Abstract
Background
Emotional neglect is associated with multiple negative outcomes, particularly increased risk for depression. Motivated by increasing evidence of reward-related ventral striatum (VS) dysfunction in depression, we investigated the role of developmental changes in VS activity on the emergence of depressive symptomatology as a function of emotional neglect.
Methods
We examined relationships between longitudinal neuroimaging of reward-related VS activity, assessments of mood, and measures of emotional neglect in 106 participants first scanned between ages 11–15 and then two years later.
Results
We found that greater levels of emotional neglect were associated with blunted development of reward-related VS activity between the first and second assessments (as indexed by lower residualized change scores). Additionally, we found that decreases in this reward-related VS activity were related to greater depressive symptomatology, and partially mediated the association between emotional neglect and subsequent depressive symptomatology.
Discussion
Our results provide an important demonstration that blunted development of reward-related VS activity as a function of emotional neglect predicts the emergence of depressive symptoms in adolescents. Further, our results are consistent with emerging evidence for the importance of reward-related VS dysfunction in the etiology and pathophysiology of depression. These results are a first step toward developing the ability to predict, prevent, and treat stress-related psychopathology through the targeting of specific neural phenotypes.
Keywords: emotional neglect, fMRI, early life stress, depression, neurodevelopment, longitudinal
Introduction
Early life stress (ELS) is associated with compromised physical and mental development as well as long-term physical and mental difficulties (1). In regards to mental health, meta-analyses suggest over a 65% increase in the risk for major depressive disorder (MDD) following ELS such as abuse or neglect (2). Though well-studied and well-replicated in psychological and epidemiological research, the exact neurobiological mechanisms mediating the association between such adverse experiences and later depression remain unclear. ELS is associated with sensitization of the neuroendocrine stress response (3) and such differences may be amplified by genetic variation (4). However, further mechanistic clarification is crucial for advancing efforts to establish predictive biomarkers of relative risk and resilience. Targeted neurobiological investigations, particularly those employing neuroimaging, could also aid in developing the next generation of intervention strategies.
In considering the various forms of ELS, psychological maltreatment, such as emotional neglect (EN) may be of particular concern, as this adversity is prevalent, often goes unreported, and is associated with a twofold increase in adolescent mental illness (5–7). Emotional neglect, which is characterized by emotional unresponsiveness, unavailability, and limited emotional interactions between parent and child (8), has been associated with the development of a host of psychological difficulties including increased shame, humiliation, anger, and feelings of worthlessness (9). Furthermore, EN has been found to be one of the “most predictively potent maltreatment type(s)” with strong links to symptoms of MDD (5;10).
To date, the majority of investigations into neural mechanisms of risk related to forms of ELS have focused on dysfunction of brain circuits involved with threat processing and stress responsiveness such as the amygdala (11;12). However, emerging research further implicates dysfunction of reward-related neural circuitry in the pathophysiology of depression (13). Central in this neural circuitry is the ventral striatum (VS), a subcortical structure supporting reward responsiveness and learning (14). VS dysfunction has been theorized to underlie symptoms of MDD (including anhedonia and apathy) and neuroimaging studies have reported decreased reward-related VS activity in depressed individuals (15;16). Furthermore, there is evidence that psychological factors protective against MDD, including maintaining optimism and a positive self-concept, are associated with increased activity of the VS and interconnected neural circuitry (17). In support of these clinical studies, preclinical animal models have found associations between depressive behavior and VS functioning as assessed by levels of transcription factors and gene-expression (18;19). Further work has found anti-depressive-like effects after manipulation of VS neurobiology such as changing the levels of transcription factors or the firing rates of neurons that project to the VS (20;21), again underscoring a central role of the functioning of this brain region in the pathophysiology of depression.
Despite the links suggested by the work described above, few investigations have directly examined associations between EN, VS dysfunction, and depression. One study has reported behavioral deficits in reward processing in children who suffered maltreatment (22). A small number of additional descriptive studies have noted lower VS activity in samples of children (23;24) or in adults that have suffered maltreatment (25). The dearth of clinical research in this area is further surprising given robust findings from preclinical models linking ELS to alterations in reward-related neural circuitry, particularly dopaminergic modulation of VS activity (26;27). Additionally, changes in reward-related behaviors, such as weakened conditioned place preferences, have been consistently noted in these preclinical models of ELS (28).
The limited available research though informative, has not investigated whether differences in reward-related VS function specifically predict later mood disturbances related to EN or other types of ELS. Prospective work is critically needed particularly during adolescence. Initial episodes of depression are likely to occur during this developmental transition (29;30), conferring greater risk for MDD in adulthood (31). Furthermore, focusing on reward during this time period may be particularly important given that anhedonia and low positive affect in adolescence predicts later MDD (32–34). Emotionally unresponsive caregiving during childhood and adolescence could influence the development of this circuitry, leading to difficulties in emotion processing and regulation (35;36). When examining these possible developmental pathways, it will be important to carefully consider seminal studies examining structural brain development, which have found that trajectories of neurobiological change may be more predictive of outcomes than a single measurement at one time point (37). Here, we report on such a prospective study. Specifically, we used longitudinal neuroimaging and behavioral data to test the hypothesis that changes in reward-related VS activity would mediate the relationship between emotional neglect and the emergence of later depressive symptomatology. We predicted that higher levels of EN would be related to greater decreases in reward-related VS activity over time and that this change in activity would partially explain the association between depressive symptoms and EN. Finally, we examined neural responses to specific valences of feedback (i.e., positive or negative) in order to more fully understand potential relationships between EN, symptoms of depression, and VS activity. Based on past theoretical and empirical reports linking lower VS activity to anhedonic elements of depression, we predicted lower VS activity to positive feedback in individuals reporting higher levels of EN.
Methods And Materials
Participants
Longitudinal data were available for 106 adolescents (51 female; Mean Age at Scan 1=13.67 years; range at Scan 1=11.88–15.45 years of age) who were initially recruited as part of a study designed to investigate factors contributing to risk for psychopathology, with an emphasis on depression and alcohol use disorders. After providing consent/assent, adolescent participants without MRI contraindications (e.g., braces) then completed in-person interviews, self-report behavioral assessments, and MRI scanning. Participants were re-contacted annually to complete diagnostic interviews and questionnaires, and also underwent a follow-up MRI scanning session (Mean time between scanning sessions=2.09±.37 years; Range=1.32–3.13 years; Age Range at Scan 2=13.77–18.25 years). A distinctive feature of our recruitment and sampling strategy was an ability to capture increases in rates of depression between adolescence and early adulthood (38;39), while also recognizing that symptoms of MDD (which predict later full-blown diagnoses) often emerge prior to age 14 (40).
Inclusion criteria required that all participants be free of psychopathology, with the exception of anxiety disorder diagnoses, at the baseline. Diagnoses were assessed using structured clinical interviews (41). Sixteen participants had an anxiety disorder at the start of the project and nine participants developed MDD between neuroimaging scans. Within the study population, participants with a family history of MDD were over-sampled. Those with both a first- and second-degree relative with a history of MDD were classified as high risk, and those with no first- or second-degree relatives with a history of MDD as low risk.
Measures of Depression and Anxiety
Depressive symptoms were measured with the child-report version of the Mood and Feelings Questionnaire (MFQ;42). Anxiety symptoms were assessed with the child version of Screen for Child Anxiety Related Disorders (SCARED;43). Both of these self-report measures have high internal consistency and test-retest reliability (MFQ:44); SCARED:43).
Emotional Neglect
EN was assessed using the Childhood Trauma Questionnaire (CTQ;45), which ascertains the experience of different trauma types. In line with prior research in this sample (46), the EN subscale exhibited greater variability in scores. This measure was collected at the baseline scanning session and the second scanning session and then averaged together to yield a composite measure of EN. Analyses were also conducted related to stressful life events occurring during the past year assessed using the interviewer-based Stressful Life Events Schedule (SLES;47) and are presented in our Supplemental materials.
Ventral Striatum Activity Paradigm
To probe reward circuitry, participants completed an fMRI card-guessing paradigm consisting of three blocks each of predominantly positive feedback (80% correct guess), predominantly negative feedback (20% correct guess), and no feedback. Participants were told that their performance on this game would determine a monetary reward to be received and were unaware of the fixed outcome probabilities associated with each block. Instead, all participants received $10. One incongruent trial was included within each task block (e.g., 1 of 5 trials during positive feedback blocks was incorrect, resulting in negative feedback) and all blocks were pseudorandomly ordered to minimize expectancy effects and to increase participant engagement throughout the task. A number of past studies have employed this blocked-designed task to elicit robust VS activity associated with positive feedback in contrast to negative feedback within the broader context of monetary reward (48;49). For additional details, see Supplemental Materials.
fMRI Data Acquisition and Analyses
Blood oxygen level-dependent (BOLD) functional neuroimaging data were acquired for each participant and then processed in Statistical Parametric Mapping 8 (SPM8; University College London, United Kingdom) using standard preprocessing parameters. Following preprocessing, linear contrasts employing canonical hemodynamic response functions were used to estimate the effects of different forms of feedback (e.g., positive;negative) for each individual. Next, a second-level whole-brain analysis ANOVA (flexible factorial) with a 2 (positive feedback, negative feedback) x 2 (Scan 1, Scan 2) design was utilized to isolate regions consistently activated in the contrast of positive feedback>negative feedback. This analytic strategy was employed because of the heterogeneity of reward-related VS activation reported across adolescence, with some studies finding relative increases (50) and others decreases (51) in comparison with adults. Multiple comparison correction was conducted via cluster-size thresholding based on Monte Carlo simulation to yield a corrected p≤0.05 (details in Supplemental Materials). Additional analyses were also conducted with regions of interest (ROI) not reaching statistical significance but previously implicated with reward processing (additional details in Supplemental Materials).
Statistical Analyses
Parameter estimates were extracted for use outside of SPM8 using MarsBaR (52) by averaging across every voxel in clusters that survived multiple-comparisons correction in each region of interest. The same masks were used at Scan 1 and Scan 2 for all analyses. Prior to all analyses and similar to past studies (53), mean parameter estimates were winsorized to minimize the effects of potential outliers, with values that were greater than 3 standard deviations above or below the mean set to the closest observed value within 3 SDs of the mean. Next, to measure change in VS activity over time, linear regression models in R were constructed with parameter estimates for Scan 2 as the dependent variable and parameter estimates for Scan 1 as the independent variable. Residuals for this model (the difference between observed activity and predicted scores for Scan 2) were then saved and used in subsequent analyses. These scores carry the advantage of controlling for the influence of baseline values on change over time, and can be interpreted as changing more or less than expected (54). Negative residuals (or lower values) would therefore represent greater decreases in activity over time than expected.
Next, bivariate correlations were calculated to assess the relationship between change in activity and depressive symptoms and also EN, controlling for age and sex. To investigate the potential mediating role of change in VS activity, path analyses tested whether EN (X) was associated with depression symptomatology (Y), and whether the observed association was mediated by change in activity (M). Statistical testing of mediation was done by nonparametric bootstrapping, with 95% CI for indirect (a X b) mediation effects. Mediation modeling was completed in R and included age (at Scan 1 and Scan 2), time between scans, sex, anxiety symptoms (SCARED at Scans 1 and 2) and depressive symptomatology (Scan 1 MFQ) as covariates.
To probe the specificity of effects, mediation modeling was also completed controlling for recent life stress and other forms of trauma (details in the Supplemental Materials). Furthermore, additional analyses were also conducted a) in relation to symptoms of anxiety, b) reversing potential mediators [change in depression symptoms as the potential mediator], and c) in relation to familial history of MDD (all detailed in the Supplemental Materials).
Exploratory Analyses Related to Feedback Valance
To better understand the effects of EN on reward-related brain function, we investigated the effects of feedback valence (positive or negative) by extracting the contrasts of positive feedback>control blocks and negative feedback>control blocks for the VS clusters that survived multiple comparisons for the contrast positive>negative feedback. For these analyses, change scores were calculated for each of these metrics and bivariate correlations were computed with our variables of interest (details in the Supplemental Materials). Due to the brief nature of our task and limited past research studies focused on such effects, this analysis was considered exploratory. Analyses examining psycho-physiological interactions were also conducted and are discussed in the Supplemental Materials.
Results
Neuroimaging Quality Control And Task Validation
All participants included in our analyses had two waves of fMRI data that met stringent quality control procedures (see Supplemental Materials). Attrition and exclusion for data quality were not related to variables of interest (additional information including demographic and recruitment information available in Supplemental Materials; also in (46;55). Similar to past reports (48;49), our fMRI paradigm elicited robust reward-related (i.e., positive>negative feedback) VS activity (Main Effect of Task shown in Figure 1). In fact, the VS was the only brain area that survived whole-brain multiple comparison correction, p<.05, corrected (Max voxel x=−8, y=+14, z=−2, t=5.2, k=328 voxels). Mean BOLD parameter estimates for this VS cluster were used in all statistical analyses, conducted in R. Analyses with additional ROIs not reaching statistical significance are included in the Supplemental Materials.
Figure 1.
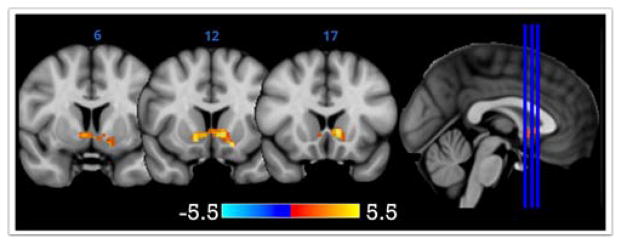
VS activity for the contrast of positive>negative feedback, corrected for multiple comparison correction, p<.05 corrected.
Association Between Stress Exposure, Depressive Symptomatology, and VS Activity
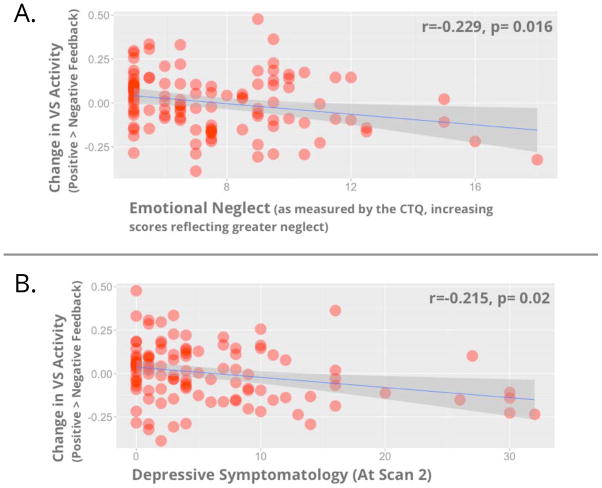
Bivariate correlations indicated EN was associated with greater depressive symptoms at Scan 2 (r=0.266, p=0.004), but not anxiety symptoms (all p’s >.08; details in Supplemental Materials). EN was associated with changes in VS activity (r=−0.234, p=0.015), with greater levels of this adversity being related to lower VS activity change (Figure 2a). This relationship remained significant when participants with any diagnosis of anxiety (p=0.044) or depression (p= 0.026) were removed from the analyses. Depressive symptomatology (at Scan 2) was associated with changes in VS activity (r=−0.215, p=0.02), with greater depressive symptomatology being related to lower VS activity change (Figure 2b). This relationship remained significant when participants with any diagnosis of anxiety (p=0.025) or depression (p=0.01) were removed from the analyses. Similar results were found using robust regression techniques to control for any potential outliers in VS activity (see Supplemental Materials). Levels of anxiety (at Scan 2) were also associated with changes in VS activity (r=−0.19, p=0.048), with greater symptoms being related to lower VS activity change. This association however became non-significant when participants with any diagnosis of anxiety (p=0.12) or depression (p=0.17) were removed from analyses. Of important note, change in VS activity was not related to recent stressful life events, familial risk or the interaction of familial risk-by-time (all p’s>.8, see Supplemental Materials). Also, no differences in levels of EN were found between these groups (p=0.845).
Figure 2.
Scatterplots showing change in VS activity (vertical axis; in both panels) and EN (horizontal axis; panel a) and depressive symptoms at Scan 2 (horizontal axis; panel b)
Statistical Mediation
After finding the associations reported above, we next investigated whether individual differences in changes in reward-related VS activity mediated the effects of EN on depression symptomatology. Similar to the correlations reported above, a linear regression model where EN predicted depressive symptomatology at Scan 2 yielded a β=0.26 (covariates: age at each scan, time between scans, and participant sex). When change in VS activity was entered into this model, the relationship between EN and depressive symptomatology at Scan 2 fell to β=0.22 (covariates: age at each scan, time between scans, participant sex, depressive symptoms at Scan 1, and anxiety symptoms at Scans 1 and 2). In support of our principal hypothesis, non-parametric bootstrapped models indicated the change in VS activity mediated 18.6% of the association between EN and depressive symptoms (variance mediated by the VS=0.186; 95% Confidence Interval (CI)=0.002–0.83, p=0.04; Figure 3). Additional mediation models employing additional covariates (e.g., pubertal status) and different metrics of VS change are detailed in the Supplemental Materials.
Figure 3.
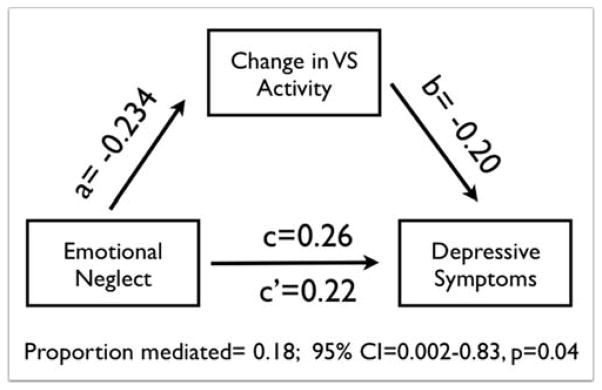
Results from a path analysis testing a model wherein changes in VS activity mediate the relationship between EN and later depressive symptoms. Standardized regression estimates are shown for the relationship between EN and VS change (path a), between VS change and depressive symptoms at Scan 2 (path b), and between EN and depressive symptoms. The latter is shown without (path c) and also while accounting for VS change (path c′)
Factors Contributing To Differences in Reward-Related VS Activity
Regarding VS activity to valence-specific feedback, we found that the change in VS activity to positive feedback was inversely related to EN (r=−0.209, p=0.03) and depressive symptoms (r=−0.259, p=0.007). Change in VS activity to negative feedback, however, was not related to either EN (r=−0.049, p=0.6) or depressive symptoms (r=−0.14, p=0.12). Analyses examining psycho-physiological Interactions with the VS and other brain regions are discussed in the Supplemental Materials.
Discussion
Our current results demonstrate that blunted development of reward-related VS activity significantly mediate the expression of depressive symptomatology as a function of emotional neglect in early life. These patterns of lower residuals of VS activity change (which can be conceptualized as greater decreases over time) are consistent with prior neuroimaging studies linking lower reward-related activity of the VS specifically with anhedonic symptoms of depression (15;16), as well as research from our group demonstrating that lower reward-related VS activity explains reductions in positive affect after stressful events in young adults (48). More broadly, our results support theoretical models (17) that posit brain reward function may be a key component of resilience to MDD following EN or other forms of ELS. In particular, VS activity may be critically related to psychological aspects of resiliency including maintaining optimism, hopefulness, and a positive self-concept after exposure to extreme stress (17;48). Our data and such ideas ally with the finding that initial episodes of MDD may be more strongly related to anhedonia and low positive affect in adolescence (32).
This work begins to fill in important gaps in the understanding of the sequelae of ELS and also the emergence of MDD. While it is clear that both stress and reward deficiencies contribute to depression, debate is ongoing regarding the sequencing of such risk factors. Stress may contribute to reward dysfunction, which then increases risk for MDD. Alternatively, preexisting reward dysfunction may amplify risk for MDD subsequent to stress (56). Our data suggest that reward-related neural dysfunction subsequent to stress exposure is associated with later depression symptomatology. However, further prospective investigations are needed to fully rule out that vulnerability to MDD is not due to the interactions between stress exposure and reward dysfunction. Further research and clarification of these pathways may have important implications for clinical practice, as adults exposed to abuse or neglect have more recurrent, persistent, and treatment-resistant depressive episodes (57). Decreases in reward-related VS activity following EN may represent a neurobiological mechanism for such clinical phenomena. Augmenting treatment with therapeutic modules related to positive affect and reward functioning may be particularly efficacious in samples exposed to EN or other forms of ELS.
In thinking about the phenomenology of EN, a paucity of emotionally responsive caregiving, especially during crucial early periods of development, could increase risk factors for MDD. Emotional unavailability and unresponsiveness in parents may result in poor emotion regulation, as parents help children learn how to adaptively respond to their environment (58). The lack of rich emotional interactions may also contribute to feelings of worthlessness and other cognitive risk factors for depression (e.g., negative attributional style). These cognitive biases may then give way to reduced optimism and greater hopelessness, processes related to VS functioning. Past investigations have found early emotional experiences and forms of emotional maltreatment significantly increase risk for symptoms of depression as well as clinical disorder (59–63). These cognitive vulnerabilities may be a unique pathway linking early EN to MDD, as opposed to other forms of ELS such as physical abuse.
The lack of associations between EN, anxiety, and VS activity in the current work is important to discuss, particularly in light of reports finding anxiety often predates MDD during development (for review, see 64). Anxiety in childhood and depression in adolescence, however, may not always reflect the same constellation of risk factors. Behavioral genetics research suggests MDD and forms of anxiety may partially reflect different genetic risk variables (65–68). Furthermore, environmental factors, such as EN or maltreatment, significantly influence the covariation of anxiety and depression symptoms during development (66;69;70). Research suggests that life stress may be more predictive of MDD, particularly the first onset of the disorder, rather than specific forms of anxiety (71–73). Such patterns could be due to cognitive or emotional vulnerabilities related to EN, noted previously. More broadly, relationships between pediatric anxiety and VS functioning have been mixed, with higher, lower, and no differences in reward-related activity reported between healthy participants and those with anxiety disorders (74;75).
As adolescence represents a period of major neurobiological change within brain reward circuitry, it is important to consider our study in the emerging sub-discipline of developmental cognitive neuroscience. A growing body of research in this field suggests major changes in VS function are a normative part of brain development (76). Initial theoretical models conceptualized increases in reward-related VS activity as an explanation for the elevated risk-taking seen during adolescence (50). More recent longitudinal work has, however, found VS increases may be “protective” in nature, with greater increases across development related to self-reports of less risky decision-making (77). This idea fits with a number of research reports by Forbes and colleagues finding that lower reward-related VS activity is related to MDD in adolescence (78–80). Additional research focused on the VS is needed to explicate these differences, particularly in regards to interactions with the medial prefrontal cortex (mPFC) as increased activity has been found in this region during reward processing for those at-risk for depression (15). In exploratory ROI analyses, we did not find any differences in the mPFC related to EN (details in Supplemental Materials). Lower VS activity during development, however, may lead to aberrant corticostriatal connectivity which is related to MDD clinical symptomatology and treatment response (81). Debate is on-going regarding these various ideas, but our data and research from other groups (15;56) supports the importance of better understanding the development of reward circuitry in the development of depression.
Our study is not without limitations, many of which suggest specific directions for future research. First, the experimental paradigm employed here assays only one facet of reward processing. Recent work has noted that such processing is a complex, non-unitary phenomenon (14; 76). Future work focused on reward anticipation, modulation, and other components of reward processing may aid in explaining the effects of ELS and/or connections with different forms of psychopathology. Second, our sample consisted of individuals with and without a familial history of MDD; this risk factor was however not related to reward activity. We discuss these null findings in relation to past research reports in our Supplemental Materials. Third, the participants in our study are still developing, and the differences highlighted here may lessen (or increase) later in adolescence and adulthood. A blunting of VS activity during development may emerge as reduced engagement in rewarding experiences or with peers, each of which put individuals at elevated risk for MDD (15). These factors may be further exacerbated during periods of stress, increasing the likelihood of MDD (82). Additional longitudinal investigations are needed, particularly those connecting earlier measures of brain functioning with later behavioral outcomes. Fourth, no information was available about the timing of EN. ELS during specific periods in development may uniquely impact facets of reward processing, further influencing stress-related affective psychopathology. Finally, the preponderance of our analyses centered on the VS and depression symptomatology. Though important, the VS does not act in isolation and interactions between the VS and interconnected corticostriatal structures, particularly the ventral tegmental area, amygdala, and mPFC, likely further mediate the effects of EN on psychopathology through alterations in reward processing. Interestingly, we find relationships between EN and VS-amygdala functional connectivity (details in Supplemental Materials). Such results connect with cross-sectional (11;12;83) and longitudinal (53;84) investigations focused on corticolimbic alterations after ELS. Conceptual models of ELS should consider differences in reward-related VS activity and also threat-related amygdala activity, as dynamic interactions between these systems may better predict maladaptive responses to stress exposure (85).
These limitations not withstanding, our results provide the first empirical demonstration that changes in reward-related VS activity as a function of EN predicts relative risk for stress-related depressive symptomatology in adolescents. Further, our results are consistent with emerging evidence for the importance of reward-related VS dysfunction in the etiology and pathophysiology of depression and suggest that targeting this neural phenotype may advance efforts for early intervention in those experiencing EN or forms of ELS. More broadly, aspects of the early environment, specifically parent-child interactions, may uniquely shape the processing of important environmental (reward) cues, creating vulnerabilities for later negative outcomes. Additional research is needed to clarify the complex relationships between ELS and related long-term physical and mental difficulties, our data are however a needed first step in the ability to predict, prevent, and treat stress-related affective psychopathology.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (Grant R01AA016274 to DEW), the Genetic and Environmental Risk endowment from the Dielmann Family (DEW), the National Institute on Drug Abuse (Grants R01DA033369 and R01DA031579 to ARH), the National Institute of Mental Health (Grant MH087493 to Consuelo Walss-Bass), a Postdoctoral Fellowship provided by the National Institute on Drug Abuse through the Center for the Study of Adolescent Risk and Resilience (Grant P30DA023026 to JLH), and a postdoctoral fellowship provided by the National Institute of Child Health and Human Development (Grant T32HD0737625) through the Center for Developmental Science, University of North Carolina at Chapel Hill, to JLH. The authors thank the Teen Alcohol Outcomes Study (TAOS) research staff and participating families for their contributions to this work.
Footnotes
Financial Disclosures: All authors report no biomedical financial interests or potential conflicts of interest.
Author Contributions
JLH performed statistical analyses and helped draft the manuscript. ARH conceived of the study, participated in study design, aided with statistical analyses, and helped draft the manuscript. DEW conceived of the study, participated in study design and data collection, aided with statistical analyses, and helped draft the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shonkoff JP, Garner AS, Siegel BS, Dobbins MI, Earls MF, McGuinn L, et al. The Lifelong Effects of Early Childhood Adversity and Toxic Stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 2.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The Long-Term Health Consequences of Child Physical Abuse, Emotional Abuse, and Neglect: A Systematic Review and Meta-Analysis. Plos Med. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Binder EB. Exp Neurol. Vol. 233. Elsevier Inc; 2012. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene–environment interactions, and epigenetics; pp. 102–111. [DOI] [PubMed] [Google Scholar]
- 5.Spinazzola J, Hodgdon H, Liang L-J, Ford JD, Layne CM, Pynoos R, et al. Unseen Wounds: The Contribution of Psychological Maltreatment to Child and Adolescent Mental Health and Risk Outcomes. Psychological Trauma: Theory, Research, Practice, and Policy. 2014;6:S18–S28. [Google Scholar]
- 6.Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A, Li S. Fourth national incidence study of child abuse and neglect (NIS-4): Report to Congress, Executive Summary. Washington, DC: U.S. Department of Health and Human Services, Administration for Children and Families; 2010. [Google Scholar]
- 7.Young R, Lennie S, Minnis H. Children’s perceptions of parental emotional neglect and control and psychopathology. J Child Psychol & Psychiat. 2011;52:889–897. doi: 10.1111/j.1469-7610.2011.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaser D. Emotional abuse and neglect (psychological maltreatment): a conceptual framework. Child Abuse Negl. 2002;26:697–714. doi: 10.1016/s0145-2134(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 9.Wright MO, Crawford E, Del Castillo D. Childhood emotional maltreatment and later psychological distress among college students: The mediating role of maladaptive schemas. Child Abuse Negl. 2009;33:59–68. doi: 10.1016/j.chiabu.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 10.McGee RA, Wolfe DA, Wilson SK. Multiple maltreatment experiences and adolescent behavior problems: adolescents’ perspectives. Dev Psychopathol. 1997;9:131–149. doi: 10.1017/s0954579497001107. [DOI] [PubMed] [Google Scholar]
- 11.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21:R947–8. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Nestler EJ, Carlezon WA., Jr The Mesolimbic Dopamine Reward Circuit in Depression. Biological Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 15.Forbes EE, Dahl RE. Research Review: altered reward function in adolescent depression: what, when and how? J Child Psychol & Psychiat. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzagalli DA. Depression, Stress, and Anhedonia: Toward a Synthesis and Integrated Model. Annu Rev Clin Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 18.Carlezon WA, Jr, Duman RS, Nestler EJ. Trends Neurosci. Vol. 28. Elsevier; 2005. The many faces of CREB; pp. 436–445. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Peng S, Zhang S, Zhang X. Stress-induced depressive behaviors are correlated with Par-4 and DRD2 expression in rat striatum. Behav Brain Res. 2011;223:329–335. doi: 10.1016/j.bbr.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. Journal of Neuroscience. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. Soc Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral Alterations in Reward System Function The Role of Childhood Maltreatment and Psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- 23.Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- 24.Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 27.Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- 28.Willner P. Chronic Mild Stress (CMS) Revisited: Consistency and Behavioural-Neurobiological Concordance in the Effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 29.Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child and Adolescent Psychiatry. 1994;33:809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, Avenevoli S, Ries Merikangas K. Mood disorders in children and adolescents: an epidemiologic perspective. Biological Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- 31.Lewinsohn PM, Rohde P, Klein DN, Seeley JR. Natural course of adolescent major depressive disorder: I. Continuity into young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:56–63. doi: 10.1097/00004583-199901000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Pine DS, Cohen E, Cohen P, Brook J. Adolescent depressive symptoms as predictors of adult depression: moodiness or mood disorder? American Journal of Psychiatry. 1999;156:133–135. doi: 10.1176/ajp.156.1.133. [DOI] [PubMed] [Google Scholar]
- 33.Joiner TE, Lewinsohn PM, Seeley JR. The core of loneliness: lack of pleasurable engagement--more so than painful disconnection--predicts social impairment, depression onset, and recovery from depressive disorders among adolescents. J Pers Assess. 2002;79:472–491. doi: 10.1207/S15327752JPA7903_05. [DOI] [PubMed] [Google Scholar]
- 34.Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee V, Hoaken PNS. Cognition, Emotion, and Neurobiological Development: Mediating the Relation Between Maltreatment and Aggression. Child Maltreat. 2007;12:281–298. doi: 10.1177/1077559507303778. [DOI] [PubMed] [Google Scholar]
- 36.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 38.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and Development of Psychiatric Disorders in Childhood and Adolescence. Arch Gen Psychiatry. 2003;60:837. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 39.Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, et al. An epidemiological study of disorders in late childhood and adolescence--I. Age- and gender-specific prevalence. J Child Psychol Psychiatry. 1993;34:851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- 40.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Angold A, Costello EJ, Messer SC, Pickles A. Int J Methods Psychiatr Res. Vol. 5. John Wiley & Sons; 1995. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents; pp. 237–249. [Google Scholar]
- 43.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Wood A, Kroll L, Moore A, Harrington R. J Child Psychol & Psychiat. Vol. 36. Wiley Online Library; 1995. Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note; pp. 327–334. [DOI] [PubMed] [Google Scholar]
- 45.Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Bogdan R, Williamson DE, Hariri AR. American Journal of Psychiatry. Vol. 169. Am Psychiatric Assoc; 2012. Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity; pp. 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J, et al. The Stressful Life Events Schedule for children and adolescents: development and validation. Psychiatry Res. 2003;119:225–241. doi: 10.1016/s0165-1781(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 48.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biological Psychiatry. 2012;72:157–163. doi: 10.1016/j.biopsych.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 49.Nikolova YS, Hariri AR. Neural responses to threat and reward interact to predict stress-related problem drinking: A novel protective role of the amygdala. Biology of Mood & Anxiety Disorders. 2012;2:19. doi: 10.1186/2045-5380-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brett M, Anton JL, Valabregue R, Poline J-B. Region of interest analysis using the MarsBar toolbox for SPM 99. NeuroImage 2002 [Google Scholar]
- 53.Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whittle S, Simmons JG, Dennison M, Vijayakumar N, Schwartz O, Yap MBH, et al. Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Developmental Cognitive Neuroscience. 2014;8:7–17. doi: 10.1016/j.dcn.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swartz JR, Williamson DE, Hariri AR. Developmental Change in Amygdala Reactivity During Adolescence: Effects of Family History of Depression and Stressful Life Events. American Journal of Psychiatry. 2014 doi: 10.1176/appi.ajp.2014.14020195. appiajp201414020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Auerbach RP, Admon R, Pizzagalli DA. Adolescent depression: stress and reward dysfunction. Harv Rev Psychiatry. 2014;22:139–148. doi: 10.1097/HRP.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nanni V, Uher R, Danese A. American Journal of Psychiatry. Vol. 169. Am Psychiatric Assoc; 2012. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis; pp. 141–151. [DOI] [PubMed] [Google Scholar]
- 58.Halberstadt A. Family socialization of emotional expression and nonverbal communication styles and skills. J Pers Soc Psychol. 1986;51:827. [Google Scholar]
- 59.Crossfield AG, Alloy LB, Gibb BE, Abramson LY. The Development of Depressogenic Cognitive Styles: The Role of Negative Childhood Life Events and Parental Inferential Feedback. J Cogn Psychother. 2002;16:487–502. [Google Scholar]
- 60.Gibb BE, Abramson LY, Alloy LB. Emotional maltreatment from parents, verbal peer victimization, and cognitive vulnerability to depression. Cogn Ther Res. 2004 doi: 10.1023/B:COTR.0000016927.18027.c2. [DOI] [Google Scholar]
- 61.Gibb B, Alloy L, Abramson L, Marx B. Childhood maltreatment and maltreatment specific inferences: A test of Rose and Abramson’s (1992) extension of the hopelessness theory. PCEM. 2003 doi: 10.1080/02699930244000237. [DOI] [Google Scholar]
- 62.Gibb BE, Butler AC, Beck JS. Childhood abuse, depression, and anxiety in adult psychiatric outpatients. Depress Anxiety. 2003;17:226–228. doi: 10.1002/da.10111. [DOI] [PubMed] [Google Scholar]
- 63.Uhrlass DJ, Gibb BE. Childhood Emotional Maltreatment and The Stress Generation Model of Depression. Journal of Social and Clinical Psychology. 2007;26:119–130. [Google Scholar]
- 64.Rutter M, Kim-Cohen J, Maughan B. Continuities and discontinuities in psychopathology between childhood and adult life. J Child Psychol & Psychiat. 2006;47:276–295. doi: 10.1111/j.1469-7610.2006.01614.x. [DOI] [PubMed] [Google Scholar]
- 65.Rice F, van den Bree MBM, Thapar A. A population-based study of anxiety as a precursor for depression in childhood and adolescence. BMC Psychiatry. 2004;4:43. doi: 10.1186/1471-244X-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silberg JL, Rutter M, Eaves L. Genetic and environmental influences on the temporal association between earlier anxiety and later depression in girls. Biological Psychiatry. 2001;49:1040–1049. doi: 10.1016/s0006-3223(01)01161-1. [DOI] [PubMed] [Google Scholar]
- 67.Rice F. Genetics of childhood and adolescent depression: insights into etiological heterogeneity and challenges for future genomic research. Genome Med. 2010;2:68. doi: 10.1186/gm189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rice F, Harold GT, Thapar A. Negative life events as an account of age-related differences in the genetic aetiology of depression in childhood and adolescence. J Child Psychol Psychiatry. 2003;44:977–987. doi: 10.1111/1469-7610.00182. [DOI] [PubMed] [Google Scholar]
- 69.Eley TC, Stevenson J. Exploring the covariation between anxiety and depression symptoms: a genetic analysis of the effects of age and sex. J Child Psychol Psychiatry. 1999;40:1273–1282. [PubMed] [Google Scholar]
- 70.Liang H, Eley TC. A monozygotic twin differences study of nonshared environmental influence on adolescent depressive symptoms. Child Dev. 2005;76:1247–1260. doi: 10.1111/j.1467-8624.2005.00848.x. [DOI] [PubMed] [Google Scholar]
- 71.Lewinsohn PM, Allen NB, Seeley JR, Gotlib IH. First onset versus recurrence of depression: differential processes of psychosocial risk. J Abnorm Psychol. 1999;108:483–489. doi: 10.1037//0021-843x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 72.Monroe SM, Rohde P, Seeley JR, Lewinsohn PM. Life events and depression in adolescence: relationship loss as a prospective risk factor for first onset of major depressive disorder. J Abnorm Psychol. 1999;108:606–614. doi: 10.1037//0021-843x.108.4.606. [DOI] [PubMed] [Google Scholar]
- 73.Pine DS, Cohen P, Johnson JG, Brook JS. Adolescent life events as predictors of adult depression. J Affect Disord. 2002;68:49–57. doi: 10.1016/s0165-0327(00)00331-1. [DOI] [PubMed] [Google Scholar]
- 74.Helfinstein SM, Fox NA, Pine DS. Approach–withdrawal and the role of the striatum in the temperament of behavioral inhibition. Developmental Psychology. 2012;48:815–826. doi: 10.1037/a0026402. [DOI] [PubMed] [Google Scholar]
- 75.Silk JS, Davis S, McMakin DL, Dahl RE, Forbes EE. Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychol Med. 2012;42:2095–2107. doi: 10.1017/S0033291712000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pfeifer JH, Masten CL, WEM, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering Adolescence: Resistance to Peer Influence, Risky Behavior, and Neural Changes in Emotion Reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neurobiology of Disease. Vol. 52. Elsevier Inc; 2013. Neural response to reward as a predictor of increases in depressive symptoms in adolescence; pp. 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, et al. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 83.Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biological Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, et al. Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology. 2014;39:1245–1253. doi: 10.1038/npp.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, Hendler T. Imbalanced Neural Responsivity to Risk and Reward Indicates Stress Vulnerability in Humans. Cerebral Cortex. 2012;23:28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.