial selectivity in CIs are prone to errors that reduce the reliability of such measures. The aim of the present article is to illustrate some of these potential confounds and to propose a method to estimate spatial selectivity that minimizes their effect.
The article is organized as follows. First, potential confounds of spatial selectivity measures are described in the remainder of this section. The proposed measure is described and tested in the next section. The following four sections report the results from four experiments designed to illustrate the potential effect of these confounds on measures of spatial selectivity. A general discussion follows in the final section.
Off-Site Listening
When the probe is detected using portions of the excitation pattern other than around its center, the subject is said to be listening “off-site.” Listening off-site can affect the spatial selectivity measured at the probe place.
Two types of off-site listening can be distinguished. One of these, which we will refer to as type 1, is analogous to the phenomenon of off-frequency listening in acoustic hearing. It occurs when the ratio of excitation produced by the masker-plus-probe and the masker alone is greatest at a place other than the center of the probe’s excitation pattern (see Figure 1(a)). In normal acoustic hearing this occurs because the excitation patterns of the masker and probe have flat tops and steep low-frequency skirts due to the asymmetry of basilar membrane vibration patterns (Moore & Glasberg, 1983). Its effects can be minimized by employing two or more maskers (on either side of the probe). Some authors have suggested that a similar phenomenon may occur with CI listeners and have recommended the use of two maskers, on apical and basal sides of the probe, respectively (Dingemanse, Frijns, & Briaire, 2006; Fielden, Kluk, & McKay, 2013). Our first two experiments studied whether off-site listening of type 1 does indeed occur but failed to find evidence for this.
Figure 1.
(a) Off-site listening of type 1: In acoustic hearing, subjects listen off-frequency when the SNR is more advantageous elsewhere than at the center of the probe excitation pattern. Adding a second masker (M2) can prevent off-frequency listening. It is not known whether this phenomenon occurs in CIs, where the excitation patterns may be different in shape. (b) Off-site listening of type 2: When neural survival is not homogeneous along the cochlea, the probe may be detected via neurons located away from the probe place. A typical instance of off-site listening of type 2 is observed when the probe is in a “dead region.” SNR = signal-to-noise ratio; CIs = cochlear implants.
Off-site listening of type 2 occurs when the probe is detected via neurons located away from the probe place because of heterogeneous neural density along the cochlea (see Figure 1(b)). A typical instance of off-site listening of type 2 is observed when the probe is in a “dead region” (Moore & Alcántara, 2001; Nelson, Donaldson, & Kreft, 2008). In this case, even if the activation functions for homogenous neural survival would indicate that the probe should be detected from the response of neurons close to the probe electrode, it will nevertheless be detected off-site. An accurate measure of spatial selectivity should be sensitive to dead regions. We present one example of type 2 off-site listening, and in Experiment 3, we show how one previously used method might obscure this phenomenon.
Methods for Measuring Spatial Selectivity
Spatial selectivity can be measured in one of four ways: (a) fixing the masker position and level and measuring the masked threshold for probes at different electrode positions (the masked excitation pattern; Kwon & van den Honert, 2006); (b) measuring the masked threshold for a probe at a fixed electrode position, as a function of the masker electrode (e.g., Dingemanse et al., 2006); (c) fixing the probe electrode position and level and varying the level of maskers at different electrode positions so as to just mask the probe (the psychophysical tuning curve [PTC]; e.g., Bierer & Faulkner, 2010); (d) fixing the probe level and masker position and measuring masker levels at thresholds (MLTs) as a function of probe position (the “output extension pattern”; Verschuure, 1981). Paradigms (a), (b), and (c) are discussed by McKay (2012, p. 1) for forward masking in CI users and, as in that article, are referred to here as Methods A, B, and C, respectively. One reason why the three methods might not yield the same result is the nonlinearity of the system; in normal hearing, this nonlinearity arises from the effects of peripheral compression. A second reason, particularly relevant to CI users, can arise from nonhomogenous neural survival in the cochlea. This may particularly affect the results of experiments obtained using Method B, which is designed to use the probe threshold as an estimate of the amount of excitation at the probe place and to do this for different masker positions. In some previous studies (Azadpour, AlJasser, & McKay, 2013; Fielden et al., 2013), the different maskers have been chosen so as to have the same loudness as each other. If one or more maskers lie in regions of poor neural survival, their levels will be increased to match the equal-loudness criterion; however, such an increase would reflect the neural health at the masker, and not the probe place. This approach can therefore distort the estimate of spatial selectivity at the probe place. We avoided this possibility by using Method C, where the masker level is varied adaptively, so as to produce a fixed amount of excitation—that is needed to just mask the probe—at the probe place. In experiments where dual-electrode maskers were used so as to limit type 1 off-site listening, the two maskers had equal physical levels. Experiment 3 compared spatial selectivity measured via excitation patterns using equal-loudness maskers (Method B) with that obtained from PTCs with equal-current maskers (Method C).
Facilitation Effect at High Stimulation Rates
The majority of experiments on spatial selectivity in CI users has used forward-masking paradigms. An alternative is to present the probe pulses interleaved with the masker pulses (Azadpour et al., 2013; Kwon & van den Honert, 2009). A potential limitation of this approach is suggested by evidence from both psychophysical and physiological experiments that the response to, or detection of, a single pulse can be enhanced by the presence of a preceding pulse (Bierer & Middlebrooks, 2004; Nelson & Donaldson, 2001; Stypulkowski & van den Honert, 1984). This “facilitation” effect (Middlebrooks, 2004) is greatest when the interval between the two pulses is very short, and there is some evidence that it is sensitive to the relative polarity of the two pulses (de Balthasar et al., 2003)—two findings that are consistent with an effect of charge summation at the cell membrane.
Charge summation can affect the spatial sensitivity measured through an interleaved masking paradigm, whereby probe and masker pulses interact to produce a reduction in masking (i.e., the probe becomes more easily detectable); this could even lead to instances of negative masking. At high stimulation rates, where the masker and probe pulses are very close in time, the masker can increase the membrane potential and lower the minimum current needed for the probe to elicit an action potential. This phenomenon may be responsible for the instances of small or no masking reported in Azadpour et al. (2013), where four out of nine CI subjects exhibited flat PTCs in interleaved masking at rates above 4,000 pps. Its effects will combine with those produced by other mechanisms, such as refractoriness. Experiment 4 aimed to provide a better understanding of the relative contributions of facilitation and refractoriness to the masking that occurs with interleaved stimulation.
Interleaved Versus Forward Masking
Both simultaneous and forward-masking paradigms are valid approaches to measure spatial selectivity in normal-hearing subjects. One important difference between these two approaches relates to the effects of nonlinear suppression, caused by the operation of the outer hair cells; it is generally agreed that the effects of suppression are reflected in simultaneous, but not in forward, masking (Moore, 1978; Oxenham & Plack, 1998). Because nonlinear suppression does not occur in the electrically stimulated cochlea, it is not a major reason to choose either forward or interleaved masking in CI experiments. Similarly, although beating between the masker and signal can affect the results of simultaneous masking experiments in acoustic hearing (Moore, Alcántara, & Dau, 1998), this need not be the case in CI experiments, where the masker and probe pulses are interleaved in time. One reason for using forward masking is that it allows one to use high masker pulse rates without possible complications arising from charge summation between masker and probe pulses (see previous section). There is, however, a potential confusion effect by using forward masking, which relates to the user’s ability to detect gaps between two stimuli. Many forward-masking studies with CI users have used the same pulse rate for the masker and probe, combined with short, or sometimes zero, silent gaps between the masker and probe (Azadpour et al., 2013; Cohen, Busby, & Clark, 1996; Cohen, Richardson, Saunders, & Cowan, 2003; Dingemanse et al., 2006; Fielden et al., 2013; Nelson et al., 2008). In such circumstances, it is likely that the probe will not be heard as a separate sound, but, instead, as a mere continuation of the masker. Such “confusion effects” have been shown to artificially sharpen PTCs in acoustic hearing, as they are greatest when the masker and probe frequencies are identical (Moore & Glasberg, 1982; Neff, 1985). A similar effect could occur in CIs, if confusion effects are greatest when the masker and probe are presented on the same, rather than on different, electrodes. For normal-hearing subjects, Neff (1985) identified three stimulus features that can reduce confusion: a different intensity level between masker and probe, a difference in frequency, or a delay between the end of the masker and the beginning of the probe stimulus. Similarly, adding an off-frequency cue that is gated on and off with the masker was shown to reduce confusion effects (Moore & Glasberg, 1982). For CI users, one potential way of minimizing confusion effects is to use a different pulse rate for the masker and probe (Macherey, van Wieringen, Carlyon, Dhooge, & Wouters, 2010). However, because CI listeners are generally insensitive to rate differences above about 300 pps, this requires either the masker or probe to have a rate at or below that value. Another way of reducing confusion effects is to use a nonzero gap between the masker and probe. However, gaps of 20 ms or less are typically used, and it is known that gap detection thresholds vary markedly across the electrode array (Bierer, Deeks, Billig, & Carlyon, 2015b; Garadat & Pfingst, 2011). Gap detection thresholds can approach 50 ms for some subjects and electrodes, even when stimuli are presented at the listener’s most comfortable level (Bierer et al., 2015b) and increase with decreasing level (Garadat & Pfingst, 2011). PTCs are usually measured at a low probe level so that, when the probe is just detectable, the effective masker excitation at the probe place is also low. Therefore, it is possible that, even when there is a silent gap of up to 20 ms between the masker and probe, that gap will not be detected; this will also be the case for some electrodes and listeners for even longer gaps. Experiment 5 is designed to gain a better understanding of the effect of the probe duration in forward-masking paradigms when the gap between probe and masker is zero.
Unit of Current
As argued by McKay (2012), changes in the cochlea produced by different input currents are better described using a ratio measure, instead of subtraction between currents expressed in amperes. This is because the important measure for perception is the amount of current at the target neurons and because the relationship between this current and that at the stimulating electrode is influenced by a number of factors including the distance from the electrode to the neural elements and the conductivity of the medium. To the extent that these factors act linearly, the proportional relationship between the current at the electrode and that at the neurons will be preserved, whereas the difference in absolute current can depend strongly on, for example, electrode-to-neuron distance. Hence, psychophysical thresholds in this study are expressed in decibel (dB).
Experiment 1: Measurement of Spatial Selectivity via PTC
Experiment 1 was designed to propose and test a measure of spatial selectivity for CI users who attempts to overcome some of the confounding factors described in the previous section.
Stimuli and Rationale for the Proposed Measure
PTCs were measured for dual-electrode maskers using interleaved stimulation. A dual-electrode masker was chosen to minimize potential instances of “type 1” off-site listening. The interleaved stimulation was preferred to forward masking to minimize confusion effects. A low pulse rate was used so that the masker and probe pulses could be separated by an interval sufficiently long to minimize charge interactions. The current level of the maskers was adaptively increased to mask a fixed-level probe (Method C as described earlier), to ensure the same amount of excitation at threshold near the probe.
Subjects and Procedure
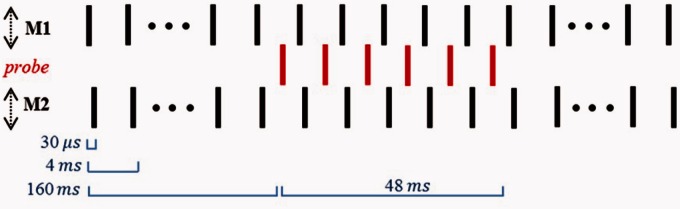
Five users of a Med-El CI (ME1, ME2, ME3, ME4, and ME5) and four users of an Advanced Bionics CI (AB1, AB2, AB3, and AB4) took part. The East of England Ethics Committee approved all procedures, and all subjects provided written informed consent. Their details are given in Table 1. There were some minor differences between the stimuli and procedure for the two devices, and these are included in the description given later. However, they were not considered important, and as it was not our aim to compare devices, they will not be discussed further. All stimuli were presented in monopolar mode in this and all other experiments described here. A three-interval two-alternative forced-choice interleaved masking paradigm was used to measure the PTC for a single-probe electrode in the array. The same current level was applied to both masking electrodes. The stimulus consisted of a 48-ms pulse train (probe) presented in the middle of a 368-ms masker (see Figure 2). A low stimulation rate of 125 pps was chosen, and the probe pulses were presented midway between successive masker pulses, leading to a 4-ms gap between masker and probe pulses, thereby minimizing charge interactions between probe and masker pulses. The electric pulses were cathodic-first biphasic pulses with zero interphase interval. For the Advanced Bionics subjects, the pulse phases were 32 µs in duration, and the two masker pulses were separated by 32 µs. For the Med-El subjects, the pulse phases were 30 µs in duration except ME4 who required 60-µs phase durations to avoid reaching compliance limits; the two masker pulses were separated by 30 µs. For Med-El subjects, the presentation order of the two masker pulses was alternated (e.g., M1 – M2 – P – M2 – M1 – P – M1…), whereas for Advanced Bionics users, the apical masker pulse always preceded the basal masker pulse. These differences between the stimuli presented to Med-El and Advanced Bionics subjects were not intentional but did not influence our conclusions as a comparison between devices was not the purpose of the present study. This is also true for some minor procedural differences described later. The probe level was fixed at a value between 15% and 20% of its dynamic range (DR) in dB, while the current level applied to each of the two masking electrodes was varied at each probe–masker electrode separation (Δx) to determine threshold. Five electrode separations were tested: Δx = 0, 1, 2, 3, and 4. Note that electrode distances are not equal for the two devices, being 2.1 mm for the Med-El implant and 1.1 mm for the Advanced Bionics implant. The two maskers were always placed symmetrically around the probe (e.g., for probe on Electrode 7 and Δx = 2, the two maskers were on electrodes 5 and 9). The probe electrode was E7 for Med-El subjects and E8 for Advanced Bionics subjects, thus both in the middle of the array. The final current level is the MLT, which is the current level necessary to mask the fixed-level probe.
Table 1.
Subjects’ Details at the Time of Testing.
| ID | Age (years) | Possible etiology | Duration of deafness (years) | Months of implantation | Took part in Experiment |
|---|---|---|---|---|---|
| ME1 | 57 | PHL after trauma, sudden after stroke | 2 | 15 | 1, 2, 4, 5 |
| ME2 | 48 | Ear infection, familial | 5 | 15 | 1, 2, 4, 5 |
| ME3 | 67 | PHL | 10 | 18 | 1, 4, 5 |
| ME4 | 55 | Born profoundly deaf, possibly genetic | 13 | 21 | 1, 2 |
| ME5 | 57 | Familial, possibly genetic | 15 | 24 | 1, 2, 4, 5 |
| AB1 | 70 | Otosclerosis, PHL, virus | 25 | 56 | 1, 2, 3 |
| AB2 | 56 | Unknown, possible ototoxicity at pregnancy | 32 | 73 | 1, 2 |
| AB3 | 68 | Otosclerosis, PHL | 23 | 56 | 1 |
| AB4 | 35 | Otosclerosis, PHL | 5 | 24 | 1 |
Note. The first two letters in the ID identify the cochlear implant maker: AB = Advanced Bionics; ME = Med-El; PHL = progressive hearing loss.
Figure 2.
Stimulus for Experiment 1. The basal and apical masker electrodes (M1 and M2) are always equidistant from the probe electrode. For single masker measurements of Experiments 2 and 4, either only M1 or only M2 was used.
MLTs were obtained using an adaptive three-up one-down procedure that converges on the 79.4% point of the psychometric function (Levitt, 1971). At the start of each procedure, the masker was set to a soft level, which was increased following every three consecutive correct trials and decreased after every incorrect trial. The change from increasing to decreasing level or vice versa was termed a reversal. The step size was 1 dB for the first two reversals and 0.25 dB thereafter. Each run continued until six reversals had occurred, and the MLT was calculated from the mean of the last four reversals. The final MLT for a given condition was obtained by averaging four individual MLT measurements. For Med-El subjects, the second stimulus in each trial, which was accompanied by illumination of the middle button of a graphical user interface displayed on a monitor, always contained the test signal, and these subjects were instructed to indicate the interval that was the same as the middle interval. For Advanced Bionics subjects, the middle interval always contained the standard, and the subjects were instructed to indicate the interval that differed from the middle interval. Detection thresholds for the probe signal were estimated adaptively with the same number of reversals and runs as for the MLTs. Approximate levels for detection thresholds (T), comfort (C), and maximum acceptable loudness (U) of masker signals were estimated by manually increasing current levels and asking the subject to report the perceived loudness on a loudness chart supplied by Advanced Bionics (0–10 loudness levels, with T, C, and U corresponding to levels 1, 6, and 7, respectively). This procedure was repeated at least twice for each electrode, and the values obtained were compared across sessions for consistency. Differences between repeated measurements were negligible for all electrodes and subject tested.
Results and Discussion
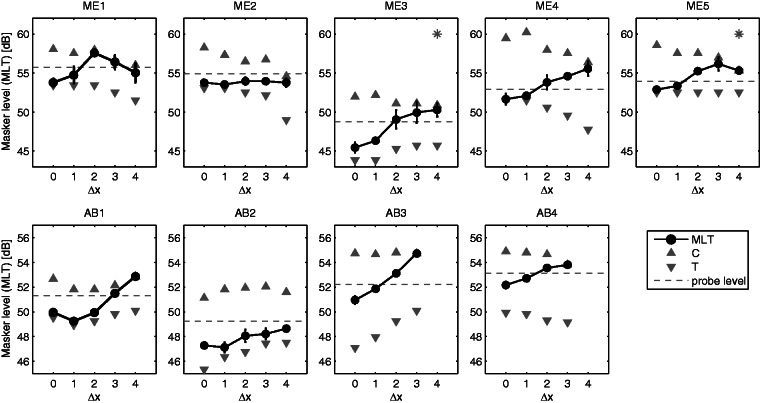
Results for the nine CI users are shown by the lines connecting filled circles in Figure 3. The values on the horizontal axis indicate the electrode separations (Δx) between the probe electrode and each of the two masker electrodes. The top and bottom rows show MLTs for the five Med-El subjects and the four Advanced Bionics subjects, respectively.
Figure 3.
Psychophysical tuning curves obtained for two maskers placed symmetrically around the probe electrode and separated from that by Δx electrodes. Data are shown for five Med-El (top row) and four Advanced Bionics (bottom row) CI users. MLT = masking level at threshold; T = absolute detection thresholds for dual-electrode masker; C = most comfortable loudness level for dual-electrode masker. The level of the probe is shown by the gray dashed lines. Asterisks refer to conditions where one or more runs hit the maximum level allowed and the procedure was interrupted. dB = decibel; CI = cochlear implant.
For most subjects, MLTs increased monotonically with increases in the probe–masker electrode separations (Δx). This can be interpreted as a reduction in masker effectiveness when the maskers are progressively distanced from the probe electrode and is in agreement with similar studies (Azadpour et al., 2013; Dingemanse et al., 2006; Fielden et al., 2013; Kwon & van den Honert, 2006). The exceptions to this trend are ME1, ME2, and AB1.
Subject ME1 showed a monotonically increasing MLT up to an electrode separation of two (Δx = 2). Lower MLTs were found at Δx = 3 and Δx = 4, indicating an increase in masking at the probe place. Although we cannot be sure why this occurred, it is consistent with cross turn or ectopic stimulation—two phenomena whereby one or more electrodes stimulate multiple nontonotopically contiguous portions of the cochlea (Finley et al., 2008; Frijns, Briaire, & Grote, 2001).
Subject ME2 shows a nearly flat PTC, indicating very little difference in masking for different Δx values. The subject reported intense tinnitus during testing sessions, which may have decreased the reliability of this subject’s performance for near-threshold tasks.
Subject AB1 shows a “hockey-stick” function, where the MLT for Δx = 1 is lower than for Δx = 0 and Δx = 2. In general, PTCs where the tip is displaced in frequency are interpreted as instances of dead regions in the cochlea (Bierer & Faulkner, 2010; Moore & Alcántara, 2001). As discussed in the Introduction section and illustrated in Figure 1, a portion of the cochlea with lower density of nerve cells may result in type 2 off-site listening; when the dual-electrode masker is on Δx = 1, it is more effective than when it is on the same electrode as the probe (Δx = 0). The masking for this listener is studied in more detail in Experiment 2.
For each subject, a summary measure of spatial sensitivity was obtained from the width of the masking function measured at 75% of the maximum masking. The average was 2.3 ± 0.5 electrodes spacing (4.9 ± 1.4 mm) for Med-El subjects and 2.7 ± 0.7 electrodes spacing (3.0 ± 0.8 mm) for Advanced Bionics subjects. This compares with Fielden et al. (2013) who reported widths in the range of 1.41 and 3.38 electrodes spacing (1.55–3.72 mm) for nine Advanced Bionics users measured in forward masking and Method B (as described in the Introduction section). When estimated in mm, the 75% PTC width for the Med-El users was significantly greater than for the Advanced Bionics subjects (p = .017). This may be due to the lateral position of the Med-El electrode array, but it is worth noting that the number of subjects was rather small and that this finding may have been unduly influenced by the data from subject ME2, whose PTC was essentially flat.
Experiment 2: Off-Site Listening
Rationale
Because the shape of the excitation pattern in acoustic and electrical hearing may be different, it is not clear whether type 1 off-frequency listening in normal hearing has its counterpart as off-site listening in CI users. If it does, methods that employ a single-electrode masker may provide an inaccurate measure of spatial selectivity. If it does not, methods that employ a single-electrode masker are likely to provide the most straightforward way of measuring spatial selectivity in each of the apical and basal directions. In Experiment 2, the slopes of MLTs measured with dual-electrode masker were compared against MLTs measured with a single-electrode masker. Using a single-electrode masker may allow off-site listening through the portion of the cochlea contralateral to probe and masker. A PTC with an artificially steeper slope is typically found in normal-hearing subjects who can listen off-frequency because this phenomenon can reduce the masking produced by off-frequency maskers. If, however, there is no off-site listening, the slopes for the single- and dual-masker functions should be similar. Specifically, to a first approximation, the MLT for a dual-electrode masker should correspond to that for the more effective of the two constituent single-electrode maskers.
Methods
MLTs were measured using a single-electrode masker (for positions both basal and apical to the probe electrode) and compared with the two-masker data shown in Figure 3. The procedure and methods were the same as for two maskers described in Experiment 1. Subjects ME1, ME2, ME4, ME5, AB1, and AB2, who had also participated in Experiment 1, took part. There were a maximum of nine conditions for each subject, corresponding to Δx values of ±4, ±3, ±2, ±1, and 0.
Results and Discussion
Figure 4 reports MLTs for dual-electrode (black symbols, redrawn from Figure 3) and single-electrode (blue, open symbols) maskers. Overall, MLTs for both single and dual maskers follow similar patterns within subject. To assess differences in the curve with respect to off-site listening, we computed the MLT for the more effective single masker condition, sMLT. For each apical and basal masker with the same absolute value of Δx, the sMLT was defined as the masker that produced the lowest MLT. The use of sMLT is necessary to factor out the contribution of ineffective masking electrodes to the sharpening of the PTC measured for dual maskers. If, for instance, an apical electrode produces little masking in the masking pair, the slope of the dual-masker PTC will be steeper than the slope of the PTC for the less effective single masker and steeper than the average of the slopes for maskers apical and basal to the probe. Note also that, in principle, the more effective masker (apical vs. basal) could be different for different values of Δx, and so the sMLT was selected from the two maskers separately for each value of Δx. Figure 5 shows sMLTs in red, shifted vertically so as to coincide with the dual-masker curve (black) at Δx = 0.
Figure 4.
Psychophysical tuning curve for dual (filled circles) and single (open circles) electrode masker from four Med-El (top row) and two AB (bottom row) cochlear implant users. Up-facing and down-facing triangles are absolute thresholds (T) and most comfortable levels (C) for the dual masker, respectively; right-facing and left-facing triangles are absolute thresholds for basal and apical electrodes, respectively. AB = Advanced Bionics; dB = decibel; MLT = masking level at threshold.
Figure 5.
MLTs for dual-electrode (filled circles) and more effective single-electrode (open circles) maskers. Data for dual masker are replotted from Figure 4 and are shown only for Δx values where MLT measurements for single masker were available. Gray symbols are MLTs for the less effective single masker obtained from basal (left-facing triangles) or apical (right-facing triangles) measurements. Single-masker MLTs were vertically shifted to match dual-masker MLTs at Δx = 0. MLT = masking level at threshold; AB = Advanced Bionics; dB = decibel; ME = Med-El.
For each subject, the normalized MLTs and sMLT repeated measurements were compared for statistical significance using a univariate analysis of variance. The MLT values for Δx = 0 were not included in the analysis, as they were used for normalization purposes. No statistically significant effect was found for masker type (dual vs. more effective single masker) for any subject. A main effect of Δx was obtained for all subjects except ME2 (p = .055, who showed a flat PTC in Figure 3) and AB1 (p = .08, who showed a “hockey-stick” function). The slopes for dual and more effective single masker were estimated using a linear regression. A z test1 of the differences between the regression coefficients of the two functions for each subject also revealed no statistical difference (p > .3). In addition, no statistically significant difference was obtained by computing single- and dual-masker widths at 75% of total masking2 (p = .13; t = −1.8; df = 5).
The similarity of the slopes for both conditions suggests the absence of “type 1” off-site listening. This finding differs from the report by Dingemanse et al. (2006), who concluded that dual-electrode masking widths were statistically significantly greater than for single-electrode maskers. However, as discussed further in the General Discussion section, our reanalysis of their data revealed no significant difference in the widths of the dual and more effective single masker excitation patterns.
Experiment 3: Comparison of Two Measures of Spatial Selectivity
Rationale
Experiment 3 was designed to elucidate an instance where the detection of an anomaly in the spatial selectivity of a CI user (e.g., a dead region or a distant electrode) can depend upon the method used. Subject AB1’s results from Experiment 1 are consistent with such an anomaly; for descriptive purposes, we will refer to this as an instance of a dead region at the probe place. Masked probe levels at threshold (MPLTs) were measured for subject AB1 using Method B (described in the Introduction section), where the maskers were fixed at equal loudness levels, and compared with MLTs measured in Experiment 1 (Method C). If both methods are equally suitable measures of spatial selectivity, a qualitatively similar masking pattern should be obtained with the two approaches.
Methods
MPLTs for AB1 were measured using the same protocol and stimuli as for Experiment 1, but the procedure was different. Instead of adaptively changing the current level applied to both maskers while keeping fixed the current on the probe electrode (Method C), the loudness of each masker was roughly equated by setting its current to 40% of its DR in dBs, and the level on the probe was adaptively reduced until it was no longer detectable (Method B). By setting individual electrodes at 40% DR, the dual-electrode maskers were roughly at most comfortable level for the four conditions tested in this experiment (Δx = 0, 1, 2, and 3), as reported by the subject on a loudness chart. Absolute detection thresholds (T levels) for masker signals were obtained by averaging four adaptive runs, as for the probe signal described in Experiment 1. Approximate most comfortable (C) and maximum loudness (U) levels were also measured using a loudness chart.
Results and Discussion
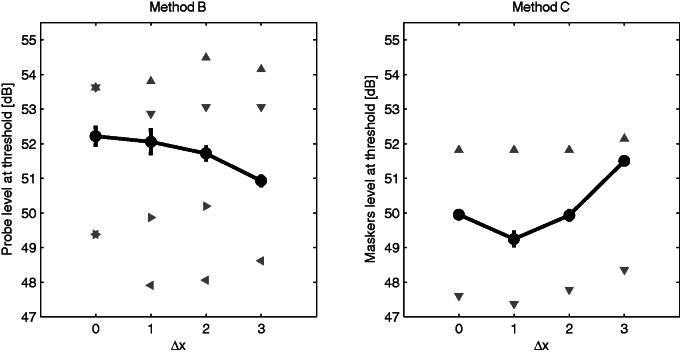
MPLTs and MLTs for AB1 are shown in the left (Method B) and right (Method C) parts of Figure 6. The T, C, and current values for different %DR are reported in Table 2.
Figure 6.
Spatial selectivity profiles for subject AB1 as calculated with Methods B and C described in Experiment 3 and briefly as follows. Method B (left): Probe level is adaptively adjusted while keeping masker level at 40% of the channel’s individual DR; T and C levels for apical single maskers are reported as down-facing and left-facing triangles, whereas T and C levels for basal single maskers are shown with up-facing and right-facing triangles. Method C (right): The probe level is kept fixed, and the current applied on masking electrodes is adaptively adjusted to just mask the probe. T and C levels for dual-electrode maskers are reported as up-facing and down-facing triangles. AB = Advanced Bionics; dB = decibel; DR = dynamic range.
Table 2.
Current Values for AB1 Measured in Experiment 3 Expressed in Units of dB Relative to 1 μA.
| MLT |
MPLT |
T |
||||||
|---|---|---|---|---|---|---|---|---|
| MΔ− | P | MΔ+ | MΔ− | P | MΔ+ | TΔ− | TΔ+ | |
| Δx = 0 | 50.2 | (51.3) | 50.2 | (51.1) | 52.2 | (51.1) | 49.4 | 49.4 |
| Δx = 1 | 49.2 | (51.3) | 49.2 | (49.9) | 52.1 | (51.5) | 47.9 | 49.9 |
| Δx = 2 | 50.0 | (51.3) | 50.0 | (50.1) | 51.7 | (49.9) | 48.0 | 50.2 |
| Δx = 3 | 51.6 | (51.3) | 51.6 | (51.6) | 50.9 | (52.3) | 48.6 | 50.9 |
Note. MLT and MPLT are the masker level and the probe level at threshold, respectively. MΔ− indicates the level of the masker electrode more apical to the probe, whereas MΔ+ indicates the level of masker more basal to the probe electrode. P indicates the level of the probe electrode. TΔ− and TΔ+ are the absolute thresholds for the masker alone apical to, or basal to the probe electrode. Values in parenthesis were not varied during the adaptive procedure, whereas values in bold represent thresholds measured adaptively. AB = Advanced Bionics; dB = decibel.
As previously stated, the “hockey-stick” function obtained using masking patterns and equal-current maskers (Method C) is consistent with an instance of a dead region. Here, a dual-electrode masker placed on Δx = ±1 produced more masking than when both probe and maskers were on the same channel (Δx = 0). Inspection of the T levels (reported as TΔ− and TΔ+ in Table 2) suggests that the probe may have been detected by neurons near the electrode at Δx = −1, where thresholds are lower than at Δx = 0. For Method C (the PTC), this would have led to the MLT for the dual masker to be lower for maskers at Δx = ±1 than at Δx = 0. However, for Method B, MPLTs would not be elevated for those maskers because, as shown in Table 2, the level of the masker at Δx = −1 (reported as MΔ− in the column MPLT) was set to a lower value than at Δx = 0.
An alternative interpretation is that the probe really was detected at electrodes close to the probe electrode, but that the electrode at Δx = −1 was closer to the modiolus than was the electrode at Δx = 0. According to this hypothesis, the difference in T between those two electrodes was due not to differences in neural survival but to differences in lateral position within the cochlea. In principle, this could have been responsible for the “hockey-stick” PTCs observed in Experiments 1 and 2 and elsewhere (Bierer & Faulkner, 2010). However, for this to have happened, the electrode at Δx = −1 would have had to be not only closer to the modiolus than the electrode at Δx = 0 but also closer to the neurons near Δx = 0 than was the electrode at Δx = 0. That is, any difference in lateral distance from the modiolus would have had to be large enough to overwhelm the 1.1-mm longitudinal separation between the two electrodes.
Experiment 4: Facilitation Effect for Different Interpulse Gaps
Rationale
Experiment 4 investigated the effect of the gap between the masker and probe pulses on masking. We varied the gap between the masker and probe pulses (masker–probe gap [MPG]) while keeping the number of probe and masker pulses fixed across conditions. This was done to examine the effects of charge interaction separately from the effect of overall pulse rate. Our approach differs from some previous studies that varied the pulse rate and in which the differences in masker–probe pulses gap covaried with the number of pulses presented (Bonnet, Boermans, Avenarius, Briaire, & Frijns, 2012; Pfingst, De Haan, & Holloway, 1991). At shorter MPGs, the probe and masker pulses are hypothesized to interact according to two main mechanisms: refractoriness, which will increase masking, and charge summation, which may reduce it.
Methods
Four Med-El users took part: ME1, ME2, ME3, and ME5. The same procedure adopted for Experiment 2 with a single-electrode masker was implemented here: Maskers and the probes were 368 and 48 ms in duration, respectively, and were interleaved. The masker and probe were presented on the same electrode (Δx = 0) and had individual pulse rate of 125 pps. Fourteen interpulse delays between the masker and probe pulses (MPG) values were tested (μs): ±30, ±60, +120, +240, ±480, ±960, ±1,920, and ±4,000. Positive MPG values indicate gaps between the end of the masker pulse and the beginning of the probe pulse; conversely, negative MPGs indicate gaps between the end of the probe pulse and the beginning of the masker pulse. The duration of the individual phases in the biphasic pulses was 120 μs for ME4 and 60 μs for the other subjects.
Results and Discussion
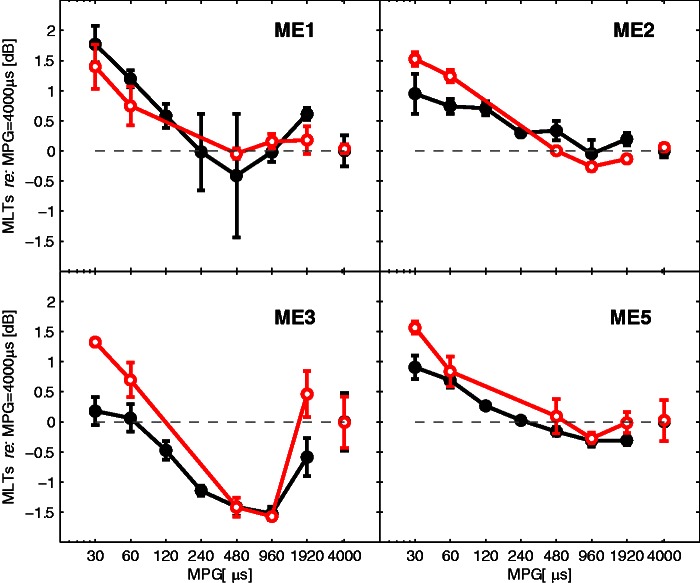
Figure 7 shows the MLTs for positive (black, filled symbols) and negative (red, open symbols) MPGs measured in the four subjects, relative to MLT value at an MPG of 4,000 μs. The MLTs at the 4,000 µs were (dB re: 1 µA): 56.0 (Subject ME1), 55.6 (ME2), 52.7 (ME3), and 55.5 (ME5). At a 4,000-μs MPG, any charge interaction between pulses would be expected to be minimal (Bierer & Middlebrooks, 2004; Nelson & Donaldson, 2001). Positive relative MLTs indicate conditions where pulse interactions produced lesser masking relative to the 4,000-μs condition, and higher current levels were necessary to mask the probe. Conversely, portions of the curve where the relative MLT is negative indicate that more masking was produced and that smaller current levels were required to mask the probe.
Figure 7.
MLTs as a function of masker–probe gaps relative to the MLT for the reference condition (Δx = 4,000 μs) for four Med-El subjects (Experiment 4). MLTs below zero (dotted line) are indicative of a condition that produced more masking relative to the reference condition; positive MLTs indicate conditions that produced less masking. MLTs for values of MPG where the probe pulse followed the masker pulse are shown as filled black symbols; conversely, MLTs for negative MPG (where the probe pulse preceded the masker pulse) are shown as open red symbols. MLT = masking level at threshold; MPG = masker–probe gap; dB = decibel; ME = Med-El.
The masking trends shown in Figure 7 are generally similar for the four subjects. Positive relative MLTs are found at shorter MPGs. This is consistent with the “neural facilitation” phenomenon due to charge summation that we described earlier. This reduction in masker effectiveness could also explain the finding in Azadpour et al. (2013) of negligible masking for high stimulation rates. Although we tested only at Δx = 0, it is reasonable to assume that current spread along the cochlea would allow a similar facilitative effect to occur at values of Δx other than zero, although a higher masker level would presumably be required to compensate for the increased distance between masker and probe electrodes (Bierer & Middlebrooks, 2004).
MLTs decreased as MPG was increased from 20 µs to about 480 to 960 µs, corresponding to an increase in masking effectiveness. This is consistent with the beneficial effect of facilitation decreasing over this range, at a rate faster than any recovery from refractoriness. MLTs increased for conditions where MPG was increased beyond 960 µs, consistent with the change in masker effectiveness over this range being dominated by a recovery from refractoriness.
Experiment 5: Duration Discrimination and Confusion Effects in Forward Masking
Rationale
In normal hearing, forward masking can be artificially increased when there are insufficient quality difference cues between the masker and probe. An example of this occurs with a narrowband masker followed, after a zero or short silent interval, by a brief tone pip whose frequency is the same as the masker center frequency. Under such circumstances, the probe is not heard as a separate object, but as a continuation of the masker, and masking can be reduced by the presence of an off-frequency or contralateral copy of the masker (Moore & Glasberg, 1982). One factor that determines the extent to which this confusion effect influences forward-masking paradigms depends on the listener’s sensitivity to differences in the masker duration. Specifically, it is more likely to have a large effect when the minimum detectable duration difference is longer than the duration of the probe-plus-silent gap. Experiment 5 measured duration discrimination using stimuli based on those used in the forward-masking experiment described by Dingemanse et al. (2006). In addition, an electrode remote from the probe channel was used to cue the detection of the probe, to determine whether this would reduce confusion effects and thereby reduce masking. Adding a remote masker to cue detection of the probe has been shown to reduce confusion effect in studies with normal-hearing listeners (Moore & Glasberg, 1982).
Methods
The same four Med-El subjects from Experiment 4 were recruited. The stimuli were chosen to replicate those used in the study by Dingemanse et al. (2006). The stimulation rate was 312 pps, and pulses were biphasic with 30-µs duration per phase (60 µs for ME4). A single 300-ms masker stimulus was immediately followed by a probe that could have one of seven possible durations (ms): 5, 10, 20, 30, 40, 80, 160, and 320. The method of constant stimuli, combined with a two-interval two-alternative forced-choice task, was used, where the probe occurred in only one of the two intervals on each trial. Both masker and probe signals were presented on Electrode 7 (Δx = 0) and had the same amplitude. The amplitude roughly corresponded to the subject’s most comfortable loudness for a 20-ms probe signal and was determined prior to the experiment. In each probe-duration condition, the subjects were told that one interval could appear longer in duration but that they were encouraged to employ whichever cue they found more salient to perform the task. A number of training runs were administered with feedback until the subject felt comfortable with the requirements of the task. Four runs of 50 trials each were presented per probe-duration condition, and the final percent recognition score was averaged across runs. Data were fitted with sigmoidal curves and plotted in Figure 8.
Figure 8.
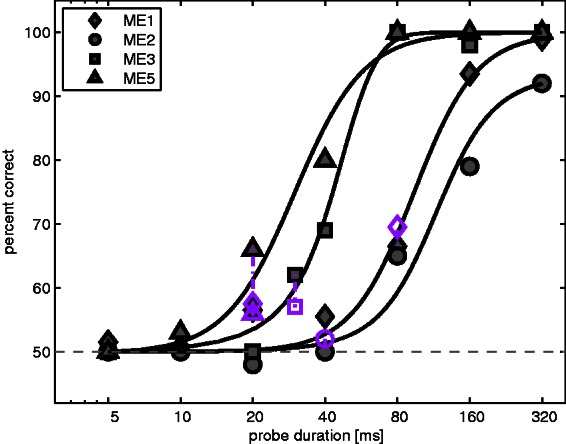
Psychometric functions for four Med-El subjects, as described in Experiment 5. Open symbols indicate scores for conditions with a remote masker (Electrode 11) stimulated at soft level to aid detection of the probe onset. Open symbols are vertically connected with the subject- and probe duration-matched condition without remote masker. The levels applied to the remote masker were (dB re: 1 µA) as follows: 53.5 (ME1), 55.1 (ME2), 39.5 (ME3), and 45.5 (ME5). dB = decibel; ME = Med-El.
Finally, the effect of a remote electrode to cue the detection of the probe was investigated as follows. For each subject, Electrode 11 (the “remote cue”) was stimulated at soft loudness and for duration equal to that of the main, forward masker. The level of the remote cue was determined by repeatedly increasing the current level from zero and asking the subjects to report when a sound additional to the masker-plus-probe was clearly detectable. During the test, subjects were told that the same additional sound was present in the mixture and that it could indicate when the probe might occur. This remote cue was identical in the standard and the signal intervals. Because it could be used to identify the onset of the probe signal, it was hypothesized that it could improve detection by reducing the confusion effect. One or more of these remote-cue conditions were measured choosing duration values between floor and ceiling within each sigmoidal fitting.
Results and Discussion
Figure 8 shows the scores for the four subjects together with sigmoidal fitting obtained with a least-squares error minimization criterion. The just-noticeable differences at 75% correct are (ms) as follows: 94 (subject ME1), 126 (ME2), 43 (ME3), and 30 (ME5). In a study with normal-hearing listeners, Abel (1972) reported duration just-noticeable differences (75% correct) of approximately 35 ms for 300-ms reference stimuli, roughly in line with results of the present study for CI users ME3 and ME5. Subjects ME1 and ME2, conversely, showed much worse discrimination abilities.
The percent correct for the condition where a remote “cue” was added is shown with open symbols and is connected through vertical lines to the score obtained without the remote cue. These values were not consistently higher than those obtained without a cue; hence, we have no evidence that this cue effectively reduced any confusion effects. In the absence of a cue, there was no way in which the probe could be heard as a separate perceptual object from the masker. Hence, the absence of a cueing effect means either that masking was dominated by other factors, such as adaptation or refractoriness, or that listeners were unable to exploit differences in the masker and probe excitation patterns to reduce their confusion. Both interpretations suggest that for our stimuli, and hence those of Dingemanse et al. (2006), it is unlikely that the estimates of spatial selectivity were unduly influenced by confusion effects. It remains possible, however, that confusion effects could influence measures of spatial selectivity with different stimulus parameters.
General Discussion
Off-Site Listening
Results from Experiment 2 provided no evidence of type 1 off-site listening. This is consistent with some findings in literature and in contrast with others.
Fielden et al. (2013) found that using dual-electrode maskers in forward masking led to masking patterns comparable with those shown with single-electrode masker paradigms (Bierer & Faulkner, 2010; Landsberger, Padilla, & Srinivasan, 2012; Zhu, Tang, Zeng, Guan, & Ye, 2012).
Conversely, Dingemanse et al. (2006) reported evidence for off-site listening. They provided us with the raw data from their Figure 4, which we then reanalyzed by computing half widths for the dual-electrode and more effective single-electrode masker. This differs from their approach, as briefly described later. In Dingemanse et al. (2006), the half width of the masking profile (probe threshold as a function of masker–probe distance; Method B) was defined as the electrode distance between masker and probe where the threshold shift is half the maximum shift. The full width of the masking profile was calculated as the sum of the apical and basal half widths, or twice the half width estimated for the dual-electrode condition. This computation could not be applied for the apical shifts of subject S3, and the basal half width was used instead.
Our reanalysis of their data followed a similar approach to estimate the half width of the more effective single-electrode threshold shift. This was obtained by selecting either the apical or the basal threshold shift for each of the four masker–probe distances, whichever was larger. Larger threshold shifts are indicative of more masking. For subject S3, because the half width of the threshold shift due to the more effective single masker could not be computed, the half width was set to the maximum masker–probe distance tested, 3. This is a conservative choice and larger half widths could be expected if more masker–probe distances had been tested. The half widths for more effective single masker and those obtained for dual masker were then compared for statistical significance. Both analyses excluded data from subject S4. No statistically significant difference was obtained following our method (p = .27; t = −1.29; df = 4).
Finally, we reanalyzed Dingemanse et al.’s data after conversion to a dB scale. The use of linear scale to express threshold shifts may not be ideal, as discussed in the present article and in McKay (2012), and does not take into account across-subject variability caused by differences in residual forward masking (Fielden et al., 2013). Nonetheless, similar patterns of results as those obtained with computation in linear units of amperes were obtained.
The lack of “type 1” off-site listening in CI users could be a consequence of the different excitation pattern in acoustic and electric hearing. It is possible that excitation patterns with shallower off-site decays (or steeper tips) in CIs reduce the maximum difference in signal-to-noise ratio that can be found relative to the center of the excitation pattern. If the better signal-to-noise ratio cannot be found off-site, CI users will be expected to gain no advantage from listening through portions of the cochlea contralateral to the masker.
General Considerations When Choosing Measures of Spatial Selectivity
Experiments 1 and 2 described a method that reduced the influence of some of the potential confounds that can influence measures of spatial selectivity. However, every method will have its own limitations, and, as argued later, the best choice of method will depend on the question that one is trying to answer. We illustrate the limitations of different methods with respect to two important choices, namely forward versus interleaved masking, and masked excitation patterns (Method A) versus PTCs (Method C).
Forward versus interleaved masking
Interleaved masking, arguably, most closely resembles the situation experienced by CI users when listening through their everyday speech-processing strategy. However, as shown in Experiment 4, masking in these circumstances can be influenced by charge interactions when the MPG is less than about 1 ms, with the largest effects observed for MPGs below 240 µs. Charge interactions might also account for the results of a single-pulse forward-masking experiment by Nelson and Donaldson (2001), in which 19 out of 24 electrodes tested showed nonmonotonicity in the recovery functions for small interpulse intervals. For interleaved masking, our results suggest that, to obtain a measure of selectivity that is uncontaminated by such effects, and with a single masker, it is necessary to use a pulse rate lower than about 1,000 pps, which would allow for an MPG of 500 µs. This is roughly consistent with the conclusions of Kwon and van den Honert (2009), who argued that charge summation had a substantial effect on the growth of loudness functions measured with interleaved masking at rates above, but not below, 500 pps. Certainly, in everyday situations, multiple electrodes will be stimulated concurrently, and the interpulse intervals between successive pulses will often be considerably less than 500 µs. This, in turn, means that charge interactions may cause high-rate strategies to distort the representation of spectral shape because the amount of charge interaction between two electrodes at a given place in the cochlea will depend on the relative amount of charge produced by those electrodes at that place, and this in turn will depend on the distance between the two electrodes. When multiple electrodes are stimulated, the interactions may be different from those obtained with a single masker and probe and may depend on the relative levels of the pulses, which again may differ from those obtained in a masking experiment. The researcher may therefore decide to avoid such charge interactions to obtain a “clean” measure of spatial selectivity. As noted earlier, this will limit the range of pulse rates that can be used.
An obvious alternative is to use a forward-masking paradigm, which has the advantage of allowing the experimenter to use high pulse rates whilst avoiding charge interactions between the masker and probe pulses. (Of course, the masker pulses may well interact with each other, but they will do so in a way that is largely independent of the spatial separation between the masker and probe.) McKay (2012) has discussed a number of complications in the interpretation of forward-masking experiments, such as differences in the rate of decay of forward masking from different parts of a masker’s excitation pattern. However, at least for the measurement of PTCs (Method A), this has only a small effect on measures of spatial selectivity. This is because, for the PTC, the amount of masker excitation at the probe place when the masker is at its MLT is assumed to be independent of masker position. A potentially greater limitation is, as we have pointed out, the likely existence of confusion effects, whereby the probe is perceived not as a separate auditory object but as a continuation of the masker. The crucial question is whether these effects decrease with increases in spatial separation between the masker and probe; if so, they could overestimate spatial selectivity. Experiment 5 found no evidence that adding a cue to the masker can reduce confusion effects, but it is possible that a reduction in such effects could occur with other stimuli, for example with those that produce more focussed excitation patterns. In addition, it possible that a difference in the positions of the masker and probe excitation pattern peaks provides a more effective cue than the addition of low-level stimulation of a remote electrode. If so, then our use of an off-site cue may not have effectively simulated the release from confusion effects that may occur in a masking paradigm when the masker is presented on a different electrode to the probe. However, even when confusion effects do occur, this may not matter when the goal is to compare selectivity between two different forms of stimulation, such as tripolar versus monopolar modes or high versus low rates. In that case, the method will still correctly identify the stimulus that results in sharper spatial selectivity, even though the size of the differences between the two stimuli may be exaggerated.
To summarize, when the experimental question does not require pulse rates faster than about 1,000 pps, and when there is only one masker, the interleaved masking paradigm described in Experiments 1 and 2 can provide an accurate measure of spatial selectivity. When higher pulse rates are required, forward masking should be used. If the aim is to use forward masking to provide a quantitative measure of selectivity, rather than simply to compare two stimuli, it may be prudent either to limit or to check for confusion effects. The use of different pulse rates for the masker and probe (Macherey et al., 2010), or of a long MPG, may reduce confusion effects. Adding an off-place “cue” provides one way of checking for the influence of such effects, although it should be noted that we have not demonstrated the effectiveness of such a cue.
Masked excitation patterns versus PTCs
Experiments 1 and 2 measured spatial selectivity using the PTC (Method C). A commonly used alternative, the masked excitation pattern (Method A), suffers from a number of complications. The aim of the masked excitation pattern is to measure the amount of excitation produced by the masker at different positions along the cochlea. One issue concerns the way in which the efficiency of probe detection depends on the absolute level of neural excitation. In normal, acoustic hearing, it is assumed that Weber’s law holds for each part of the excitation pattern, or “frequency channel.” Although some exceptions have been observed, this assumption has generally been validated by a large amount of psychophysical data and computational modeling (e.g., Florentine & Buus, 1981). Hence, in an acoustic masking experiment where the probe is detected from a very restricted region of the excitation pattern, the masked threshold of the probe can be taken as a good estimate of the amount of masker excitation at the probe place.
For CIs, however, Weber’s law may not hold for a given cochlear place. For example, Nelson, Schmitz, Donaldson, Viemeister, and Javel (1996) reported that Weber fractions (10 log (ΔI/I)) for the detection of level differences decreased by an average of 8 dB across the DR and suggested that this was due to a shift in the site of auditory-nerve excitation from peripheral processes, where rate-level functions are shallow, to the central axons, where those functions are steeper. A shift from peripheral to central sites of activation has also been proposed as an explanation for the “kneepoint” level, above which there is a marked increase in the rate of loudness growth with increasing current level (McKay, Henshall, Farrell, & McDermott, 2003). In the context of a masked excitation pattern, this might reduce masked thresholds for electrodes close to the peak of the excitation pattern (where the overall excitation level is high) and increase those for electrodes on the skirts. This flattens the measured excitation pattern. In addition, when comparing masked excitation patterns for two different masker types (e.g., monopolar vs. bipolar stimuli) then, if one stimulus produces more masking overall, it will not necessarily be valid to compare the two patterns simply by scaling one relative to the other (cf. McKay, 2012). Nelson et al. (1996) also found that Weber fractions could differ substantially across electrodes, which is another factor that could distort masked excitation patterns as a measure of spatial selectivity.
In contrast to masked excitation patterns, PTCs measure masking at a fixed probe position, thereby avoiding complications arising from different “detector efficiency” at different cochlear sites. Furthermore, because the probe level is fixed, the excitation at the probe place, produced by each masker at its MLT, is the same for all maskers, avoiding complications associated with differences in detector efficiency across level. We therefore believe that PTCs provide the most straightforward measure of spatial selectivity in CI for a given current level of the probe. While this level is generally in the range of those used in everyday continuous interleaved stimulations, it is possible that different spatial selectivity profiles are produced by different probe levels. It may well be that, to process real-world sounds such as speech, the listener needs good spatial selectivity at higher overall levels of excitation—such as occurs near the formants. For this purpose, masked excitation patterns might be more appropriate and can be useful provided that one is primarily interested in determining which of two masker types produces the more selective excitation pattern, rather than in obtaining an “absolute” measure of selectivity. To do so, the two masker levels should be adjusted so that they produce the same masked threshold for a probe on the same electrode as the masker (Macherey et al., 2010). The masker for which detection thresholds decays more steeply with increasing Δx will then be the one that produces the sharper excitation pattern.
Clinical Relevance
Overall, the measure proposed in Experiment 1 can provide information about the spatial selectivity of channels for CI users. It has a potential application as a clinical tool for identification of electrodes with poor electrode-to-neuron interface and subsequent development of appropriate patient-specific stimulation strategies. At present, it is time-consuming, but the inclusion of fast techniques based on Bekesy tracking (e.g., Bierer, Bierer, Kreft, & Oxenham, 2015a) could add clinical relevance to this approach.
Spatial selectivity has been used as a measure of goodness of the electrode-to-neuron interface, which has led the design of patient-based stimulation strategies. In Noble, Gifford, Hedley-Williams, Dawant, and Labadie (2014), for instance, computed tomography scans were used to deactivate electrodes that did not excite a unique portion of the cochlea. This led to a small but significant improvement in performance across a large population of CI users. Computed tomography scans, however, are not available for all subjects, and a behavioral measure of spatial selectivity could provide the information necessary to design the appropriate stimulation strategy.
Acknowledgments
The authors thank Dr. Jeroen Briaire for generously providing us with the raw data from the article by Dingemanse et al. (2006).
Notes
A modified z test was used as discussed in Paternoster, Brame, Mazerolle, and Piquero (1998).
For PTC data, McKay (2012) suggests to compute widths as percent of the total amount of masking. A different computation of the widths may be possible for thresholds shifts, as in Dingemanse et al. 2006.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Medical Research Council (program number: MC-A060-5PQ70). SC was funded by Action on Hearing Loss (grant number: MC-A060-5PQ75).
References
- Abel S. M. (1972) Duration discrimination of noise and tone bursts. The Journal of the Acoustical Society of America 51: 1219–1223. [DOI] [PubMed] [Google Scholar]
- Azadpour M., AlJasser A., McKay C. M. (2013) Place specificity measured in forward and interleaved masking in cochlear implants. The Journal of the Acoustical Society of America 134: EL314–EL320. [DOI] [PubMed] [Google Scholar]
- Bierer J. A., Bierer S. M., Kreft H. A., Oxenham A. J. (2015a) A fast method for measuring psychophysical thresholds across the cochlear implant array. Trends in Hearing 19: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer J. A., Deeks J. M., Billig A. J., Carlyon R. P. (2015b) Comparison of signal and gap-detection thresholds for focused and broad cochlear implant electrode configurations. Journal of the Association for Research in Otolaryngology 16: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer J. A., Faulkner K. F. (2010) Identifying cochlear implant channels with poor electrode-neuron interface: Electrically-evoked auditory brainstem responses measured with the partial tripolar configuration. Ear and Hearing 32: 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer J. A., Middlebrooks J. C. (2004) Cortical responses to cochlear implant stimulation: Channel interactions. Journal of the Association for Research in Otolaryngology 5: 32–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet R. M., Boermans P.-P. B. M., Avenarius O. F., Briaire J. J., Frijns J. H. M. (2012) Effects of pulse width, pulse rate and paired electrode stimulation on psychophysical measures of dynamic range and speech recognition in cochlear implants. Ear and Hearing 33: 489–496. [DOI] [PubMed] [Google Scholar]
- Cohen L. T., Busby P. A., Clark G. M. (1996) Cochlear implant place psychophysics. Audiology and Neurotology 1: 278–292. [DOI] [PubMed] [Google Scholar]
- Cohen L. T., Richardson L. M., Saunders E., Cowan R. S. C. (2003) Spatial spread of neural excitation in cochlear implant recipients: Comparison of improved ECAP method and psychophysical forward masking. Hearing Research 179: 72–87. [DOI] [PubMed] [Google Scholar]
- de Balthasar C., Boëx C., Cosendai G., Valentini G., Sigrist A., Pelizzone M. (2003) Channel interactions with high-rate biphasic electrical stimulation in cochlear implant subjects. Hearing Research 182: 77–87. [DOI] [PubMed] [Google Scholar]
- Dingemanse J. G., Frijns J. H. M., Briaire J. J. (2006) Psychophysical assessment of spatial spread of excitation in electrical hearing with single and dual electrode contact maskers. Ear and Hearing 27: 645–657. [DOI] [PubMed] [Google Scholar]
- Fielden C. A., Kluk K., McKay C. M. (2013) Place specificity of monopolar and tripolar stimuli in cochlear implants: The influence of residual masking. The Journal of the Acoustical Society of America 133: 4109–4123. [DOI] [PubMed] [Google Scholar]
- Finley C. C., Holden T. A., Holden L. K., Whiting B. R., Chole R. A., Neely G. J., Skinner M. W. (2008) Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otology & Neurotology 29: 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentine M., Buus S. (1981) An excitation-pattern model for intensity discrimination. The Journal of the Acoustical Society of America 70: 1646–1654. [Google Scholar]
- Frijns J. H. M., Briaire J. J., Grote J. J. (2001) The importance of human cochlear anatomy for the results of modiolus-hugging multichannel cochlear implants. Otology & Neurotology 22: 340–349. [DOI] [PubMed] [Google Scholar]
- Garadat S. N., Pfingst B. E. (2011) Relationship between gap detection thresholds and loudness in cochlear-implant users. Hearing Research 275: 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B. J., van den Honert C. (2006) Effect of electrode configuration on psychophysical forward masking in cochlear implant listeners. The Journal of the Acoustical Society of America 119: 2994–3002. [DOI] [PubMed] [Google Scholar]
- Kwon B. J., van den, Honert C. (2009) Spatial and temporal effects of interleaved masking in cochlear implants. Journal of the Association for Research in Otolaryngology 10: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberger D. M., Padilla M., Srinivasan A. G. (2012) Reducing current spread using current focusing in cochlear implant users. Hearing Research 284: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H. (1971) Transformed up-down methods in psychoacoustics. The Journal of the Acoustical Society of America 49: 467–477. [PubMed] [Google Scholar]
- Macherey O., van Wieringen A., Carlyon R. P., Dhooge I., Wouters J. (2010) Forward-masking patterns produced by symmetric and asymmetric pulse shapes in electric hearing. The Journal of the Acoustical Society of America 127: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay C. M. (2012) Forward masking as a method of measuring place specificity of neural excitation in cochlear implants: A review of methods and interpretation. The Journal of the Acoustical Society of America 131: 2209–2224. [DOI] [PubMed] [Google Scholar]
- McKay C. M., Henshall K. R., Farrell R. J., McDermott H. J. (2003) A practical method of predicting the loudness of complex electrical stimuli. The Journal of the Acoustical Society of America 113: 2054–2063. [DOI] [PubMed] [Google Scholar]
- Middlebrooks J. C. (2004) Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. The Journal of the Acoustical Society of America 116: 452–468. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J. (1978) Psychophysical tuning curves measured in simultaneous and forward masking. The Journal of the Acoustical Society of America 63: 524–532. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Alcántara J. I. (2001) The use of psychophysical tuning curves to explore dead regions in the cochlea. Ear and Hearing 22: 268–278. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Alcántara J. I., Dau T. (1998) Masking patterns for sinusoidal and narrow-band noise maskers. The Journal of the Acoustical Society of America 104: 1023–1038. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Glasberg B. R. (1982) Contralateral and ipsilateral cueing in forward masking. The Journal of the Acoustical Society of America 71: 942–945. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Glasberg B. R. (1983) Suggested formulae for calculating auditory-filter bandwidths and excitation patterns. The Journal of the Acoustical Society of America 74: 750–753. [DOI] [PubMed] [Google Scholar]
- Neff D. L. (1985) Stimulus parameters governing confusion effects in forward masking. The Journal of the Acoustical Society of America 78: 1966–1976. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Donaldson G. S. (2001) Psychophysical recovery from single-pulse forward masking in electric hearing. The Journal of the Acoustical Society of America 109: 2921–2933. [DOI] [PubMed] [Google Scholar]
- Nelson D. A., Donaldson G. S., Kreft H. A. (2008) Forward-masked spatial tuning curves in cochlear implant users. The Journal of the Acoustical Society of America 123: 1522–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. A., Schmitz J. L., Donaldson G. S., Viemeister N. F., Javel E. (1996) Intensity discrimination as a function of stimulus level with electric stimulation. The Journal of the Acoustical Society of America 100: 2393–2414. [DOI] [PubMed] [Google Scholar]
- Noble J. H., Gifford R. H., Hedley-Williams A. J., Dawant B. M., Labadie R. F. (2014) Clinical evaluation of an image-guided cochlear implant programming strategy. Audiology and Neurotology 19: 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham A. J., Plack C. J. (1998) Suppression and the upward spread of masking. The Journal of the Acoustical Society of America 104: 3500–3510. [DOI] [PubMed] [Google Scholar]
- Paternoster R., Brame R., Mazerolle P., Piquero A. (1998) Using the correct statistical test for the equality of regression coefficients. Criminology 36: 859–866. [Google Scholar]
- Pfingst B. E., De Haan D. R., Holloway L. A. (1991) Stimulus features affecting psychophysical detection thresholds for electrical stimulation of the cochlea. I: Phase duration and stimulus duration. The Journal of the Acoustical Society of America 90: 1857–1866. [DOI] [PubMed] [Google Scholar]
- Stypulkowski P., van den Honert C. (1984) Physiological properties of the electrically stimulated auditory nerve I. Compound action potential recordings. Hearing Research 14: 205–223. [DOI] [PubMed] [Google Scholar]
- Verschuure J. (1981) Pulsation patterns and nonlinearity of auditory tuning: I. Psychophysical results. Acta Acustica united with Acustica 49: 288–295. [Google Scholar]
- Zhu Z., Tang Q., Zeng F.-G., Guan T., Ye D. (2012) Cochlear-implant spatial selectivity with monopolar, bipolar and tripolar stimulation. Hearing Research 283: 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]