Abstract
Background
Rewarming from hypothermia during cardiopulmonary bypass (CPB) may compromise cerebral oxygen balance potentially resulting in cerebral ischemia. The purpose of this study was to evaluate whether CPB rewarming rate is associated with cerebral ischemia assessed by the release of the brain injury biomarker glial fibrillary acidic protein (GFAP).
Methods
Blood samples were collected from 152 patients after anesthesia induction and after CPB for the measurement of plasma GFAP levels. Nasal temperatures were recorded every 15 minutes. A multivariate estimation model for post-operative plasma GFAP level was determined that included the baseline GFAP levels, rewarming rate, CPB duration, and patient age.
Results
The mean rewarming rate during CPB was 0.21±0.11°C/min; the maximum temperature was 36.5±1.0°C (range, 33.1-38.0°C). Plasma GFAP levels increased after compared with before CPB (median, 0.022 ng/ml vs. 0.035 ng/ml, p<0.001). Rewarming rate (p=0.001), but not maximum temperature (p=0.77), was associated with higher plasma GFAP levels after CPB. In the adjusted estimation model, rewarming rate was positively associated with postoperative plasma log GFAP levels (Coef, 0.261; 95% Confidence Intervals, 0.132 to 0.390; p<0.001). Six (3.9%) patients experienced a post-operative stroke. Rewarming rate was higher (0.3±0.09°C/min vs. 0.2±0.11°C/min, p=0.049) in the patients with stroke compared with those without a stroke.
Conclusions
Rewarming rate during CPB was correlated with evidence of brain cellular injury documented with plasma GFAP levels. Modifying current practices of patient rewarming might provide a strategy to reduce the frequency of neurological complications after cardiac surgery.
Keywords: Cardiopulomonary bypass, Cerebral protection, Brain injury, Perioperative care
Introduction
Hypothermia is a common practice during cardiac surgery using cardiopulmonary bypass (CPB) for protecting the brain and other organs from ischemic injury. Despite substantial laboratory data supporting its neuroprotective effects, there is no conclusive evidence that reducing body temperature during CPB results in improved neurological outcomes for patients undergoing cardiac surgery.(1-4) Cerebral hyperthermia has been demonstrated to exacerbate the extent of experimental and clinical ischemic injury. (5-7) Explanations for the failure of hypothermia to provide clinical neuroprotective benefits, thus, have included either too rapid patient rewarming and/or inadvertent cerebral hyperthermia before separation from CPB.(8-10) For the most part, investigations that have evaluated the benefits versus risk of hypothermia and patient rewarming during CPB on neurological outcomes have focused mainly on clinical stroke and/or postoperative cognitive end-points. (10-15) Many factors can contribute to cognitive decline after cardiac surgery potentially confounding this outcome as an end-point of clinical investigations.(16, 17) Further, clinical stroke as an end-point of neuroprotective investigations requires large patient sample sizes and may underestimate the extent of ischemic brain injury compared with more precise brain MRI imaging. (18, 19)
A sensitive blood biomarker of brain injury would be of value for the early detection of neurological complication of cardiac surgery possibly allowing for institution of strategies to limit the extent of ischemic injury. Prior investigations have measured S100β and neuron-specific enolase levels as biomarkers of brain injury, but extra-cranial contamination of these two proteins limit their specificity. (20) Glial fibrillary acidic protein (GFAP) is an astrocyte specific protein found to be released into the plasma of adults with traumatic brain injury or stroke. (20-22) Monitoring plasma GFAP levels, thus, provides a sensitive means for detecting brain injury from CPB that would be useful in developing strategies for improving neurological outcomes. The purpose of this study was to evaluate whether there is a relationship between rewarming rate during CPB and plasma GFAP levels in adults undergoing cardiac surgery.
Patients and Methods
From October 2009 to April 2013, patients at high risk for neurological complication based on a Johns Hopkins encephalopathy risk > 0.05 or stroke risk >0.02 who were undergoing cardiac surgery were enrolled in a prospective study with an aim to verify usage of cerebral autoregulation monitoring during CPB to reduce postoperative neurological complications (NCT00981474). (23) All study procedures including the measurement of plasma GFAP levels were approved by the Institutional Review Board of The Johns Hopkins Medical Institutions (initial approval, August 4, 2009 with annual re-approval) and all patients provided written informed consent for participation.
Perioperative Care
The patient received routine institutional perioperative care that included general anesthesia with a combination of midazolam, fentanyl, isoflurane. Pancronium or vecuronium was given for skeletal muscle relaxation. Following the administration of heparin to achieve an ACT of > 480s, CPB was instituted using non-pulsatile flow between 2.0L/min/m2 to 2.4L/min/m2 and a membrane oxygenator. The patients were managed with alpha-stat pH management; oxygenation and normocarbia were ensured with continuous in-line arterial blood gas monitoring calibrated hourly with arterial sampling. Body temperature and timing of rewarming from hypothermia during CPB was at the discretion of the operating surgeon. Institutional guideline for rewarming included maintaining the gradient between the venous inlet and arterial outlet within 10°C. Arterial outlet temperature was controlled not to exceed 37°C. A circulating water blanket, Medi-Therm III (Gaymar Industries, Inc. Orchard Park, NY, USA), placed under the patient was turned on after aortic cross clamp removal, and when the bladder temperature reached 32°C.
Plasma GFAP Assays
Arterial blood samples for measuring plasma GFAP levels were collected after anesthesia induction, and at the completion of protamine administration after termination of cardiopulmonary bypass. These samples were collected into EDTA containing glass tubes and then processed within 2 hours of collection by centrifugation at 1500g for 8 min at 4°C. The plasma was pipetted into plastic cryotubes then stored at −70°C until batch analysis. Assays were c arried out in duplicate using an electrochemiluminescent sandwich immunoassay platform (MesoScale Discovery [MSD], Gaithersburg, MD) and were analyzed on a Sector Imager 2400 (MSD) according to the manufacturer’s protocol. (24, 25) The lower limit of detection for GFAP, assay was 0.011 ng/mL.
Data Analysis
The normality of the data was tested using Kolmogorov-Smirnov test. Normally distributed continuous data were analyzed with Student’s T test and data that were not normally distributed were analyzed with the Mann-Whitney test when logarithmic transformation was not applicable. Plasma GFAP levels that were below the lower limit of detection were assigned a value of the lower limit of detection. The association between postoperative plasma GFAP levels and rewarming rate and maximum nasopharyngeal temperature were analyzed by Pearson’s correlation analysis. Mean rewarming rate was calculated in 15 minutes block intervals from the start of rewarming, and the maximum rewarming rate among these intervals was used for the analysis. A multivariate estimation model for post-operative plasma GFAP level was calculated that included preoperative plasma GFAP level, rewarming rate, duration of CPB and patient age as possible confounding variables. (26, 27) P-values less than 0.05 were considered statistically significant. All analysis was performed using Stata (Version 13.1, Stata Corp, College Station, TX) and Prism 5 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Among 214 patients enrolled, plasma GFAP levels were not available in 62 patients, and these patients were excluded from this study. The characteristics for the 152 patient included in this analysis are shown in Table 1. The average minimum temperature during CPB was 30.8±2.9°C (range, 20.3-35.1°C) and the maximum temperature was 36.5±1.0°C (range, 33.1-38.0°C). The average duration of rewarming was 42.6 ±17.4 minutes (range, 10-81) and average maximum CPB arterial perfusate during rewarming was 37°C (range 33.0-38.0°C). The mean rewarming rate was 0.21±0.11°C/min (range, 0-0.50°C) and the mean gradient between CPB perfusate to nasopharyngeal temperature was 2.9±1.9°C (range, 0.1-9.0°C).
Table 1.
Demographic and medical information for the patients included in the study. Data are listed as mean±standard deviation or as number and percent of patients for dichotomous variables
| Variables | N=152 |
|---|---|
| Age (yr)† | 71±7.9 |
| Male gender* | 99 (65.1%) |
| JHH Risk Scoreठ| 0.09 (0.06-0.11) |
| Chronic obstructive pulmonary disease (%)* | 19 (12.5%) |
| Diabetes* | 75 (49.3%) |
| Coronary artery disease* | 118 (77.6%) |
| Congestive heart failure* | 25 (16.5%) |
| Prior myocardial infarction* | 41 (27.0%) |
| Hypertension* | 124 (81.6%) |
| Baseline estimated glomerular filtration rate (mL×min-1×1.73m-2) † |
76.5±25.2 |
| Angiotensin-converting enzyme inhibitors-I* | 56 (36.8%) |
| Statins* | 97 (63.8%) |
| Aspirin* | 115 (75.7%) |
| Nitrates* | 28 (18.4%) |
| Calcium channel blocker* | 40 (26.3%) |
| Beta-adrenergic receptor blocker* | 84 (55.3%) |
| Diuretics* | 69 (45.4%) |
| Current smoker* | 18 (11.8%) |
| Prior cardiac surgery* | 17 (11.2%) |
| Prior carotid endarterectomy* | 8 (5.3%) |
| Prior cerebral vascular accident* | 15 (9.9%) |
| Prior TIA* | 10 (6.6%) |
| Peripheral vascular disease* | 28 (18.4%) |
| Surgical Procedure | |
| CABG* | 82 (53.9%) |
| CABG+AVR/MVR* | 34 (22.4%) |
| AVR/MVR* | 28 (18.4%) |
| Aortic Root* | 5 (3.3%) |
| LVAD/Aortic* | 3 (2.0%) |
| Cardiopulmonary bypass duration (min)† | 110.7±40.9 |
| Cross Clamp (min)† | 70.0±29.1 |
| Rewarming Time (min)† | 42.6±17.4 |
| Cooling rate (degrees celcius/min)† | 0.2±0.13 |
| Rewarming rate (degrees celcius/min)† | 0.2±0.11 |
| Maximum Temperature (degrees celcius)† | 36.5±1.02 |
| Minimum Temperature (degrees celcius)† | 30.8±2.91 |
| Maximum arterial perfusate temperature (°C)degrees‡ | 37 (36-37) |
| Nasopharyngeal-arterial perfusate temperature gradient (°C)† |
2.9±1.9°C |
| Temperature upon ICU admission (°C)† | 35.5±0.75 |
n(%);
Mean ± SD;
Median, interquartile range;
Stroke. 2006;37(2):562-71. (24)
Aortic = aortic surgery; AVR = aortic valve replacement; CABG = coronary artery bypass graft; LVAD = left ventricular assisting device placement; MVR = mitral valve replacement or repair; TIA = transient ischemic attack
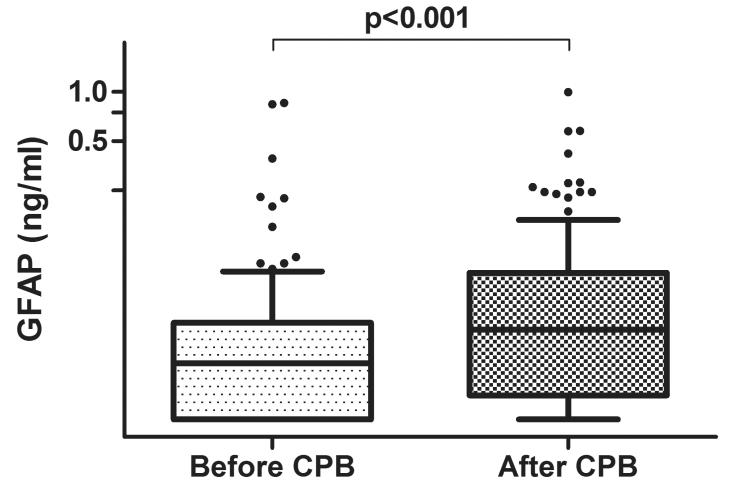
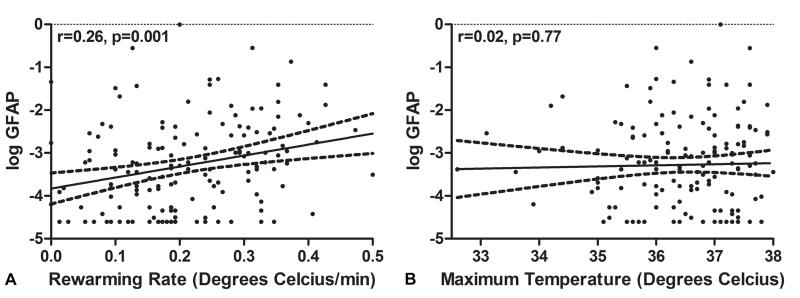
Plasma biomarker levels obtained after anesthesia induction before CPB and those measured after termination of CPB are shown in Figure 1. There was an increase in plasma GFAP levels after surgery compared with the levels measured after induction of anesthesia (median; interquartile range [IQR]: 0.022 ng/ml; 0.010 to 0.039 vs. 0.035 ng/ml; 0.014 to 0.078; p<0.001). The relationships between plasma GFAP levels and rewarming rate during CPB and the maximum temperature are shown in Figure 2. There was a positive correlation between CPB rewarming rate and plasma GFAP levels measured after surgery (r=0.26, 95% Confidence Interval [CI], 0.11 to 0.41, p=0.001), (Figure 2A) but there was no relationship between plasma GFAP levels and maximum temperature (r=0.02; 95%CI, −0.14 to 0.18; p=0.77). (Figure 2B) Minimum temperature was strongly correlated with rewarming rate (r=−0.71, 95%CI −0.78 to −0.62, p<0.0001) and with increases in plasma GFAP level (r=−0.32, 95%CI −0.46 to −0.17, p<0.001).
Figure 1.
Box and whiskers plots showing the changes in plasma glial fibrillary acidic protein (GFAP) levels from the measurements obtained before and after cardiopulmonary bypass (CPB). The horizontal line in the shaded box represents the median value, and the shaded box represents the interquartile range. The error bars below and above the shaded area represents ±1.5× the interquartile range; points beyond the error bar are outliers.
Figure 2.
Scatter plot of glial fibrillary acidic protein (GFAP) with rewarming rate (A) and maximum temperature (B). The long dash line represent 95% confidence interval for the fitted line. There was a significant correlation between log GFAP and rewarming rate (r=0.26, 95%CI, 0.11 to 0.41, p=0.001). There were no significant correlation between log GFAP and maximum temperature (r=0.02; 95%CI, −0.14 to 0.18; p=0.77).
The results of the multivariate estimation modeling are shown in Table 2. After adjusting for plasma log GFAP levels obtained after anesthesia induction, the duration of CPB, patient age, and CPB rewarming rate was significantly associated with plasma log GFAP levels measured after surgery (Coef, 0.261; 95%CI, 0.132 to 0.390; p<0.001; adjusted R2 =0.42). That is, there was a 29.8% (95%CI, 14.1 to 47.8%) increase in plasma GFAP (ng/ml) after surgery for every 0.1°C/min increase in rewarming rate.
Table 2.
Multivariate estimation model for the primary variable rewarming rate with potential confounding variables; age and duration of cardio-pulmonary bypass on the postoperative GFAP levels (logarithmically transformed) adjusted to pre-operative GFAP levels. The adjusted R2 for the model is 0.42.
| Coef | Standard Error |
95% Confidence Interval |
p-value | |
|---|---|---|---|---|
| Rewarming rate (0.1°/min) | 0.261 | 0.065 | 0.132 to 0.390 | <0.001 |
| Age (yr) | 0.006 | 0.009 | −0.011 to 0.023 | 0.52 |
| Total Bypass Time (min) | 0.003 | 0.002 | −0.001 to 0.007 | 0.11 |
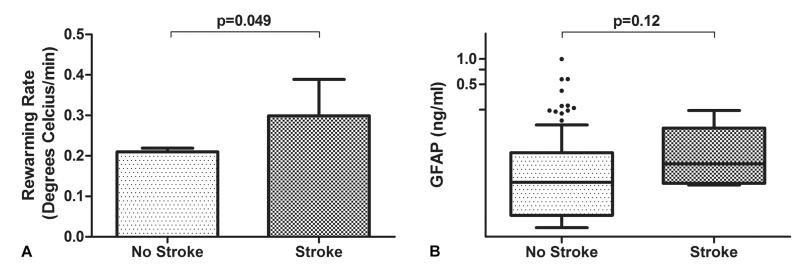
Six patients (3.9%) experienced a stroke after surgery. The CPB rewarming rate was higher in patients with a stroke compared with patients without stroke (0.3±0.09°C/min vs. 0.2±0.11°C/min, p=0.049). (Figure 3A) There was a trend for plasma GFAP levels in patients with a stroke to be higher than those without a stroke (median; IQR: 0.051; 0.031-0.099 vs. 0.039; IQR, 0.020 to 0.077, p=0.12) as shown in Figure 3B.
Figure 3.
Bar graph (A) and box and whisker plot (B) showing the relationship between rewarming rate (p=0.048) and plasma glial fibrillary acidic protein (GFAP) levels for patients with and without postoperative stroke. For the latter, the horizontal line in the shaded box for the box and whisker plot represents the median value, and the shaded box represents the interquartile range. The error bars below and above the shaded area represents ±1.5× the interquartile range; points beyond the error bar are outliers.
Comment
The results of this study are that rewarming rate from hypothermia during CPB is positively correlated with plasma GFAP levels measured after surgery. In contrast, peak temperature during patient rewarming was not significantly associated with plasma GFAP levels.
There are substantial laboratory data supporting the robust neuroprotective effects of hypothermia via multiple mechanisms including a reduction in cerebral metabolic rate for oxygen, diminished excitatory neurotransmitter release, attenuation of calcium accumulation in ischemic cells, enhanced recovery of protein synthesis, reduction in reactive oxygen specie generation, and others mechanisms. (1-5) Further, hypothermia has been reported to improve outcomes in patients after cardiac arrest. (28, 29) Nonetheless, there is a long-standing debate regarding the relative benefits of hypothermia for providing neuroprotection during cardiac surgery using CPB. (9) In randomized trials of hypothermic versus normothermic CPB, for example, there were no beneficial effects of hypothermia on the risk for stroke. (12, 13, 30-32) Nathan et al. (33) randomized patients undergoing CABG surgery with CPB at 32°C to a rewarming temperature of either 37°C or 34°C. They found that the incidence of cognitive dysfunction at hospital discharge was 48% in the hypothermic group compared with 62% in the normothermic group (p=0.048).
The rate of rewarming and/or unintentional cerebral hyperthermia during rewarming has been an important confounding factor in assessing any benefits of hypothermia on neurological outcomes after cardiac surgery. (8, 13) In a study of 165 patients, Grigore et al. (10), for example, reported that patients randomized to slow rewarming (CPB perfusate to nasopharyngeal temperature gradient kept ≤ 2°C) from hypothermic CPB (28°C to 32°C) had improved neurocognitive outcomes 6 weeks after surgery compared with a control group receiving conventional rewarming (p=0.047). Further, Boodhwani et al(15) randomized patients undergoing CABG surgery with CPB to a nasopharyngeal temperature of either 34°C or 37°C maintained using a water-circulating thermal control pads (ie, no active central rewarming). There was no differences in the frequency of neurocognitive deficits between the hypothermic and normothermic groups at hospital discharge (controls, 45% vs hypothermia group 49%, p=0.49) or 3 months after surgery (controls 8% vs hypothermia group 4%, p=0.28). These latter data suggest that avoidance of excessive rewarming and not hypothermia per se may reduce the incidence of neurocognitive dysfunction after surgery.
Glial fibrillary acidic protein is a brain specific biomarker that arises from the cytoskeleton of astrocytes. Astrocytes are the most abundant cell in the brain that have multiple functions including modulating synaptic plasticity, re-uptake of the excitatory neurotransmitter glutamate, promoting neuronal repair after ischemic injury, and maintenance of blood brain barrier integrity.(20, 34) In a prior study in pediatric patients undergoing congenital heart surgery (median age, 0.67 years; range, 0.005-17.2 years), our group has reported that plasma GFAP levels were elevated during and after CPB in 70% of cases, peaking during the rewarming phase (p=0.0001). (35) Elevation of plasma GFAP levels are associated with the extent of brain injury in adults with acute stroke and pediatric patients with hypoxic ischemic injury.(22, 27) Detection of plasma GFAP, thus may allow for the early identificatoin of brain injury in a variety of settings. Measurement of this biomarker obviates many limitations associated with other methods for assessing neurological end-points in patients undergoing cardiac surgery such as cognitive decline or clinical stroke. (16-19) Rapid availability of plasma GFAP results might foster the development of care strategies to identify patients suffering subtle brain injury from surgery in whom neuroprotective or rescue therapies might be instituted.
The association between faster rewarming rate, elevated plasma GFAP levels, and stroke in our study is in agreement with laboratory studies that report cytotoxicity and cellular death from rewarming. In cell cultures, rewarming is known to induce cellular stress and activate pro-apoptotic pathways.(36) An increase in cleaved caspase-3, an effector enzyme of apoptosis, was observed in the hippocampus and basal ganglia of swine that received deep hypothermic cardiopulmonary bypass with selective cerebral perfusion and rewarming.(37) Moreover, rewarming promoted cortical apoptosis (including among astrocytes) in a piglet model of hypoxic brain injury, therapeutic hypothermia, and rewarming above that observed in piglets that remained hypothermic. Rapid rewarming may activate caspase-3 to a greater degree than slow rewarming.(38) In our study, we most likely observed the cytotoxicity and promotion of cell death mechanisms associated with rewarming as increases in plasma GFAP and greater risk of stroke in patients that received faster rewarming rates. This suggests that rapid rewarming could negate the neuroprotective benefits of hypothermia during cardiopulmonary bypass.
There are several limitations to this study including its observational design and the relative small sample size. Thus, we cannot exclude whether residual confounding variables not accounted for in our multivariate modeling could influence our findings. For example, more extensive surgery in patients at higher risk for brain injury may have been associated with lower body temperature and longer and more rapid rewarming during CPB. Duration of CPB in our analysis, however, was not associated with plasma GFAP levels. Nevertheless, randomization of patients undergoing similar surgery to different rewarming rates might better define the effect of rate of rewarming on plasma GFAP levels. We found that rewarming rate was higher for patients developing stroke than for those without stroke. The plasma GFAP levels, though, were no different between those with versus without stroke. We did not obtain serial plasma GFAP levels after surgery. It is possible that some of the strokes in this series occurred after the measurement of plasma GFAP levels. (39) It is also possible that we did not observe the peak GFAP levels due to our limited blood sampling. However, limiting the analysis to plasma GFAP levels measured after protamine administration focuses the analysis of potential factors associated with its release to the CPB period of surgery and not postoperative factors. We have recently reported, for example, that excursion of blood pressure outside the limits of cerebral autoregulation after surgery in the ICU are associated with plasma GFAP levels measured on postoperative day 1.(40)
In conclusion, rewarming rate during CPB was correlated with evidence of brain cellular injury documented with plasma GFAP levels. Modifying current practices of patient rewarming during CPB might provide a strategy to reduce the frequency of neurological complications after cardiac surgery.
Acknowledgments
This work was supported in part by Grant-in-Aid Number [103363 from the Mid-Atlantic Affiliate of the American Heart Association]; grants [R01HL092259 from National Institute of Health].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke. 1989;20(7):904–10. doi: 10.1161/01.str.20.7.904. [DOI] [PubMed] [Google Scholar]
- 2.Kil HY, Zhang J, Piantadosi CA. Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. J Cereb Blood Flow Metab. 1996;16(1):100–6. doi: 10.1097/00004647-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 3.White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179(S1-2):1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 4.Winfree CJ, Baker CJ, Connolly ES, Jr, Fiore AJ, Solomon RA. Mild hypothermia reduces penumbral glutamate levels in the rat permanent focal cerebral ischemia model. Neurosurgery. 1996;38(6):1216–22. doi: 10.1097/00006123-199606000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Castillo J, Davalos A, Noya M. Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc Dis. 1999;9(1):22–7. doi: 10.1159/000015891. [DOI] [PubMed] [Google Scholar]
- 6.Reith J, Jorgensen HS, Pedersen PM, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet. 1996;347(8999):422–5. doi: 10.1016/s0140-6736(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich WD, Busto R, Valdes I, Loor Y. Effects of normothermic versus mild hyperthermic forebrain ischemia in rats. Stroke. 1990;21(9):1318–25. doi: 10.1161/01.str.21.9.1318. [DOI] [PubMed] [Google Scholar]
- 8.Cook D, Orszulak T, Daly R, Buda DA. Cerebral hyperthermia during cardiopulmonary bypass in adults. J Thorac Cardiovasc Surg. 1996;111(1):268–9. doi: 10.1016/S0022-5223(96)70425-7. [DOI] [PubMed] [Google Scholar]
- 9.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103(1):21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 10.Grigore AM, Grocott HP, Mathew JP, et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg. 2002;94(1):4–10. doi: 10.1097/00000539-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Rees K, Beranek-Stanley M, Burke M, Ebrahim S. Hypothermia to reduce neurological damage following coronary artery bypass surgery. Cochrane Database Syst Rev. 2001;1:CD002138. doi: 10.1002/14651858.CD002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regragui I, Birdi I, Izzat MB, et al. The effects of cardiopulmonary bypass temperature on neuropsychologic outcome after coronary artery operations: a prospective randomized trial. J Thorac Cardiovasc Surg. 1996;112(4):1036–45. doi: 10.1016/s0022-5223(96)70105-8. [DOI] [PubMed] [Google Scholar]
- 13.Grigore AM, Mathew J, Grocott HP, et al. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95(5):1110–9. doi: 10.1097/00000542-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Nathan HJ, Parlea L, Dupuis JY, et al. Safety of deliberate intraoperative and postoperative hypothermia for patients undergoing coronary artery surgery: a randomized trial. J Thorac Cardiovasc Surg. 2004;127(5):1270–5. doi: 10.1016/j.jtcvs.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Boodhwani M, Rubens F, Wozny D, Rodriguez R, Nathan HJ. Effects of sustained mild hypothermia on neurocognitive function after coronary artery bypass surgery: a randomized, double-blind study. J Thorac Cardiovasc Surg. 2007;134(6):1443–50. doi: 10.1016/j.jtcvs.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Hogue CW, Jr, Hershey T, Dixon D, et al. Preexisting cognitive impairment in women before cardiac surgery and its relationship with C-reactive protein concentrations. Anesth Analg. 2006;102(6):1602–8. doi: 10.1213/01.ANE.0000219591.10826.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366(3):250–7. doi: 10.1056/NEJMra1100109. [DOI] [PubMed] [Google Scholar]
- 18.Wityk R, Goldsborough M, Hillis A, et al. Diffusion- and perfusion-weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery. Arch Neurol. 2001;58(4):571–6. doi: 10.1001/archneur.58.4.571. [DOI] [PubMed] [Google Scholar]
- 19.Cook DJ, Huston J, 3rd, Trenerry MR, Brown RD, Jr, Zehr KJ, Sundt TM., 3rd Postcardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83(4):1389–95. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 20.Seco M, Edelman JJ, Wilson MK, Bannon PG, Vallely MP. Serum biomarkers of neurologic injury in cardiac operations. Ann Thorac Surg. 2012;94(3):1026–33. doi: 10.1016/j.athoracsur.2012.04.142. [DOI] [PubMed] [Google Scholar]
- 21.Kaneko T, Kasaoka S, Miyauchi T, et al. Serum glial fibrillary acidic protein as a predictive biomarker of neurological outcome after cardiac arrest. Resuscitation. 2009;80(7):790–4. doi: 10.1016/j.resuscitation.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31(11):2670–7. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- 23.McKhann GM, Grega MA, Borowicz LM, Jr, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006;37(2):562–71. doi: 10.1161/01.STR.0000199032.78782.6c. [DOI] [PubMed] [Google Scholar]
- 24.Stewart A, Tekes A, Huisman TA, et al. Glial fibrillary acidic protein as a biomarker for periventricular white matter injury. Am J Obstet Gynecol. 2013;209(1):e1–7. doi: 10.1016/j.ajog.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savage WJ, Everett AD, Casella JF. Plasma glial fibrillary acidic protein levels in a child with sickle cell disease and stroke. Acta Haematol. 2011;125(3):103–6. doi: 10.1159/000321791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bembea MM, Savage W, Strouse JJ, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(5):572–9. doi: 10.1097/PCC.0b013e3181fe3ec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ennen CS, Huisman TA, Savage WJ, et al. Glial fibrillary acidic protein as a biomarker for neonatal hypoxic-ischemic encephalopathy treated with whole-body cooling. Am J Obstet Gynecol. 2011;205(3):251, e1–7. doi: 10.1016/j.ajog.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard SA, Gray TW, Buist MD, et al. Treatment of Comatose Survivors of Out-of-Hospital Cardiac Arrest with Induced Hypothermia. N Engl J Med. 2002;346(8):557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 29.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 30.Parodi E, Lijoi A, Scarano F, et al. Normothermic versus hypothermic perfusion during cardiopulmonary bypass. A randomized study on 132 patients. Minerva Cardioangiol. 2000;48(12):435–40. [PubMed] [Google Scholar]
- 31.Luehr M, Bachet J, Mohr FW, Etz CD. Modern temperature management in aortic arch surgery: the dilemma of moderate hypothermia. Eur J Cardiothorac Surg. 2014;45(1):27–39. doi: 10.1093/ejcts/ezt154. [DOI] [PubMed] [Google Scholar]
- 32.Algarni KD, Yanagawa B, Rao V, Yau TM. Profound hypothermia compared with moderate hypothermia in repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2014;148(6):2888–94. doi: 10.1016/j.jtcvs.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Nathan HJ, Wells GA, Munson JL, Wozny D. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: a randomized trial. Circulation. 2001;104(12 Suppl 1):I85–91. doi: 10.1161/hc37t1.094710. [DOI] [PubMed] [Google Scholar]
- 34.Middeldorp J, Hol EM. GFAP in health and disease. Prog Neurobiol. 2011;93(3):421–43. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Brunetti MA, Jennings JM, Easley RB, et al. Glial fibrillary acidic protein in children with congenital heart disease undergoing cardiopulmonary bypass. Cardiol Young. 2014;24(4):623–31. doi: 10.1017/S1047951113000851. [DOI] [PubMed] [Google Scholar]
- 36.Neutelings T, Lambert CA, Nusgens BV, Colige AC. Effects of mild cold shock (25 degrees C) followed by warming up at 37 degrees C on the cellular stress response. PLoS One. 2013;8(7):e69687. doi: 10.1371/journal.pone.0069687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Argueta-Morales IR, Munro HM, Olsen M, et al. Effects of phentolamine infusion during selective cerebral perfusion in neonatal piglets. Ann Thorac Surg. 2013;96(6):2203–9. doi: 10.1016/j.athoracsur.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Armstrong JS, Lee JH, et al. Rewarming from therapeutic hypothermia induces cortical neuron apoptosis in a swine model of neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2014.245. doi: 10.1038/jcbfm.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogue CW, Jr, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100(6):642–7. doi: 10.1161/01.cir.100.6.642. [DOI] [PubMed] [Google Scholar]
- 40.Hori D, Ono M, Rappold TE, et al. Hypotension after cardiac surgery based on autoregulation monitoring leads to brain cellular injury. Ann Thorac Surg. 2015 doi: 10.1016/j.athoracsur.2015.03.036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]