Abstract
Background
Although prescription opioid use disorder has recently increased sharply in the United States, relatively little is known about the general well-being of this population. Assessment of quality of life in patients with substance use disorders has been recommended to improve clinical care.
Objectives
Health-related quality of life was examined in prescription opioid-dependent patients at entry to a national multi-site clinical trial, to compare quality of life scores in the study sample to other populations; further, background variables associated with quality of life in the literature were examined.
Methods
Prescription opioid-dependent patients (N=653) were compared to general populations on the Medical Outcome Study Short Form-36 (SF-36) quality of life measure; and the association between patient background variables and quality of life was examined.
Results
Compared to a general population, the current sample of prescription opioid-dependent patients had worse physical (−1.7 points, p<.001) and mental quality of life (−12.3 points, p<.001) as measured by the SF-36, similar to other opioid-use disorder populations. Within our sample, women showed more impairment than men in mental quality of life (−4.3 points, p<.001); older patients scored worse on physical (−5.2 points, p<.001), but not mental, quality of life. Chronic pain was associated with poorer physical quality of life (−9.0 points, p<.001).
Conclusions and scientific significance
The growing focus on wellness underscores the importance of measuring quality of life in addition to substance use outcomes. Routine assessment of health-related quality of life can add an important dimension to overall evaluation of patients’ treatment response.
INTRODUCTION
Rates of prescription opioid abuse and dependence have increased markedly in the United States during the past decade; over 1.5 million people had a prescription opioid use disorder in 2002, increasing to over 2 million people in 2012.1 Reasons proposed for this increase include new formulations of prescription opioids, a lower threshold on the part of many physicians to prescribe opioids for pain, and easier access to these medications.2 Calls to address the assessment needs of this growing population of patients who misuse primarily prescription opioids rather than heroin2,3 have been followed by research comparing prescription opioid-dependent patients to heroin users.4–7 For example, in comparison to heroin users, prescription opioid users have been found to be younger,5 more likely to be white,5 and more likely to report chronic pain4 and a history of psychiatric treatment4. They are also less likely to have ever used opioids intravenously7 or to have hepatitis C5 than heroin users.
Although these findings contribute to an understanding of prescription opioid dependent patients, relatively little is known about their general well-being. Over the last few decades, health-related quality of life has been found to predict subsequent mortality or physical pain in various disease-specific populations such as patients with liver disease8 and chronic pain.9 This evidence, combined with the national Healthy People 2010 goals for wellness,10 suggests that examination of health-related quality of life is important and timely and should be included in the assessment of drug-dependent patients.11 Indeed, health-related quality of life is an emerging area of research in the addiction field.12–14
Early studies of health-related quality of life in substance use disorder (SUD) patients reported worse scores compared to general populations15–17 and similar16 or worse17 scores compared to populations with other chronic diseases, based on one of the most widely used health-related quality of life measures, the Medical Outcome Study Short Form-36 (SF-36, or, alternatively, a 12- or 20-item version). These findings were based on samples with a variety of primary substances of abuse, potentially limiting their generalizability to specific SUD populations.
Studies focused on health-related quality of life exclusively in patients with opioid use disorders have consistently found worse scores for physical and mental domains compared to norms for the general population.18–23 Studies of the differences between those with opioid use disorders and patients with non-SUD psychiatric disorders have yielded mixed results and are limited to fewer domains.21,23 As in studies of health-related quality of life in other SUD populations, demographic variables may be associated with quality of life scores among opioid-dependent patients: studies generally suggest that women,19,24–26 whites,26 and older individuals19,20,24,27 exhibit worse quality of life.
While these studies suggest significantly impaired health-related quality of life in patients with opioid use disorders, most such studies either consider only those who primarily use heroin or fail to separate findings by heroin vs. prescription opioid use. One study that did focus on quality of life in prescription opioid abusers reported lower health-related quality of life compared to population norms on all domains of the SF-36: vitality, physical and social function, physical and emotional role limits, mental health, general health, and bodily pain; with women exhibiting more impairment than men.28 That study was limited, however, by its targeted sampling of patients in private treatment centers, limiting generalizability. A recent opioid-dependence treatment study found no difference in mental or physical domains between heroin-dependent patients and prescription opioid-dependent patients,6 although this study included only a small sample of prescription opioid users (n=61).
Because health-related quality of life measures have been useful in other SUD research but have received little attention to date in patients with prescription opioid-use disorders, utilization of these measures in this burgeoning population has been recommended. This represents an effort to evaluate the effect of treatments beyond a mere measure of amount of drug use, to assess such domains as consequences of use and overall functioning.29 Indeed, research shows that standard measures of drug consumption have not been associated with quality of life scores.15,23 In accordance with recommendations to assess quality of life in patients with SUD, we examined health-related quality of life in prescription opioid-dependent patients at entry to a large, national clinical trial.30 This study is novel in extending a validated measure to a new population, comparing scores of prescription opioid-dependent patients to general populations, and examining correlates of health-related quality of life.
METHODS
Data were collected as part of the Prescription Opioid Addiction Treatment Study sponsored by the National Drug Abuse Treatment Clinical Trials Network, a multi-site randomized controlled trial (N=653) that examined different lengths of buprenorphine-naloxone treatment and varying intensities of counseling in the treatment of patients with prescription opioid dependence (see details of the parent study30). At the time that the main trial was designed, most studies examining the role of counseling in patients receiving opioid agonist treatment had focused on heroin-dependent patients receiving methadone maintenance treatment. Only one study had examined different intensities of counseling in patients receiving office-based buprenorphine-naloxone; most were heroin users (86%).31 The only prior treatment study of prescription opioid users was a small feasibility study (n=15 nonrandomized patients), 7 of whom also used heroin.32 It is not clear that findings regarding either length of pharmacotherapy or role of counseling from previous studies could be generalized to patients seeking office-based buprenorphine-naloxone treatment for prescription opioid dependence. In light of the increased prevalence of prescription opioid dependent patients1 and the increasing use of buprenorphine (most commonly as buprenorphine-naloxone) by physicians in office-based treatment33, we decided to study different durations of buprenorphine-naloxone and different intensities of counseling in the treatment of patients dependent upon prescription opioids. After approval by the Institutional Review Board at each of ten sites across the United States, patients entering treatment for prescription opioid dependence at the study sites were recruited.
Participants from the Prescription Opioid Addiction Treatment Study were all included in this analysis (N=653); they met DSM-IV criteria for current opioid dependence and were at least 18 years of age. Key exclusion criteria included heroin use on ≥4 days in the past month, a lifetime diagnosis of opioid dependence due to heroin alone, a history of ever injecting heroin, a need for continued pain management with opioids, currently unstable psychiatric illness, or concurrent formal SUD treatment (see details30).
Measures
Quality of life was measured by the Medical Outcome Study Short Form-36 (SF-36) Health Survey,34 which was administered to all patients at intake to the treatment study. This measure was designed for a variety of uses, from policy evaluation to clinical practice, and has been widely used in a variety of populations; strong psychometric properties have been reported (e.g., McHorney and colleagues35). As expected, internal consistency was strong in our sample (Cronbach’s alpha=.88). A user’s manual36 presents scores derived from a national probability sample, balanced geographically, with a 68% response rate, for a general population (N=7003) and for subsets of that population, including a healthy population (n=1963) and disease-specific populations (e.g., depression; n=1006). Scores from our sample of prescription opioid users were compared to the population scores.
The Standard Version, which covers quality of life over the past four weeks, was used in the current study. Patients responded to 36 Likert-scaled items covering eight domains: vitality, physical and social function, physical and emotional role limits, mental health, general health, and bodily pain. Physical and Mental Component Summary scores were then computed. Scores are norm-based and range from 0–100 after transformation, with higher scores indicating better quality of life.
Additional standardized assessments were administered to all participants. The Composite International Diagnostic Interview37 was used to diagnose SUDs and other selected psychiatric disorders (major depressive disorder and posttraumatic stress disorder). The Pain and Opiate Analgesic Use History, developed for this study, was administered at baseline to assess opioid use history, with a focus on the relationship between pain and opioid use. The Fagerstrom Test for Nicotine Dependence38 is a 6-item measure with scores that range from 0–10, with higher scores indicating greater severity. The Addiction Severity Index-Lite (ASI)39 is a semi-structured interview that measures the severity of problems related to substance use across seven different domains: alcohol, drug, legal, medical, psychiatric, family/social, and employment. ASI composite scores range from 0 to 1, with higher scores indicating greater problem severity; this measure was used to assess external validity for the SF-36 in this new population.
Statistical analysis
Comparisons to other populations36 used z-tests, and associations between background variables and health-related quality of life used independent t-tests and analysis of variance; to adjust for multiple testing, we report only differences significant at the .001 level. Data were analyzed using SPSS v.20.40
RESULTS
Sample description
Most of the 653 study participants (91.3%) were white, and 40.0% were female. At baseline, mean age was 33.2 (sd=10.2), and mean years of education was 13.0 (sd=2.2). About half (49.9%) had never been married, and most (62.9%) were employed full-time. Approximately one-quarter (23.0%) reported lifetime heroin use; current chronic pain was common (42.0%). Oxycodone, extended-release, was the most commonly-abused opioid in the month prior to study entry (35.2% of the study population), followed by hydrocodone (32.3%), oxycodone, immediate-release, (18.7%), methadone (6.4%), morphine (2.1%), and other (5.3%).
Comparison to other populations
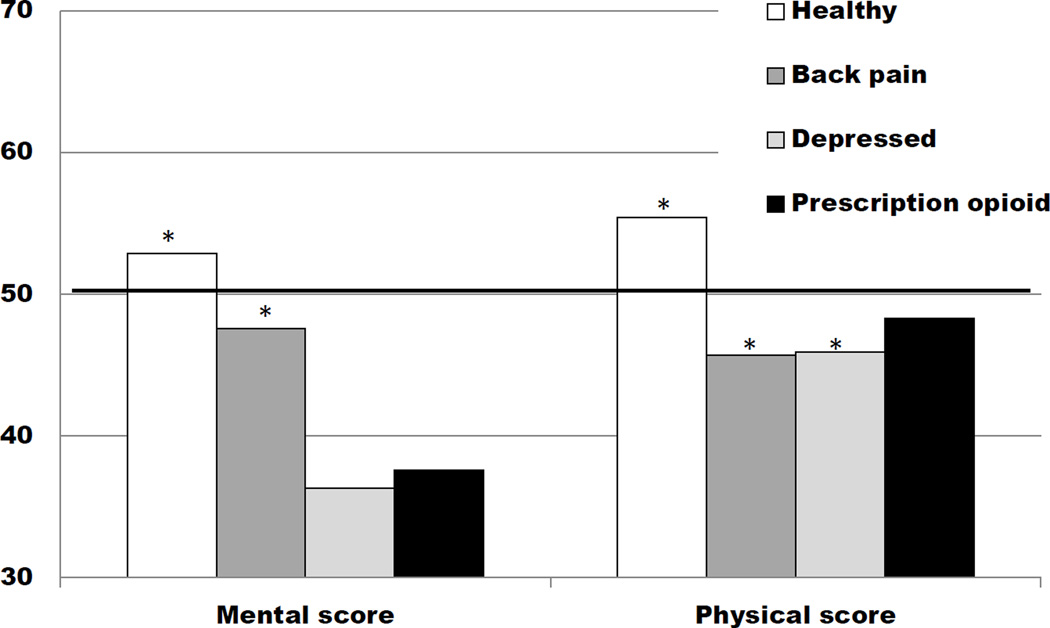
The SF-36 has been administered to large samples of the general population, as well as specific populations, permitting comparison of our sample to others. As expected, this sample of prescription opioid-dependent patients scored significantly worse (p<.001) than a healthy population and a general population on both the Mental (−15.3 points vs. healthy population; −12.3 vs. general population) and Physical Summary (−7.1 points vs. healthy population; −1.7 points vs. general population) scores; the differences were discernibly greater for the Mental Summary score (Fig. 1). Further, this sample scored significantly worse than a population with back pain, but similar to a depressed population on the Mental Summary score (−10.0 points vs. population with back pain, p<.001; +1.3 points vs. depressed population), and significantly better than both of these comparison populations on the Physical Summary score (+2.6 points vs. population with back pain; +2.4 points vs. depressed population; Fig. 1). The subset of patients in our study with a lifetime diagnosis of major depressive disorder (n=226) scored significantly worse on the Mental Summary score compared to a depressed population but were similar on the Physical Summary score (−5.2 points for Mental; and +1.5 points for Physical Summary scores).
Figure 1.
Quality of life Mental and Physical Component Summary scores for various populations29 compared to the prescription opioid samplea
aThe horizontal black line indicates general population means=50 (sd=10.0), by definition.
*p value <.001 compared to the prescription opioid sample
Male prescription opioid-dependent patients in our study sample scored significantly worse on the Physical Summary score than their gender-matched counterparts in a general population (−2.0 points, p<.001); they were similar on the Mental Summary score. Women in our study sample scored considerably worse on the Mental Summary score than a general population of women (−13.7 points, p<.001), but were similar on the Physical Summary score (−1.7 points).
Next, our patient sample was compared by age to a general population, using categories reported in the SF-36 manual.36 Only 1 patient was >64 years old, so the oldest age category was dropped. For each age category, the Physical and Mental Summary scores were significantly worse for the prescription opioid-dependent patients (with mean differences ranging from −3.6 to −5.0 points for Physical Summary scores and from −7.1 to −13.4 points for Mental Summary scores; all p values <.001), with one exception: the oldest age category (i.e., 55–64) was similar for the Physical Summary score. These age differences were also seen when men and women were examined separately.
Correlates of quality of life
Background characteristics that have been reported to be correlated with quality of life in other samples were examined in this population (Table 1). Men scored better than women on Mental Summary scores. Quality of life did not vary by race. Older age was associated with worse Physical Summary scores, as expected; age was not associated with Mental Summary score.
Table 1.
Quality of life Physical and Mental Component Summary scores (SF-36)a at baseline by background characteristics among prescription opioid-dependent patients (N=653)
| Background characteristics | Physical | Mental | |
|---|---|---|---|
| Gender | Male (392) | 49.0 (9.1) | 39.5 (12.3) |
| Female (261) | 47.3 (10.8) | 35.2 (13.0) b | |
| White race | Yes (596) | 48.5 (9.8) | 37.7 (12.6) |
| No (56) | 46.5 (10.5) | 38.1 (14.5) | |
| Age | 18–24 (150) | 49.9 (9.1) | 39.0 (12.2) |
| 25–34 (279) | 49.1 (9.6) | 37.2 (12.2) | |
| 35–44 (115) | 47.7 (10.7) | 36.7 (13.4) | |
| 45–54 (93) | 44.7 (10.1) c | 38.8 (14.6) | |
| 55–64 (15) | 46.5 (7.3) | 38.2 (10.6) | |
| Chronic pain | Yes (274) | 43.1 (9.6) | 37.6 (13.0) |
| No (379) | 52.1 (8.2) d | 37.9 (12.5) | |
| Non-opioid SUD | Yes (103) | 47.3 (9.7) | 32.5 (12.4) |
| No (550) | 48.5 (9.9) | 38.8 (12.6) e | |
| Heroin use, lifetime | Yes (150) | 49.4 (9.4) | 38.2 (12.9) |
| No (503) | 48.0 (10.0) | 37.6 (12.7) | |
| Smoker | Yes (489) | 48.2 (9.8) | 38.1 (12.7) |
| No (164) | 48.8 (10.2) | 36.8 (12.9) | |
| Major depressive disorder, lifetime | Yes (226) | 46.9 (9.8) | 31.1 (11.9) |
| No (427) | 49.1 (9.8) | 41.3 (11.7) f | |
| Major depressive disorder, past year | Yes (141) | 46.5 (9.5) | 27.3 (10.6) |
| No (512) | 48.8 (9.9) | 40.7 (11.7) g | |
Higher SF-36 scores indicate better quality of life, with a population average set to 50.
t(651)=−4.35, p<.001
F(4, 647)=4.91, p<.001; Post hoc tests showed that scores were worse for 45–54 year olds compared to the two youngest age categories (i.e., 18–24 and 25–34)
t(530.2)=12.59, p<.001
t(651)=4.65, p<.001
t(651)=10.56, p<.001
t(651)=12.25, p<.001
Other background characteristics of particular interest in understanding prescription opioid-dependent patients were examined, including chronic pain and use of other substances (Table 1). Patients with chronic pain scored worse on the Physical Summary score than those without chronic pain. Patients with an SUD other than opioid dependence scored worse on the Mental Summary score compared to patients dependent only on opioids. Quality of life did not vary by lifetime heroin use. While the dichotomous measure of smoking (yes or no) was not related to quality of life scores, the correlation between the Physical Summary score and the Fagerstrom Test for Nicotine Dependence among smokers was significant, with better quality of life scores (r=−.19, p<.001) associated with lower severity of nicotine dependence. Patients with major depressive disorder scored worse on the Mental Summary score, but not the Physical Summary score, whether this was a past-year or lifetime diagnosis.
Because the most consistent associations with SF-36 scores in this sample were for gender and chronic pain, we considered whether these variables might be associated. This was not the case: 44.4% of women and 40.3% of men had chronic pain (χ2(1)=1.10, p=.29). Further evidence of the independent association of gender and chronic pain with quality of life was provided by analyses adjusted for both variables.
DISCUSSION
This secondary analysis of a multi-site study of prescription opioid-dependent patients (N=653) evaluated their health-related quality of life, thus providing insight into the overall functioning of these patients.
Comparison to other populations
The current sample of prescription opioid-dependent patients had worse physical and mental quality of life than a healthy population or a general population,36 similar to other opioid-use disorder populations.18,20–22 However, they had better self-reported physical quality of life and similar mental quality of life compared to a depressed population, partially contradicting studies showing decrements in some physical and mental subscores among other opioid-use disorder populations20,23 when compared to depressed patients. This may reflect the relatively short opioid use histories of our sample and the fact that 68% of them were seeking opioid dependence treatment for the first time. Patients in the current sample with a lifetime diagnosis of major depressive disorder in addition to prescription opioid dependence, however, did score worse on the Mental Summary scale than the depressed comparison population, similar to reports on patients with a mix of SUDs.41
Correlates of quality of life
The association between quality of life and other baseline characteristics was largely consistent with existing literature on health-related quality of life in patients with SUD. In the current sample, women showed more impairment in health-related quality of life than men, although only Mental Summary scores were significant; this is similar to findings in the general population,36 as well as samples of patients with opioid dependence,26 prescription opioid abusers,24 patients receiving methadone maintenance treatment,25 and patients with a variety of other SUDs.17,42 Not surprisingly, given the small number of non-whites in this sample, scores were not significantly different across race. Other research has shown that older age was associated with worse physical and mental quality of life,19,24,27 whereas, in the current sample, older patients scored worse on the Physical Summary score only, similar to reports on heroin-dependent patients.20
Our finding of worse physical quality of life in patients with chronic pain is not surprising since people with chronic pain have more physical problems. This supports earlier studies showing that health-related quality of life is worse in patients with chronic pain compared to the general population.9,43 Other studies have shown that those with severe chronic pain were more likely to have mood and anxiety disorders,44 suggesting worse mental quality of life as well, unlike the patients in our study; perhaps patients with moderate pain such as those in our sample are less likely to have lower mental quality of life than those with severe pain. Further, chronic pain has been implicated as a significant motivating factor for initiating opioid use among patients with prescription opioid dependence.45
A lifetime history of heroin use was not associated with poorer health-related quality of life at baseline, perhaps because our sample excluded regular heroin users. This finding supports earlier literature demonstrating discrepancies between other measures of drug use severity (i.e., heroin use) and quality of life15 and adds to evidence suggesting that measures of health-related quality of life may provide additional useful information in determining functional status of patients.15,29
Although the presence or absence of current smoking was not related to health-related quality of life in this sample, higher scores on the Fagerstrom Test for Nicotine Dependence were associated with worse health-related quality of life. In contrast to our finding, other studies of health-related quality of life have shown that current smokers exhibit worse quality of life than nonsmokers on at least some domains of the SF-36.46,47 Our findings, however, lend support to studies (e.g., Laaksonen and colleagues46) showing that this difference is driven by heavy smokers.
Limitations
The SF-36 is one of the most widely-used health-related quality of life measures. Advantages include ease of administration (i.e., self-report, similar to other quality of life measures) and low time burden (i.e., 5–10 minutes for completion). Some researchers argue that an expanded construct of quality of life overall, not simply health-related quality of life, constitutes the most important measure of outcome.48 The current sample provides a broader perspective on the decrements in health-related quality of life among prescription opioid-dependent patients, but cannot be generalized to patients who abuse both heroin and prescription opioids regularly or to samples from different sociodemographic statuses.
Research & clinical implications
Examination of health-related quality of life has been shown to be useful over the last two decades due to its contribution to the quantification of costs,49 impairment,50 and changes in health status10 for a wide variety of illnesses. Indeed, health-related quality of life is one of the key patient-reported outcomes that have gained prominence in the evaluation of the impact of new treatments.51 Perhaps patient self-perception can be useful in assessing level of functional impairment and targeting areas to facilitate recovery in the clinical care of prescription opioid-dependent patients. This can be particularly germane for prescription-opioid dependent patients with chronic pain; while the proximal target of treatment may be the cessation or reduction of opioid use, the ultimate goal is, of course, improved functioning. Correlating reduction in opioid use with changes in functioning can help guide an overall treatment strategy. Indeed, research in depression and anxiety disorders52 has shown that quality of life assessments can add an important dimension to overall evaluation of patients’ treatment response, as symptom measures do not always present a complete picture of a patient’s overall functioning or recovery. Furthermore, brief, widely-used measures of health-related quality of life such as the SF-36 enable comparisons of these factors between different patient populations that can inform both clinical treatment selection and policy decisions about the allocation of health care resources.
Acknowledgements
This work was supported by National Institute on Drug Abuse (Rockville, MD) CTN grants U10 DA015831 (RDW), K24 DA022288 (RDW), and DA029115 (KPH).
We thank the staff and participants at the community treatment programs and regional research and training centers of the National Institute on Drug Abuse Clinical Trials Network for their involvement in this project, including Chestnut Ridge Hospital, San Francisco General Hospital, St. Luke’s Roosevelt Hospital, Long Island Jewish Medical Center-Addiction Recovery Services, Bellevue Hospital Center, McLean Hospital, East Indiana Treatment Center, Adapt Inc, UCLA Integrated Substance Programs, Behavioral Health Service of Pickens County, and Providence Behavioral Health Services. We also thank the staff of the Clinical Coordinating Center (The EMMES Corporation, Rockville, MD) and the Data and Statistics Center (The Duke Clinical Research Institute, Durham, NC), and the staff of the Center for the Clinical Trials Network at the National Institute on Drug Abuse (Rockville, MD) for their work on this project.
Dr. Weiss has served as a consultant to Titan Pharmaceuticals and Reckitt Benckiser.
Footnotes
Declaration of interest
The remaining authors report no conflicts of interest.
References
- 1.Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- 2.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on Problems of Drug Dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug Alcohol Depend. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 4.Brands B, Blake J, Sproule B, Gourlay D, Busto U. Prescription opioid abuse in patients presenting for methadone maintenance treatment. Drug Alcohol Depend. 2004;73:199–207. doi: 10.1016/j.drugalcdep.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen S, Hillhouse M, Mooney L, Fahey J, Ling W. Comparing buprenorphine induction experience with heroin and prescription opioid users. J Subst Abuse Treat. 2012;43:285–290. doi: 10.1016/j.jsat.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sigmon SC. Characterizing the emerging population of prescription opioid abusers. Am J Addict. 2006;15:208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- 8.Kanwal F, Gralnek IM, Hays RD, et al. Health-related quality of life predicts mortality in patients with advanced chronic liver disease. Clin Gastroenterol Hepatol. 2009;7:793–799. doi: 10.1016/j.cgh.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Bergman S, Jacobsson LT, Herrstrom P, Petersson IF. Health status as measured by SF-36 reflects changes and predicts outcome in chronic musculoskeletal pain: a 3-year follow up study in the general population. Pain. 2004;108:115–123. doi: 10.1016/j.pain.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Zahran HS, Kobau R, Moriarty DG, Zack MM, Holt J, Donehoo R. Health-related quality of life surveillance--United States, 1993–2002. MMWR Surveill Summ. 2005;54:1–35. [PubMed] [Google Scholar]
- 11.Laudet AB. The case for considering quality of life in addiction research and clinical practice. Addict Sci Clin Pract. 2011;6:44–55. [PMC free article] [PubMed] [Google Scholar]
- 12.Garner BR, Scott CK, Dennis ML, Funk RR. The relationship between recovery and health-related quality of life. J Subst Abuse Treat. 2014;47:293–298. doi: 10.1016/j.jsat.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karow A, Verthein U, Pukrop R, et al. Quality of life profiles and changes in the course of maintenance treatment among 1,015 patients with severe opioid dependence. Subst Use Misuse. 2011;46:705–715. doi: 10.3109/10826084.2010.509854. [DOI] [PubMed] [Google Scholar]
- 14.Pud D, Zlotnick C, Lawental E. Pain depression and sleep disorders among methadone maintenance treatment patients. Addict Behav. 2012;37:1205–1210. doi: 10.1016/j.addbeh.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Morgan TJ, Morgenstern J, Blanchard KA, Labouvie E, Bux DA. Health-related quality of life for adults participating in outpatient substance abuse treatment. Am J Addict. 2003;12:198–210. [PubMed] [Google Scholar]
- 16.Smith KW, Larson MJ. Quality of life assessments by adult substance abusers receiving publicly funded treatment in Massachusetts. Am J Drug Alcohol Abuse. 2003;29:323–335. doi: 10.1081/ada-120020517. [DOI] [PubMed] [Google Scholar]
- 17.Stein MD, Mulvey KP, Plough A, Samet JH. The functioning and well being of persons who seek treatment for drug and alcohol use. J Subst Abuse. 1998;10:75–84. doi: 10.1016/s0899-3289(99)80142-4. [DOI] [PubMed] [Google Scholar]
- 18.Astals M, Domingo-Salvany A, Buenaventura CC, et al. Impact of substance dependence and dual diagnosis on the quality of life of heroin users seeking treatment. Subst Use Misuse. 2008;43:612–632. doi: 10.1080/10826080701204813. [DOI] [PubMed] [Google Scholar]
- 19.Deering D, Frampton C, Horn J, Sellman D, Adamson S, Potiki T. Health status of clients receiving methadone maintenance treatment using the SF-36 health survey questionnaire. Drug Alcohol Rev. 2004;23:273–280. doi: 10.1080/09595230412331289428. [DOI] [PubMed] [Google Scholar]
- 20.Millson PE, Challacombe L, Villeneuve PJ, et al. Self-perceived health among Canadian opiate users: a comparison to the general population and to other chronic disease populations. Can J Public Health. 2004;95:99–103. doi: 10.1007/BF03405775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien S, Mattick RP, White J, et al. Maintenance pharmacotherapy for opioid dependence and SF-36 health status: a comparison with general population norms and other chronic disorders. Addict Disord Their Treat. 2006;5:155–164. [Google Scholar]
- 22.Rosen D, Smith ML, Reynolds CF., 3rd The prevalence of mental and physical health disorders among older methadone patients. Am J Geriatr Psychiatry. 2008;16:488–497. doi: 10.1097/JGP.0b013e31816ff35a. [DOI] [PubMed] [Google Scholar]
- 23.Ryan CF, White JM. Health status at entry to methadone maintenance treatment using the SF-36 health survey questionnaire. Addiction. 1996;91:39–45. doi: 10.1046/j.1360-0443.1996.911397.x. [DOI] [PubMed] [Google Scholar]
- 24.Cicero TJ, Surratt HL, Kurtz S, Ellis MS, Inciardi JA. Patterns of prescription opioid abuse and comorbidity in an aging treatment population. J Subst Abuse Treat. 2012;42:87–94. doi: 10.1016/j.jsat.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haug NA, Sorensen JL, Lollo ND, Gruber VA, Delucchi KL, Hall SM. Gender differences among HIV-positive methadone maintenance patients enrolled in a medication adherence trial. AIDS Care. 2005;17:1022–1029. doi: 10.1080/09540120500100882. [DOI] [PubMed] [Google Scholar]
- 26.Wu LT, Ling W, Burchett B, Blazer DG, Shostak J, Woody GE. Gender and racial/ethnic differences in addiction severity, HIV risk, and quality of life among adults in opioid detoxification: results from the National Drug Abuse Treatment Clinical Trials Network. Subst Abuse Rehabil. 2010;2010:13–22. doi: 10.2147/SAR.S15151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millson P, Challacombe L, Villeneuve PJ, et al. Determinants of health-related quality of life of opiate users at entry to low-threshold methadone programs. Eur Addict Res. 2006;12:74–82. doi: 10.1159/000090426. [DOI] [PubMed] [Google Scholar]
- 28.Cicero TJ, Lynskey M, Todorov A, Inciardi JA, Surratt HL. Co-morbid pain and psychopathology in males and females admitted to treatment for opioid analgesic abuse. Pain. 2008;139:127–135. doi: 10.1016/j.pain.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Tiffany ST, Friedman L, Greenfield SF, Hasin DS, Jackson R. Beyond drug use: a systematic consideration of other outcomes in evaluations of treatments for substance use disorders. Addiction. 2012;107:709–718. doi: 10.1111/j.1360-0443.2011.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 32.Sigmon SC, Dunn KE, Badger GJ, Heil SH, Higgins ST. Brief buprenorphine detoxification for the treatment of prescription opioid dependence: a pilot study. Addict Behav. 2009;34:304–311. doi: 10.1016/j.addbeh.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arfken CL, Johanson CE, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: National surveys of physicians. J. Subst. Abuse Treat. 2010;39:96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 35.McHorney CA, Ware JE, Jr, Rogers W, Raczek AE, Lu JFR. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30:MS253–MS265. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 36.Ware JE, Jr, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User's Manual for the SF-36v2 Health Survey. 2nd ed. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- 37.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 38.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 39.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 40.SPSS Statistics for Windows, Version 20.0 [computer program] Armonk, NY: IBM Corp; 2011. [Google Scholar]
- 41.Richter D, Eikelmann B, Berger K. Use of the SF-36 in the evaluation of a drug detoxification program. Qual Life Res. 2004;13:907–914. doi: 10.1023/B:QURE.0000025589.07313.46. [DOI] [PubMed] [Google Scholar]
- 42.Garg N, Yates WR, Jones R, Zhou M, Williams S. Effect of gender, treatment site and psychiatric comorbidity on quality of life outcome in substance dependence. Am J Addict. 1999;8:44–54. doi: 10.1080/105504999306072. [DOI] [PubMed] [Google Scholar]
- 43.Gormsen L, Rosenberg R, Bach FW, Jensen TS. Depression, anxiety, health-related quality of life and pain in patients with chronic fibromyalgia and neuropathic pain. Eur J Pain. 2010;14:e1.127–e8.127. doi: 10.1016/j.ejpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 44.McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106:127–133. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- 45.Weiss RD, Potter JS, Griffin ML, et al. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J Subst Abuse Treat. 2014;47:140–145. doi: 10.1016/j.jsat.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laaksonen M, Rahkonen O, Martikainen P, Karvonen S, Lahelma E. Smoking and SF-36 health functioning. Prev Med. 2006;42:206–209. doi: 10.1016/j.ypmed.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Sarna L, Bialous SA, Cooley ME, Jun HJ, Feskanich D. Impact of smoking and smoking cessation on health-related quality of life in women in the Nurses' Health Study. Qual Life Res. 2008;17:1217–1227. doi: 10.1007/s11136-008-9404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Maeyer J, Vanderplasschen W, Broekaert E. Quality of life among opiate-dependent individuals: A review of the literature. Int J Drug Policy. 2010;21:364–380. doi: 10.1016/j.drugpo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Bastida J, Linertova R, Oliva-Moreno J, Posada-de-la-Paz M, Serrano-Aguilar P. Social economic costs and health-related quality of life in patients with systemic sclerosis in Spain. Arthritis Care Res (Hoboken) 2014;66:473–480. doi: 10.1002/acr.22167. [DOI] [PubMed] [Google Scholar]
- 50.Stewart AL, Greenfield S, Hays RD, et al. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907–913. [PubMed] [Google Scholar]
- 51.Emery MP, Perrier LL, Acquadro C. Patient-reported outcome and quality of life instruments database (PROQOLID): frequently asked questions. Health Qual Life Outcomes. 2005;3:12. doi: 10.1186/1477-7525-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.IsHak WW, Mirocha J, Christensen S, et al. Patient-reported outcomes of quality of life, functioning, and depressive symptom severity in major depressive disorder comorbid with panic disorder before and after ssri treatment in the star*d trial. Depress Anxiety. 2014;31:707–716. doi: 10.1002/da.22152. [DOI] [PMC free article] [PubMed] [Google Scholar]