Abstract
Roux-en-Y gastric bypass (RYGB) surgery has negative effects on bone, mediated in part by effects on nutrient absorption. Not only can RYGB result in vitamin D malabsorption, but the bypassed duodenum and proximal jejunum are also the predominant sites of active, transcellular, 1,25(OH)2D-mediated calcium (Ca) uptake. However, Ca absorption occurs throughout the intestine, and those who undergo RYGB might maintain sufficient Ca absorption, particularly if vitamin D status and Ca intake are robust. We determined the effects of RYGB on intestinal fractional Ca absorption (FCA) while maintaining ample 25OHD levels (goal ≥30 ng/mL) and Ca intake (1200 mg daily) in a prospective cohort of 33 obese adults (BMI 44.7 ± 7.4 kg/m2). FCA was measured preoperatively and 6 months postoperatively with a dual stable isotope method. Other measures included calciotropic hormones, bone turnover markers, and BMD by DXA and QCT. Mean 6-month weight loss was 32.5 ± 8.4 kg (25.8% ± 5.2% of preoperative weight). FCA decreased from 32.7% ± 14.0% preoperatively to 6.9% ± 3.8% postoperatively (p < 0.0001), despite median (interquartile range) 25OHD levels of 41.0 (33.1 to 48.5) and 36.5 (28.8 to 40.4) ng/mL, respectively. Consistent with the FCA decline, 24-hour urinary Ca decreased, PTH increased, and 1,25(OH)2D increased (p ≤ 0.02). Bone turnover markers increased markedly, areal BMD decreased at the proximal femur, and volumetric BMD decreased at the spine (p < 0.001). Those with lower postoperative FCA had greater increases in serum CTx (ρ = −0.43, p = 0.01). Declines in FCA and BMD were not correlated over the 6 months. In conclusion, FCA decreased dramatically after RYGB, even with most 25OHD levels ≥30 ng/mL and with recommended Ca intake. RYGB patients may need high Ca intake to prevent perturbations in Ca homeostasis, although the approach to Ca supplementation needs further study. Decline in FCA could contribute to the decline in BMD after RYGB, and strategies to avoid long-term skeletal consequences should be investigated.
Keywords: CALCIUM ABSORPTION, VITAMIN D, NUTRITION, BARIATRIC SURGERY, GASTRIC BYPASS SURGERY, BIOCHEMICAL MARKERS OF BONE TURNOVER
Introduction
Obesity is a chronic disease of major proportions: 35% of U.S. adults are obese, and 6% are extremely obese, with BMI ≥40 kg/m2.(1) Because weight loss through diet and exercise is difficult to attain and maintain, there has been an escalating demand for bariatric surgery, including the Roux-en-Y gastric bypass (RYGB). Bariatric surgery produces substantial weight loss, improves comorbidities, and reduces mortality.(2–4)
Despite its metabolic benefits, increasing evidence indicates that RYGB has detrimental effects on the skeleton, including early and sustained increases in bone turnover and declines in BMD.(5,6) As a result, there is a growing concern that those who undergo RYGB will be at risk for osteoporosis and fracture-related disability more frequently and earlier than their peers. Indeed, a higher fracture incidence rate was recently documented among bariatric surgery patients, compared to the general population.(7) The skeletal effects of RYGB are likely multifactorial, and contributing factors may include decreased skeletal loading, changes in levels of fat-secreted hormones or estrogen, body composition changes (ie, fat versus lean mass loss, or visceral versus subcutaneous fat loss), and nutritional factors.(8,9)
Nutritional changes after RYGB are dramatic. The RYGB procedure involves the creation of a small gastric pouch and bypasses of the entire duodenum and the proximal jejunum. As a result, the procedure can cause vitamin D malabsorption, resulting in a high incidence of postoperative vitamin D deficiency and even in osteomalacia in the most severe cases.(10,11) Additionally, the bypassed duodenum and proximal jejunum, which no longer come into contact with food, are usually the predominant sites of active, transcellular, 1,25(OH)2D-mediated calcium (Ca) uptake. However, Ca absorption does occur throughout the intestine,(12) and those who have undergone RYGB might maintain sufficient Ca absorption, particularly if vitamin D status and Ca intake are robust. In a prior study of 21 women undergoing RYGB, fractional Ca absorption (FCA) decreased postoperatively from 36% to 24%. However, over one-half of the women had 25OHD levels <25 ng/mL, and mean postoperative Ca intake was under 1000 mg with large variability.(13)
We determined the effects of RYGB on intestinal FCA in a cohort of severely obese women and men, in the setting of a target 25OHD level of at least 30 ng/mL and Ca intake of 1200 mg daily. We hypothesized that maintaining the robust vitamin D status and controlled Ca intake would mitigate postoperative declines in FCA.
Patients and Methods
Study population
Women and men 25 to 70 years of age were recruited from two academic bariatric surgery centers (the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center), where surgeons used the same standardized surgical approach to RYGB. Potential participants were eligible if they were scheduled for an upcoming RYGB procedure. Eligibility for RYGB at our institutions was based upon 1991 NIH consensus BMI guidelines (BMI >40 kg/m2 or BMI >35 kg/m2 with obesity-related comorbidities)(14) and failure to lose weight with medical management. Women were excluded from the study if they were perimenopausal (defined as last menses >3 months but <5 years ago), and women or men were excluded if they used medications known to impact bone metabolism, including bisphosphonates or teriparatide (in the last year or for >12 months ever), oral glucocorticoids (>5 mg prednisone equivalent daily for >10 days in the last 3 months), and thiazolidinediones. Premenopausal women on stable hormonal contraception, postmenopausal women on stable estrogen/progestin therapy, and men on stable testosterone therapy were eligible. Other exclusion criteria included prior bariatric surgery, estimated glomerular filtration rate <30 mL/min/1.73m2, and weight >350 pounds (the weight limit of the DXA scanner).
The institutional review board approved the study protocol. All participants provided written informed consent. The study was registered at www.clinicaltrials.gov (NCT01330914).
Calcium and vitamin D supplementation
Vitamin D and chewable Ca citrate supplements were supplied upon enrollment and throughout the study. At enrollment, low 25OHD levels were repleted with a target level ≥30 ng/mL, and vitamin D supplements were dosed individually to maintain that target level. Also upon enrollment, each participant’s daily Ca intake was brought to 1200 mg through individualized Ca citrate dosing, based on estimation of dietary Ca intake with a validated questionnaire.(15) During postoperative follow-up, 25OHD levels and estimated dietary Ca intake were monitored, and each participant’s supplement doses were adjusted to maintain our vitamin D and Ca intake goals.
FCA
Preoperatively and 6 months postoperatively, FCA was measured by a standardized dual stable isotope technique, with one stable Ca isotope administered orally to label dietary Ca and the other intravenously (i.v.) to measure Ca removal from the blood.(16,17) After an overnight fast, participants emptied their bladders, blood was drawn, and a 24-hour urine collection was initiated. Each participant ate a standardized test breakfast consisting of 300 mg Ca. The nutrient composition of the breakfast was identical for the preoperative and postoperative tests, and all participants consumed all of each meal. A 10-mg dose of oral 44Ca was administered mid-breakfast. A 3-mg dose of 43Ca was infused i.v. 60 min later. Blood was drawn 24 hours later, when the orally and i.v.-administered isotopes would be tracking together.(18) Isotope enrichment was determined by high-resolution, inductively coupled plasma mass spectrometry (ELEMENT-2, ThermoFinnigan Corp., Bremen, Germany) at Purdue University (West Lafayette, IN, USA), using an Aridus Desolvating Sample Introduction system with a T1H nevulizer (Cetac Technologies, Omaha, NE, USA). FCA was determined as the ratio of oral to i.v. isotope in the blood sample, adjusted for the dose of each isotope.(19) Urine specimens from the 24-hour urine collection were used for isotope enrichment and FCA determination to confirm values from blood specimens.
Other measures
Preoperatively and 6 months postoperatively, BMI was calculated as weight/height2 (kg/m2). Waist circumference was measured in the mid-axillary line at the level of the lowest rib, and hip circumference at the maximum extension of the buttocks, viewed from the side. Whole-body fat and lean mass (g) and areal BMD (aBMD, g/cm2) were measured preoperatively and 6 months postoperatively by DXA (Hologic Discovery W densitometer; Hologic, Bedford, MA, USA). Modified half-body scans were employed if a participant’s body dimensions exceeded the width of the scanning area.(20) Spinal volumetric BMD (vBMD, g/cm3) at the L3 and L4 vertebrae was assessed by QCT (General Electrics VCT64 scanner; General Electric, Milwaukee, WI, USA). Findings on QCT were evaluated according to methods as described (Mindways Software, Austin, TX, USA).(21,22) Visceral adipose tissue area (cm2) was measured by CT using a single axial slice at the mid-L4 vertebra.
Serum samples were collected preoperatively and 6 months postoperatively after an overnight fast. In premenopausal women, attempts were made to schedule preoperative and postoperative blood draws at similar times in the menstrual cycle. Chemistries including PTH and 25OHD were measured, then serum was stored at −70°C until batch analysis was done for other analytes in a central laboratory (Maine Medical Center Research Institute, Scarborough, ME, USA). Serum 1,25(OH)2D, serum CTx (a marker of bone resorption), P1NP (a marker of bone formation), osteocalcin (OC; a marker of bone formation), bone-specific alkaline phosphatase (BAP; a marker of bone formation), IGF-1, and IGF-binding protein 3 (IGFBP-3, the predominant carrier protein of IGF-1) were measured by automated immunoassay (iSYS; Immunodiagnostic Systems, Scottsdale, AZ, USA), with interassay and intraassay coefficients of variation (CVs) of 11.2% and 8.0%, 6.2% and 3.2%, 4.6% and 2.9%, 6.1% and 2.5%, 7.3% and 1.6%, 5.1% and 2.2%, and 6.4% and 1.9%, respectively. Total estradiol was measured by ELISA (Alpco Diagnostics, Salem, NH, USA), with interassay and intraassay CVs of 8.7% and 7.8%, respectively. The adipokines adiponectin and leptin were measured by ELISA (R&D Systems, Minneapolis, MN, USA), with interassay and intraassay CVs of 6.5% and 3.5% for adiponectin and 4.4% and 3.2% for leptin.
At each time point, comprehensive estimates of dietary intakes were obtained using the Block 60-item food frequency questionnaire.(23)
Surgical approach
The RYGB procedure was performed by experienced bariatric surgeons at both academic bariatric surgery centers in a standardized laparoscopic fashion. This included a 30-mL gastric pouch, a gastrojejunal anastomosis created with a 25-mm circular stapler, an antecolic and antegastric Roux limb 100 to 150 cm in length, and an end-to-side jejunostomy.
Statistical analysis
Means ± SDs and medians (interquartile ranges [IQRs]) were calculated for baseline characteristics, as appropriate. Daily absorbed calcium (mg) was calculated as FCA × 1200 mg, the total daily Ca intake. Spearman’s rank correlation and Mann-Whitney tests were used to characterize the relationships between baseline FCA and other study parameters. Paired t tests or Wilcoxon signed-rank tests were used as appropriate to determine whether study outcomes changed between preoperative and 6-month postoperative time points. To explore which factors might influence the extent to which FCA changes after RYGB, Spearman’s rank correlation test was used to characterize the relationships between the change in FCA and changes in other study parameters considered potential determinants of FCA change. Linear models were then employed to estimate adjusted associations, with covariates selected from those variables associated with baseline FCA. Normalizing log transformations were used when needed. In addition, the relationships between postoperative (final) FCA, changes in biochemical markers of bone turnover, and changes in BMD were characterized, as were the relationships between changes in BMD and other study parameters. Data were analyzed using Stata 12 software (StataCorp, College Station, TX, USA).
Results
Baseline participant characteristics and correlations
Participants were 45.4 ± 12.8 (mean ± SD) years old (Table 1). Of the 33 participants, 19 (58%) were premenopausal women, 6 (18%) were postmenopausal women, and 8 (24%) were men. Sixty-four percent were white. Mean preoperative weight was 125.3 ± 17.8 kg, and mean BMI was 44.7 ± 7.4 kg/m2.
Table 1.
Baseline Characteristics of Study Participants (n = 33)
| Characteristic | Value |
|---|---|
| Age (years) | 45.4 ± 12.8 |
| Women, n (%) | 25 (76%) |
| Premenopausal | 19 (76%) |
| Postmenopausal | 6 (24%) |
| Race, n (%) | |
| White | 21 (64%) |
| Black | 6 (18%) |
| Asian | 2 (6%) |
| Hispanic/Latino | 2 (6%) |
| Hawaiian/Pacific Islander | 2 (6%) |
| Diabetes, n (%) | 15 (45%) |
| Weight (kg) | 125.3 ± 17.8 |
| BMI (kg/m2) | 44.7 ± 7.4 |
| Percentage body fat (%) | 46.7 ± 6.3 |
| Waist circumference (cm) | 122.3 ± 13.3 |
| Waist-hip ratio | 0.90 ± 0.10 |
| 25OHD upon enrollment (ng/mL) | 23.6 (18.5–29.0) |
| 25OHD at preoperative study visit (ng/mL) | 41.0 (33.1–48.5) |
| 24-hour urinary calcium (mg)a | 191.4 (92.7–246.9) |
| PTH (pg/mL)a | 41.3 (32.0–53.1) |
| 1,25(OH)2D (pg/mL)a | 37.1 (33.6–45.6) |
| Areal BMD (DXA) (g/cm2) | |
| Femoral neck | 0.973 ± 0.132 |
| Total hip | 1.157 ± 0.140 |
| Lumbar spine | 1.189 ± 0.140 |
| Volumetric BMD (QCT) (g/cm3) | |
| Spine (L3–L4) | 0.162 ± 0.040 |
| Fractional calcium absorption (%) | 32.7 ± 14.0 |
Values are mean ± SD, median (IQR), or count (percentage).
IQR = interquartile range.
Values are from measurements made at the preoperative study visit, following vitamin D repletion.
Upon initial enrollment, median 25OHD level was 23.6 (IQR, 18.5 to 29.0) ng/mL. With vitamin D repletion, median 25OHD rose to 41.0 (IQR, 33.1 to 48.5) ng/mL at the time of preoperative FCA measurement. Mean cholecalciferol supplement dose at the time of FCA measurement was 2636 ± 822 IU daily, in combination with variable repletion courses of ergocalciferol. Three of 33 participants’ 25OHD levels at the preoperative study visit fell short of the target level of ≥30 ng/mL. At the time of preoperative FCA measurement, median PTH level was 41.3 (IQR, 32.0 to 53.1) pg/mL, and median 24-hour urinary Ca level was 191.4 (IQR, 92.7 to 246.9) mg.
Mean preoperative FCA was 32.7% ± 14.0%. At baseline, there was a correlation between age and FCA (ρ = −0.42, p = 0.02), such that older participants had lower FCA. Preoperatively, FCA was lower in white participants than in nonwhite participants (27.1% versus 45.5%, p < 0.01), and in women compared to men (30.9% versus 38.2%, p = 0.18 in bivariate analysis, p < 0.01 after adjustment for age). Despite the role played by 1,25(OH)2D in active calcium absorption, preoperative 1,25(OH)2D level was not significantly associated with preoperative FCA.
Changes in body composition, metabolic, and dietary parameters after RYGB
All participants lost weight during the 6 months after RYGB, with a mean loss of 32.5 ± 8.4 kg, or a 25.8% ± 5.2% decline from preoperative weight (p < 0.0001, Table 2). Total fat mass declined 40.3% ± 9.0% from its preoperative baseline, and total lean mass declined 11.3% ± 5.2% (p < 0.0001 for both). Glycated hemoglobin (HbA1c) and leptin levels decreased, and adiponectin level increased (p < 0.001 for all). There was no statistically significant change in estradiol level over the 6-month period. IGF-1 level increased over the 6 months (p = 0.03). Dietary intakes of macronutrients and micronutrients decreased significantly (Table 3).
Table 2.
Changes in Body Composition and Metabolic Parameters 6 Months After Roux-en-Y Gastric Bypass
| Parameter | Preoperation | 6-month postoperation | % Change | p |
|---|---|---|---|---|
| Body composition | ||||
| Weight (kg) | 125.3 ± 17.8 | 92.8 ± 13.9 | −25.8% ± 5.2% | <0.0001 |
| BMI (kg/m2) | 44.7 ± 7.4 | 33.4 ± 5.8 | −25.4% ± 5.0% | <0.0001 |
| Total body fat (kg) | 56.7 ± 11.4 | 34.0 ± 9.3 | −40.3% ± 9.0% | <0.0001 |
| Total body lean (kg) | 61.8 ± 11.0 | 54.7 ± 9.7 | −11.3% ± 5.2% | <0.0001 |
| Percentage body fat (%) | 46.7 ± 6.3 | 37.0 ± 7.4 | −21.3% ± 7.8% | <0.0001 |
| Visceral fat (cm2) | 199.7 ± 93.5 | 107.2 ± 59.4 | −45.2% ± 17.9% | <0.0001 |
| Waist circumference (cm) | 122.3 ± 13.3 | 98.4 ± 10.5 | −19.1% ± 8.5% | <0.0001 |
| Hip circumference (cm) | 137.3 ± 14.5 | 113.9 ± 12.7 | −17.0% ± 4.1% | 0.0001 |
| Laboratory parameters | ||||
| HbA1c (%) | 5.6 (5.3–6.6) | 5.2 (4.9–5.5) | −10.6% (−17.9% to −6.0%) | <0.0001 |
| Adiponectin (ng/mL) | 4905 (2574–6903) | 8208 (5293–10,100) | +66.0% (+36.2% to +102.9%) | <0.0001 |
| Leptin (ng/mL) | 49.7 (34.5–63.7) | 11.4 (7.0–23.1) | −71.9% (−86.2% to −62.2%) | <0.0001 |
| Estradiol (pg/mL) | 59.4 (44.2–72.9) | 64.7 (42.7–90.0) | +13.7% (−21.5% to +80.0%) | 0.27 |
| IGF-1 (ng/mL) | 108.7 (73.4–138.3) | 128.5 (92.4–153.2) | +8.2% (−3.1% to +48.4%) | 0.03 |
| IGFBP-3 (ng/mL) | 4155 (3227–4907) | 3601 (3262–4158) | −9.6% (−16.4% to −1.7%) | <0.001 |
Values are mean ± SD or median (IQR). Bold p values are significant.
HbA1c = glycated hemoglobin; IGFBP-3 = IGF-binding protein 3; IQR = interquartile range.
Table 3.
Dietary Intake Parameters, Before and 6 Months After Roux-en-Y Gastric Bypass
| Parameter | Preoperation | 6-months postoperation | p |
|---|---|---|---|
| Dietary intake, per day | |||
| Total kcal | 1947 ± 769 | 1012 ± 433 | <0.0001 |
| Protein (g) | 78 ± 32 | 48 ± 20 | <0.0001 |
| Total fat (g) | 90 ± 38 | 47 ± 23 | <0.0001 |
| Carbohydrate (g) | 206 ± 91 | 102 ± 50 | <0.0001 |
| Calcium (mg) | 829 ± 336 | 594 ± 295 | <0.0001 |
| Phosphorus (mg) | 1287 ± 497 | 815 ± 361 | <0.0001 |
| Sodium (mg) | 3448 ± 1402 | 1771 ± 745 | <0.0001 |
| Potassium (mg) | 2603 ± 1090 | 1668 ± 649 | <0.0001 |
| Fiber (g) | 16.5 ± 7.3 | 9.4 ± 4.9 | <0.0001 |
| Soluble fiber (g) | 5.5 ± 2.6 | 3.0 ± 1.6 | <0.0001 |
| Percentage of total kcal | |||
| Fat (%) | 41.4 ± 6.9 | 40.9 ± 7.8 | 0.86 |
| Protein (%) | 16.1 ± 2.6 | 19.7 ± 4.6 | <0.001 |
| Carbohydrate (%) | 42.8 ± 7.7 | 40.3 ± 8.3 | 0.16 |
Values are mean ± SD. Bold p values are significant.
Changes in fractional calcium absorption and calciotropic hormones
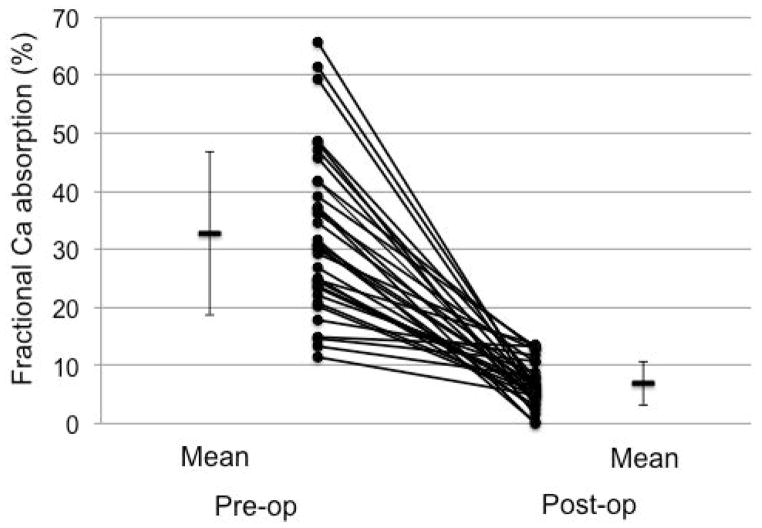
After RYGB, FCA decreased from 32.7% ± 14.0% preoperatively to 6.9% ± 3.8% postoperatively (p < 0.0001, Fig. 1). Mean difference between time points was −25.8% ± 15.3%, and mean percentage decline was 73.1% ± 21.6% from the preoperative baseline. This corresponded to a decline in daily absorbed Ca from 392.2 ± 168.1 mg to 82.3 ± 45.0 mg. Median preoperative and postoperative 25OHD levels were 41.0 (IQR, 33.1 to 48.5) ng/mL and 36.5 (IQR, 28.8 to 40.4) ng/mL, respectively. Mean cholecalciferol supplement dose at the 6-month postoperative visit was 3121 ± 1111 IU daily. In a sensitivity analysis excluding the 3 participants with postoperative 25OHD levels <20 ng/mL and the 6 participants with 25OHD between 20 and 30 ng/mL (ie, not attaining or maintaining target 25OHD despite our repletion/supplementation protocol), FCA still declined from mean 29.6% ± 13.5% to 7.3% ± 3.3% (p < 0.0001).
Fig. 1.
Fractional calcium absorption before and 6 months after Roux-en-Y gastric bypass surgery (n = 33). Values are mean ± SD.
Concurrently, 24-hour urinary Ca level decreased from a median of 191.4 (IQR, 92.7 to 246.9) mg to 109.0 (IQR, 59.9 to 159.4) mg (p < 0.001). PTH level increased from a median of 41.3 (IQR, 32.0 to 53.1) pg/mL to 48.4 (IQR, 39.3 to 59.1) pg/mL (p = 0.02), and 1,25(OH)2D increased from 37.1 (IQR, 33.6 to 45.6) pg/mL to 51.4 (IQR, 41.0 to 63.3) pg/mL (p < 0.001). When we restricted our analysis to the 24 participants with 25OHD levels ≥30 ng/mL at both time points, these changes in urinary Ca and calciotropic hormones were still evident: 24-hour urinary calcium declined from a median of 192.6 (IQR, 87.4 to 252.7) mg preoperatively to 104.7 (IQR, 59.0 to 164.9) mg postoperatively (p < 0.01), PTH increased from a median of 41.8 (IQR, 28.6 to 54.9) pg/mL preoperatively to 47.0 (IQR, 37.7 to 59.7) pg/mL postoperatively (p = 0.056), and 1,25(OH)2D increased from 37.7 (IQR, 31.1 to 45.6) pg/mL to 54.2 (IQR, 44.8 to 62.9) pg/mL (p < 0.001).
In bivariate analysis, there was a trend toward a correlation between percentage change in weight and change in FCA (ρ = 0.34, p = 0.05), such that those with greater percentage weight loss had greater declines in FCA. After adjustment for age, sex, and race, the association remained statistically significant (p = 0.02). Changes in IGFBP-3 and the adipokine adiponectin were associated with greater declines in FCA in bivariate analysis, but these relationships were no longer statistically significant after adjustment for age, sex, race, and percentage weight loss. Changes in other metabolic parameters, calciotropic hormones, and nutritional intakes did not correlate with change in FCA.
Changes in bone turnover markers and BMD
Bone turnover markers increased after RYGB (Table 4). Serum CTx, a marker of bone resorption, increased by a median 276% (IQR, +167% to +381%, p < 0.0001). Median percentage increases in serum P1NP, OC, and BAP (markers of bone formation) were +104%, +135%, and +15%, respectively (p < 0.001 for all). aBMD decreased at the proximal femur over the 6-month period, with declines of 4.6% ± 4.6% and 4.7% ± 5.0% at the femoral neck and total hip, respectively (p < 0.0001). There was no statistically significant change in spinal aBMD over the 6 months. Spinal vBMD decreased by 6.5% ± 5.1% (p < 0.0001).
Table 4.
Changes in Fractional Calcium Absorption, Calciotropic Hormones, Biochemical Markers of Bone Turnover, and BMD 6 Months After Roux-en-Y Gastric Bypass
| Parameter | Preoperation | 6-months postoperation | % Change | p |
|---|---|---|---|---|
| Fractional Ca absorption (%) | 32.7 ± 14.0 | 6.9 ± 3.8 | −73.1% ± 21.6% | <0.0001 |
| Daily absorbed Ca (mg)a | 392.2 ± 168.1 | 82.3 ± 45.0 | −73.1% ± 21.6% | <0.0001 |
| Measures of calcium homeostasis | ||||
| 25OHD (ng/mL) | 41.0 (33.1–48.5) | 36.5 (28.8–40.4) | −15.1% (−24.7% to −1.0%) | <0.01 |
| 24-hour urinary calcium (mg) | 191.4 (92.7–246.9) | 109.0 (59.9–159.4) | −35.0% (−54.3% to +13.4%) | <0.001 |
| PTH (pg/mL)b | 41.3 (32.0–53.1) | 48.4 (39.3–59.1) | +23.2% (−2.5% to +47.1%) | 0.02 |
| 1,25(OH)2D (pg/mL)b | 37.1 (33.6–45.6) | 51.4 (41.0–63.3) | +45.9% (+7.2% to +91.1%) | <0.0001 |
| Bone turnover markersb | ||||
| CTx (ng/mL) | 0.293 (0.167–0.347) | 0.975 (0.802–1.278) | +276% (+167% to +381%) | <0.0001 |
| BAP (μg/L) | 12.9 (10.1–15.0) | 15.7 (12.4–19.0) | +14.6% (−2.6% to +31.8%) | <0.001 |
| OC (ng/mL) | 10.8 (7.7–13.9) | 24.6 (20.3–33.3) | +135% (+89% to 202%) | <0.0001 |
| P1NP (ng/mL) | 30.8 (25.1–42.7) | 75.4 (55.5–91.7) | +104% (+72% to +153%) | <0.0001 |
| BMD | ||||
| Areal BMD (DXA) (g/cm2) | ||||
| Femoral neck | 0.973 ± 0.132 | 0.925 ± 0.110 | −4.6% ± 4.6% | <0.0001 |
| Total hip | 1.157 ± 0.140 | 1.102 ± 0.138 | −4.7% ± 5.0% | <0.0001 |
| Lumbar spine | 1.184 ± 0.142 | 1.188 ± 0.149 | −0.1% ± 4.5% | 0.92 |
| Volumetric BMD (QCT) (g/cm3) | ||||
| Spine (L3–L4) | 0.162 ± 0.040 | 0.152 ± 0.039 | −6.5% ± 5.1% | <0.0001 |
Values are mean ± SD or median (IQR). Bold p values are significant.
BAP = bone-specific alkaline phosphatase; OC = osteocalcin; IQR = interquartile range.
Daily absorbed Ca in mg calculated as fractional Ca absorption × 1200 mg, the total daily Ca intake.
95% reference intervals provided by the test manufacturers: PTH, 14 to 72 pg/mL; 1,25(OH)2D, 26.1 to 95.0 pg/mL; CTx, 0.112 to 0.738 ng/mL; BAP, 4.7 to 27.0 μg/L; OC, 10.4 to 45.6 ng/mL; and P1NP, 27.7 to 127.6 ng/mL.
Participants with greater percentage declines in FCA or lower postoperative FCA level had greater increases in bone resorption marker serum CTx (ρ = −0.35, p = 0.04 and ρ = −0.43, p = 0.01, respectively; Fig. 2). FCA change and postoperative FCA were not correlated with change in bone formation marker P1NP (ρ = −0.01, p = 0.96 and ρ = −0.02, p = 0.92, respectively) or the other markers of bone formation. Although FCA, proximal femur aBMD, and spinal vBMD declined substantially, there was not a statistically significant correlation between the extent of FCA and BMD changes, nor between postoperative FCA level and change in BMD over the 6-month study period. Change in PTH level was associated with percentage change in aBMD at the femoral neck, such that those with greater increases in PTH had greater declines in femoral neck aBMD (ρ = −0.38, p = 0.03 in bivariate analysis); this association remained statistically significant after adjustment for age, sex, and race (p = 0.01). Those with higher postoperative CTx levels had greater percentage declines in total hip aBMD and in spinal vBMD (ρ = −0.42, p = 0.02 and ρ = −0.52, p < 0.01, respectively).
Fig. 2.
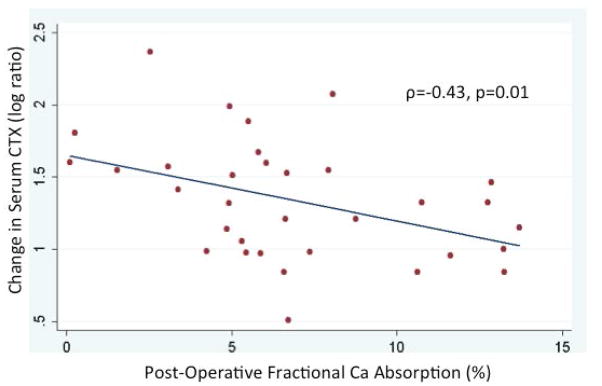
Correlation between postoperative fractional calcium absorption (final 6-month postoperative value) and change in serum CTx after Roux-en-Y gastric bypass surgery.
Discussion
The RYGB bariatric surgical procedure involves the creation of a small gastric pouch and the bypass of the duodenum and proximal jejunum, which are the sites of most active, transcellular Ca absorption in the intact intestine. The procedure results in impressive weight loss and in changes in dietary and hormonal factors implicated in the ability of the intestine to absorb Ca.(24,25) In a cohort of 33 severely obese adults, we used a dual stable isotope method to determine the effects of RYGB on intestinal FCA. We found that mean preoperative FCA was 32.7%, which was within the range expected for this largely female, middle-aged group.(24,26) Postoperatively, FCA decreased dramatically to a mean of 6.9%. Consistent with the decline in FCA, 24-hour urinary Ca level decreased, PTH increased, and 1,25(OH)2D increased. Those with lower postoperative FCA had greater increases in the bone resorption marker serum CTx.
This dramatic decline in FCA occurred despite careful attention to maintaining robust 25OHD status and stable Ca intake. Our approach was in contrast to most studies of bone and mineral metabolism after bariatric surgery, in which calcium and vitamin D supplementation are part of clinical care and ancillary to the study protocol. Vitamin D deficiency is common in obesity(27,28) and after RYGB,(11,29) and inadequacy of the metabolite 1,25(OH)2D is thought to impair Ca uptake in those situations. Our goal was to understand to what extent the portion of the gastrointestinal tract still in contact with food and supplements can participate in active, transcellular, 1,25(OH)2D-mediated Ca absorption and passive, paracellular Ca transport. We hypothesized that FCA would decrease, but that the 25OHD levels of ≥30 ng/mL and the Ca intake of 1200 mg daily would mitigate the postoperative decline. Instead, we observed a precipitous drop in FCA. In a sensitivity analysis excluding those participants not attaining or maintaining target 25OHD levels despite our repletion/supplementation protocol, the precipitous drop still occurred (from 29.6% to 7.3% instead of 32.7% to 6.9% in the full cohort). Urinary Ca levels still declined markedly in this sensitivity analysis.
The observed FCA decline presumably resulted from the marked alteration to the gastrointestinal tract with RYGB, with the duodenum and proximal jejunum no longer in contact with ingested food and supplements. We further explored which additional factors may explain the variability in FCA decline, hypothesizing that the extent to which particular dietary intakes (eg, dietary protein, fat, fiber), hormone levels (eg, estradiol, IGF-1), or other metabolic parameters (eg, weight) change postoperatively might be important. We found that participants with greater percentage weight loss had greater declines in FCA. This finding is consistent with the published observation that nonsurgical weight loss results in a decline in FCA compared to weight maintenance.(30,31) Our findings may also be confounded by details of the surgical approach: Those with slightly more intestine bypassed (longer nonabsorptive biliopancreatic limbs) may have experienced, as a result, greater weight loss and also a greater impact on Ca absorption. However, this is purely speculative, because we were not able to document such confounding statistically. We observed correlations with other parameters that reflect weight loss (adiponectin and IGFBP-3, a protein that decreased in concert with weight loss and may denote nutritional status). Otherwise, our search for nonsurgical determinants of FCA decline after RYGB was largely unrevealing. We suspect that the surgical alteration to the gastrointestinal tract was so profound that it overshadowed subtle effects of other potential determinants of FCA.
Our postoperative findings are different than those of the one other published study of FCA after RYGB. Riedt and colleagues(13) studied 21 women before and 6 months after RYGB and found that FCA decreased from 36% to 24%. Over one-half of the women in that study had 25OHD levels <25 ng/mL, and we anticipated that if our participants’ vitamin D status was more robust, the FCA decline might be attenuated. Further, mean postoperative total daily Ca intake in that study was under 1000 mg with large variability, making passive, paracellular Ca uptake unlikely for most participants. Instead of observing an attenuated FCA decline, we observed much lower postoperative FCA values. With their 1200 mg daily Ca intake, our participants’ mean daily absorbed Ca fell from 392 mg to 82 mg, in contrast to the decline from 417 mg to 227 mg in the study by Riedt and colleagues.(13) The reason for the discrepancy in FCA results is unclear. The women in the study by Riedt and colleagues(13) were of very similar mean age to our mostly female participants, and although they were more obese at baseline and follow-up than our participants, both cohorts’ weight loss was dramatic. Because participants in the study by Riedt and colleagues(13) had lower mean postoperative total Ca intake, and because low Ca intake increases FCA as a compensatory measure,(32) it is possible that habitually low Ca intake elevated postoperative FCA in some participants in that study but not in ours. With regard to the surgical approach, Roux limb length (ie, distance from gastric pouch to the junction with biliopancreatic secretions) was similar between studies, but we cannot rule out differences in biliopancreatic limb length (ie, the length of proximal jejunum bypassed). Biliopancreatic limb length, or the distance from the ligament of Treitz at which the proximal jejunum is transected, is not as precisely measured and recorded as Roux limb length is. If the most proximal aspect of the jejunum (just distal to the ligament of Treitz) is critical for Ca absorption, and if biliopancreatic limb length is longer at our institution, a potential result could be lower postoperative FCA in our cohort. In fact, a very long biliopancreatic limb (200 cm, much longer than that performed at our institution) has been associated with more secondary hyperparathyroidism than a 60-cm limb.(33)
With respect to methodological differences between our study and that of Riedt and colleagues,(13) our participants consumed a test meal with a larger Ca load than theirs (300 mg versus 200 mg). However, Heaney and colleagues(34) have defined the inverse correlation between FCA and ingested Ca test load, and this difference in load would only explain a small part of the difference between our study’s postoperative FCA values. (In the healthy adult women studied by Heaney and colleagues,(34) FCA would be 37.8% with a 200 mg test load versus 33.9% with a 300 mg load.) We used higher stable isotope doses than Riedt and colleagues,(13) and we used the same isotope doses preoperatively and postoperatively, whereas they used weight-based doses.(35) Regardless of the reason that the postoperative FCA values were much lower in our study, we can certainly conclude that maintaining robust 25OHD status and Ca intake does not mitigate the FCA decline after RYGB.
A decline in FCA after RYGB, especially a decline as dramatic as the one we observed, raises concern for bone loss and, ultimately, for possible increased fracture risk. The impaired ability of the intestine to absorb Ca may cause skeletal fragility by means of defective bone mineralization or high bone resorption. Indeed, in a large cohort of older postmenopausal women, those with lower FCA were more likely to sustain a subsequent hip fracture; this association was most striking for those with low Ca intakes.(36) In our study, we observed striking increases in biochemical markers of bone turnover and decreases in BMD at the proximal femur. Serum CTx, a marker of bone resorption, increased by a median of 276%, and P1NP, a marker of formation, increased by 104%. Those participants with greater declines in FCA or with lower postoperative FCA had greater increases in serum CTx over the 6 months of the study, suggesting that the low FCA could be implicated in RYGB’s effects on bone metabolism.
aBMD assessed by DXA decreased almost 5% at the proximal femur over only 6 months, an impressively large decline that is on par with the 6-month declines reported in other studies of RYGB and BMD.(37,38) vBMD at the spine assessed by QCT also decreased substantially, by 6.5%. This was in contrast to spinal aBMD, for which a change was not observed. Discordance between DXA-assessed and QCT-assessed BMD in the settings of obesity and changing body composition is well documented,(38–40) and this discordance presumably reflects the artifacts that may have affected the two imaging modalities in these settings.(5) We did not observe a statistically significant correlation between the extent of FCA and BMD changes. Although this likely reflects the effects of other important factors (eg, mechanical, hormonal, and nutritional) on BMD, it does not preclude a contribution of the FCA decline to the BMD decline. Of note, those with greater increases in PTH did have greater declines in femoral neck aBMD (ρ = −0.38, p = 0.03 in bivariate analysis), as has been reported.(41) Further, an effect of the reduced FCA on BMD may be manifest over a period of time longer than 6 months.
Our findings have direct implications for the preoperative and postoperative care of RYGB patients. For the general adult population up to age 70 years, the Recommended Daily Allowance for Ca is 1000 mg.(42) Given concerns about Ca malabsorption after bariatric surgery, professional organizations of surgeons and endocrinologists have recommended daily Ca intakes of 1200 to 1500 mg from diet and Ca citrate supplements.(43,44) Our participants experienced decreases in 24-hour urinary Ca level and increases in PTH level despite intakes of 1200 mg daily. Therefore, our findings suggest that RYGB patients may need even higher Ca intakes in order to prevent perturbations in Ca homeostasis. However, the approach to Ca supplementation needs to be studied, ideally through FCA studies on a range of doses.
A limitation of our study is that its observational design prevents the assessment of causality with respect to predictors of change in FCA or the relationship between changes in FCA, bone turnover markers, and BMD. The study is limited by its 6-month duration because we are unable to determine the longer-term effects of the decline in FCA on skeletal health. We also do not know the longer-term effects of RYGB on FCA; the postsurgical anatomy is permanent, so the severely low FCA may be permanent, but it is possible that the intestine adapts over time and increases its Ca absorptive capacity. Methodologically, we measured FCA with a rigorous, standardized dual stable isotope technique(16,17) that assesses FCA over 24 hours, a time period ample for the complete absorption of Ca in the normal gastrointestinal tract.(12) Although we cannot rule out that the complete absorption of Ca in the RYGB patient requires a longer period of time, a small study of RYGB patients found oral-cecal transit time similar to that of obese controls.(45) Full Ca balance studies would be required to confirm the negative balance between intake and excretion implied by our results.
In conclusion, intestinal FCA decreased dramatically 6 months after RYGB, even with most 25OHD levels ≥30 ng/mL and with Ca intake 1200 mg daily. Those participants who lost the most weight had the most severe declines in FCA. Our findings suggest that RYGB patients may need higher Ca intakes than those currently recommended for this population in order to prevent perturbations in calcium homeostasis, although the approach to Ca supplementation needs to be studied. Six months postoperatively, those participants with the lowest FCA had the greatest postoperative increases in bone resorption marker serum CTx. The decline in FCA could be one of the determinants of the decline in BMD observed after RYGB, and strategies to avoid long-term skeletal consequences should be investigated.
Acknowledgments
This study was supported by the US Department of Veterans Affairs, VHA, CSR&D Service (Career Development Award-2 5 IK2 CX000549-04, to ALS, SFVAMC). Additional support was provided by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI grant UL1 TR000004, by NIH grant 5 K12 HD052163-15, and by an Endocrine Society Summer Research Fellowship. The contents of the article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Laboratory assay kits were provided by Immunodiagnostic Systems (IDS), and calcium citrate supplements were supplied by Bariatric Advantage. We thank Berdine Martin, PhD, for her work in isotope ratio assessment; Barbara Arnold, BA, for study coordination and data collection; Viva Tai, RD, MPH, for test meal preparation and DXA scan acquisition; Lisa Palermo, MA, and Lucy Wu, BA, for their work in data management; Nooshin Yashar, MD, for study support; Saunak Sen, PhD, for biostatistical advice; and Dimitry Petrenko, BA, and Thomas Lang, PhD, for CT image analysis.
Authors’ roles: Study design: ALS, CMW, DMB, DMS, and DES. Study conduct: ALS, ALW, HC, GVS, LS, SJR, JTC, AMP, DMS. Data collection: ALS, ALW, HC, GVS. Data analysis: ALS, CMW. Data interpretation: ALS, CMW, DMB, DMS, DES. Drafting manuscript: ALS. Revising manuscript content: All authors. Approving final version of manuscript: All authors. ALS takes responsibility for the integrity of the data analysis.
Footnotes
Presented in part at the Annual Meeting American Society for Bone and Mineral Research (ASBMR); September 12–15, 2014; Houston, TX, USA. Schafer A, Sellmeyer D, Weaver C, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery, despite optimization of vitamin D status. J Bone Miner Res. 2014;29(Suppl 1). Presentation Number: 1077. Available from: http://www.asbmr.org/education/AbstractDetail?aid=3d1b4052-e216-4cc2-b356-e501dc744430.
Public clinical trial registration: http://clinicaltrials.gov/show/NCT01330914. Effects of Gastric Bypass Surgery on Calcium Metabolism and the Skeleton.
Disclosures
CMW has served as scientific advisor to Pharmavite and has received grants from Nestlé and Tate & Lyle. DMB has consulted for and has received grants from Amgen, Lilly, Merck, Novartis, and Radius. The other authors state that they have no conflicts of interest.
References
- 1.Fryar CD, Carroll MD, Ogden CL. NCHS Health EStat. Hyattsville, MD, USA: National Center for Health Statistics; 2014. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960–1962 through 2011–2012. Available from: http://www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.pdf. [Google Scholar]
- 2.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien PE, MacDonald L, Anderson M, Brennan L, Brown WA. Long-term outcomes after bariatric surgery: fifteen-year follow-up of adjustable gastric banding and a systematic review of the bariatric surgical literature. Ann Surg. 2013;257:87–94. doi: 10.1097/SLA.0b013e31827b6c02. [DOI] [PubMed] [Google Scholar]
- 5.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29:1507–18. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2:165–74. doi: 10.1016/S2213-8587(13)70183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura KM, Haglind EG, Clowes JA, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int. 2014;25:151–8. doi: 10.1007/s00198-013-2463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilarrasa N, Gomez JM, Elio I, et al. Evaluation of bone disease in morbidly obese women after gastric bypass and risk factors implicated in bone loss. Obes Surg. 2009;19:860–6. doi: 10.1007/s11695-009-9843-5. [DOI] [PubMed] [Google Scholar]
- 9.Wucher H, Ciangura C, Poitou C, Czernichow S. Effects of weight loss on bone status after bariatric surgery: association between adipokines and bone markers. Obes Surg. 2008;18:58–65. doi: 10.1007/s11695-007-9258-0. [DOI] [PubMed] [Google Scholar]
- 10.Al-Shoha A, Qiu S, Palnitkar S, Rao DS. Osteomalacia with bone marrow fibrosis due to severe vitamin D deficiency after a gastrointestinal bypass operation for severe obesity. Endocr Pract. 2009;15:528–33. doi: 10.4158/EP09050.ORR. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JM, Maher JW, DeMaria EJ, Downs RW, Wolfe LG, Kellum JM. The long-term effects of gastric bypass on vitamin D metabolism. Ann Surg. 2006;243:701–4. doi: 10.1097/01.sla.0000216773.47825.c1. discussion 704–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barger-Lux MJ, Heaney RP, Recker RR. Time course of calcium absorption in humans: evidence for a colonic component. Calcif Tissue Int. 1989;44:308–11. doi: 10.1007/BF02556309. [DOI] [PubMed] [Google Scholar]
- 13.Riedt CS, Brolin RE, Sherrell RM, Field MP, Shapses SA. True fractional calcium absorption is decreased after Roux-en-Y gastric bypass surgery. Obesity (Silver Spring) 2006;14:1940–8. doi: 10.1038/oby.2006.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consensus Development Conference Panel. Gastrointestinal surgery for severe obesity: Consensus Development Conference statement. Ann Intern Med. 1991;115:956–61. [PubMed] [Google Scholar]
- 15.Hacker-Thompson A, Robertson TP, Sellmeyer DE. Validation of two food frequency questionnaires for dietary calcium assessment. J Am Diet Assoc. 2009;109:1237–40. doi: 10.1016/j.jada.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeGrazia JA, Ivanovich P, Fellows H, Rich C. A double isotope method for measurement of intestinal absorption of calcium in man. J Lab Clin Med. 1965;66:822–9. [PubMed] [Google Scholar]
- 17.Yergey AL, Abrams SA, Vieira NE, Aldroubi A, Marini J, Sidbury JB. Determination of fractional absorption of dietary calcium in humans. J Nutr. 1994;124:674–82. doi: 10.1093/jn/124.5.674. [DOI] [PubMed] [Google Scholar]
- 18.Smith DL, Atkin C, Westenfelder C. Stable isotopes of calcium as tracers: methodology. Clin Chim Acta. 1985;146:97–101. doi: 10.1016/0009-8981(85)90129-9. [DOI] [PubMed] [Google Scholar]
- 19.Weaver CM, Heaney RP. Calcium in human health. Totowa, NJ: Humana Press; 2006. [Google Scholar]
- 20.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–4. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 21.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–7. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Khoo BC, Brown K, Cann C, et al. Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporos Int. 2009;20:1539–45. doi: 10.1007/s00198-008-0820-y. [DOI] [PubMed] [Google Scholar]
- 23.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 24.Wolf RL, Cauley JA, Baker CE, et al. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr. 2000;72:466–71. doi: 10.1093/ajcn/72.2.466. [DOI] [PubMed] [Google Scholar]
- 25.Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Brolin RE, Taich L. Hormonal and dietary influences on true fractional calcium absorption in women: role of obesity. Osteoporos Int. 2012;23:2607–14. doi: 10.1007/s00198-012-1901-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heaney RP, Recker RR. Distribution of calcium absorption in middle-aged women. Am J Clin Nutr. 1986;43:299–305. doi: 10.1093/ajcn/43.2.299. [DOI] [PubMed] [Google Scholar]
- 27.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 28.Goldner WS, Stoner JA, Thompson J, et al. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–50. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 29.Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol. 2012;8:544–56. doi: 10.1038/nrendo.2012.48. [DOI] [PubMed] [Google Scholar]
- 30.Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA. Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr. 2004;80:123–30. doi: 10.1093/ajcn/80.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapses SA, Sukumar D, Schneider SH, et al. Vitamin D supplementation and calcium absorption during caloric restriction: a randomized double-blind trial. Am J Clin Nutr. 2013;97:637–45. doi: 10.3945/ajcn.112.044909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989;4:469–75. doi: 10.1002/jbmr.5650040404. [DOI] [PubMed] [Google Scholar]
- 33.Nergaard BJ, Leifsson BG, Hedenbro J, Gislason H. Gastric bypass with long alimentary limb or long pancreato-biliary limb-long-term results on weight loss, resolution of co-morbidities and metabolic parameters. Obes Surg. 2014;24:1595–602. doi: 10.1007/s11695-014-1245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaney RP, Weaver CM, Fitzsimmons ML. Influence of calcium load on absorption fraction. J Bone Miner Res. 1990;5:1135–8. doi: 10.1002/jbmr.5650051107. [DOI] [PubMed] [Google Scholar]
- 35.Field MP, Shapses S, Cifuentes M, Sherrell RM. Precise and accurate determination of calcium isotope ratios in urine using HR-ICP-SFMS. J Anal At Spectrom. 2003;18:727–33. [Google Scholar]
- 36.Ensrud KE, Duong T, Cauley JA, et al. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;132:345–53. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 37.Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19:41–6. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 38.Yu EW, Bouxsein ML, Roy AE, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29:542–50. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–24. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonaretti S, Carpenter RD, Saeed I, et al. Novel anthropomorphic hip phantom corrects systemic interscanner differences in proximal femoral vBMD. Phys Med Biol. 2014;59:7819–34. doi: 10.1088/0031-9155/59/24/7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (IOM) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for vitamin D and calcium. Washington, DC: National Academies Press; 2011. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/pdf/TOC.pdf. [PubMed] [Google Scholar]
- 43.Mechanick JI, Youdim A, Jones DB, et al. American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic and Bariatric Surgery. Obesity (Silver Spring) 2013;21(Suppl 1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Brethauer S on behalf of the ASMBS Clinical Issues Committee; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. Position Statement. Metabolic bone changes after bariatric surgery. Surg Obes Relat Dis. doi: 10.1016/j.soard.2014.03.010. Forthcoming. Epub 2014 Apr 14. [DOI] [Google Scholar]
- 45.Carswell KA, Vincent RP, Belgaumkar AP, et al. The effect of bariatric surgery on intestinal absorption and transit time. Obes Surg. 2014;24:796–805. doi: 10.1007/s11695-013-1166-x. [DOI] [PubMed] [Google Scholar]