Abstract
Background
In patients with atrial fibrillation(AF), the addition of surgical ablation to aortic valve replacement(AVR) does not increase procedural morbidity or mortality. However, efficacy in this population has not been carefully evaluated. This study compared outcomes between patients undergoing stand-alone Cox-Maze IV to those undergoing surgical ablation and concomitant AVR.
Methods
From January 2002 to May 2014, 188 patients received a stand-alone Cox-maze IV(n=113) or surgical ablation with concomitant AVR(n=75). In the concomitant AVR group, patients underwent Cox-maze IV(n=58), left-sided Cox-maze IV(n=3), or pulmonary vein isolation(n=14). Thirty-one perioperative variables were compared. Freedoms from AF on and off antiarrhythmic drugs were evaluated at 3, 6, 12, and 24 months.
Results
Follow up was available in 97% of patients. Freedom from AF on and off antiarrhythmic drugs in patients receiving a stand-alone Cox-maze IV vs. concomitant AVR was not significantly different at any time point. The concomitant AVR group had more comorbidities, paroxysmal AF, pacemaker implantations(24% vs. 5%, p=0.002), and complications(25% vs. 5%, p<0.001). Freedoms from AF off antiarrhythmic drugs for patients receiving an AVR and pulmonary vein isolation at 1 year was only 50%, which was significantly lower than patients receiving an AVR and Cox-maze IV(94%, p=0.001).
Conclusions
A Cox-maze IV with concomitant AVR is as effective as a stand-alone Cox-maze IV in treating AF, even in an older population with more comorbidities. Pulmonary vein isolation was not as effective and is not recommended in this population. A Cox-maze IV should be considered all in patients undergoing AVR with a history of AF.
Keywords: Atrial Fibrillation, Aortic Valve Replacement, Arrhythmia Therapy, Ablation
INTRODUCTION
Atrial fibrillation (AF) is the most prevalent sustained arrhythmia and currently affects an estimated 1–2% of the general population. The prevalence has nearly doubled in the last 15 years, and conservative estimates predict it will double again by mid-century.(1) In patients undergoing cardiac surgery, AF has been associated with increased perioperative morbidity and mortality and worse late survival.(2–4) The Cox-Maze procedure (CMP) was developed in 1987 and has evolved into the gold standard for surgical AF ablation.(5, 6) The current iteration, the CMPIV, utilizes a combination of cryoablation and bipolar radiofrequency ablation to replace most of the surgical incisions of the traditional cut-and-sew technique, which has resulted in a significant decrease in procedural morbidity without sacrificing efficacy.(7, 8)
Based on the 2012 Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS) Expert Consensus Statement, it is appropriate to consider all patients with symptomatic AF undergoing other cardiac surgery for AF ablation.(11) The majority of surgical ablations in the U.S. are performed in a concomitant setting, so it is imperative to evaluate outcomes in this population.(10) While the role of surgical ablation in concomitant mitral valve and coronary artery bypass surgery have been frequently examined,(6, 12–17) the role of surgical ablation in concomitant aortic valve replacement (AVR) is less studied. Moreover, in an analysis of the Society of Thoracic Surgeons (STS) National Database, only 28% of patients undergoing AVR with a history of AF underwent a concomitant surgical ablation.(10) There may be multiple factors responsible for this underutilization including concerns for increased morbidity and mortality. However, recent studies have suggested that adding surgical ablation to AVR does not increase morbidity.(18, 19)
Even though the procedural outcomes of adding surgical ablation to AVR has been evaluated, the efficacy of surgical ablation of AF in the AVR population has not been carefully evaluated, particularly in regards as to whether the CMPIV or pulmonary vein isolation (PVI) have similar efficacy in this group. The goal of this study was to directly compare outcomes between patients undergoing stand-alone CMPIV to those undergoing surgical ablation and concomitant AVR.
PATIENTS AND METHODS
This study was approved by the Washington University School of Medicine Institutional Review Board. Written informed consent was obtained from each patient prior to enrollment. All data were entered prospectively into a longitudinal database maintained at our institution. The database contained more than 400 demographic and perioperative variables.
Patient Selection
A total of 188 consecutive patients who received either a stand-alone CMP or surgical ablation with concomitant AVR from January 2002 to May 2014 were retrospectively reviewed. These patients were divided into two groups based on whether or not they underwent a concomitant AVR. All 113 patients in the stand-alone CMP group underwent standard CMPIV lesion set (Figure 1).(20) In the concomitant AVR group, 75 patients underwent AVR with concomitant surgical ablation. This group was further subdivided into three ablation sets: CMPIV (n=58), left-sided CMPIV (n=3), or PVI (n=14). All patients undergoing other concomitant valvular surgery or any other incision besides a full sternotomy were excluded.
Figure 1.
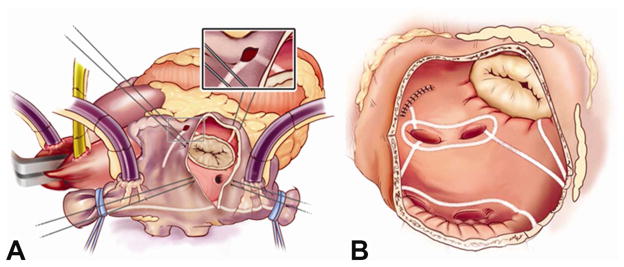
Cox-Maze IV Right (A) and Left (B) Atrial Lesion Sets. In the right atrium, radiofrequency (RF) ablation lines (white lines) extend from SVC to IVC and along the RA free wall down to tricuspid valve annulus. In the left atrium, RF ablation lines (white lines) are created including pulmonary vein isolation, pulmonary vein roof and floor connecting lesions, lesion from left superior pulmonary vein and amputated atrial appendage, and lesion from inferior atriotomy to mitral valve annulus.
Adapted from Weimar T, Bailey MS, Watanabe Y et al. The cox-maze IV procedure for lone atrial fibrillation: A single center experience in 100 consecutive patients. Journal of interventional cardiac electrophysiology: an international journal of arrhythmias and pacing 2011;31(1):47–54 [20], with kind permission from Springer Science and Business Media.
Thirty-two preoperative and perioperative variables (Table 1 and 2) were selected for comparison after preliminary analysis of all the variables collected between the STS and our institutional AF database. Major complications, which were defined as pneumonia, mediastinitis, need for intraaortic balloon pump, permanent stroke, reoperation for bleeding, and renal failure requiring dialysis, were compared between groups. Selected demographic variables were also compared between patients who underwent AVR and either CMPIV or PVI (Table 3).
Table 1.
Preoperative Demographics
| Demographic | Stand-alone CMPIV (N=113) | Concomitant AVR (N=75) | p value |
|---|---|---|---|
| Age (years) | 56.6 ± 10.6 | 70.5 ± 8.2 | p<0.001 |
| Male | 86/113 (76%) | 50/75 (67%) | p=0.157 |
| Paroxysmal | 28/113 (25%) | 45/75 (60%) | p<0.001 |
| Persistent | 7/113 (6%) | 10/75 (13%) | p<0.001 |
| Long-Standing Persistent | 78/113 (69%) | 20/75 (27%) | p<0.001 |
| Length of Time in AF (months) | 89 ± 77 | 84 ± 113 | p=0.746 |
| LA Size (cm) | 4.9 ± 1.1 | 4.9 ± 0.8 | p=0.984 |
| Failed Catheter Ablation | 53/113 (47%) | 4/75 (5%) | p<0.001 |
| Preoperative Pacemaker | 13/113 (12%) | 12/75 (16%) | p=0.374 |
| NYHA 3/4 | 32/113 (28%) | 55/75 (73%) | p<0.001 |
| LVEF | 51 ± 13 | 55 ± 13 | p=0.035 |
| PVD | 6/113 (5%) | 12/75 (16%) | p=0.015 |
| Hypertension | 68/113 (60%) | 56/75 (75%) | p=0.040 |
| Dyslipidemia | 58/113 (51%) | 55/75 (73%) | p=0.003 |
| Chronic Lung Disease (Moderate-Severe) | 3/113 (3%) | 6/75 (8%) | p=0.093 |
| Diabetes | 12/113 (11%) | 30/75 (40%) | p<0.001 |
| Renal Failure | 0/113 (0%) | 3/75 (4%) | p=0.032 |
Mean ± standard deviation for continuous variables
Table 2.
Perioperative Data – Stand-alone CMPIV vs Concomitant AVR
| Perioperative Data | Stand-alone CMPIV (N=113) | Concomitant AVR (N=75) | p value |
|---|---|---|---|
| Box Lesion | 92/113 (81%) | 59/75 (79%) | p=0.642 |
| CPB Time (min) | 126 ± 28 | 195 ± 46 | p<0.001 |
| Cross-clamp Time (min) | 40 ± 14 | 106 ± 23 | p<0.001 |
| Post Op Permanent Pacemaker | 9/113 (8%) | 18/75 (24%) | p=0.002 |
| Overall Major Complications | 6/113 (5%) | 20/75 (27%) | p<0.001 |
| Pneumonia | 5/113 (4%) | 11/75 (15%) | p=0.014 |
| Mediastinitis | 0/113 (0%) | 1/75 (1%) | p=0.198 |
| IABP | 0/113 (0%) | 3/75 (4%) | p=0.032 |
| MI | 0/113 (%) | 1/75 (1%) | p=0.218 |
| Stroke | 1/113 (1%) | 2/75 (3%) | p=0.340 |
| Reoperation for Bleeding | 0/113 (0%) | 7/75 (9%) | p=0.001 |
| Dialysis | 0/113 (10%) | 6/75 (8%) | p=0.002 |
| Median Hospital LOS, d(range) | 8 (4–53) | 11 (4 – 52) | p<0.001 |
| Median Total ICU LOS, d(range) | 2 (1 – 35) | 5 (1 – 32) | p<0.001 |
| 30-d Mortality | 1/113 (1%) | 3/75 (4%) | p=0.147 |
Mean ± standard deviation for continuous variables, unless noted
Table 3.
Concomitant AVR Subgroup Perioperative Data
| Demographic | CMPIV+AVR (N=58) | PVI+AVR (N=14) | p value |
|---|---|---|---|
| Age (years) | 72.1 ± 10.0 | 65.4 ± 6.6 | p=0.031 |
| Male | 39/58 (67%) | 10/14 (71%) | p=0.763 |
| Paroxysmal | 34/58 (58%) | 8/14 (57%) | p=0.920 |
| Persistent | 7/58 (12%) | 3/14 (21%) | p=0.363 |
| Long-Standing Persistent | 17/58 (29%) | 3/14 (21%) | p=0.555 |
| Length of Time in AF (months) | 86 ± 100 | 89 ± 167 | p=0.947 |
| LA Size (cm) | 5.0 ± 0.8 | 5.0 ± 0.7 | p=0.907 |
| Failed Catheter Ablation | 4/58 (7%) | 0/14 (0%) | p=0.312 |
| Post Op Permanent Pacemaker | 13/58 (22%) | 5/14 (36%) | p=0.302 |
Mean ± standard deviation for continuous variables, unless noted
Patients were discharged on class I or III antiarrhythmic drugs and warfarin, unless contraindicated; antiarrhythmic agents were discontinued 2 months postoperatively if patients were in normal sinus rhythm. Calcium channel blockers or b-blockers were not considered as antiarrhythmic drugs.
Follow up
Patients were prospectively followed at 3, 6, 12, and 24 months postoperatively. At each follow-up, patients underwent an electrocardiogram (ECG) or 24-hour cardiac monitoring (CM) in the form of Holter monitoring, pacemaker interrogation, or interrogation of implantable loop recorders.(11, 21) Starting in 2008, at least 24-hour CM was routinely obtained in all patients in accordance with the 2007 HRS/EHRA/ECAS consensus statement on catheter ablation and surgical ablation of AF.(21) Freedom from atrial tachyarrhythmias (ATAs, including atrial flutter and AF) on and off antiarrhythmic drugs (AADs) were evaluated at each time point following a 3-month blanking period as defined by the HRS/EHRA/ECAS consensus statements.(11, 21) Recurrence was defined as any episode of AF, atrial flutter, or ATA that lasted longer than 30 seconds.(21) Freedom from anticoagulation was also assessed.
Follow up was available in 97% of patients at any time point with an average follow up time of 3.0 ± 2.5 years. At 1 and 2 years following surgery, follow up was 80% (129/162) and 59% (90/153), respectively. After 2007, CM follow up was obtained in 71% and 68% of patients at 1 and 2 years, respectively.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or as median with range. Categorical variables were expressed as frequencies and percentages with outcomes compared using the χ2 or the Fisher exact test. Continuous outcomes were compared using the t-test for means of normally distributed continuous variables and the Mann-Whitney U nonparametric test for skewed distributions. All data analyses were performed using SYSTAT 13 software (Systat Software, Inc., Chicago, IL).
RESULTS
Demographics
Of the 17 preoperative demographics reviewed (Table 1), there were no significant differences in gender, length of time in AF, left atrial size, preoperative pacemaker implantation, or chronic lung disease between the stand-alone CMPIV and concomitant AVR groups. However, the concomitant AVR group was significantly older with more comorbidities and had a higher percentage of patients with paroxysmal AF when compared to the stand-alone CMPIV group. When comparing the demographics of those patients that underwent AVR with CMPIV to those that had an AVR with PVI, there were no significant differences except for age (Table 3).
Perioperative Results
Fifteen perioperative variables were selected and compared (Table 2). There were no significant differences in number of patients having a box lesion set or in 30 day mortality. As expected, the concomitant AVR group also had more overall major complications. Interestingly, the concomitant AVR group also had a higher incidence of postoperative pacemaker implantation. In half of the patients (9/18) who received postoperative pacemaker implantation in the concomitant AVR group, the indication for postoperative pacemaker implantation was 3rd degree heart block.
Freedom from Atrial Tachyarrhythmias
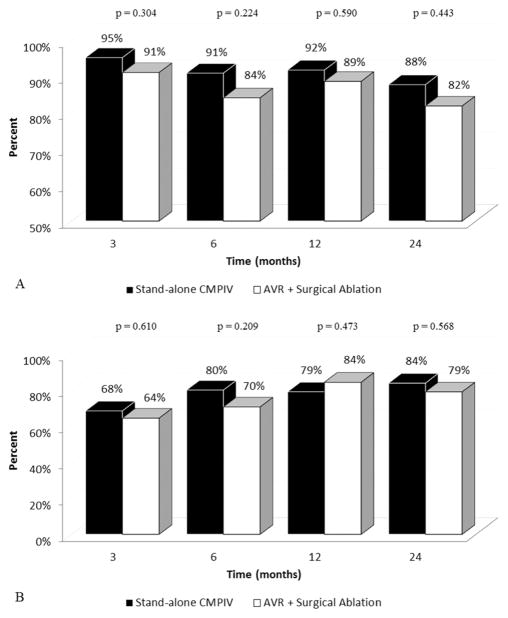
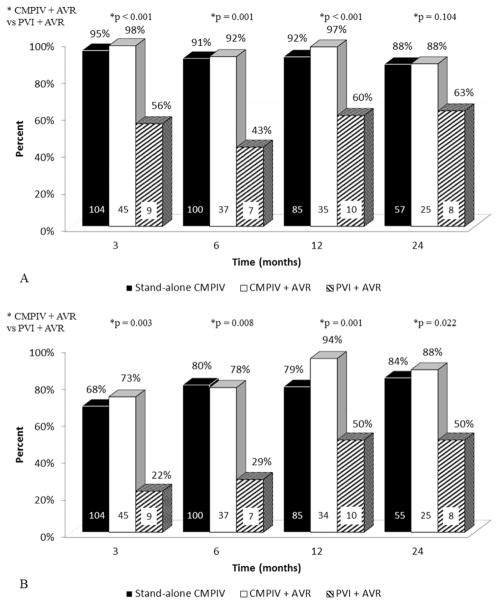
There were no significant differences in overall freedom from ATAs on or off AADs among patients who underwent stand-alone CMPIV vs. concomitant AVR at any time point (Figure 2A+2B). In subgroup analyses, patients who underwent only PVI with concomitant AVR had significantly lower freedoms from ATAs at 3, 6, and 12 months than those who underwent CMPIV with concomitant AVR (Figure 3A). Patients who underwent only PVI with concomitant AVR also had significantly lower freedoms from ATAs and AADs at 3, 6, 12, and 24 months than those who CMPIV with concomitant AVR (22%, 29%, 50%, and 50% vs. 73%, 78%, 94%, and 88%, respectively; p < 0.022 for all; Figure 3B). Freedom from anticoagulation at 12 months was 77% (66/86), 76% (26/34), and 50% (3/6) in the stand-alone CMPIV, CMPIV with concomitant AVR, and PVI with concomitant AVR, respectively.
Figure 2.
(A) Overall freedom from ATAs. (B) Overall freedom from ATAs off AADs. ATA, Atrial tachyarrhythmia; AAD, antiarrhythmic drugs
Figure 3.
Outcomes in patients undergoing stand-alone CMPIV, CMPIV and AVR, and PVI and AVR. (A) Freedom from ATAs; (B) Freedom from ATAs and AADs. ATA, Atrial tachyarrhythmia; AAD, antiarrhythmic drugs.
COMMENT
In order to examine the effect of concomitant AVR on the outcomes of surgical ablation, we examined our consecutive series of patients undergoing AVR with concomitant surgical ablation between 2002 and 2014, and compared these outcomes to a consecutive series of patients undergoing stand-alone CMPIV at our institution. In the concomitant AVR group, three types of surgical ablation were included (CMPIV, left-sided CMPIV, and PVI) in order to increase overall numbers and allow for subgroup analyses. Despite having equivalent efficacy to those undergoing CMPIV via sternotomy,(22) all patients who underwent a minimally invasive CMPIV were excluded to provide a better perioperative comparison given that all patients in the concomitant AVR group underwent sternotomy.
As expected, a comparison of preoperative variables showed that the groups differed in several important characteristics. The concomitant AVR group was older, had more paroxysmal and less longstanding persistent AF, worse New York Heart Association (NYHA) class, and more comorbidities. Not surprisingly, the concomitant AVR group had a significantly higher major complication rate, primarily attributed to increased incidences of pneumonia, reoperation for bleeding, and renal failure requiring dialysis. Longer cardiopulmonary bypass and cross-clamp times may also have contributed to the increased complication rate.
The high incidence of pacemaker implantation in this group has not been previously reported. This was much higher than we saw in patients undergoing a stand-alone CMPIV.(23) During the same time period our incidence of pacemaker implantation in patients undergoing isolated AVR was 6.7%. This high incidence may be explained by the older patient population and the complexity of the surgery performed in these patients. Half of the patients requiring postoperative pacemaker implantation in this group were indicated for 3rd-degree heart block, which is rarely seen following the stand-alone CMPIV.(23) Though there is some evidence to suggest patients with sinus node dysfunction improve after the CMP lesion sets,(24, 25) further studies will be needed to assess this complication.
Despite these differences, the efficacy of surgical ablation in patients undergoing concomitant AVR is similar to stand-alone CMPIV with respect to freedom from ATAs and AADs at both one and two years. This is surprising given that multiple groups have correlated increased age and worse preoperative comorbidities with increased AF recurrence following surgical ablation.(26, 27) However, late AF recurrence has also been associated with increased left atrial size, duration of AF, and failure to fully isolate the posterior left atrium,(28–32) which were similar between the two groups. Moreover, most patients in both groups underwent a biatrial CMP, which has been shown to have high efficacy in virtually all types of patients with AF.(20)
In a subgroup analysis, freedom from ATAs and AADs in patients who underwent AVR and concomitant PVI were less than those who underwent AVR and concomitant CMPIV. This has not been previously reported in the AVR group, but it is consistent with previous results in other groups of patients(33, 34) indicating that PVI is not as durable as more extensive lesion sets.
Previous studies from our institution and others have documented high rates of restoration of sinus rhythm without increasing morbidity following surgical ablation with concomitant procedures including coronary artery bypass and mitral valve surgery.(6, 12–17) Few studies have carefully evaluated the efficacy of surgical ablation in the AVR population. Two groups have demonstrated improved restoration of sinus rhythm in patients undergoing surgical ablation and AVR compared to patients undergoing lone AVR.(19, 35) Malaisrie et al demonstrated a reduced need for anticoagulation, but the majority of patients lacked complete lesion sets and were not compared to the stand-alone CMP.(35) Yoo et al also demonstrated a decrease in requirements for anticoagulation, and also importantly noted that AF ablation did not affect perioperative morbidity or mortality, while improving echocardiographic results in patients undergoing AVR.(19)
This study has several important limitations. This was a retrospective review of prospectively collected data, which introduces selection bias. However, this is tempered by the inclusion of consecutive patients. The non-randomized nature of the review also may have introduced potential unidentified confounding factors that could have affected the results. There were also major differences that were previously noted between the two groups in their preoperative and perioperative variables, including the type of AF. The concomitant AVR group had significantly more paroxysmal AF, which may affect the success of surgical ablation. Further, the increased morbidity of the concomitant AVR group was thus likely representative of a sicker cohort of patients. However, the efficacy of surgical ablation was equivalent between the two groups.
The number of patients undergoing PVI was small, indicating a bias towards performing a CMPIV in these patients. Thus, the comparison between CMPIV and PVI groups need to be interpreted cautiously. A randomized trial would best differentiate the two procedures.
Lastly, this study is limited by being a single institutional experience with a large experience in surgical ablation of AF, and as a result, might not be translatable to other institutions.
Conclusion
The addition of surgical ablation in patients with AF undergoing AVR is as effective at restoring sinus rhythm as the stand-alone CMPIV, even in an older population with more comorbidities. Concomitant PVI with AVR was not as successful at restoring sinus rhythm as the CMPIV, and is therefore not recommended in routine cases. A CMPIV should be considered in all patients undergoing AVR with a history of AF, when it can be performed without adding to procedural morbidity and mortality.
Acknowledgments
Support in part by National Institutes of Health grants R01 HL032257 and T32 HL007776.
ABBREVIATIONS AND ACRONYMS
- AAD
antiarrhythmic drugs
- AF
atrial fibrillation
- ATA
atrial tachyarrhythmia
- AVR
aortic valve replacement
- CPB
cardiopulmonary bypass
- CM
continuous monitoring
- CMP
Cox-maze procedure
- CMPIV
Cox-maze IV procedure
- ECAS
European Cardiac Arrhythmia Society
- ECG
electrocardiogram
- EHRA
European Heart Rhythm Assocation
- HRS
Heart Rhythm Society
- IABP
intraaortic balloon pump
- ICU
intensive care unit
- LA
left atrial
- LOS
length of stay
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NYHA
New York Heart Association
- PVD
peripheral vascular disease
- PVI
pulmonary vein isolation
- STS
Society of Thoracic Surgery
Footnotes
Presented at the Society for Thoracic Surgeons 51st Annual Meeting, San Diego, CA, January 24-28, 2015
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circulation research. 2014;114(9):1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: The framingham heart study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Banach M, Mariscalco G, Ugurlucan M, Mikhailidis DP, Barylski M, Rysz J. The significance of preoperative atrial fibrillation in patients undergoing cardiac surgery: Preoperative atrial fibrillation--still underestimated opponent. Europace: European pacing, arrhythmias, and cardiac electrophysiology: journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2008;10(11):1266–1270. doi: 10.1093/europace/eun273. [DOI] [PubMed] [Google Scholar]
- 4.Ngaage DL, Schaff HV, Barnes SA, et al. Prognostic implications of preoperative atrial fibrillation in patients undergoing aortic valve replacement: Is there an argument for concomitant arrhythmia surgery? The Annals of thoracic surgery. 2006;82(4):1392–1399. doi: 10.1016/j.athoracsur.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Cox JL, Ad N, Palazzo T, et al. Current status of the maze procedure for the treatment of atrial fibrillation. Seminars in thoracic and cardiovascular surgery. 2000;12(1):15–19. doi: 10.1016/s1043-0679(00)70011-6. [DOI] [PubMed] [Google Scholar]
- 6.Prasad SM, Maniar HS, Camillo CJ, et al. The cox maze iii procedure for atrial fibrillation: Long-term efficacy in patients undergoing lone versus concomitant procedures. The Journal of thoracic and cardiovascular surgery. 2003;126(6):1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 7.Lall SC, Melby SJ, Voeller RK, et al. The effect of ablation technology on surgical outcomes after the cox-maze procedure: A propensity analysis. The Journal of thoracic and cardiovascular surgery. 2007;133(2):389–396. doi: 10.1016/j.jtcvs.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Melby SJ, Zierer A, Bailey MS, et al. A new era in the surgical treatment of atrial fibrillation: The impact of ablation technology and lesion set on procedural efficacy. Annals of surgery. 2006;244(4):583–592. doi: 10.1097/01.sla.0000237654.00841.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ad N, Suri RM, Gammie JS, Sheng S, O’Brien SM, Henry L. Surgical ablation of atrial fibrillation trends and outcomes in north america. The Journal of thoracic and cardiovascular surgery. 2012;144(5):1051–1060. doi: 10.1016/j.jtcvs.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 10.Gammie JS, Haddad M, Milford-Beland S, et al. Atrial fibrillation correction surgery: Lessons from the society of thoracic surgeons national cardiac database. The Annals of thoracic surgery. 2008;85(3):909–914. doi: 10.1016/j.athoracsur.2007.10.097. [DOI] [PubMed] [Google Scholar]
- 11.Calkins H, Kuck KH, Cappato R, et al. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the european heart rhythm association (ehra), a registered branch of the european society of cardiology (esc) and the european cardiac arrhythmia society (ecas); and in collaboration with the american college of cardiology (acc), american heart association (aha), the asia pacific heart rhythm society (aphrs), and the society of thoracic surgeons (sts). Endorsed by the governing bodies of the american college of cardiology foundation, the american heart association, the european cardiac arrhythmia society, the european heart rhythm association, the society of thoracic surgeons, the asia pacific heart rhythm society, and the heart rhythm society. Heart rhythm: the official journal of the Heart Rhythm Society. 2012;9(4):632–696. e621. doi: 10.1016/j.hrthm.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Saint LL, Bailey MS, Prasad S, et al. Cox-maze iv results for patients with lone atrial fibrillation versus concomitant mitral disease. The Annals of thoracic surgery. 2012;93(3):789–794. doi: 10.1016/j.athoracsur.2011.12.028. discussion 794–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stulak JM, Sundt TM, 3rd, Dearani JA, Daly RC, Orsulak TA, Schaff HV. Ten-year experience with the cox-maze procedure for atrial fibrillation: How do we define success? The Annals of thoracic surgery. 2007;83(4):1319–1324. doi: 10.1016/j.athoracsur.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Abreu Filho CA, Lisboa LA, Dallan LA, et al. Effectiveness of the maze procedure using cooled-tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation. 2005;112(9 Suppl):I20–25. doi: 10.1161/CIRCULATIONAHA.104.526301. [DOI] [PubMed] [Google Scholar]
- 15.Ad N, Holmes SD, Massimiano PS, Pritchard G, Stone LE, Henry L. The effect of the cox-maze procedure for atrial fibrillation concomitant to mitral and tricuspid valve surgery. The Journal of thoracic and cardiovascular surgery. 2013;146(6):1426–1434. doi: 10.1016/j.jtcvs.2013.08.013. discussion 1434–1425. [DOI] [PubMed] [Google Scholar]
- 16.Damiano RJ, Jr, Gaynor SL, Bailey M, et al. The long-term outcome of patients with coronary disease and atrial fibrillation undergoing the cox maze procedure. The Journal of thoracic and cardiovascular surgery. 2003;126(6):2016–2021. doi: 10.1016/j.jtcvs.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Doukas G, Samani NJ, Alexiou C, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: A randomized controlled trial. Jama. 2005;294(18):2323–2329. doi: 10.1001/jama.294.18.2323. [DOI] [PubMed] [Google Scholar]
- 18.Ad N, Henry L, Hunt S, Holmes SD. Do we increase the operative risk by adding the cox maze iii procedure to aortic valve replacement and coronary artery bypass surgery? The Journal of thoracic and cardiovascular surgery. 2012;143(4):936–944. doi: 10.1016/j.jtcvs.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Yoo JS, Kim JB, Ro SK, et al. Impact of concomitant surgical atrial fibrillation ablation in patients undergoing aortic valve replacement. Circulation journal: official journal of the Japanese Circulation Society. 2014;78(6):1364–1371. doi: 10.1253/circj.cj-13-1533. [DOI] [PubMed] [Google Scholar]
- 20.Weimar T, Bailey MS, Watanabe Y, et al. The cox-maze iv procedure for lone atrial fibrillation: A single center experience in 100 consecutive patients. Journal of interventional cardiac electrophysiology: an international journal of arrhythmias and pacing. 2011;31(1):47–54. doi: 10.1007/s10840-011-9547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Heart Rhythm A, European Cardiac Arrhythmia S, American College of C et al. Hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Heart rhythm: the official journal of the Heart Rhythm Society. 2007;4(6):816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Lawrance CP, Henn MC, Miller JR, et al. A minimally invasive cox maze iv procedure is as effective as sternotomy while decreasing major morbidity and hospital stay. The Journal of thoracic and cardiovascular surgery. 2014;148(3):955–961. doi: 10.1016/j.jtcvs.2014.05.064. discussion 962–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson JO, Cuculich PS, Saint LL, et al. Predictors and risk of pacemaker implantation after the cox-maze iv procedure. The Annals of thoracic surgery. 2013;95(6):2015–2020. doi: 10.1016/j.athoracsur.2013.03.064. disussion 2020–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasic M, Musci M, Siniawski H, Edelmann B, Tedoriya T, Hetzer R. Transient sinus node dysfunction after the cox-maze iii procedure in patients with organic heart disease and chronic fixed atrial fibrillation. Journal of the American College of Cardiology. 1998;32(4):1040–1047. doi: 10.1016/s0735-1097(98)00358-1. [DOI] [PubMed] [Google Scholar]
- 25.Pasic M, Musci M, Siniawski H, et al. The cox maze iii procedure: Parallel normalization of sinus node dysfunction, improvement of atrial function, and recovery of the cardiac autonomic nervous system. The Journal of thoracic and cardiovascular surgery. 1999;118(2):287–295. doi: 10.1016/S0022-5223(99)70219-9. [DOI] [PubMed] [Google Scholar]
- 26.Bakker RC, Akin S, Rizopoulos D, Kik C, Takkenberg JJ, Bogers AJ. Results of clinical application of the modified maze procedure as concomitant surgery. Interactive cardiovascular and thoracic surgery. 2013;16(2):151–156. doi: 10.1093/icvts/ivs440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osmancik P, Budera P, Straka Z, Widimsky P. Predictors of complete arrhythmia free survival in patients undergoing surgical ablation for atrial fibrillation. Prague-12 randomized study sub-analysis. International journal of cardiology. 2014;172(2):419–422. doi: 10.1016/j.ijcard.2014.01.104. [DOI] [PubMed] [Google Scholar]
- 28.Ad N, Holmes SD. Prediction of sinus rhythm in patients undergoing concomitant cox maze procedure through a median sternotomy. The Journal of thoracic and cardiovascular surgery. 2014;148(3):881–886. doi: 10.1016/j.jtcvs.2014.04.050. discussion 886–887. [DOI] [PubMed] [Google Scholar]
- 29.Damiano RJ, Jr, Schwartz FH, Bailey MS, et al. The cox maze iv procedure: Predictors of late recurrence. The Journal of thoracic and cardiovascular surgery. 2011;141(1):113–121. doi: 10.1016/j.jtcvs.2010.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrance CP, Henn MC, Miller JR, Sinn LA, Schuessler RB, Damiano RJ., Jr Comparison of the stand-alone cox-maze iv procedure to the concomitant cox-maze iv and mitral valve procedure for atrial fibrillation. Annals of cardiothoracic surgery. 2014;3(1):55–61. doi: 10.3978/j.issn.2225-319X.2013.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pecha S, Schafer T, Subbotina I, Ahmadzade T, Reichenspurner H, Wagner FM. Rhythm outcome predictors after concomitant surgical ablation for atrial fibrillation: A 9-year, single-center experience. The Journal of thoracic and cardiovascular surgery. 2014;148(2):428–433. doi: 10.1016/j.jtcvs.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 32.Gaynor SL, Schuessler RB, Bailey MS, et al. Surgical treatment of atrial fibrillation: Predictors of late recurrence. The Journal of thoracic and cardiovascular surgery. 2005;129(1):104–111. doi: 10.1016/j.jtcvs.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 33.Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (fast): A 2-center randomized clinical trial. Circulation. 2012;125(1):23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 34.Tada H, Ito S, Naito S, et al. Long-term results of cryoablation with a new cryoprobe to eliminate chronic atrial fibrillation associated with mitral valve disease. Pacing and clinical electrophysiology: PACE. 2005;28 (Suppl 1):S73–77. doi: 10.1111/j.1540-8159.2005.00011.x. [DOI] [PubMed] [Google Scholar]
- 35.Malaisrie SC, Lee R, Kruse J, et al. Atrial fibrillation ablation in patients undergoing aortic valve replacement. The Journal of heart valve disease. 2012;21(3):350–357. [PubMed] [Google Scholar]