Abstract
There is inconsistent evidence for increased stress exposure among individuals at clinical high risk (CHR) for psychosis. Yet, similar to patients with a diagnosed psychotic illness, the preponderance of evidence suggests that CHR individuals tend to experience stressful life events (LE) and daily hassles (DH) as more subjectively stressful than healthy individuals. The present study utilizes data from the North American Prodrome Longitudinal Study Phase 2 (NAPLS-2) to test the hypotheses that 1) CHR individuals manifest higher self-reported stress in response to both LE and DH, when compared to healthy controls (HC), 2) group differences in self-reported stress increase with age, 3) baseline self-reported stress is associated with follow-up clinical status, and 4) there is a sensitization effect of LE on the response to DH. In contrast to some previous research, the present findings indicate that the CHR group (N= 314) reported exposure to more LE when compared to the HC group (N=162). As predicted, CHR participants rated events as more stressful, and those who progressed to psychosis reported a greater frequency of LE and greater stress from events compared to those whose prodromal symptoms remitted. There was also some evidence of stress-sensitization; those who experienced more stress from LE rated current DH as more stressful. The results indicate that the “prodromal” phase is a period of heightened stress and stress sensitivity, and elevated cumulative lifetime exposure to stressful events may increase reactions to current stressors.
Keywords: Clinical High Risk, Prodrome, Stress, PERI Life Events Scale, Daily Stress Inventory, Daily Hassles
1. Introduction
Etiological theories have posited that patients with psychotic disorders are vulnerable to psychosocial stress due to a congenital diathesis. Despite the theoretical assumption of a causal role for general life stress in the course of psychosis, Norman and Malla (1993) noted that exposure to life stress would not necessarily be expected to differ between diagnosed patients and controls, as patients are assumed to have an elevated vulnerability to psychosis, and hence require lower levels of stress to precipitate a psychotic episode. Further, among patients, prolonged hospitalizations and reduced social and occupational activities would be expected to decrease exposure to some life events (LE) (Heila et al., 1999).
Indeed, contemporary reviews suggest no consistent cross-sectional evidence that individuals with psychosis experience more recent LE (past 3 months to 1 year) than those without psychosis (Holtzman et al., 2012; Norman and Malla, 1993; Phillips et al., 2007). Yet, several retrospective and prospective studies have revealed elevations in psychosocial stressors preceding psychosis (Bebbington et al., 1993; Canton and Fraccon, 1985; Castine et al., 1998; van Winkel et al., 2008), although others do not (Horan et al., 2005). Thus, the results generally suggest that patients with psychosis are not necessarily exposed to more stressful LE (e.g., moving to a worse neighborhood, social exclusion), but may be more sensitive to them when they occur (Holtzman et al., 2012). Further, in the domain of negative life events (NLE) or ‘trauma,’ there is evidence that risk for psychosis is heightened among individuals who have experienced childhood trauma, such as abuse, with cumulative trauma exposure increasing risk (Galletly et al., 2011; Holtzman et al., 2013; Shevlin et al., 2008).
The evidence to date for increased exposure to stressful LE and daily hassles (DH) in clinical high risk (CHR) samples is also inconsistent (Aiello et al., 2012; Holtzman et al., 2013). Yet, similar to the findings with diagnosed patients, the preponderance of findings indicate that CHR individuals tend to experience stressful LE and DH as more subjectively stressful than healthy samples. In a review, Aiello and colleagues (2012) concluded that CHR groups manifest greater stress sensitivity than controls, as indexed by multiple measures (e.g., Experience Sampling Methods, metabolic stressor, and cortisol). Further, like diagnosed patients, research on CHR samples has shown a higher rate of self-reported childhood trauma exposure (Holtzman et al., 2013).
The present study utilizes data from the North American Prodrome Longitudinal Study, Phase 2 (NAPLS-2) to investigate stressful events and the subjective stress response in CHR participants. NAPLS-2 is a multi-site prospective longitudinal study of prodromal syndromes aimed at enhancing psychosis prediction and uncovering neural mechanisms of conversion (Addington et al., 2012). A recent study using this sample revealed significantly elevated cortisol levels in CHR individuals relative to healthy controls (HC) participants (Walker et al., 2013). Baseline cortisol levels were also found to be associated with interim clinical status; CHR participants in NAPLS-2 who progressed to psychosis had significantly higher baseline cortisol than those whose prodromal symptoms remitted.
In this report, we test the following hypotheses. First, based on the past literature, it is predicted that CHR individuals will manifest higher self-reported stress than HC in response to both LE and DH. Second, it is predicted that group differences in self-reported stress will increase with age through adolescence and young adulthood. Age-related increases in stress exposure (particularly trauma exposure) have been demonstrated in clinical and healthy samples (Finkelhor et al., 2009), likely due to increased opportunity to experience stressors as development progresses and role responsibilities broaden (Aldwin, 2011). Third, it is predicted that higher baseline stress will be associated with poorer clinical status at follow-up. Finally, the current research examines the potential sensitization effect of LE on subjective stress from DH (van Winkel, Stefanis, & Myin-Germeys, 2008).
2. Method
2.1 Sample
Participants were recruited as part of NAPLS-2 (Addington et al., 2012), which at the halfway mark included 540 individuals. This study presented here included those subjects with baseline self-report ratings of LE and DH. These data were available for 476 participants; 314 CHR participants (58.6% male) who met prodromal syndrome criteria and 162 HC participants (48.3% male). The age range of participants at baseline was 12 to 35 years, with a mean age of 18.99 years (SD 4.18) for the CHR group and 19.54 years (SD 4.77) for the HC group. The protocol was approved by Institutional Review Boards at all NAPLS sites (Addington et al., 2012). All participants provided informed consent or assent.
As of this writing, 296 individuals in the present CHR group where either followed at least 24-months without conversion to psychosis or were documented to have developed psychosis within the follow-up period or subsequent to it. Thus the outcome classification is based on the most recently available data on conversion for the present sample. CHR participants were classified as manifesting prodromal stabilization or progression (i.e., exhibiting symptoms in the prodromal range [3-5 in severity] on the SOPS), psychotic (i.e., currently meeting criteria for a psychotic disorder or evidencing scores of 6 on one or more SOPS positive symptoms), or in remission (i.e., scores of 2 or less on the five SOPS positive symptoms scales). Clinical status data yielded the following groups: remission=91; prodromal stabilization or progression= 160; and psychotic= 45.
2.2 Assessment Procedures and Measures
Participants were interviewed using the Structured Interview for Prodromal Syndromes (SIPS) (Miller et al., 2003). Interview responses were then quantified by trained interviewers on the Scale of Prodromal Symptoms (SOPS, Miller et al., 2003). The SOPS provides an index of symptom severity that ranges from 0 (absent) to 6 (severe, psychotic).
A detailed description of the study measures and procedures is presented elsewhere (Addington et al., 2012). In brief, general exclusions included an Axis I psychotic disorder, substance dependence, neurological disorder or full scale IQ <70. HC were excluded if they had a first-degree relative with a current or past psychotic disorder, or met prodromal criteria.
Study participants completed a modified version of the Psychiatric Epidemiology Research Interview Life Events Scale (LES) (Dohrenwend et al., 1978) and the Daily Stress Inventory (DSI) (Brantley et al., 1987) at baseline. The LES was modified to exclude items that would be of unlikely relevance to the adolescent/young adult age range included in this study (e.g., getting a divorce, encountering serious financial loss). The modified version of the LES included 59-items pertaining to significant events or life changes that could conceivably be experienced at any of the ages included in the study sample. Events on the LES have been designated as “independent” of or “dependent” on an individual's characteristics. Items are also classified as positive or negative (Dohrenwend et al., 1978). Participants indicated whether the LE occurred at any point in their life. Interviewers queried participants about their level of subjective stress for each LE endorsed on a 7-point Likert scale ranging from “occurred, but was not very stressful” to “caused me to panic.”
The Daily Stress Inventory (DSI) is a 58-item measure of minor, common DH occurring within the past 24 hours. Examples of such items include “was interrupted during task/activity,” “was criticized or verbally attacked,” and “had your sleep disturbed.” Participants indicated if the event occurred and rated each endorsed DH on a same 7-point Likert scale as described above.
2.3 Data Analyses
Statistical analyses were conducted with PASW statistics 18 statistical software (SPSS Inc., Chicago, Illinois). Independent sample t tests or chi-square tests were used to compare the CHR and HC groups on demographic characteristics. Analyses of covariance (ANCOVA) were used to test group differences in the frequency of stressful LE, DH, and the self-reported stress ratings. Stress data were normalized using a logarithmic transformation. All ANCOVAS included sex as a covariate. Further, the statistical analyses of subjective stress included the frequency of LE or DH as covariates, in order to test for group differences in sensitivity to stressful events/hassles, independent of the frequency of events. For follow-up clinical status, comparisons were tested for 1) remission vs. stabilization/progression, 2) remission vs. psychotic, and 3) stabilization/progression vs. psychotic for stress measures. Because of the inclusion of covariates, analyses were conducted within the ANCOVA framework. Cohen's d effect sizes were calculated. Regression analyses were conducted to test the predictive power of the frequency of cumulative LE and subjective stress from LE on the subjective stress from DH. Analyses included sex and the frequency of DH as covariates by entry in the first block.
3. Results
3.1 Demographic Characteristics of Diagnostic Groups
Consistent with the recently published overview of NAPLS (Addington et al., 2012), the CHR and HC groups did not differ with respect to age or ethnicity (p=0.19, p=.45, respectively). CHR and HC in the current analyses significantly differed with respect to the sex ratio (p=.02), such that the CHR group included a greater proportion of males than the HC group.
3.2 Baseline Stress
As shown in Table 1, analyses revealed high positive inter-correlations among the frequency of positive negative, independent, and dependent LE endorsed. Given this, present analyses focused on the total score from the LES.
Table 1.
Correlations among Dependent, Independent, Positive, and Negative Life Event Subscales
| HC | CHR | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Dependent | --- | .489** | .842** | .853** | --- | .559** | .812** | .881** |
| Independent | --- | --- | .365** | .709** | --- | --- | .437** | .721** |
| Positive | --- | --- | --- | .550** | --- | --- | --- | .569** |
| Negative | --- | --- | --- | --- | --- | --- | --- | --- |
1=Dependent; 2=Independent; 3=Positive; 4=Negative
Significant at .01
Preliminary analyses were conducted to identify LES and DSI correlates for inclusion as covariates. Some CHR participants were on psychotropic medications at baseline that may impact self-report and self-appraisal of events. Analyses revealed significant relationships of antidepressants, antipsychotics, and benzodiazepines with stress measures; generally those on medication had higher scores (see Table 2 for medication effects). As appropriate for the dependent measure, medication was included as a covariate in subsequent analyses. Consistent with previous reports on healthy and clinical samples, preliminary analyses of sex differences revealed that female CHR participants reported more subjective stress from DH than male participants (t (262) =−1.737, p=.042). Although sex did not reach significance for any other measure, trends were in the direction of female participants reporting more stress. Sex was included as a covariate in subsequent analyses.
Table 2.
Mean difference in baseline LE and DH between those on and off medication.
| Antidepressants (18%) | Benzodiazepine (7%) | Antipsychotics (17%) | |
|---|---|---|---|
| Number of LE | −.05 | −.09 | .04 |
| Subjective stress from LE | −.12** | −.23** | −.002 |
| Number of DH | −.08* | −.13* | .09* |
| Subjective stress from DH | −.15** | −.24* | .10 |
Note: Antipsychotic=only CHR group; negative mean difference indicates higher scores in those on medication
p<.05
p<.01
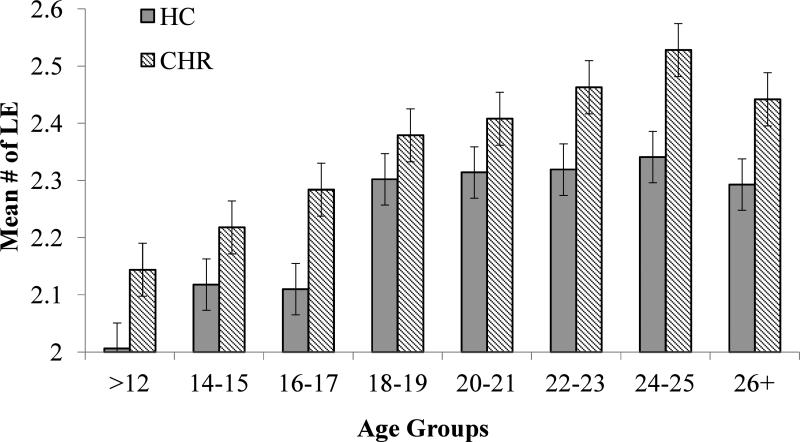
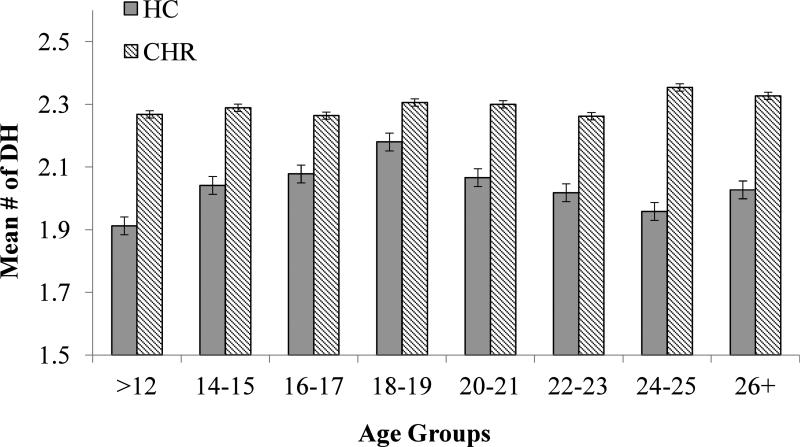
Mean LE and DH frequencies, by diagnostic group are presented in Figures 1 and 2, respectively. Analyses of covariance (ANCOVA) were conducted on the frequency of LE and DH endorsed, with sex as a covariate for LE, and sex, antidepressants, and antipsychotics for DH. Results revealed a main effect of group, such that CHR participants reported significantly more LE (F (1,459) =26.292, p< .000) and more DH (F (1,425) =52.236, p<.000) than HC participants. There was also a main effect of age on the number of self-reported LE, such that the lifetime frequency of events increased with age (F (7, 459) =10.903; p<.000) among CHR and HC participants. There was no significant Age X Group interaction for LE. In contrast, for DH frequency there was no main effect of age, nor a significant Age X Group interaction.
Figure 1.
Frequency of total LE by age in CHR and HC Groups.
Figure 2.
Frequency of DH by Age in CHR and HC Groups.
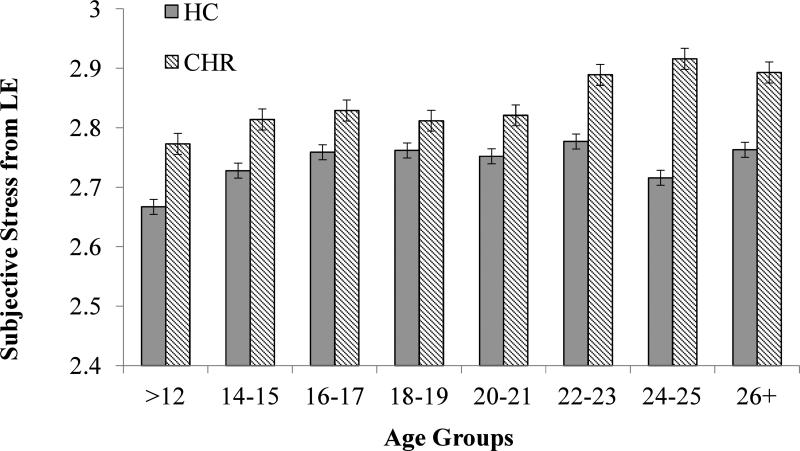
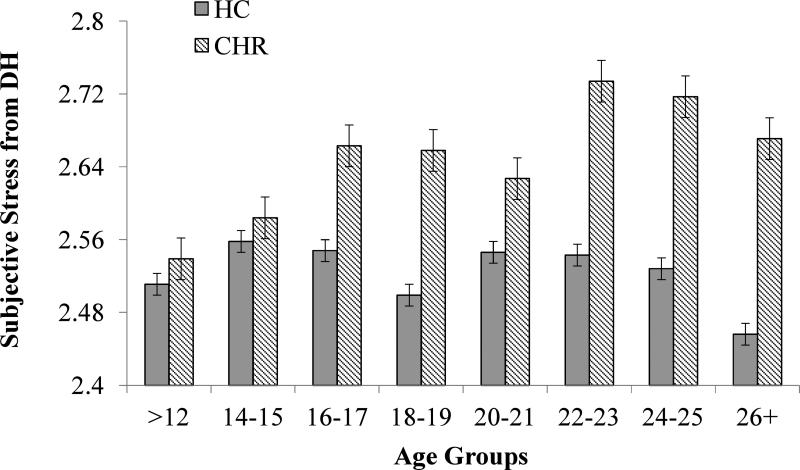
Mean subjective stress ratings for LE and DH by diagnostic group are presented in Figures 3 and 4, respectively. ANCOVA of LE stress ratings, with frequency of LE, medication, and sex as covariates, revealed main effects of group and age, but no interaction. CHR participants reported greater subjective stress from LE (F (1, 431) =37.918, p< .000) and self-reported stress increased with age for both groups (F (7, 431) =2.012, p=.052). Similarly, ANCOVA of DH subjective stress ratings, with frequency of DH, medication, and sex as covariates, revealed a main effect of group (F (1, 366) =31.432, p<.000). The Age X Group interaction showed trend-level significance (F (7, 366) =1.688, p=.111), in that CHR participants showed an age-related increase in self-reported subjective stress from DH (F (7, 251) =2.772, p=.009), whereas HC participants showed no increase related to age (F (7, 112) =.787, p=.600).
Figure 3.
Subjective stress from LE by Age in CHR and HC Groups.
Figure 4.
Subjective stress from DH by Age in CHR and HC Groups.
3.4 Follow-up Clinical Status
ANCOVA of the baseline stress measures with sex and medication revealed significant differences among follow-up clinical status groups (LE frequency: F (3,436) =8.691, p< .000; LE stress: F (3,413) =12.243, p< .000; DH Frequency: F (3,405) =18.507, p< .000; DH stress: F (3,351) =12.158, p< .000) (see effect sizes in Table 3). As shown, the remitted CHR group reported fewer LE and DH, and less stress from LE and DH compared to the prodromal stabilization/progression and psychotic groups. Those who showed a psychotic level of symptom severity at the most recent follow-up reported greater stress in response to LE and DH when compared to those who continued to exhibit prodromal level symptoms.
Table 3.
Effect sizes for CHR follow-up clinical status for differences in baseline LE and DH
| R<P | P<PS | R<PS | |
|---|---|---|---|
| Number of LE | .25* | .14 | .41* |
| Stress from LE | .36** | .25* | .63** |
| Number of DH | .37** | .15 | .50** |
| Stress from DH | .37** | .33** | .74** |
R=remission, P=prodromal progression and stabilization; PS=psychotic
p<.05
p<.01
3.5 Stress Sensitization: Cumulative LE and Current Subjective Stress
Regression analyses were conducted on stress ratings of DH, statistics for predictors are presented in Table 4. For the model that included only sex as a covariate, the frequency of total LE was a significant predictor of subjective stress from DH in HC (R2 =.129, F (2,119) =8.842, p< .000) and CHR (R2 =.063, F (2,258) =8.719, p< .000) groups. The pattern was the same for analyses with LE stress ratings as the predictor for both groups (HC: R2 =.213, F (2,117) =15.727, p< .000; CHR R2 =.135, F (2,248) =19.404, p< .000).
Table 4.
Regression of total LE and stress from LE on stress from DH.
| Covariates: | Sex | Sex, Frequency of DH | ||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| HC Group | ||||||
| Total LE Frequency | .360 | 4.206 | .000 | .068 | 1.998 | .048 |
| Subjective Stress of LE | .463 | 5.608 | .000 | .100 | 2.869 | .005 |
| CHR Group | ||||||
| Total LE Frequency | .227 | 3.771 | .000 | .029 | 1.072 | .285 |
| Subjective Stress of LE | .352 | 5.946 | .000 | .102 | 3.642 | .000 |
The pattern of results changed when both sex and the frequency of DH were entered as covariates. Although both models were significant, the frequency of total LE predicted current stress from DH for the HC group, but not for CHR (HC: R2 =.879, F (3,118) =285.563, p< .000; CHR: R2 =.815, F (3,257) =377.923, p< .000). In contrast, subjective stress from LE predicted DH stress in both groups (R2 =.884, F (3,115) =291.380, p< .000; R2 =.823, F (3,247) =382.187, p< .000).
4. Discussion
Consistent with diathesis-stress models, the present investigation found that CHR individuals report more subjective stress in response to LE and DH. In contrast to some previous reports on the frequency of recent stressors, the present findings also indicate that CHR adolescents and young adults are exposed to more cumulative LE when compared to HC.
There are several factors that may account for the discrepancy in findings with regard to the frequency of stressful event exposure. First, the present sample is larger than that used in the majority of previous studies, affording greater power for detecting significant relationships. Second, the duration of time and stage of illness selected for the measurement of LE may play an important role in study findings (Horan et al., 2005; Norman and Malla, 1993; Phillips et al., 2007). The present study focused on cumulative LE, whereas some previous reports focused on a narrow window in close proximity to illness onset, which could be problematic in illnesses characterized by a reduction in functional capacity that limits recreational and occupational activities (Harvey et al., 2009). In other words, the frequency of exposure to LE stressors may be elevated and serve as a precipitating factor in the premorbid phase, but in the later prodromal stages gradual withdrawal from activities may reduce stress exposure (Norman and Malla, 1993). In sum, it is possible that mixed findings on rates of LE stress exposure reflect changes in the likelihood of stress exposure as one progresses through the illness stages, resulting in varied patterns depending on the age and illness stage of the sample.
There is a general consensus that patients with psychosis are more susceptible than HC to subjective stress from major and minor events and hassles. Consistent with this body of work, the current investigation showed that, even after accounting for the number of events, CHR participants rate events as more subjectively stressful than HC. Further, while subjective stress from LE exposure increased with age in both groups, only the CHR group showed a trend toward an age-related increase in stress from DH. However, because the present stress data are cross sectional, rather than longitudinal, it is not possible to test for differences in stress changes over time as a function of outcome group. CHR individuals who are closer to the greatest risk period for psychosis onset may have had longstanding elevations in stress from LE and DH, or may increase in conjunction with the transition to psychosis. When longitudinal data on stress are available for the entire sample, future analyses will address the issue of changes over time in relation to outcome.
Nonetheless, it appears that both the frequency of LE and the subjective stress they generate may play a role in determining the diagnostic course for CHR individuals. Specifically, this study demonstrated that both the frequency and subjective stress from LE and DH differentiated CHR individuals who remitted from those who continued to meet prodromal criteria or progressed to a psychotic level of symptom severity. As might be expected, those who progressed to psychosis by the most recent follow-up reported greater subjective stress compared to those who remained at a prodromal level of symptom severity. These findings are consistent with the notion that studies using cross-sectional designs in the measurement of both stress and clinical status may have underestimated the link between psychosocial stress and psychosis (Walker et al., 2008).
As mentioned, Van Winkel and others (2008) proposed a sensitization effect of LE, suggesting that it is in fact the cumulative effect of stress exposure on later stress sensitivity that is important in the development of illness. For example, LE occurring in the past year predicted emotional reactivity to minor DH in diagnosed schizophrenia patients (Myin-Germeys et al., 2003). The current study yielded some support for the notion of stress sensitization in risk for psychosis; cumulative LE subjective stress, was a significant predictor of current stress from DH, for both the HC and the CHR group. Further, this held when controlling for the frequency of DH. Because the correlation between the frequency of DH and the subjective stress from DH is high (r =.92); controlling for the frequency of DH is a very conservative approach that constrains the variance in DH stress, the dependent variable. Nonetheless, consistent with previous reports in other clinical and nonclinical samples, the results suggest a stress-sensitization effect, albeit one that is not specific to the CHR sample (Monroe and Harkness, 2005), although it would be expected to be amplified in the CHR group because this group is characterized by a significantly higher overall level of LE and DH stress.
The current study improves on the extant literature on stress and psychosis risk with cumulative measurement of LE in a large CHR sample, but it is not without limitation. Like other reports, the current study relied on self-report of LE and DH. Self-report instruments are subject to recall errors and bias, which may be exacerbated by psychiatric symptoms and compromise reliability (Dohrenwend, 2006). Nonetheless, the present findings replicate and extend past findings and highlight the relevance of stress in the etiology of psychosis. As described in a recent NAPLS report on cortisol levels in CHR youth (Walker et al., 2013), it is assumed that the hypothalamic-pituitary-adrenal axis is one of the biological systems mediating the adverse effects of stress on psychiatric outcome. Future studies will test this assumption, as well as other questions related to mediating pathways in stress exposure and sensitivity. It should also be noted that the present study focuses only on the first half of the targeted NAPLS-2 sample, and the current clinical status categories only includes those non-converting participants that have been followed at least 24-months. Thus, additional conversions would be expected as more participants are followed up to and beyond the 24 month period, and this will allow for greater power in testing mediating factors.
Acknowledgments
This research was supported in part by Grant U01MHMH081988 from the National Institute of Mental Health awarded to the third author, Elaine F. Walker, Ph.D.
Glossary
- LE
life events
- DH
daily hassles
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, Cannon TD. North american prodrome longitudinal study (napls 2): Overview and recruitment. Schizophr Res. 2012;142(1-3):77–82. doi: 10.1016/j.schres.2012.09.012. 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello G, Horowitz M, Hepgul N, Pariante CM, Mondelli V. Stress abnormalities in individuals at risk for psychosis: A review of studies in subjects with familial risk or with “at risk” mental state. Psychoneuroendocrinology. 2012;37(10):1600–1613. doi: 10.1016/j.psyneuen.2012.05.003. 1016/j.psyneuen.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Bebbington P, Wilkins S, Jones P, Foerster A, Murray R, Toone B, Lewis S. LE and psychosis. Initial results from the camberwell collaborative psychosis study. The British journal of psychiatry : the journal of mental science. 1993;162:72–79. doi: 10.1192/bjp.162.1.72. [DOI] [PubMed] [Google Scholar]
- Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A daily stress inventory: Development, reliability, and validity. Journal of behavioral medicine. 1987;10(1):61–74. doi: 10.1007/BF00845128. [DOI] [PubMed] [Google Scholar]
- Canton G, Fraccon IG. LE and schizophrenia. A replication. Acta psychiatrica Scandinavica. 1985;71(3):211–216. doi: 10.1111/j.1600-0447.1985.tb01276.x. [DOI] [PubMed] [Google Scholar]
- Castine MR, Meador-Woodruff JH, Dalack GW. The role of LE in onset and recurrent episodes of schizophrenia and schizoaffective disorder. Journal of psychiatric research. 1998;32(5):283–288. doi: 10.1016/S0022-3956(98)00017-X. 10.1016/S0022-3956(98)00017-X. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful LE as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological bulletin. 2006;132(3):477–495. doi: 10.1037/0033-2909.132.3.477. 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BS, Krasnoff L, Askenasy AR, Dohrenwend BP. Exemplification of a method for scaling LE: The peri LE scale. Journal of health and social behavior. 1978;19(2):205–229. [PubMed] [Google Scholar]
- Galletly C, Van Hooff M, McFarlane A. Psychotic symptoms in young adults exposed to childhood trauma--a 20 year follow-up study. Schizophr Res. 2011;127(1-3):76–82. doi: 10.1016/j.schres.2010.12.010. 10.1016/j.schres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Helldin L, Bowie CR, Heaton RK, Olsson AK, Hjarthag F, Patterson TL. Performance-based measurement of functional disability in schizophrenia: A cross-national study in the united states and sweden. The American journal of psychiatry. 2009;166(7):821–827. doi: 10.1176/appi.ajp.2009.09010106. 10.1176/appi.ajp.2009.09010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heila H, Heikkinen ME, Isometsa ET, Henriksson MM, Marttunen MJ, Lonnqvist JK. LE and completed suicide in schizophrenia: A comparison of suicide victims with and without schizophrenia. Schizophrenia bulletin. 1999;25(3):519–531. doi: 10.1093/oxfordjournals.schbul.a033398. [DOI] [PubMed] [Google Scholar]
- Holtzman CW, Shapiro DI, Trotman HD, Walker EF. Stress and the prodromal phase of psychosis. Curr Pharm Des. 2012;18(4):527–533. doi: 10.2174/138161212799316280. [DOI] [PubMed] [Google Scholar]
- Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, Walker EF. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–191. doi: 10.1016/j.neuroscience.2012.12.017. 10.1016/j.neuroscience.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Ventura J, Nuechterlein KH, Subotnik KL, Hwang SS, Mintz J. Stressful LE in recent-onset schizophrenia: Reduced frequencies and altered subjective appraisals. Schizophr Res. 2005;75(2-3):363–374. doi: 10.1016/j.schres.2004.07.019. 10.1016/j.schres.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophrenia bulletin. 2003;29(4):703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychol Rev. 2005;112(2):417–445. doi: 10.1037/0033-295X.112.2.417. 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Krabbendam L, Delespaul PA, Van Os J. Do LE have their effect on psychosis by influencing the emotional reactivity to daily life stress? Psychol Med. 2003;33(2):327–333. doi: 10.1017/s0033291702006785. [DOI] [PubMed] [Google Scholar]
- Norman RM, Malla AK. Stressful LE and schizophrenia. I: A review of the research. The British journal of psychiatry : the journal of mental science. 1993;162:161–166. doi: 10.1192/bjp.162.2.161. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: Towards the development of new models of investigation. Clinical psychology review. 2007;27(3):307–317. doi: 10.1016/j.cpr.2006.10.003. 10.1016/j.cpr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Shevlin M, Houston JE, Dorahy MJ, Adamson G. Cumulative traumas and psychosis: An analysis of the national comorbidity survey and the british psychiatric morbidity survey. Schizophrenia bulletin. 2008;34(1):193–199. doi: 10.1093/schbul/sbm069. 10.1093/schbul/sbm069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Winkel R, Stefanis NC, Myin-Germeys I. Psychosocial stress and psychosis. A review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophrenia bulletin. 2008;34(6):1095–1105. doi: 10.1093/schbul/sbn101. 10.1093/schbul/sbn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual review of clinical psychology. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, Woods SW. Cortisol levels and risk for psychosis: Initial findings from the north american prodrome longitudinal study. Biol Psychiatry. 2013;74(6):410–417. doi: 10.1016/j.biopsych.2013.02.016. 10.1016/j.biopsych.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]