Abstract
The genus Crocodylus comprises 12 currently recognized species, many of which can be difficult to differentiate phenotypically. Interspecific hybridization among crocodiles is known to occur in captivity and has been documented between some species in the wild. The identification of hybrid individuals is of importance for management and monitoring of crocodilians, many of which are Convention on International Trade in Endangered Species (CITES) listed. In this study, both mitochondrial and nuclear DNA markers were evaluated for their use in confirming a suspected hybrid zone between American crocodile (Crocodylus acutus) and Morelet’s crocodile (Crocodylus moreletii) populations in southern Belize where individuals and nests exhibiting atypical phenotypic features had previously been observed. Patterns observed in both phenotypic and molecular data indicate possible behavioural and ecological characteristics associated with hybridization events. The results of the combined analyses found that the majority of suspected hybrid samples represent crosses between female C. acutus and male C. moreletii. Phenotypic data could statistically identify hybrids, although morphological overlap between hybrids and C. moreletii reduced reliability of identification based solely on field characters. Ecologically, C. acutus was exclusively found in saline waters, whereas hybrids and C. moreletii were largely absent in these conditions. A hypothesized correlation between unidirectional hybridization and destruction of C. acutus breeding habitats warrants additional research.
Keywords: American crocodile, Morelet’s crocodile, hybrid zone, hybridization, species' boundaries, Belize
1. Introduction
Crocodiles (Crocodylus spp.) hybridize readily in captivity [1–5], and hybridization is known or suspected to occur among wild populations of several sympatric species [6–12]. Despite hybridization being considered a potential threat to some populations of endangered crocodilians [3,4,9,10,12–14], the frequency, geographical extent and drivers of hybridization among wild crocodilians remain poorly understood [11,15].
Hybridization between the American (Crocodylus acutus) and Morelet’s (Crocodylus moreletii) crocodiles was long postulated based on observations of crocodiles with phenotypic characteristics of both species [16–24]. More recently, molecular tools have provided genetic evidence for hybridization between these species in northern Belize [8] and the Yucatán Peninsula of Mexico [10,11]. In Mexico, hybridization appears to occur primarily in coastal regions of sympatry [10,11], while in Belize hybrids were found at inland sites outside the distribution of C. acutus [8]. Hybridization between C. acutus and the endangered Cuban crocodile (Crocodylus rhombifer) has been determined to be much more extensive than previously assumed based on phenotypic data [12].
Integrating molecular, phenotypic and environmental data to elucidate patterns of crocodile hybridization in Belize is important as the International Union for Conservation of Nature (IUCN) [25,26] currently classifies C. acutus as vulnerable (globally) and C. moreletii as conservation dependent. In this study, we use mitochondrial sequence and nuclear microsatellite data to assess the correspondence between phenotypic characteristics of nests, eggs and crocodiles with genetically based species assignment for populations of C. acutus and C. moreletii in coastal regions of Belize. We also examine ecological features associated with the presence of hybrid crocodiles and potential implications for conservation of these species.
2. Material and methods
2.1. Study area
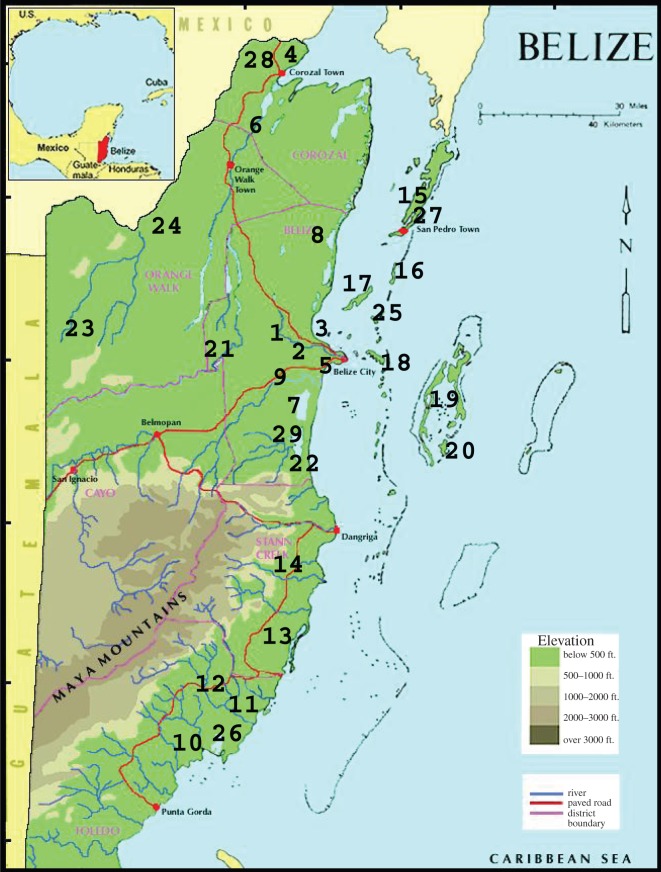
Our study was conducted in the Caribbean coastal zone of southern Belize (figure 1) [28,29]. The mainland of southern Belize (south of Belize City) is characterized by extensive mangrove swamps and a number of short, swift-flowing rivers (Monkey–Bladen–Swasey River system, and Deep, Moho, Sittee, Temash and Sarstoon rivers) draining the Maya Mountains [29,30]. The Belize barrier reef extends 220 km along the coast, separated from the mainland by the Inner Channel, which contains approximately 450 low elevation islands, or Cays, including three from this study (Turneffe and Lighthouse Atolls, and Glovers Reef). The coastal zone of Belize is described in greater detail elsewhere [28,31,32].
Figure 1.
Sampling localities for American (C. acutus) and Morelet’s (C. moreletii) crocodiles in Belize. Numbers correspond to localities listed in table 1. Adapted from [27].
2.2. Sampling
Crocodiles of both species (C. acutus and C. moreletii) were captured as part of a countrywide population survey in the coastal zone from June 1996 through to October 1997 (table 1) [33,34]. Crocodiles were captured at night with the aid of a spotlight; smaller crocodiles (total length [TL]≤100 cm) were taken by hand or dip-net, and a noose-pole was used to capture larger individuals (TL>100 cm).
Table 1.
Summary of localities for American crocodile (C. acutus) and Morelet’s crocodile (C. moreletii) samples collected in coastal mainland habitats of Belize (1996–1997) (adapted from [27]). (Numbers correspond to map in figure 1.)
| locality | C. acutus | C. moreletii |
|---|---|---|
| (1) Belize River | 0 | 1 |
| (2) Burdon Canal—FabersLagoon | 0 | 2 |
| (3) Coastline (Ladyville) | 0 | 1 |
| (4) Four-mile Lagoon | 0 | 1 |
| (5) Haulover Creek | 0 | 1 |
| (6) New River | 0 | 15 |
| (7) Northern Lagoon | 1 | 1 |
| (8) Northern River Lagoon | 3 | 0 |
| (9) SibunRiver/BurdonCanal | 0 | 3 |
| (10) Deep River | 1 | 0 |
| (11) Monkey River | 0 | 7 |
| (12) Bladen River | 0 | 7 |
| (13) Placencia Lagoon | 0 | 1 |
| (14) Sittee River | 0 | 4 |
| (15) Ambergris Cay | 7 | 0 |
| (16) Cay Caulker | 4 | 0 |
| (17) Hicks Cay | 1 | 0 |
| (18) Maps Cay | 1 | 0 |
| (19) Turneffe Atoll | 22 | 0 |
| (20) Calabash Cay | 2 | 0 |
| (21) Cox Lagoon | 0 | 1 |
| (22) Gales Point | 3 | 0 |
| (23) Gallon Jug | 0 | 3 |
| (24) Gold Button Lagoon | 0 | 3 |
| (25) Long Cay | 2 | 0 |
| (26) Payne’s Creek | 0 | 3 |
| (27) San Pedro Lagoon | 1 | 0 |
| (28) Sapote Lagoon | 0 | 4 |
| (29) Western Lagoon | 3 | 0 |
| total n=54 | 56 |
We recorded standard morphometric measurements from each crocodile, counted the number of dorsal precaudal scale rows and the scales in each row as in Platt et al. [35] and noted the presence or absence of irregular subcaudal scale groups [18,36]. Based on published keys, crocodiles exhibiting groups of irregular subcaudal scales and more than four scales in any transverse dorsal precaudal scale row were classified as C. moreletii, while those lacking groups of irregular subcaudal scales and having no more than four scales in any dorsal precaudal scale row were classified as C. acutus [36]. Crocodiles with atypical characters were classified as possible hybrids.
Approximately 1 ml of blood was drawn from the nuchal sinus of each crocodile [37] and immediately decanted into an equal amount of buffer (10 mM Tris, pH 7.6). Samples were initially stored at room temperature and later at −20°C for long-term storage. All individuals were permanently marked for future identification by notching the dorsal edge of a unique series of caudal scutes [38], and then released at the site of capture within 24 h.
In response to local reports of atypical crocodile nest mounds (described as having mixed features of both C. acutus and C. moreletii) along rivers in southern Belize [27], we searched this region from March to May during 1997 and 1998. We measured the dimensions of each nest mound and distance to the water (measured from the centre of the mound). We then carefully opened the nest, determined the clutch size and measured (length and width to nearest 0.1 mm) and weighed (±1.0 g) each egg. Egg viability was determined by the presence of opaque bands, and the date of oviposition was estimated by the extent of banding [39]. A single egg was sacrificed from each nest and the embryo preserved in 75% ethanol for later genetic analysis.
2.3. Laboratory procedures
Total genomic DNA was extracted from 56 C. moreletii and 54 C. acutus samples collected from throughout the coastal zone of Belize. Additionally, total genomic DNA was extracted from seven embryos collected at atypical nests found along the Bladen, Swasey and Monkey rivers, and Paynes Creek in southern Belize. For extractions, we used 2 μl of whole blood from adults and a small piece of heart muscle dissected from embryos and isolated DNA using the Qiagen DNeasy Blood and Tissue Kit. Resulting DNA was quantified via gel electrophoresis on a 1% agarose gel and subsequently diluted as necessary to ensure amplification.
We sequenced mtDNA gene regions including 12 s rRNA, 16 s and control region (Dloop) using primers described in Hekkala et al. [40]. PCR amplifications were carried out in 25 μl reaction volume containing 1 μl each 10 mM primer and 2 μl template with GE PCR-ready-to-go beads pre-loaded in a 0.2 μl tube to which 21 μl ddH20 was added. Thermocycler parameters for all gene regions consisted of a 5 min denaturation at 94°C for 30 s, 49°C for 30 s and 72°C for 45 s, followed by an extension period of 5 min at 72°C. Bands were visualized on a 1% agarose gel and PCR products were cleaned according to the manufacturer’s instructions using Qiagen PCR cleanup kit. Double-stranded PCR products were sequenced on an ABI 3730XL automated DNA sequencer, and edited and aligned in Sequencher v. 4.5 (Gene Codes, Ann Arbor, MI, USA).
We screened 18 Crocodylus-derived dinucleotide microsatellites [14] (table 2) in an 8 μl reaction volume consisting of 1 μl Taq polymerase (Perkins Elmer), 0.3 μl of each primer (10 mM), 2.5 μl Taq buffer containing 15 μM MgCl2, 2.5 μl dNTPs and 0.5 μl of diluted (1:10) template DNA. Amplicons were visualized by electrophoresis in 1.5% agarose and diluted 1:2 with distilled water. A 2 μl aliquot of dilute (1:3) sample was added to a total volume of 8 μl formanide and ROX size standard and run on Applied Biosystems 3100 or 3730 DNA Analyzer. Fragments were analysed using Genemapper® 4.0 (Applied Biosystems).
Table 2.
Mitochondrial primers [40] and microsatellite primers characterized by Fitzsimmons et al. [14] tested for use in identifying hybrids between C. acutus and C. moreletii. (Fixed marker indicates alleles unique to parental species. Variable marker indicates frequency variation in alleles between parental species.)
| primer name | primer sequence (5′–3′) | species | repeat motif | amplification | fixed | variable | |
|---|---|---|---|---|---|---|---|
| mtDNA | 12s | F: CCGTCTTTGACAGTC | |||||
| R: ATGTTCCAAGCACACCTTCC | |||||||
| 16s | F: AAGGTAGCGTAATCATTTG | ||||||
| R: GGGGATTGCGCTGTTATCCCTG | |||||||
| Dloop | F: GCCGACATTCTTATTAAACTAC | ||||||
| R: GCAGATAAATGAATGCCTTAT | |||||||
| microsatellite | Cj119 | F: GTTTGCTGTGGAATGTTTCTAC | C. johnsoni | (CA)14 | yes | yes | yes |
| R: CGCTATATGAAACGGTGGCTG | |||||||
| C391 | F: ATGAGTCAGGTGGCAGGTTC | C. acutus | (CA)22 | yes | no | yes | |
| R: CATAAATACACTTTTGAGCAGCAG | |||||||
| Cj104 | F: TCCTTCCATGCATGCACGTGTG | C. johnsoni | (CA)12 | yes | yes | yes | |
| R: GTTTCAGTGTCTGGTATTGGAGAAGG | |||||||
| Cj105 | F: CAACAGAAAGTGCCACCTCAAG | C. johnsoni | (CA)14 | multiple bands | no | no | |
| R: GTTTGATTATGAGACACCGCCACC | |||||||
| Cj107 | F: ACCCCGCATTCTGCCAAGGTG | C. johnsoni | (CA)16 | multiple bands | no | no | |
| R: GTTTATTGCCATCCCCACTGTGTC | |||||||
| Cj122 | F: GTTTCATGCTGACTGTTTCTAATCACC | C. johnsoni | (CA)ls | yes | mono | mono | |
| R: GGAACTACAATTGGTCAACCTCAC | |||||||
| Cj127 | F: CCCATAGTTTCCTGTTACCTG | C. johnsoni | (CT)7TT(CT)12 (CA)16 | yes | no | yes | |
| R: GTTTCCCTCTCTGACTTCAGTGTTG | |||||||
| Cj128 | F: ATTGGGGCAGATAAGTGGACTC | C. johnsoni | (CA)22 | no | no | no | |
| R: GTTTCTGCTTCTCTTCCCTACCTGG | |||||||
| Cj35 | F: GTTTAGAAGTCTCCAAGCCTCTCAG | C. johnsoni | (CT)7TA(CA)17(CT)12 | yes | yes | yes | |
| R: CTGGGGCAAGGATTTAACTCTC | |||||||
| Cj101 | F: ACAGGAGGAATGTCGCATAATTG | C. johnsoni | (CA)12 | yes | no | yes | |
| R: GTTTATACCGTGCCATCCAAGTTAG | |||||||
| Cj131 | F: GTTTGTCTTCTTCCTCCTGTCCCTC | C. johnsoni | (CA)14 | yes | yes | yes | |
| R: AAATGCTGACTCCTACGGATGG | |||||||
| Cjl6 | F: CATGCAGATTGTTATTCCTGATG | C. johnsoni | (CA)20 | yes | unknown | unknown | |
| R: TGTCATGGTGTCAATTAAACTC | |||||||
| Cjl8 | F: ATCCAAATCCCATGAACCTGAGAG | C. johnsoni | unpublished | yes | yes | yes | |
| R: CCGAGTGCTTACAAGAGGCTGG | |||||||
| Cp10 | F: GATTAGTTTTACGTGACATGCA | C. porosus | (CA)ls | yes | mono | mono | |
| R: ACATCAAGTCATGGCAGGTGAG | |||||||
| CUD68 | F: GCTTCAGCAGGGGCTACC | C. acutus | (CA)13 | only C. acutus | plus/minus | no | |
| R: TGGGGAAACTGCACTTTAGG | |||||||
| CUC20 | F: GATCTGCAGTGCAAGAAAG | C. acutus | unpublished | yes | yes | yes | |
| R: GGTTTAGCGGTCACAGTAAC | |||||||
| CUD78 | F GAAGTGAATGCCATCTATCA | C. acutus | (CA)15 | yes | mono | mono | |
| R AATTGCATCCCCTTTTG | |||||||
| CUI 108 | F: ACTGGCCACAGCTGGGGTA | C. acutus | (CA)20 | multiple bands | no | no | |
| R: CCAGCAGCGTGGAGAGCTG |
2.4. Molecular analytical approaches
We initially identified diagnostic markers (fixed mtDNA haplotypes and private microsatellite alleles) for parental species using samples from populations of each species from outside of the purported hybrid zone. Subsequently, individuals from the purported hybrid zone were examined for specific patterns of admixture in the distribution of haplotypes, private alleles and phenotype. We used Bayesian assignment methods including Newhybrids v. 1.1 [41] and Structure v. 2.3.4 [42] to infer ancestry and to identify putative hybrids. The Structure analysis was implemented with an admixture model with uncorrelated allele frequencies and without including sample location as a prior. We used 20 replicates for each value of K (genetic cluster) ranging from K=1−7, with 10 000 000 Markov chain Monte Carlo replicates following an initial burn-in of 1 000 000. We chose a threshold for parental species membership in a cluster at 0.0–0.05 or more than 0.95–1.0 and for hybrids between these boundaries.
We used Anderson & Thompson’s [41] Bayesian method of detecting hybrids that more directly attempts to detect hybrid individuals between two parent species as implemented in Newhybrids v. 1.1. This model infers each individual’s genotype frequency class, or hybrid category, thus providing posterior probabilities that reflect the level of certainty that an individual belongs to a given hybrid class (e.g. F1, backcross, purebred). Unlike in Structure, here the parameter of interest (q) is a discrete variable with up to six genotype frequency classes (i.e. purebred, F1, F2, backcross). Individuals were assigned to pure C. acutus, pure C. moreletii and hybrids (F1, F2 and both F1 backcrosses). Results were based on the average of 10 independent runs each with 1 000 000 iterations following a 100 000 step burn-in using Jeffrey’s priors (following preliminary runs indicating similar results with uniform priors). As in Structure, individuals were identified as purebred based on a qi>0.95. To determine the ability of Newhybrids to identify purebred and hybrid individuals, simulated genotypes were created using Hybridlab [43].
Genotypes were selected from pure C. moreletii and C. acutus individuals identified in the initial Newhybrids analysis (qi>0.95). Alleles were randomly drawn from the pool of parental genotypes to create 100 simulated purebred C. moreletii and C. acutus individuals. These new parental genotypes were then used to simulate F1, F2 and backcrossed populations (100 of each hybrid class). These 600 simulated genotypes were then analysed in Newhybrids under the same protocols described above. Power (number of correctly identified individuals for a category over the actual number of individuals of that category) and accuracy (number of correctly identified individuals for a category over the total number of individuals assigned to that category) were calculated for six Tq values (0.95, 0.9, 0.8, 0.7, 0.6, 0.5).
For all downstream environmental and phenotypic analyses reliant upon hybrid identification a priori, we used the more conservative estimate of species identification (ID) from Structure results.
2.5. Environmental analyses
Once individuals were identified using molecular data and mapped using GPS coordinates, environmental characters associated with parental species and hybrids in Belize were evaluated using distributional modelling (MAXENT). We compared niche models developed for each parental species against models developed for hybrids and evaluated highest ranking environmental variables for each species’ model for statistical differences [44].
The variables used for niche modelling were then used to test whether or not the habitats of parental crocodile species and hybrids differed in their bioclimatic envelopes, as well as the salinity of their habitats. A site’s environmental variables were assigned to only one individual per crocodile species or hybrid per site. The BIOCLIM data [45] used were acquired from the WorldClim database [45] and were formatted for use in R with the raster package using the raster and extract functions [46,47]. All tests were conducted using R [47], with multivariate statistics relying on the vegan package [48]. The majority of environmental variables were not normally distributed; accordingly, non-parametric tests were used. Differences between the habitats of the species and hybrid categories of crocodile were tested using PERMANOVA with the function adonis [48,49]. Individual environmental variables were then compared between crocodile groups with Kruskal–Wallis (KW) tests (function kruskal.test), followed by pairwise comparisons (function pairwise.wilcox.test). All KW and pairwise comparison p-values were adjusted for multiple testing with the false discovery rate (FDR) correction [50].
2.6. Phenotypic data
We examined field collected morphological data on head shape and scalation patterns for differences between genetically determined parent species and hybrids. Specifically, we tested the following variables: the head width to length ratio; the snout width at the fifth maxilla to the snout width at the anterior orbit ratio; the presence, reduction or absence of irregular subcaudal scales; and the mean number of scales in the transverse scale rows. As data were largely non-parametric, FDR corrected Kruskal–Wallis and follow up pairwise comparisons were conducted following procedures for environmental analyses. We also compared egg mass from nests containing genetically identified hybrids to parental species egg mass using an ANOVA. Data were tested for violations of normality and homogeneity of variances [51]. Mean values are presented as ±1 s.d. and results were considered significant at p≤0.05.
3. Results
3.1. Genetic characterization of phenotypically identified hybrid crocodiles
Samples from 110 crocodiles were sequenced for three mtDNA gene regions totalling 1374 nucleotides (12 s=365 bp, 16 s=319 bp, control region=690 bp). For analyses of nuclear markers, we were unable to consistently amplify all six loci in 34 individuals resulting in a reduced sample size for nuclear analyses (n=76).
As in prior phylogenetic analyses [52], sequences from pure C. acutus and C. moreletii samples exhibited fixed differences between parental species at multiple sites (12 s=7 bp, 16 s=4 bp, control region=21 bp), which were subsequently considered to be diagnostic characters. Sequenced mtDNA haplotypes revealed 24 individuals field identified as C. moreletii or possible hybrids that exhibited C. acutus fixed mtDNA markers. The majority of these crocodiles (80%) had been characterized as putative hybrids in the field on the basis of nest type or scale counts.
Of the 18 microsatellite loci tested, 13 produced reliable amplification products in both species and one (Cu68) amplified only in C. acutus (table 2). After an initial examination of allelic distribution between C. moreletii and C. acutus, seven loci (Cj18, Cj131, Cj119, Cj127, Cj35, Cj101 and Cj104) exhibited fixed markers for each parental species and thereafter were characterized for the remainder of the samples.
The Bayesian clustering analyses performed on simulated genotypes in Newhybrids resulted in clearly identifiable partitions among parental species and hybrids (table 3 and figure 2, upper panel). Power and accuracy were consistently high for both purebred categories across all Tq values, meaning nearly all assigned genotypes were correctly assigned to each parental species (table 4). Accuracy remained near 0.9 across all classes examined for more conservative Tq values (i.e. greater than or equal to 0.8), though power tended to be low for F2 and backcrosses. When all hybrid classes were considered together (‘hybrid’), simulations exhibited high levels of power and accuracy in identifying hybrid individuals. Given the number of loci used, our limited ability to correctly assign individuals to F2 and backcross populations is expected. Accordingly, we combined posterior probabilities of all hybrid classes as an estimate for the detection of hybrids. We used a conservative threshold of 0.95 to assign individuals as pure or hybrid. At this threshold, our analyses identified 27 hybrids (approx. 35%). These results were similar to those of our Structure analyses (table 3 and figure 2, lower panel). Both Bayesian clustering approaches assigned the majority of parental individuals (97%) to their own species with 99–100% certainty (table 4). Individuals of each parental species were never identified as the other parental species. Only C. moreletii (11%) and hybrids (9%) were mis-assigned and then only infrequently (6%). Accuracy of Field ID relative to genetic assignment was highest for C. acutus and lower for both C. moreletii (82%) and hybrids (92%) (table 5). The accuracy of field ID’d hybrids is probably owing to targeted sampling for those individuals and may not reflect ease of identification in regular surveys.
Table 3.
Proportions and frequencies of pure and admixed C. acutus and C. moreletii individuals inferred using Bayesian clustering (Structure) and assignment (Newhybrids) methods. (Only strict assignment (Tq=0.95) to parental and admixed classes included.)
| C. acutus | admixed | C. moreletii | |
|---|---|---|---|
| Newhybrids | 29 (38.2%) | 27 (35.5%) | 20 (26.3%) |
| Structure | 29 (38.2%) | 23 (30.3%) | 24 (31.6%) |
Figure 2.
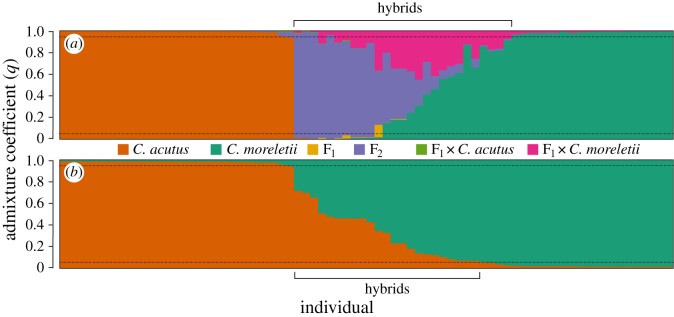
Bayesian assignments of 76 C. acutus, C. moreletii and hybrid individuals computed by Newhybrids ((a) K=6, number of genotype frequency classes) and Structure ((b) K=2, number of species). Each individual is represented by a single vertical line broken into segments whose length is proportional to the estimated membership (probability qi) in the clusters. The ‘hybrid’ identification includes individuals that fall in the 0.05<qi<0.95 range.
Table 4.
Power and accuracy of Newhybrids to detect pure and hybrid individuals across six Tq values. (All six pure and hybrid classes (excluding ‘hybrid’) consisted of 100 simulated genotypes. The ‘hybrid’ class was created by summing the assignment probabilities of the four hybrid categories and was used to assess the ability of Newhybrids to identify generic ‘hybrid’ individuals. Power is defined as the number of correctly identified individuals for a category over the actual number of individuals of that category and accuracy as the number of correctly identified individuals for a category over the total number of individuals assigned to that category.)
|
Tq=0.95 |
Tq=0.9 |
Tq=0.8 |
Tq=0.7 |
Tq=0.6 |
Tq=0.5 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| class | power | accuracy | power | accuracy | power | accuracy | power | accuracy | power | accuracy | power | accuracy |
| C. acutus | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.97 | 0.94 | 0.97 | 0.94 | 0.99 | 0.93 |
| C. moreletii | 0.93 | 0.98 | 0.94 | 0.98 | 0.98 | 0.97 | 1.00 | 0.97 | 1.00 | 0.97 | 1.00 | 0.96 |
| F1 | 0.51 | 0.98 | 0.84 | 0.97 | 0.96 | 0.96 | 0.97 | 0.94 | 0.97 | 0.94 | 0.98 | 0.94 |
| F2 | 0.51 | 1.00 | 0.55 | 1.00 | 0.62 | 1.00 | 0.63 | 0.98 | 0.65 | 0.98 | 0.65 | 0.98 |
| F1×C. acutus | 0.00 | — | 0.10 | 0.91 | 0.50 | 0.89 | 0.70 | 0.88 | 0.79 | 0.89 | 0.87 | 0.90 |
| F1×C. moreletii | 0.00 | — | 0.21 | 1.00 | 0.61 | 0.84 | 0.75 | 0.81 | 0.87 | 0.82 | 0.91 | 0.81 |
| hybrid | 0.92 | 1.00 | 0.93 | 1.00 | 0.94 | 1.00 | 0.96 | 1.00 | 0.97 | 1.00 | 0.97 | 1.00 |
Table 5.
Accuracy of field ID relative to genetic assignment for each category of species ID for pure and admixed C. acutus and C. moreletii individuals.
| field ID | gene ID | % accuracy |
|---|---|---|
| C. acutus | C. acutus | 100 |
| C. moreletii | C. moreletii | 82 |
| hybrid | hybrid | 92 |
| C. acutus | hybrid | n.a. |
| C. moreletii | hybrid | 17 |
| hybrid | C. acutus | n.a. |
| hybrid | C. moreletii | 8 |
Overall, the results of the Newhybrids assignment and the comparison of diagnostic nuclear alleles with mtDNA haplotypes revealed a consistent pattern of hybridization between female C. acutus and male C. moreletii indicting unidirectional outcrossing. Five of six embryos from atypical nests and several hybrid adults exhibited combined mtDNA haplotypes and multilocus genotypes consistent with F2 backcrossing, and thus hybrid viability.
3.2. Phenotypic and reproductive attributes of hybrids
Genetic probability of assignment to parental species versus hybrid was strongly associated with morphological characters relating to head shape and scalation pattern were significant (figure 4): the head width to length ratio (KW χ2=20.7; FDR corrected, p<0.001); the snout width at the fifth maxilla to the snout width at the anterior orbit ratio (KW χ2=23.1; FDR corrected, p<0.001); the presence, reduction or absence of subcaudals (KW χ2=48.2; FDR corrected, p<0.001); and the mean number of scales in the transverse scale rows (KW χ2=23.8; FDR corrected, p<0.001; figure 4). Most significant was the presence, reduction or absence of subcaudal scale rows; presence was fixed for C. moreletiiand absence was fixed for C. acutus, while hybrids tended to be present or reduced (rarely absent). In addition, the mean number of scales in transverse scale rows was the most significant continuous variable. Pairwise comparisons found C. moreletiiand the hybrids to significantly differ from C. acutus for all variables, while hybrids were significantly different for the presence, reduction or absence of the subcaudal scales (p=0.005)
Figure 4.
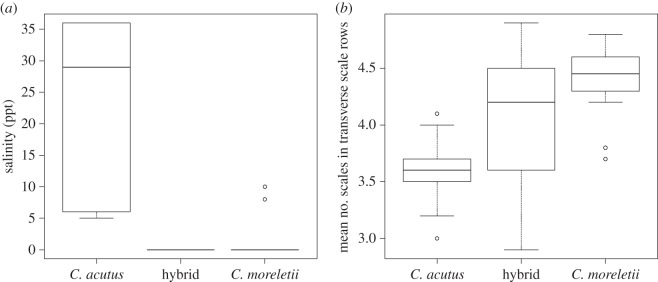
(a,b) Box-and-whisker plots of the most significant environmental (FDR corrected, p=0.012) and continuous morphological variables (FDR corrected, p< 0.001) associated with genetically determined species ID for C. moreletii and C. acutus in Belize using Kruskal–Wallis tests. The box contains the middle two quartiles (separated by the median), the whiskers are the extreme values up to 1.5 times the interquartile range, and the dots represent outliers.
Nesting occurred during the dry season and the mean estimated laying date was 22 April ± 8 days (range=10–30 April). During nesting season, 11 atypical crocodile nests were observed along Payne’s Creek (n=3), and Monkey (n=2), Bladen (n=3), Swasey (n=2), Sennis (n=1) rivers in southern Belize during field surveys in 1997 and 1998. Nests along Monkey and Bladen River were found beside oxbow lakes adjacent to the river, while the remaining nests were constructed on sandbars along the main river channel. All of the nests we examined were mound-type nests, although nest material varied depending on microhabitat. Nests at oxbow lakes were constructed of soil, leaf litter and woody debris, while those along main river channels were composed almost wholly of sand. Mounds (n=11) averaged 158±60 cm wide (range=95–300 cm) and 61±24 cm high (range=30–100 cm), and the distance to water ranged from 110 to 1260 cm.
Ten of the 11 nests we examined contained eggs, while one nest had been depredated prior to our arrival. Mean clutch size was 31.3±11.7 eggs (range=15–48 eggs). We measured the linear dimensions of 311 eggs (including three eggs from which the contents had leaked); mean length and width were 77.8±6.5 mm (range=61.3–95.0 mm) and 45.9±3.7 mm (range=36.9–51.1 mm), respectively. Three hundred and eight intact eggs were weighed; mean egg mass was 105.1±20.9 g (n=308; range=59–142 g) and 17 (5.5%) were non-viable. Mean clutch size of hybrid containing nests in southern Belize was significantly greater than values reported for either C. moreletii or C. acutus (table 6; F2,96=3.97; Tukey–Kramer minimum significant difference; p<0.05). Likewise, mean egg mass of hybrid containing nests was significantly greater than reported for either C. moreletii or C. acutus [53,54] (table 5; paired t-test 14.554; p<0.01). Seventeen (5.5%) of 308 intact eggs were non-viable.
Table 6.
(a) Crocodile egg mean weights (g) for eggs from typical (C. acutus and C. moreletii) and atypical crocodile nests. (b) ANOVA for all groups indicating significant differences, paired t-tests indicated differences between eggs from either parental species and those found in atypical, purported hybrid nests (p<0.001).
| (a) species | n | s.d. | range | mean (g) | |
|---|---|---|---|---|---|
| C. acutus[53] | 280 | 9.7 | 61.5–111.0 | 85.6 | |
| C. moreletii[21] | 1702 | 9.4 | 46.2–91.1 | 69.0 | |
| ‘hybrid’ nests | 308 | 20.3 | 59–142 | 105.1 |
| (b) source of variation | sum of squares | d.f. | variance | F | p |
|---|---|---|---|---|---|
| between groups | 369 990.0 | 2 | 184 995.0 | 1396.0 | <0.001 |
| within groups | 303 063.1 | 2287 | 132.5 | ||
| total | 673 053.1 | 2289 |
3.3. Niche conservatism in parental and hybrid crocodiles
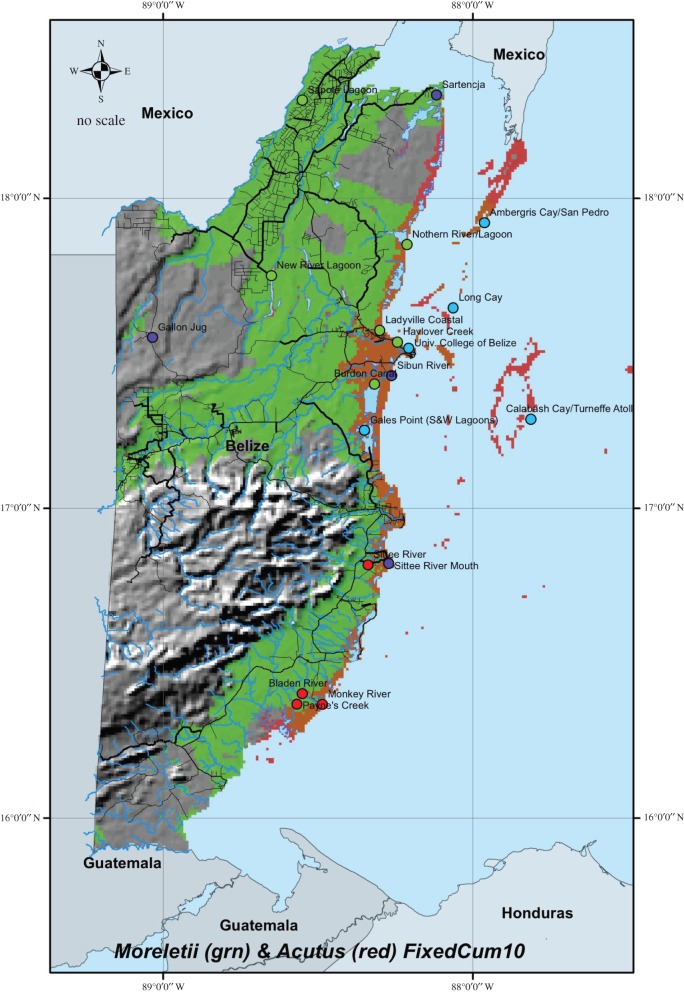
Although distribution mapping of genetically identified C. acutus and C. moreletii results in narrow areas of overlap (figure 3), overall, species and hybrids did not differ based on combined habitat environmental features (r2=0.188; p=0.111). None of the BIOCLIM environmental variables were significant after correcting for multiple testing. However, BC6 (minimum temperature of coldest month) and BC7 (temperature annual range) were significant before FDR corrections, while BC2 (mean diurnal range) and BC15 (precipitation seasonality) approached significance before correcting. Salinity was the only environmental variable to remain significant after FDR corrections for multiple testing (KW χ2=14.7; FDR corrected, p=0.012; figure 4). Habitat salinity for C. acutuswas significantly different from that found for C. moreletii (p=0.010) and for hybrids (p=0.003), while the latter two groups did not differ significantly (p=0.222).
Figure 3.
Maximum entropy (MAXENT) species distribution model (SDM) for genetically identified C. moreletii (in green) and C. acutus (in red) in Belize. Sampling localities for C. moreletii (green dots), C. acutus (blue dots), hybrids (red dots) and both hybrids and parental (purple dots).
4. Discussion
Analysis of mtDNA from individuals collected outside of areas of sympatry in Belize, as well as data published elsewhere [10,11,40,52], indicates that there are fixed, diagnostic, haplotypic differences between C. moreletii and C. acutus, which can be used as DNA barcodes. Additionally, nuclear microsatellite loci exhibit both frequency differences and private alleles useful in differentiating between the two species of Crocodylus in Belize. These and published species descriptions based on phenotypic characters clearly support their continued recognition as two species.
Our combined use of mtDNA and nuclear markers indicate that hybridization between C. moreletii and C. acutus has occurred in two regions of Belize: the lower reaches of New River and Rio Bravo around Chetumal Bay, and several coastal rivers in southern Belize, south of Gales Point (figure 3). Similarly, Ray et al. [8] detected C. acutus haplotypes among crocodiles that phenotypically resembled C. moreletii in the New River and Belize River watersheds in northern Belize and concluded hybridization was also occurring in these regions. The presence of discordant species-specific mtDNA haplotypes, multilocus genotypes and phenotypic characteristics confirms the presence of hybrids in these areas. Unlike other studies that found hybrids were cryptic and not readily distinguished on the basis of morphology [8], we found morphologically intermediate characters and atypical nests as relatively reliable indicators of the presence of hybrid crocodiles (table 5 and figure 4); for example, a reduction in subcaudal scales is diagnostic for hybrids when present.
Our use of biparental and maternally inherited markers indicates unidirectional hybridization in southern Belize with male C. moreletii crossing with female C. acutus. This contrasts with results from Mexico, where Cedeño-Vázquez et al. [10] found that hybridization between C. moreletii and C. acutus is bidirectional, and occurs in about the same proportion in each direction. Asynchrony in courtship and mating in Belize’s crocodile populations may contribute to the observed pattern in our data, where breeding in C. acutus occurs during February and March [53], while C. moreletii breeds in April and May [54]. We speculate that male C. moreletii establish territory during the latter part of C. acutus breeding season and breed with female C. acutus before female C. moreletii enter a reproductive state.
Other factors influencing directionality of geneflow may be behavioural. Unidirectional hybridization is frequent [55] when one species is larger than the other, and males of the larger species usually mate with females of the smaller species [56]. Mating between females of the larger species and males of the smaller species generally does not occur because females rarely select smaller males as mates [56]. Crocodilians engage in elaborate courtship and mating rituals that involve female choice based primarily on the size of male suitors [1]. During courtship and mating, larger males typically dominate breeding groups and drive off or even inflict injuries on smaller subdominant males [1]. Although male C. acutus are known to reach maximum TLs of 6–7 m [57], in Belize males rarely attain lengths of over 3 m [33]. By contrast, male C. moreletii can reach lengths of 3.6–4.0 m [58,59] and possibly larger [60], suggesting that large male C. moreletii would probably displace male C. acutus during courtship for access to female C. acutus.
Our distribution models indicate that distributions of C. moreletii and C. acutus are largely related to water salinity (figure 4). Published natural history data suggest that high salinities (approx. 36 ppt) restrict C. moreletii to freshwater and mainland coastal habitats [34,61], while C. acutus occurs in marine habitats, with lower numbers found in mainland coastal habitats [24,33]. In our study area, populations of C. moreletii occur in freshwater wetlands, while C. acutus is found primarily on offshore islands and atolls. While the two species occasionally co-occur in brackish mangrove swamps of the coastal mainland [33,34,62–64], our niche models suggest a latitudinal gradient within Belize in niche overlap, with southern populations exhibiting the steepest shift between species (figure 3).
Although our knowledge of the historic distribution remains problematic, a lack of specimen-based records [63] suggest it was absent from this region until recently [64]. An ongoing range expansion by C. moreletii into southern Belize may have occurred when populations rebounded rapidly following legal protection in 1981 [34]. Expanding C. moreletii populations in Belize are heavily biased in favour of males [34,59,65].
Observed niche conservatism, in combination with known coastal development suggests that hybridization between C. moreletii and C. acutus in southern Belize may be driven at least partially by recent anthropogenic factors. The ongoing development of coastal and offshore nesting beaches used by C. acutus [33,66] might result in the dispersal of female C. acutus to less disturbed habitats in southern Belize where contact with male C. moreletii would be more likely.
Hybridization in animals is generally regarded as maladaptive because the fitness of hybrid progeny is often reduced [67]. However, in our study, hybrid crocodiles deposited significantly larger clutches than either C. moreletii or C. acutus, and despite considerable overlap in egg mass, eggs produced by hybrids were significantly larger than eggs of either parental species. Among hybrid Crocodylus in captivity, there is no evidence of decreased fitness or dysgenesis; in fact, hybrids produce high-quality skins, grow faster, exhibit enhanced survivorship and produce larger clutches than parental species [3,10]. Because egg size is positively correlated with hatchling size in crocodilians [68–70] and larger hatchlings exhibit accelerated growth and increased survivorship when compared to smaller hatchlings [71], the large eggs deposited by hybrid crocodiles in southern Belize may produce neonates with greater fitness than either C. moreletii or C. acutus hatchlings. Larger eggs often also contain more water, an advantage for dry season nesting when dehydration can reduce fitness if it interferes with embryonic development near the end of incubation [72,73]. Furthermore, hybridization of C. moreletii and C. acutus might impart increased salinity tolerance to the offspring, an obvious advantage for crocodiles living in coastal habitats [10]; however, hybrids found in this study exclusively were found in non-saline environments.
The reproductive consequences of hybridization in crocodilians are poorly understood [10]. While genetic data indicate backcrossing in the wild [12], we are unaware of any published reports describing nesting ecology among known hybrid crocodiles in the wild. We found that nest construction and nesting phenology of hybrid crocodiles in southern Belize had elements in common with both C. moreletii (mound) and C. acutus (sand), while egg and clutch attributes differed from either parental species. In Belize, C. acutus generally deposits clutches in shallow holes excavated in the deep sand, while C. moreletii constructs mound nests using adjacent vegetation [53,66]. Mound nesting behaviour has occasionally been noted among Florida populations of C. acutus [74] and is thought to be an adaptive response to nesting in areas where the probability of flooding is high [57].
Our genetic data for adults and embryos indicate that hybrid crocodiles in southern Belize are fertile and actively reproducing. Contrary to many hypotheses regarding reduced viability in hybrids, our data indicated that only 5% of eggs in hybrid nests were not viable as compared to a range of 8–10% for pure C. acutus [33] and 8% for pure C. morelleti [54]. Our data appear congruent with Rodriguez et al. [11] who concluded that hybrids were not being selected against.
4.1. Conservation implications
In Belize, populations of C. acutus and C. moreletii were nearly extirpated owing to over-harvesting by commercial skin hunters [60,75]. Legal protection was afforded to both species in 1981 and C. moreletii populations quickly rebounded [34]. However, recovery of C. acutus populations has been slow, largely owing to the continuing destruction of critical nesting habitat [33,66] occurring on the Atlantic and Pacific coasts of Mexico, Central America, as well as the Caribbean Islands of Cuba, Jamaica, Hispaniola and the southern tip of Florida, USA [57]. Despite the more restricted distribution of C. moreletii in the Atlantic and Caribbean lowlands of Mexico, Guatemala and Belize [3], recovery has been rapid.
In many instances, natural hybridization is part of the evolutionary process [76]. However, introgression between C. moreletii and C. acutus in southern Belize is potentially being driven by a combination of anthropogenic factors related to uneven rates of recovery for each species and ongoing destruction of C. acutus nesting habitat. Coastal land development and recreational use by tourists occurs predominantly along stretches of raised sandy beach ideal for nesting by C. acutus. The accelerated pace of development in coastal Belize over the past two decades may have displaced breeding females to less optimal nesting areas in the south.
Because of asymmetric breeding seasons, such displacement would result in female C. acutus in breeding condition encountering male C. moreletii prior to the onset of breeding condition in female C. moreletii [77]. While introgression of C. acutus genomic elements into C. moreletii populations appears unrelated to viability, our data suggest that hybridization may act as a sink for C. acutus populations if there is differential survival of hybrid offspring [78].
Hybridization is especially problematic when rare species come into contact with other species that are more abundant, and can result in the formation of localized hybrid swarms and eventual genetic swamping of the rarer species [76,79,80]. Given the rarity of C. acutus in the region [81], we agree with Cedeño-Vázquez et al. [10] that conservation efforts should be focused on this species, but at present management options to prevent further genetic introgression in southern Belize appear limited. Perhaps the best measure is to maintain and attempt to increase C. acutus populations on offshore islands and atolls where high salinities preclude the encroachment of C. moreletii. Population recruitment of C. acutus in Belize is best accomplished through protection of existing nesting beaches and associated nursery habitats that are critical for the survival of hatchlings [33,66] where anthropogenic occupation and alteration of brackish and estuarine waterways may impact important nursery areas for developing young.
Offshore populations of C. acutus are expected to remain genetically pure, and expanding populations could act as a source for dispersers destined for the mainland. However, if hybrid progeny exhibit increased salinity tolerance as suggested by Cedeño-Vázquez et al. [10], then offshore populations may not function as a genetic refuge for pure C. acutus in Belize.
Because of the potential consequences, additional studies to clarify the extent of hybridization and the forces driving hybridization in southern Belize are warranted. New surveys of southern Belize focused on identifying previously marked individuals and hybrid nests are necessary to determine the persistence of hybridization and survival rates of hybrids in the 15 years since surveys and sampling took place. Ultimately, hybridization presents a management problem for New World crocodiles and complicates identification of species based on morphology alone [5]. We concur with Rodriguez et al. [11] that future conservation of crocodilians will require genetic identification of pure populations, and ultimately management of those populations, while carefully considering the implications of both natural and anthropogenically reinforced hybridization.
Acknowledgements
Coral Cay Conservation, University College of Belize, Oceanic Society, Richard and Carol Foster, and Monkey Bay Wildlife Sanctuary generously provided logistic support. Comments from members of the IUCN Crocodile Specialist Group and two anonymous reviewers from AXIOS reviews improved an earlier draft of this manuscript. We dedicate this work to our friend and mentor John (T) Thorbjarnarson.
Ethics
Protocols for animal handling and collecting biological samples were approved by the Field Veterinary Program of the Wildlife Conservation Society. Research permits were issued by Emil Cano and Raphael Manzanero of the Conservation Division, Forest Department, Belmopan, Belize.
Data accessibility
The data supporting this article are available at the Dryad digital repository: http://dx.doi.org/10.5061/dryad.32h8t.
Authors' contributions
E.H., S.P., G.A. and J.T. conceived and facilitated the study; S.P., T.R. and J.T. conducted the fieldwork; E.H., S.P., T.R, M.T. and S.C. wrote the manuscript. E.H and S.C. conducted the genetic analyses; M.T., S.P. and C.T. conducted statistical analyses; all authors contributed to the revised manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding for this project was provided by the United Nations Development Program; The Wildlife Conservation Society; an EPA Star Fellowship to E.H.; The Sackler Institute for Comparative Genomics and Fordham University.
References
- 1.Lang JW. 1987. Crocodilian behaviour: implications for management. In Wildlife management: crocodiles and alligators (eds GJW Webb, SC Manolis, PJ Whitehead), pp 273–294.Sydney, Australia: Surrey Beatty & Sons. [Google Scholar]
- 2.Honegger R, Hunt R. 1990. Breeding crocodiles in zoological gardens outside the species range, with some data on the general situations in European zoos, 1989. In Crocodiles.Proc. of the 10th Working Meeting of the IUCN/SSC Crocodile Specialist Group, Gainesville, FL, USA, 1990, pp. 200–228. Cambridge, UK: IUCN.
- 3.Thorbjarnarson JB, Messel H, King FW, Ross JP. 1992. Crocodiles: an action plan for their conservation. Cambridge, UK: IUCN. [Google Scholar]
- 4.Fitzsimmons NN, Buchan JC, Lam PV, Polet G, Hung TT, Thang NQ, Gratten J. 2002. Identification of purebred Crocodylus siamensis for reintroduction in Vietnam. J. Exp. Zool. 294, 373–381. (doi:10.1002/jez.10201) [DOI] [PubMed] [Google Scholar]
- 5.Weaver JP, Rodriguez D, Venegas-Anaya M, Cedeño-Vázquez JR, Forstner MR, Densmore LD. 2008. Genetic characterization of captive Cuban crocodiles (Crocodylus rhombifer) and evidence of hybridization with the American crocodile (Crocodylus acutus). J. Exp. Zool. A Ecol. Genet. Physiol. 309, 649–660. (doi:10.1002/jez.471) [DOI] [PubMed] [Google Scholar]
- 6.Varona LS. 1987. The status of Crocodylus acutus in Cuba. Caribbean J. Sci. 23, 256–259. [Google Scholar]
- 7.Ramos R, de Buffrenil V, Ross J. 1994Current status of the Cuban crocodile, Crocodylus rhombifer, in the wild. In Crocodiles, Proc. of the 12th Working Meeting of the Crocodile Specialist Group, 1994, pp. 113–140. Darwin, Australia: Crocodile Specialist Group.
- 8.Ray DA, et al. 2004. Low levels of nucleotide diversity in Crocodylus moreletii and evidence of hybridization with C. acutus. Conserv. Genet. 5, 449–462. (doi:10.1023/B:COGE.0000041024.96928.fe) [Google Scholar]
- 9.Russello M, Brazaitis P, Gratten J, Watkins-Colwell G, Caccone A. 2007. Molecular assessment of the genetic integrity, distinctiveness and phylogeographic context of the saltwater crocodile (Crocodylus porosus) on Palau. Conserv. Genet. 8, 777–787. (doi:10.1007/s10592-006-9225-7) [Google Scholar]
- 10.Cedeño-Vázquez JR, Rodriguez D, Calme S, Ross JP, Densmore LD, Thorbjarnarson JB. 2008. Hybridization between Crocodylus acutus and Crocodylus moreletii in the Yucatan Peninsula: I. Evidence from mitochondrial DNA and morphology. J. Exp. Zool. A Ecol. Genet. Physiol. 309, 661–673. (doi:10.1002/jez.473) [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez D, Cedeño-Vázquez JR, Forstner MR, Densmore LD. 2008. Hybridization between Crocodylus acutus and Crocodylus moreletii in the Yucatan Peninsula: II. Evidence from microsatellites. J. Exp. Zool. A Ecol. Genet. Physiol. 309, 674–686. (doi:10.1002/jez.499) [DOI] [PubMed] [Google Scholar]
- 12.Milián-García Y, Ramos-Targarona R, Pérez-Fleitas E, Sosa-Rodríguez G, Guerra-Manchena L, Alonso-Tabet M, Espinosa-López G, Russello M. 2014. Genetic evidence of hybridization between the critically endangered Cuban crocodile and the American crocodile: implications for population history and in situ/ex situ conservation. Heredity 114, 272–280. (doi:10.1038/hdy.2014.96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross JP, Espinosa E. 1998. Crocodiles: status survey and conservation action plan. Gland, Switzerland: IUCN. [Google Scholar]
- 14.FitzSimmons N, Tanksley S, Forstner M, Louis E, Daglish R, Gratten J, Davis S. 2001. Microsatellite markers for Crocodylus: new genetic tools for population genetics, mating system studies and forensics. In Crocodilian biology and evolution (eds GC Grigg, F Seebacher, CE Franklin), pp. 51–57. Chipping Norton, UK: Surrey Beatty and Sons. [Google Scholar]
- 15.Hekkala E. 2004. Conservation genetics at the species boundary: case studies from African and Caribbean crocodiles (Genus: Crocodylus). New York, NY: Columbia University. [Google Scholar]
- 16.Schmidt KP. 1924. Notes on Central American crocodiles. Chicago, IL: University of Illinois. [Google Scholar]
- 17.Powell J. 1972. The Morelet’s crocodile: an unknown quantity. Smith III. Animal Kingdom. [Google Scholar]
- 18.Ross CA, Ross FD. 1974. Caudal scalation of central American Crocodylus. Proc. Biol. Soc. Wash. 87, 231–234. [Google Scholar]
- 19.Abercrombie CL, Davidson D, Hope CA, Scott DE. 1980. Status of Morelet’s crocodile Crocodylus moreleti in Belize. Biol. Conserv. 17, 103–113. (doi:10.1016/0006-3207(80)90040-3) [Google Scholar]
- 20.Ross FD, Mayer GC. 1983. On the dorsal armor of the Crocodilia. Smith VII. In Advances in herpetology and evolutionary biology (eds A Rhodin, K Miyata), pp. 305–331. Cambridge, MA: Museum of Comparative Zoology. [Google Scholar]
- 21.Platt SG. 1996. The ecology and status of Morelet’s crocodile in Belize. PhD dissertation, Clemson University, Clemson, SC, USA.
- 22.Sigler L. 1998. A Crocodylus acutus with the appearance of a C. moreletii. Crocodile Spec. Group Newslett. 173, 9–11. [Google Scholar]
- 23.Villegas A. 2005. Phenotypic characteristics of Crocodylus acutus and C. moreletii in South Quintana Roo. Crocodile Spec. Group Newslett. 24,8–9. [Google Scholar]
- 24.Cedeño-Vázquez JR, Ross JP, Calmé S. 2006. Population status and distribution of Crocodylus acutus and C. moreletii in southeastern Quintana Roo, Mexico. Herpetol. Nat. History 10, 17–30. [Google Scholar]
- 25.Nature IU. f. C. o., Resources N. 2008. IUCN red list of threatened species. Cambridge, UK: International Union for Conservation of Nature and Natural Resources. [Google Scholar]
- 26.Ross JP, Espinosa E. 1998. Crocodiles: status survey and conservation action plan, 2nd edn. Gland, Switzerland: IUCN/SSC Crocodile Specialist Group. [Google Scholar]
- 27.Platt S, Thorbjarnarson J. 1997. Status and life history of the American crocodile in Belize. Belize coastal zone management project BZE/92 G. 31.
- 28.Platt SG, Meerman JC, Rainwater TR. 1999. Diversity, observations and conservation of the herpetofauna of Turneffe, Lighthouse, and Glovers Atolls, Belize. Br. Herpetol. Soc. Bull. 66, 1–13. [Google Scholar]
- 29.McField M, Wells S, Gibson J. 1996. State of the coastal zone report, Belize, 1995. Coastal Zone Management Programme. Belize City: Belize: CZMAI. [Google Scholar]
- 30.Hartshorn G, et al. 1984. Belize country profile: a field study. Belize City, Belize: USAID and Robert Nicolait and Assoc., Ltd. [Google Scholar]
- 31.Stoddart DR. 1962. Three Caribbean atolls: Turneffe Islands, Lighthouse Reef, and Glover’s Reef. British Honduras: DTIC Document. [Google Scholar]
- 32.Zisman S, Planning F. 1992. Mangroves in Belize: their characteristics, use and conservation.Forest Planning and Management Project. Belmopan, Belize: Belize Forest Department. [Google Scholar]
- 33.Platt SG, Thorbjarnarson JB. 2000. Status and conservation of the American crocodile, Crocodylus acutus, in Belize. Biol. Conserv. 96, 13–20. (doi:10.1016/S0006-3207(00)00038-0) [Google Scholar]
- 34.Platt SG, Thorbjarnarson JB. 2000. Population status and conservation of Morelet’s crocodile, Crocodylus moreletii, in northern Belize. Biol. Conserv. 96, 21–29. (doi:10.1016/S0006-3207(00)00039-2) [Google Scholar]
- 35.Platt S. 2008. Scalation of Morelet’s crocodile (Crocodylus moreletii) from Northern Belize. Herpetol. Rev. 39, 293. [Google Scholar]
- 36.Platt S, Rainwater T. 2005. A review of morphological characters useful for distinguishing Morelet’s crocodile (Crocodylus moreletii) and American crocodile (Crocodylus acutus) with an emphasis on populations in the coastal zone of Belize. Bull. Chicago Herpetol. Soc. 40, 25–29. [Google Scholar]
- 37.Olson G, Hessler J, Faith R. 1975. Technics for blood collection and intravascular infusion of reptiles. Lab. Anim. Sci. 25, 783–786. [PubMed] [Google Scholar]
- 38.Jennings ML, David DN, Portier KM. 1991. Effect of marking techniques on growth and survivorship of hatchling alligators. Wildl. Soc. Bull. 19, 204–207. [Google Scholar]
- 39.Ferguson MW. 1985. Reproductive biology and embryology of the crocodilians. Biol. Reptilia 14, 329–491. [Google Scholar]
- 40.Hekkala E, et al. 2011. An ancient icon reveals new mysteries: mummy DNA resurrects a cryptic species within the Nile crocodile. Mol. Ecol. 20, 4199–4215. (doi:10.1111/j.1365-294X.2011.05245.x) [DOI] [PubMed] [Google Scholar]
- 41.Anderson E, Thompson E. 2002. A model-based method for identifying species hybrids using multilocus genetic data. Genetics 160, 1217– 1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155, 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nielsen EE, Bach LA, Kotlicki P. 2006. HYBRIDLAB (version 1.0): a program for generating simulated hybrids from population samples.Mol. Ecol. Notes 6, 971–973. (doi:10.1111/j.1471-8286.2006.01433.x) [Google Scholar]
- 44.Pearson RG, Raxworthy CJ. 2009. The evolution of local endemism in Madagascar: watershed versus climatic gradient hypotheses evaluated by null biogeographic models. Evolution 63, 959–967. (doi:10.1111/j.1558-5646.2008.00596.x) [DOI] [PubMed] [Google Scholar]
- 45.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. (doi:10.1002/joc.1276) [Google Scholar]
- 46.Hijmans RJ, van Etten J. 2012. Raster: geographic data analysis and modeling. R package v. 2.1–49.See https://cran.r-project.org/web/packages/raster/index.html.Illinois. [Google Scholar]
- 47.R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 48.Oksanen J. 2013. Vegan: ecological diversity. See hokudai. ac. jp (accessed 15 August 2014).
- 49.Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 26, 32–46. [Google Scholar]
- 50.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300. [Google Scholar]
- 51.Zar JH. 1999. Biostatistical analysis. The University of Michigan, MI: Pearson Education India. [Google Scholar]
- 52.Meredith RW, Hekkala ER, Amato G, Gatesy J. 2011. A phylogenetic hypothesis for Crocodylus (Crocodylia) based on mitochondrial DNA: evidence for a trans-Atlantic voyage from Africa to the New World. Mol. Phylogenet. Evol. 60, 183–191. (doi:10.1016/j.ympev.2011.03.026) [DOI] [PubMed] [Google Scholar]
- 53.Platt SG, Thorbjarnarson JB, Price A. 2000. Nesting ecology of the American crocodile in the coastal zone of Belize. Copeia. 2000,869–873. (doi:10.1643/0045-8511%282000%29000%5B0869%3ANEOTAC%5D2.0.CO%3B2) [Google Scholar]
- 54.Platt SG, Rainwater TR, Thorbjarnarson JB, McMurry ST. 2008. Reproductive dynamics of a tropical freshwater crocodilian: Morelet’s crocodile in northern Belize. J. Zool. 275, 177–189. (doi:10.1111/j.1469-7998.2008.00426.x) [Google Scholar]
- 55.Wirtz P. 1999. Mother species–father species: unidirectional hybridization in animals with female choice. Anim. Behav. 58, 1–12. (doi:10.1006/anbe.1999.1144) [DOI] [PubMed] [Google Scholar]
- 56.Grant PR, Grant BR. 1997. Mating patterns of Darwin’s finch hybrids determined by song and morphology. Biol. J. Linn. Soc. 60, 317–343. (doi:10.1111/j.1095-8312.1997.tb01499.x) [Google Scholar]
- 57.Thorbjarnarson JB. 1989. Ecology of the American crocodile, Crocodylus acutus. In Crocodiles, their ecology, management and conservation, a special publication of the Crocodile Specialist Group, pp. 228–259, Gland, Switzerland: IUCN Publications. [Google Scholar]
- 58.Perez-Higareda G, Rangel-Rangel A, Smith H. 1991. Maximum sizes of Morelet’s and American crocodiles. Bull. MD Herpetol. Soc. 27, 34–37. [Google Scholar]
- 59.Platt SG, Rainwater TR, Thorbjarnarson JB, Finger AG, Anderson TA, McMurry ST. 2009. Size estimation, morphometrics, sex ratio, sexual size dimorphism, and biomass of Morelet’s crocodile in northern Belize. Caribb. J. Sci. 45, 80–93. [Google Scholar]
- 60.Frost MD. 1974. A biogeographical analysis of some relationships between man, land, and wildlife in Belize (British Honduras). Corvallis, OR: Oregan State University. [Google Scholar]
- 61.Escobedo-Galvan A, Palacios-Chavez V, Vovides-Tejera A. 2008. Crocodylus moreletii (Morelet’s Crocodile) salinity tolerance. Herpetol. Rev. 39, 346–347. [Google Scholar]
- 62.Meerman J. 1992. The status of crocodiles in the eastern Corozal District. Occas. Papers Belize Nat. Hist. Soc. 1, 1–5. [Google Scholar]
- 63.Lee JC. 1996. The amphibians and reptiles of the Yucatan Peninsula. Ithaca, NY: Comstock. [Google Scholar]
- 64.Platt SG, Thorbjarnarson JB, Rainwater TR. 1999. Distribution of Morelet’s crocodile (Crocodylus moreletii) in southern Belize. Southwestern Nat. 44, 395–398. [Google Scholar]
- 65.Rainwater T, Platt S, McMurry S. 1998. A population study of Morelet’s crocodile (Crocodylus moreletii) in the New River watershed of northern Belize. In Proc. of the 14th Working Meeting of the Crocodile Specialist Group, 1998, pp. 206–220. Darwin, Australia: Crocodile Specialist Group.
- 66.Platt SG, Rainwater TR, Nichols S. 2004. A recent population assessment of the American crocodile (Crocodylus acutus) in Turneffe Atoll, Belize. Herpetolog. Bull. 89, 26–32. [Google Scholar]
- 67.Arnold ML. 1997. Natural hybridization and evolution: Oxford series in ecology and evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 68.Staton MA, Dixon JR. 1977. Breeding biology of the spectacled caiman, Caiman crocodilus crocodilus, in the Venezuelan Llanos. Darwin, Australia: Crocodile Specialist Group, Department of the Interior, US Fish and Wildlife Service. [Google Scholar]
- 69.Deitz DC, Hines TC. 1980. Alligator nesting in north-central Florida. Copeia 1980, 249–258. (doi:10.2307/1444001) [Google Scholar]
- 70.Campos Z, Magnusson W. 1995. Relationships between rainfall, nesting habitat and fecundity of Caiman crocodilus yacare in the Pantanal, Brazil. J. Trop. Ecol. 11, 351–358. (doi:10.1017/S0266467400008828) [Google Scholar]
- 71.Messel H, Vorlicek GC. 1989. Ecology of Crocodylus porosus in northern Australia. In Crocodiles: their ecology, management and conservation, pp. 164–183. Gland, Switzerland: IUCN. [Google Scholar]
- 72.Packard GC, Packard MJ, Miller K, Boardman TJ. 1987. Influence of moisture, temperature, and substrate on snapping turtle eggs and embryos. Ecology 68, 983–993. (doi:10.2307/1938369) [Google Scholar]
- 73.Campos Z, Magnusson W, Sanaiotti T, Coutinho M. 2008. Reproductive trade-offs in Caiman crocodilus crocodilus and Caiman crocodilus yacare: implications for size-related management quotas. Herpetol. J. 18, 91–96. [Google Scholar]
- 74.Kushlan JA, Mazzotti FJ. 1989. Population biology of the American crocodile. J. Herpetol. 23, 7–21. (doi:10.2307/1564310) [Google Scholar]
- 75.Charnock-Wilson J. 1970. Manatees and crocodiles. Oryx 10, 236–238. (doi:10.1017/S0030605300008498) [Google Scholar]
- 76.Allendorf FW, Leary RF, Spruell P, Wenburg JK. 2001. The problems with hybrids: setting conservation guidelines. Trends Ecol. Evol. 16, 613–622. (doi:10.1016/S0169-5347(01)02290-X) [Google Scholar]
- 77.Aguilar X, Casas-Andreu G. 1991. Crocodylus acutus (American crocodile) reproduction. Life history notes Herp. Rev. 22, 98. [Google Scholar]
- 78.Ralls K, Harvey PH, Lyles AM. 1986. Inbreeding in natural populations of birds and mammals. In Conservation biology: the science of scarcity and diversity (ed. ME Soule), pp. 35–56. Sunderland, MA: Sinauer Associates.
- 79.Rhymer JM, Simberloff D. 1996. Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 27, 83–109. (doi:10.1146/annurev.ecolsys.27.1.83) [Google Scholar]
- 80.Olden JD, Poff NL, Douglas MR, Douglas ME,Fausch KD. 2004. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19, 18–24. (doi:10.1016/j.tree.2003.09.010) [DOI] [PubMed] [Google Scholar]
- 81.Thorbjarnarson J, et al. 2006. Regional habitat conservation priorities for the American crocodile. Biol. Conserv. 128, 25–36. (doi:10.1016/j.biocon.2005.09.013) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this article are available at the Dryad digital repository: http://dx.doi.org/10.5061/dryad.32h8t.