Abstract
The shaping of a multicellular body, and the maintenance and repair of adult tissues require fine-tuning of cell adhesion responses and the transmission of mechanical load between the cell, its neighbors and the underlying extracellular matrix. A growing field of research is focused on how single cells sense mechanical properties of their micro-environment (extracellular matrix, other cells), and on how mechanotranduction pathways affect cell shape, migration, survival as well as differentiation. Within multicellular assemblies, the mechanical load imposed by the physical properties of the environment is transmitted to neighboring cells. Force imbalance at cell-cell contacts induces essential morphogenetic processes such as cell-cell junction remodeling, cell polarization and migration, cell extrusion and cell intercalation. However, how cells respond and adapt to the mechanical properties of neighboring cells, transmit forces, and transform mechanical signals into chemical signals remain open questions.
A defining feature of compact tissues is adhesion between cells at the specialized Adherens Junction (AJ) involving the cadherin super-family of Ca2+-dependent cell-cell adhesion proteins (e.g., E-cadherin in epithelia). Cadherins bind to the cytoplasmic protein β-catenin, which in turn binds to the filamentous (F)-actin binding adaptor protein α-catenin, which can also recruit vinculin, making the mechanical connection between cell-cell adhesion proteins and the contractile actomyosin cytoskeleton.
The cadherin-catenin adhesion complex is a key component of the AJ, and contributes to cell assembly stability and dynamic cell movements. It has also emerged as the main route of propagation of forces within epithelial and non-epithelial tissues. Here, we discuss recent molecular studies that point toward force-dependent conformational changes in α-catenin that regulate protein interactions in the cadherin-catenin adhesion complex, and show that α-catenin is the core mechanosensor that allows cells to locally sense, transduce and adapt to environmental mechanical constrain.
Introduction
As formulated a century ago by D'Arcy Thomson in his treatise “On Growth and Form”, morphogenesis could be explained in part by forces and motion - in other words by mechanics [1]. Tissue-scale mechanics are not only important in morphogenesis [2-5] but also in tissue repair [6, 7] and tumor progression [8, 9]. However “cell and tissue mechanics” were neglected for decades and has only recently been investigated in depth to develop a detailed mechanistic understanding.
Substantial mechanical forces propagate across cells in tissues through cell-cell junctions to drive large scale tissue remodelling (epithelial bending), coordinated cell movements (wound healing), apical cell constriction, tissue elongation, dorsal closure, cell extrusion, cell intercalation and cell migration [10-12]. Such cellular mechanics work with known biochemical signaling cascades and genetic/epigenetic regulation of gene expression. Therefore, it is important to understand: 1) how cells sense, transmit and adapt to mechanical forces imposed by neighboring cells and the extracellular matrix (ECM), and 2) how this mechanical signal is transduced as a biochemical signal to elicit cellular responses resulting from the integration of both biochemical and mechanical pathways.
Tissue mechanics rely on cell-ECM interactions [13], the rheology of each cell [14], their active motors [15, 16], and on the transmission and distribution of the mechanical stress between cells [2, 17, 18]. Apart from well-studied mechanotransduction that takes place at the cell-ECM interface (reviewed in [19, 20]), cells exert mechanical forces on each other at sites of cell-cell adhesion through cadherins [10, 21]. Indeed, it has been reported almost 10 years ago that cadherin-associated adhesions transmit mechanical stress [22] and adapt to the environment stiffness [23]. Nevertheless, major questions were how cadherin adhesions adapt to mechanical forces at molecular and cellular levels, and how such adaptation contributes to force transmission, adaptive cell-cell cohesion, and eventually to tissue-scale mechanics. We report here on recent studies to understand the magnitude of forces transmitted at cell-cell contacts, and how mechanical stress regulates the architecture of cell-cell adhesion complexes and the dynamics of cell-cell contacts. These data identify the adaptor protein α-catenin as the central protein of the core molecular mechanosensor at work at cell-cell contacts.
From cell-cell adhesion to mechanotransduction
Although individual cells within a multicellular organism can be considered as functional units by themselves, they must interact with each other to maintain tissue cohesion. This is an ancestral acquisition required for the emergence of multicellularity during evolution [24, 25]. This cell adhesion principle was recognized at the cellular level a century ago [26] and at molecular level in the 1970's (reviewed in [27]) as not only causing cells to adhere to each other, but also to exchange signals that regulate cell fate and function. Although the existence of a feed-back loop encompassing expression of specific genes coding for cell adhesion molecules that in turn regulated master genes required for cell fate determination was proposed in the 1980's [28], such hypothesis has only been supported recently by experimental data for cell-ECM adhesion [29], and likely for cell-cell adhesion although there is less direct evidence. Interestingly, the main signal downstream of adhesion complexes seems to be the internal mechanical tension imposed on cells by ECM stiffness. This poses the central problem of how mechanical forces are sensed and transmitted from the outside to the inside of cells [30, 31].
On short time scales (typically 10-30 min), cells respond to mechanical force through changes in internal tension imposed by non-muscle myosin (Myosin II) on the F-actin network [32]). Anchoring of the actomyosin cytoskeleton to adhesion sites is mediated by adaptor proteins that link F-actin to transmembrane cell adhesion receptors, thereby allowing mechanical coupling between the intracellular and extracellular compartments. This dynamic coupling, which is well described for integrin-mediated cell-ECM adhesions, allows cells to sense, signal, and respond to physical changes in the environment [13, 20, 33]. The whole process not only allows pulling forces applied to and by cells to equilibrate, but also involves the transduction of the mechanical signal into intracellular biochemical signals that cause actomyosin cytoskeleton re-organization and adhesion complexes recruitment, thereby directing tension-dependent growth of these adhesions [34-36]. Mechanotranduction pathways associated with integrin-based cell-ECM adhesion have been extensively studied over the past 20 years and reviewed elsewhere [19, 20]. Thus, we will focus here on mechanisms of force sensing and mechanical signal transduction at sites of cell-cell adhesion.
The architecture of the cadherin-based intercellular adhesions
The main cell adhesion receptors forming intercellular adhesive structures in all non-circulating cells belong to the cadherin super-family [37]. Desmosomal cadherins, which will not be discussed here, link the intercellular junction to non-contractile intermediate filaments in mammalian epithelial tissues [38]. Classical type I cadherins are more ubiquitously expressed in epithelial (E-cadherin, P-cadherin), endothelial (VE-cadherin) and all other non-epithelial cells (N-cadherin) where they link the adherens-type intercellular junction (AJ) to actin filaments [37, 39]. Regardless of the family member expressed in each tissue, cadherin intercellular junctions differ in their ultrastructural organization, stability, and the topology and organization of the associated F-actin. These differences likely endow these junctions with different mechanical and mechanotransduction properties. The zonula adherens is associated with a circumferential belt of actomyosin filaments as part of the apical junction complex around polarized cells of non-stratified, so-called simple layer, epithelia in Drosophila and mammals [25, 40, 41]. In other epithelia and endothelial cells, a less structured linear AJ is associated with actin filaments tangential to the membrane [42]. At early stages of epithelial cell-cell contact, and in fibroblastic and myoblastic cells, even less organized focal AJs are formed of puncta of cadherins linked to actin filaments perpendicular to the contacting membranes [39, 42-44]. A similar topology has been described for cadherin adhesions formed by cells artificially adhering to cadherin-coated surfaces [45, 46]. The transition from cadherin adhesions (or focal AJs) to a linear AJ is observed during the maturation of junctions of stratified epithelia [47]. A reversal in the transition in junction organization may occur during dissociation of endothelial intercellular junctions under specific conditions [46].
Cardiac myocytes, which are subjected to repeated mechanical load due to cyclic contraction/relaxation, are mechanically coupled by specific cadherin-based junctions termed fascia adherens which combine proteins of AJs and desmosomes [48]. At the opposite end of the junctional organization spectrum, migrating neuronal precursors [49, 50] and their growth cones establish poorly structured and unstable cadherin adhesions that are loosely associated with retrograde movements of the F-actin meshwork [51]. Nevertheless, mechanical coupling of N-cadherin to acto-myosin is required for cell migration [52].
All of these intercellular junctions share a core molecular composition of a cadherin-catenin complex that binds actin filaments maintained under tension by myosin II. Differences in maturation, stability and mechanical strength of these junctions may result from the extent and topology of association of cadherin-catenin complexes to F-actin that are controlled by mechanotransduction pathways discussed below.
Molecular organization of the cadherin-catenin complex and its association with F-actin
Classical cadherins found at the AJ are Ca2+-dependent cell-cell adhesion molecules composed of an extracellular domain with five cadherin repeats, a transmembrane domain and a C-terminal cytoplasmic domain [10]. The extracellular domain is responsible for homophilic interactions with cadherin expressed at the surface of neighboring cells. While interacting in trans, the cadherin extracellular domain is thought to cluster by cis-interaction to form oligomeric arrays bridging the plasma membranes of the two opposing cells [53, 54]. Extracellular domain interactions trigger interactions of proteins associated with the conserved cytoplasm C-terminal domain with actin filaments [39, 55]. During cadherin synthesis in the ER, the arm family proteins p120-catenin and β-catenin (alternatively γ-catenin in some cell types) assemble onto the cytoplasmic domain. While p120-catenin has an essential function in regulating the stability of cadherin-catenin complexes at the plasma membrane [56], β-catenin interacts with the actin binding protein α-catenin. The integrity of the cadherin-catenin complex as well as its correct association with the actin cytoskeleton was recognized very early on as a prerequisite for cell-cell adhesion [57]. It has also been known for some time that the α-catenin/β-catenin heterodimer binds in a stoichiometric complex to cadherins, and to actin filaments [58]. Subsequent biochemical studies challenged the direct linkage between the cadherin-catenin complex and F-actin [59]. However, recent data obtained by manipulating single molecules under force reconciled these opposite views by considering the role of force as a central element required for binding the cadherin-catenin complex to F-actin [60, 61].
Cadherin adhesions transmit and adapt locally to forces
The first direct indication of mechanical coupling of cadherin to actin came from the observation that N-cadherin-coated beads attached to the cell surface were dragged laterally along the plasma membrane [55]. Subsequently, direct measurement of forces applied on cadherin-coated PDMS arrays revealed that cells apply, through cadherin adhesions, tension in the range of 4-5 nN/μm2 [22, 23], similar to integrin-dependent forces applied to the ECM (5.5 nN/μm2) [34]. Further determination of the force applied through N-cadherin and E-cadherin as a function of the compliance of the adhesive surface (Table I) demonstrated that stress increased with stiffness at low stiffness comparable to those of soft tissues, and reached a plateau above 90 kPa [23, 62-64]. Together, these data demonstrate that cadherin adhesions both transmit and adapt to mechanical load.
Table I.
Stress experimentally measured at cadherin adhesions
| Stiffness of the cadherin surface | Cadherin (Cell type) | ref | |||||
|---|---|---|---|---|---|---|---|
| 1 kPa | 8.5-9 kPa | 34 kPa | 95 kPa | 120 kPa | |||
| Stress developed at cadherin adhesions | - | 150 pN/μm2 | 1300 pN/μm2 | 4 nN/μm2 | 4 nN/μm2 | N-cad (C2C12) | [22, 23] |
| <10 pN/μm2 | - | 500 pN/μm2 | - | - | N-cad (MDA-MB-435) | [64] | |
| 43 pN/μm2 | - | 160 pN/μm2 | - | - | E-cad (MDCK, DLD) | [62] | |
| <10 pN/μm2 | - | 550 pN/μm2 | - | - | E-cad (MDCK) | [64] | |
| - | 100 pN/μm2 | - | - | - | E-cad (MDCK) | [63] | |
Measurements have been obtained for cells spread on deformable cadherin-coated substrates of controlled stiffness, either micropillars [22, 23] or polyacrymamide gels [62-64]. As a matter of comparison, 1 kPa is equivalent to lung tissue stiffness [62], and stiffness of renal tissue ranges from 4.3 to 6.8 kPa from the cortex to the renal sinus [109].
These results implied that cadherin-catenin adhesion complexes were under direct tension. To test this, Borghi et al. used a E-cadherin FRET-based molecular tension sensor, in which the FRET signal is in inverted relation with the unfolding of a well described peptide sequence flanked by the two fluorescent proteins and inserted within the cytoplasmic domain of E-cadherin. The results demonstrated that indeed the cytoplasmic domain of E-cadherin in epithelial cells is under tension that required binding of E-cadherin to catenins, as well as actomyosin contractile activity [65]. These forces were in the order of 2-3 pN at resting cell-cell contacts, and at contact-free membranes, and were further increased by ~1 pN at cell-cell contacts by artificially stretching cell doublets. More recently, Schwartz and collaborators using a similar approach showed that VE-cadherin is under tension at the junctions between endothelial cells and that this tension was modulated by fluid shear stress [66]. Altogether these findings point to a role of cadherins in transducing mechanical forces. Furthermore, they show that the degree of tension per molecule or the number of molecules under tension at cell-cell contacts increases with tension. Using a similar approach in Drosophila embryos, Cai et al. showed that during border cell migration in the germarium, E-cadherin tension is asymmetrically distributed within the border cell cluster with more tension at the front of the migrating cluster of cells [67]. Taken together, these cellular data demonstrate that the cadherin-catenin adhesion complex is under tension, and that force transmitted through cadherin-mediated contacts is dependent on local forces applied by/on the cell-cell contact.
General principles of molecular mechanosensing
These recent data indicate that, like cell-ECM adhesions, cell-cell adhesions are responsible for transmission of mechanical forces between neighboring cells. The molecular mechanism underlying cell-ECM mechanosensing processes are partially understood and remains a working example for studying mechanosensing at cell-cell contacts. Although cell-ECM mechanosensing relies on global adaptation of the actomyosin viscoelastic networks [68], integrin-associated cytoplasmic proteins also undergo conformational changes in response to actomyosin-induced forces including p130Cas [69] and talin [70, 71], which may then initiate force-dependent building of adaptor complexes linking cell-ECM adhesions to tension-generating actomyosin network. At the single molecule level, these events rely on a very simple principle built from general thermodynamic rules that dictate the folding of proteins and other macromolecules at their minimum of energy in a given environment. As reviewed by Pruitt et al., changes in catalytic activity or affinity for binding partners that are dictated by force-induced alteration in protein conformation result from the addition in their folding environment of an extra form of energy - the mechanical work generated by myosin (in the range of 2 pN/ single molecule) [72]. Similar “thermodynamic” rules will apply at cell-cell contacts to regulate force-dependent conformational changes of single molecules that will then determine their interaction with other partners in the cadherin-catenin complex. As for cell-ECM adhesions [73], these new interactions, some of which could be reversible, and others not, will modify the association/dissociation equilibrium within a macro-molecular complex thereby changing the response to further force application. By such iterative modifications, the whole complex and its association with actin filaments would drastically evolve with traction forces.
Molecular mechanisms of mechanosensing at cell-cell adhesions
At present, the mechanosensitive pathways at cell-cell contacts are too complex to be studied directly in cellulo. Thus, in vitro systems have been used to identify pathways with purified proteins, including single-molecule force-clamp spectroscopy which has led to a major breakthrough in the understanding of the force response of cadherin-catenin complexes [60, 61]. These studies have been important in reconciling previous biochemical studies that did not find binding of the reconstituted cadherin/β-catenin/α-catenin complex to F-actin in the absence of force [59], with studies of cells and tissues that indicated a direct functional linkage between the cadherin/β-catenin/α-catenin complex and actin filaments [65]. On the other hand, cellular studies have revealed that the recruitment of vinculin observed during cell-cell contact remodeling is both myosin II and α-catenin-dependent [46, 74-76], while this protein fails to efficiently associate to its partner α-catenin in solution [74].
These apparent differences between biochemical and cellular approaches could be reconciled soon. Indeed the results of the two recent single-molecule studies independently reporting on the force-dependent binding of α-catenin to F-actin [61] and the force-dependent unfolding of α-catenin and its binding to vinculin [60] suggest that α-catenin may undergo force-dependent conformational changes that regulate binding of the minimal cadherin-catenin complex to an actin filament under force. An actin filament was suspended between 2 optical traps above a complex of α-catenin/β-catenin/cytoplasmic domain of E-cadherin bound to a platform. The platform was moved back and forth to induce force-dependent interactions between the cadherin-catenin complex and the actin filament. Force stabilized the formation of cadherin-catenin/F-actin bond that could not form in solution in the absence of force [59]. Bond dissociation kinetics could be explained by a 2-step catch bond in which force shifted the α-catenin/F-actin bond from a weak, to a strongly bound state, likely as the result of a conformational conversion of the F-actin binding domain of the molecule. The force threshold (4.5 pN) of this switch was in the range of forces developed by a few myosin II motors (2-3 pN, [72]). This tension-dependent intramolecular transition may stabilize the association of the cadherin adhesion complex to actin filaments (Figure 1A), and thereby account for the mechanosensing properties of the cadherin-catenin adhesion complex without participation of a biochemical (activating) signaling pathway.
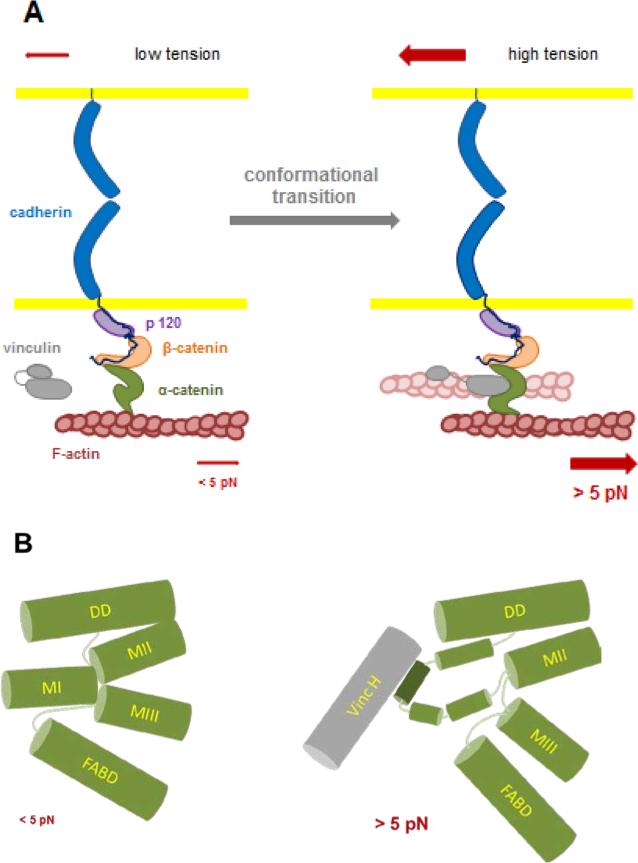
Figure 1. schematics integrating the two tension-dependent conformational changes in α-catenin single molecules elicited at ~ 5 pN.
A: Two force-dependent reversible transitions in α-catenin conformation have been described recently at the single molecule level: one affects the unfolding of the central domain allowing the binding of vinculin [60], the other affects the binding of the C-terminus domain of the molecule to F-actin [61]. The typical force needed for these transitions is around ~ 5 pN. In the cells, this force would be generated by a few Myosin II motors pulling via F-actin on α-catenin tethers held under tension by their association with the cadherin-catenins complex in hemophilic interaction with cadherins present at the surface of a neighboring cell. The two transitions have been characterized independently with a partial complex, and further studies will be required to determine whether vinculin head binding, blocking α-catenin in its open conformation, [60] also stabilizes the F-actin binding domain of the molecule in its (F)-actin high affinity conformation [61]. Whether this transition activates vinculin by head to tail dissociation and provides a second (F)-actin binding event (shaded filament) will require further investigation.
B: α-catenin has a compact structure comprising four α-helix bundle domains: DD (Dimerization and β-catenin binding Domain), MI to MIII (Modulation domains I to III and FABD (F-Actin Binding Domain). The MI domain is the Vinculin Binding Domain (VDB); MII and MIII are the auto-inhibitory domains. Under zero force MI, MII and MIII domain form a tightly packed λ-shape arrangement of helix bundles blocking access to the vinculin binding site. Upon application of forces > 5 pN, MI, MII and MIII domain interactions are lost and the MI domain reconfigurates to expose the vinculin binding α helix allowing vinculin head binding.
A long postulated mechanism for cell-cell adhesion mechanosensing is the tension-dependent recruitment of the actin-binding protein, vinculin, which was recognized early as a marker of the mature AJ [77]. More recently, its recruitment at cell-cell contact has been reported to be dependent on myosin II activity [46, 74]. Although vinculin may bind β-catenin [78], its binding to a central domain of α-catenin named MI or VBD (Vinculin Binding Domain) has been well demonstrated [77, 79], while two adjacent domains, MII and MIII, have been reported to inhibit vinculin binding to the VBD domain [74, 80]. In addition vinculin is recruited to cell-cell contacts upon cell stretching, and vinculin as well as α-catenin are required for strengthening of cell-cell contacts over time [76]. Together these data supported the hypothesis that vinculin is recruited in a force-dependent manner to the cadherin/catenin complex upon actomyosin force-dependent unfolding of the α-catenin central domain [74]; this process would be similar to the binding of vinculin to the talin rod domain upon tension-dependent unfolding [70, 71], which is thought to be central in cell-ECM adhesion mechanosensing.
Single-molecule force-clamp spectroscopy experiments using magnetic tweezers performed on the central domain of α-catenin have provided direct evidence of force-dependent unfolding of α-catenin and its role in vinculin/α-catenin binding [60]. A single α-catenin molecule stretched with magnetic tweezers unfolded in three characteristic steps including a reversible step at ~4.8pN. This conformational change triggered vinculin head binding to α-catenin in a 1:1 molar ratio with nanomolar affinity [60]. This stretch-induced conformational changes in α-catenin caused unfolding of the VBD domain, by destabilizing the interaction between the helix bundle MI containing the vinculin binding α-helix and the two inhibitory helix bundles constituting the domains MII and MIII [81], resulting in a 1000-fold increase in the affinity for vinculin (Figure 1 B). This resulted in very stable binding of α-catenin and vinculin head even after force was released, and inhibited α-catenin returning to its open conformation. Interestingly, the force-dependent binding of vinculin head to α-catenin was biphasic, and was optimized in a force range of 5-10 pN. The binding was strongly inhibited at forces < 5 pN at which MI exists in a stable autoinhibitory bundle of α-helices, or > 30 pN at which the α-helix conformation of the vinculin binding site bound to a vinculin head domain was destabilized. Thus, as in the case of α-catenin/F-actin binding, vinculin binding to α-catenin was dependent on a mechanical signal that caused changes in the conformational equilibrium of α-catenin with no involvement of a biochemical signaling pathway.
These results provided the first direct evidence for how the cadherin-catenin complex transduces mechanical forces into a long lasting biochemical signal through two intramolecular tension-dependent reconfigurations of α-catenin folding (Figure 1A). Further analysis at the single molecule level will test a more integrated model in which the two transitions could be cooperative and vinculin binding therefore could stabilize F-actin binding and vice versa. Another question is the exact role of vinculin in the cadherin complex: does it just stabilize a conformation of α-catenin through its head binding, or does it provide an additional site for the complex to bind F-actin? Ongoing experiments suggest that the binding α-catenin to vinculin is of sufficient affinity to force vinculin head-to-tail dissociation (Yao et al., unpublished data). Further studies will be required to determine whether this binding increases binding of vinculin tail for F-actin (so-called vinculin activation, [82, 83]).
Mechanosensing beyond single α-catenin molecule unfolding
The two intramolecular transitions in α-catenin described above are likely to have additional consequences, and very likely are not the only molecular reconfigurations to take place in the cadherin-catenin- F-actin complex under mechanical load. α-Catenin binds other actin binding proteins, including ZO-1 [84, 85], afadin [86], α-actinin [87] and formin-1 [88], through sites distributed in the central part of the molecule, and to EPLIN [89] at the C-terminal. It will be interesting in future studies to determine whether the affinity of α-catenin for these proteins is regulated by the force-dependent unfolding of the molecule. Many of these interactions may direct the recruitment of more actin filaments or bundles of filaments to the cadherin complex, thereby accounting for the increased local accumulation of actin observed during cell contact maturation and strengthening. Additional force-dependent conformational changes in the cadherin-catenin complex may complete this molecular mechanosensing machinery. β-Catenin single molecule force spectroscopy has been performed using AFM at a pulling rate of 400 nm/s, but failed to reveal near-equilibrium transitions at low force and only unfolding at forces > 50 pN were observed [90], although this does not rule-out a physiological mechanosensor role of this protein. Finally, the cadherin extracellular domain has been described as forming catch bonds during homophilic trans interactions with a typical transition critical force of ~ 30 pN [91], which is well above the ~ 5 pN transition of α-catenin intramolecular transitions.
Dynamic analysis of α-catenin conformational changes in cellulo has been reported using a FRET-based sensor [92]. However, this approach could not relate molecular unfolding events with the measurement of forces developed locally through the cell-cell contact. This study revealed that vinculin recruitment at cell-cell contacts was delayed compare to α-catenin unfolding. Although α-catenin unfolding is central in force sensing, the delayed recruitment of vinculin may not be surprising since there is no evidence so far indicating that the vinculin-bound, open conformation, of α-catenin is the stable configuration in mature contacts. The high affinity binding of unfolded α-catenin to vinculin could be a transient state, needed for sequential recruitment of additional actin binding proteins as those described above, and local actomyosin organization and dynamics. Activated vinculin may itself act as an F-actin bundling proteins [93] or an anchor for other proteins that support junctional actin assembly such as Mena/VASP [94], which would stimulate the recruitment of F-actin at adhesion sites. The co-recruitment of additional cadherin/catenin complexes could result from a positive feedback action of this F-actin re-organization [23, 39].
Together, these molecular processes would allow cell-cell contact architecture to evolve according to forces exchanged locally between two neighboring cells. Only with the development of experimental approaches that measure simultaneously forces developed at cell-cell contacts, molecular unfolding and single protein recruitment will we be able to determine the time sequence of the tension-dependent elaboration of the cadherin/catenin/F-actin complex. The limited knowledge we have today on these sequential events may explain the apparent controversy raised by the induced recruitment of vinculin both during cell-cell contact maturation [46, 74, 76] and cell-cell contact dissociation [42]. Further studies will also be needed to address additional interesting questions such as the influence on these processes of the cooperative adhesive interactions of cadherin ectodomains [52], the cooperative binding of α-catenin to F-actin [59], the local regulation of actin dynamics by α-catenin [109], the regulation of clusters size by F-actin [42, 108].
Mechanics of cell-cell contact rearrangement and tissue shaping
The sequential intermolecular interactions initiated by α-catenin unfolding that may tightly adjust the recruitment and association to F-actin with cadherin-catenin complexes, may thus locally regulate the transmission of forces that drive morphogenetic processes (reviewed in [17]). A well-documented example of such a process is embryonic germ band extension in Drosophila larvae, during which the early epithelium is elongated by cell intercalation. This tissue elongation requires a planar polarized remodeling of AJs under the control of myosin II-based cell contraction [95]. α-Catenin has been shown to be essential in this AJ remodeling [96]. Recently, Lenne et al., developed a method to deform the cell-cell interfaces and measure tension at the cell junctions using optical tweezers [97]. They could show that a tension in the 100 pN range, which could be powered by a few tens of molecular motors, is sufficient to produce significant deformation of the cell-cell contact. Whether an asymmetric modification of the linkage of a few E-cadherin complexes to actomyosin via α-catenin on one side of AJ would create enough asymmetry in myosin-II powered tension to induce interface deformation and cell shape fluctuation has not been addressed yet.
Morphogenetic processes in vertebrates also rely on the tension-dependent reorganization of cell-cell contacts. Although, data on force transmission at intercellular contacts in vivo are not available, they are accumulating on mammalian cells in culture [98-104]. Intercellular junctions are permanently displaced to adapt their position in order to minimize intracellular forces imposed by cell-ECM interactions and intercellular forces [99]. Transmission of forces through cell-cell junctions is a key regulator of coordinated movements of epithelial tissues in vitro [103, 105, 106] and large scale coordinated movements of epithelial cells is strongly altered in cells in which the cadherin/catenin complex and AJ organization are disrupted, and in cancerous cell types. However, a direct measurement of such forces as well as of the stress components is particularly challenging. On average, the stress experienced by cell-cell junctions (1 nN/μm2) [98-102] is in the range of the stress measures through cadherin adhesions (Table I), with some unexplained discrepancies for evaluations made on the softer substrates. On the other hand, recent sub-resolution localization studies have provided quite precise estimation of the distribution and number of cadherin–catenin complexes populating an AJ [44], fitting well the estimation made in Drosophila [107]. These estimates can be used to compare forces measured at intercellular contacts with those sensed by individual proteins. According to super-resolution quantitative microscopy [44], an AJ is composed of broadly size-dispersed nanoclusters containing less than ten cadherin molecules (median= 6 molecules). Within these nanoclusters, a force of 30 pN would be sufficient to unfold cadherin-bound α-catenin and link the cluster to F-actin. However, these clusters are interspaced from each other and the overall cadherin density in an AJ fell to 2000 molecules/μm2 at maximum. The tension needed to unfold the corresponding α-catenin would thus be in the range of 10 nN/μm2, which is one order of magnitude above the tension measured at cell-cell contacts [98-102]. The lower stress value measured at cell-cell contacts could be due to the partial engagement of the population of cadherin/catenin complexes in adhesive interactions, an hypothesis supported by FRAP data [59]. Nevertheless, given these uncertainties, there is a good agreement between the range of forces experienced by intercellular contacts and the forces required to trigger tension-dependent remodeling of individual cadherin/catenin/F-actin links.
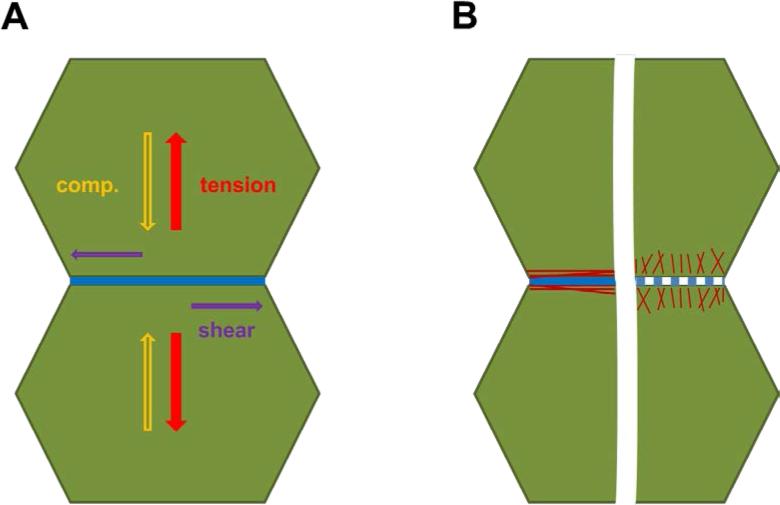
However, in the future a more detailed inspection of the orientation of the forces applied to the cell-cell contacts, as well as the consideration of the topology of actin filament orientation will be required (Figure 2). Indeed the AJ is subjected to various mechanical forces that can be due to tensile, compressive or even shear forces, in particular during collective cell movements [108]. As such, the linkages between adherent cells, particularly cadherin-related cell-cell junctions, contribute to friction forces between neighboring cells as they adhere and move relatively to each other. Moreover, as discussed above, depending on the type of AJs formed in the different types of cells and/or of the maturation of these contacts, the F-actin is clearly differently orientated compared to the orientation of the adhesions (either parallel to adhesions in zonula adherens or perpendicular in focal AJs). This orientation as well as the type of forces considered would differentially influence the response of cadherin-catenin adhesion complexes. Thus, there is room for strong modulation of the mechano-response of the cadherin-catenin-F-actin link to small changes in mechanical forces as well as for a differential regulation of this response in function of the cell-type specific architecture of AJs.
Figure 2. Variety of forces (A) and orientations of actin filaments encountered at cell-cell contacts.
A: Cell-cell contacts (Blue) are subject to various mechanical stresses that can be due to tensile (red), compressive (yellow) or shear (violet) forces in particular during collective cell movements.
B: Actin filaments orientation (red) and AJ morphology (blue) both vary with the cell type considered and/or the maturation of the cell-cell contacts.
In conclusion, the reversible unfolding of the mechanical switch protein α-catenin upon physiologically-relevant forces is likely to be central in the adaptation of cell-cell contacts to mechanical force distribution in cell layers. Coupled to the force generating actomyosin system, this mechanotransduction machinery is a key player in morphogenenic processes. Further progress in its characterization at the molecular, cellular and tissue levels, and in living model organisms, will bring new information on the control of cell and tissue mechanics, and on its interplay with biochemical and genetic regulations required for proper morphogenesis and its evolution along the animal kingdom.
Supplementary Material
Insight Box.
The cadherin-catenin adhesion complex is a key component of the intercellular Adherens junction that contributes to epithelial and non-epithelial tissue stability and dynamic cell movements. The cadherin adhesion complex bridges neighboring cells and the actin-myosin cytoskeleton, and thereby contributes to mechanical coupling between cells which drives many morphogenesis events and tissue repair. Mechanotransduction at cadherin adhesions enables cells to sense, signal, and respond to physical changes in the environment. We discuss in this review recent breakthroughs in understanding cellular and molecular aspects of this mechanotranduction process that is centered on tension-dependent conformational switches in the F-actin binding protein α-catenin.
Acknowledgements
R.M.M., W.J.N, B.L, and J.Y are jointly supported by the Human Frontier Science Program (HFSP grant RPG0040/2012). In addition: R.M.M. was supported by grants from CNRS, and Agence Nationale de la Recherche (ANR 2010 Blan1515); B.L. was supported by grants from CNRS, Mechanobiology Institute, Agence Nationale de la Recherche (ANR 2010 Blan1515) and European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement n° 617233; J.Y. was supported by the National Research Foundation of Singapore through the Mechanobiology Institute Singapore; and W.J.N. was supported by the National Institutes of Health (GM-035527). We thank Delphine Delacour and Marc-Antoine Fardin for discussions and editing.
References
- 1.Thompson DW. On growth and form. Cambridge University Press; Cambridge, UK: 1917. [Google Scholar]
- 2.Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1185–1189. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 3.Cetera M, Ramirez-San Juan GR, Oakes PW, Lewellyn L, Fairchild MJ, Tanentzapf G, Gardel ML, Horne-Badovinac S. Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat Commun. 2014;5:5511. doi: 10.1038/ncomms6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitre JL, Berthoumieux H, Krens SF, Salbreux G, Julicher F, Paluch E, Heisenberg CP. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338:253–256. doi: 10.1126/science.1225399. [DOI] [PubMed] [Google Scholar]
- 5.Heisenberg CP, Bellaiche Y. Forces in tissue morphogenesis and patterning. Cell. 2013;153:948–962. doi: 10.1016/j.cell.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Anon E, Serra-Picamal X, Hersen P, Gauthier NC, Sheetz MP, Trepat X, Ladoux B. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc Natl Acad Sci U S A. 2012;109:10891–10896. doi: 10.1073/pnas.1117814109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vedula SR, Hirata H, Nai MH, Brugues A, Toyama Y, Trepat X, Lim CT, Ladoux B. Epithelial bridges maintain tissue integrity during collective cell migration. Nat Mater. 2014;13:87–96. doi: 10.1038/nmat3814. [DOI] [PubMed] [Google Scholar]
- 8.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat Med. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Mouw JK, Weaver VM. Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol. 2011;21:47–56. doi: 10.1016/j.tcb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeichi M. Dynamic contacts: rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol. 2014;15:397–410. doi: 10.1038/nrm3802. [DOI] [PubMed] [Google Scholar]
- 11.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 12.Montell DJ. Morphogenetic cell movements: diversity from modular mechanical properties. Science. 2008;322:1502–1505. doi: 10.1126/science.1164073. [DOI] [PubMed] [Google Scholar]
- 13.Ladoux B, Nicolas A. Physically based principles of cell adhesion mechanosensitivity in tissues. Rep Prog Phys. 2012;75:116601. doi: 10.1088/0034-4885/75/11/116601. [DOI] [PubMed] [Google Scholar]
- 14.Mitrossilis D, Fouchard J, Pereira D, Postic F, Richert A, Saint-Jean M, Asnacios A. Real-time single-cell response to stiffness. Proc Natl Acad Sci U S A. 2010;107:16518–16523. doi: 10.1073/pnas.1007940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munjal A, Lecuit T. Actomyosin networks and tissue morphogenesis. Development. 2014;141:1789–1793. doi: 10.1242/dev.091645. [DOI] [PubMed] [Google Scholar]
- 16.Mitrossilis D, Fouchard J, Guiroy A, Desprat N, Rodriguez N, Fabry B, Asnacios A. Single-cell response to stiffness exhibits muscle-like behavior. Proc Natl Acad Sci U S A. 2009;106:18243–18248. doi: 10.1073/pnas.0903994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao Y, Baum B. Tug of war-The influence of opposing physical forces on epithelial cell morphology. Dev Biol. 2015 doi: 10.1016/j.ydbio.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Ishihara S, Sugimura K, Cox SJ, Bonnet I, Bellaiche Y, Graner F. Comparative study of non-invasive force and stress inference methods in tissue. Eur Phys J E Soft Matter. 2013;36:9859. doi: 10.1140/epje/i2013-13045-8. [DOI] [PubMed] [Google Scholar]
- 19.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 20.Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18:472–481. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Leckband DE, de Rooij J. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol. 2014;30:291–315. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- 22.Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, Ladoux B. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 23.Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mege RM. Strength dependence of cadherin-mediated adhesions. Biophys J. 2010;98:534–542. doi: 10.1016/j.bpj.2009.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulpiau P, Gul IS, van Roy F. New insights into the evolution of metazoan cadherins and catenins. Prog Mol Biol Transl Sci. 2013;116:71–94. doi: 10.1016/B978-0-12-394311-8.00004-2. [DOI] [PubMed] [Google Scholar]
- 25.Miller PW, Clarke DN, Weis WI, Lowe CJ, Nelson WJ. The evolutionary origin of epithelial cell-cell adhesion mechanisms. Curr Top Membr. 2013;72:267–311. doi: 10.1016/B978-0-12-417027-8.00008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson HV. On some phenomena of coalescence and regeneration in sponges. J. Exp. Zool. 1907;5:245–258. [Google Scholar]
- 27.Steinberg MS. Adhesion in development: an historical overview. Dev Biol. 1996;180:377–388. doi: 10.1006/dbio.1996.0312. [DOI] [PubMed] [Google Scholar]
- 28.Edelman GM. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986;2:81–116. doi: 10.1146/annurev.cb.02.110186.000501. [DOI] [PubMed] [Google Scholar]
- 29.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 31.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 32.Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15:825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz US, Safran SA. Physics of adherent cells. Rev. Mod. Phys. 2013;85:1327–1372. [Google Scholar]
- 34.Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 35.Stricker J, Aratyn-Schaus Y, Oakes PW, Gardel ML. Spatiotemporal constraints on the force-dependent growth of focal adhesions. Biophys J. 2011;100:2883–2893. doi: 10.1016/j.bpj.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franke WW. Discovering the molecular components of intercellular junctions--a historical view. Cold Spring Harb Perspect Biol. 2009;1:a003061. doi: 10.1101/cshperspect.a003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nekrasova O, Green KJ. Desmosome assembly and dynamics. Trends Cell Biol. 2013;23:537–546. doi: 10.1016/j.tcb.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 41.Kobielak A, Fuchs E. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol. 2004;5:614–625. doi: 10.1038/nrm1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- 43.Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Kanchanawong P, Zaidel-Bar R. Actin-delimited adhesion-independent clustering of e-cadherin forms the nanoscale building blocks of adherens junctions. Dev Cell. 2015;32:139–154. doi: 10.1016/j.devcel.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Gavard J, Lambert M, Grosheva I, Marthiens V, Irinopoulou T, Riou JF, Bershadsky A, Mege RM. Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J Cell Sci. 2004;117:257–270. doi: 10.1242/jcs.00857. [DOI] [PubMed] [Google Scholar]
- 46.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–1115. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 48.Kartenbeck J, Franke WW, Moser JG, Stoffels U. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 1983;2:735–742. doi: 10.1002/j.1460-2075.1983.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luccardini C, Hennekinne L, Viou L, Yanagida M, Murakami F, Kessaris N, Ma X, Adelstein RS, Mege RM, Metin C. N-cadherin sustains motility and polarity of future cortical interneurons during tangential migration. J Neurosci. 2013;33:18149–18160. doi: 10.1523/JNEUROSCI.0593-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bard L, Boscher C, Lambert M, Mege RM, Choquet D, Thoumine O. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giannone G, Mege RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475:510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lambert M, Choquet D, Mege RM. Dynamics of ligand-induced, Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J Cell Biol. 2002;157:469–479. doi: 10.1083/jcb.200107104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peifer M, Yap AS. Traffic control: p120-catenin acts as a gatekeeper to control the fate of classical cadherins in mammalian cells. J Cell Biol. 2003;163:437–440. doi: 10.1083/jcb.200310090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- 59.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mege RM, et al. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun. 2014;5:4525. doi: 10.1038/ncomms5525. [DOI] [PubMed] [Google Scholar]
- 61.Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science. 2014;346:1254211. doi: 10.1126/science.1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barry AK, Tabdili H, Muhamed I, Wu J, Shashikanth N, Gomez GA, Yap AS, Gottardi CJ, de Rooij J, Wang N, et al. alpha-catenin cytomechanics--role in cadherin-dependent adhesion and mechanotransduction. J Cell Sci. 2014;127:1779–1791. doi: 10.1242/jcs.139014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maruthamuthu V, Gardel ML. Protrusive activity guides changes in cell-cell tension during epithelial cell scattering. Biophys J. 2014;107:555–563. doi: 10.1016/j.bpj.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabdili H, Langer M, Shi Q, Poh YC, Wang N, Leckband D. Cadherin-dependent mechanotransduction depends on ligand identity but not affinity. J Cell Sci. 2012;125:4362–4371. doi: 10.1242/jcs.105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell. 2014;157:1146–1159. doi: 10.1016/j.cell.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trichet L, Le Digabel J, Hawkins RJ, Vedula SR, Gupta M, Ribrault C, Hersen P, Voituriez R, Ladoux B. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci U S A. 2012;109:6933–6938. doi: 10.1073/pnas.1117810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao M, Goult BT, Chen H, Cong P, Sheetz MP, Yan J. Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation. Sci Rep. 2014;4:4610. doi: 10.1038/srep04610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pruitt BL, Dunn AR, Weis WI, Nelson WJ. Mechano-transduction: from molecules to tissues. PLoS Biol. 2014;12:e1001996. doi: 10.1371/journal.pbio.1001996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nature cell biology. 2010;12:533–542. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 75.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas WA, Boscher C, Chu Y-S, Cuvelier D, Martinez-Rico C, Seddiki R, Heysch J, Ladoux B, Thiery JP, Mege R-M, et al. α-Catenin and vinculin cooperate to promote high E-cadherin-based adhesion strength. The Journal of biological chemistry. 2013;288:4957–4969. doi: 10.1074/jbc.M112.403774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peng X, Cuff LE, Lawton CD, DeMali KA. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J Cell Sci. 2010;123:567–577. doi: 10.1242/jcs.056432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Choi HJ, Pokutta S, Cadwell GW, Bobkov AA, Bankston LA, Liddington RC, Weis WI. alphaE-catenin is an autoinhibited molecule that coactivates vinculin. Proc Natl Acad Sci U S A. 2012;109:8576–8581. doi: 10.1073/pnas.1203906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishiyama N, Tanaka N, Abe K, Yang YJ, Abbas YM, Umitsu M, Nagar B, Bueler S.a., Rubinstein JL, Takeichi M, et al. An Autoinhibited Structure of α-catenin and Its Implications for Vinculin Recruitment to Adherens Junctions. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M113.453928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rangarajan ES, Izard T. The cytoskeletal protein alpha-catenin unfurls upon binding to vinculin. J Biol Chem. 287:18492–18499. doi: 10.1074/jbc.M112.351023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cohen DM, Chen H, Johnson RP, Choudhury B, Craig SW. Two distinct head-tail interfaces cooperate to suppress activation of vinculin by talin. The Journal of biological chemistry. 2005;280:17109–17117. doi: 10.1074/jbc.M414704200. [DOI] [PubMed] [Google Scholar]
- 83.Bois PRJ, O'Hara BP, Nietlispach D, Kirkpatrick J, Izard T. The vinculin binding sites of talin and alpha-actinin are sufficient to activate vinculin. The Journal of biological chemistry. 2006;281:7228–7236. doi: 10.1074/jbc.M510397200. [DOI] [PubMed] [Google Scholar]
- 84.Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A. Functional domains of alpha-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol. 1999;144:1311–1322. doi: 10.1083/jcb.144.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Itoh M. Involvement of ZO-1 in Cadherin-based Cell Adhesion through Its Direct Binding to alpha Catenin and Actin Filaments. The Journal of Cell Biology. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pokutta S, Drees F, Takai Y, Nelson WJ, Weis WI. Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. The Journal of biological chemistry. 2002;277:18868–18874. doi: 10.1074/jbc.M201463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci. 1997;110(Pt 8):1013–1022. doi: 10.1242/jcs.110.8.1013. [DOI] [PubMed] [Google Scholar]
- 88.Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nature cell biology. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abe K, Takeichi M. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci U S A. 2008;105:13–19. doi: 10.1073/pnas.0710504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valbuena A, Vera AM, Oroz J, Menendez M, Carrion-Vazquez M. Mechanical properties of beta-catenin revealed by single-molecule experiments. Biophys J. 2012;103:1744–1752. doi: 10.1016/j.bpj.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rakshit S, Zhang Y, Manibog K, Shafraz O, Sivasankar S. Ideal, catch, and slip bonds in cadherin adhesion. Proc Natl Acad Sci U S A. 2012;109:18815–18820. doi: 10.1073/pnas.1208349109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim TJ, Zheng S, Sun J, Muhamed I, Wu J, Lei L, Kong X, Leckband DE, Wang Y. Dynamic Visualization of alpha-Catenin Reveals Rapid, Reversible Conformation Switching between Tension States. Curr Biol. 2015;25:218–224. doi: 10.1016/j.cub.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chinthalapudi K, Rangarajan ES, Patil DN, George EM, Brown DT, Izard T. Lipid binding promotes oligomerization and focal adhesion activity of vinculin. J Cell Biol. 2014;207:643–656. doi: 10.1083/jcb.201404128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol. 2014;24:1689–1699. doi: 10.1016/j.cub.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–1114. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 96.Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- 97.Bambardekar K, Clement R, Blanc O, Chardes C, Lenne PF. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1418732112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9949. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tseng Q, Duchemin-Pelletier E, Deshiere A, Balland M, Guillou H, Filhol O, Thery M. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc Natl Acad Sci U S A. 2012;109:1506–1511. doi: 10.1073/pnas.1106377109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ng MR, Besser A, Brugge JS, Danuser G. Mapping the dynamics of force transduction at cell-cell junctions of epithelial clusters. Elife. 2014;4 doi: 10.7554/eLife.03282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater. 2011;10:469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saez A, Anon E, Ghibaudo M, du Roure O, Di Meglio JM, Hersen P, Silberzan P, Buguin A, Ladoux B. Traction forces exerted by epithelial cell sheets. J Phys Condens Matter. 2010;22:194119. doi: 10.1088/0953-8984/22/19/194119. [DOI] [PubMed] [Google Scholar]
- 105.Doxzen K, Vedula SR, Leong MC, Hirata H, Gov NS, Kabla AJ, Ladoux B, Lim CT. Guidance of collective cell migration by substrate geometry. Integr Biol (Camb) 2013;5:1026–1035. doi: 10.1039/c3ib40054a. [DOI] [PubMed] [Google Scholar]
- 106.Vedula SR, Peyret G, Cheddadi I, Chen T, Brugues A, Hirata H, Lopez-Menendez H, Toyama Y, Neves de Almeida L, Trepat X, et al. Mechanics of epithelial closure over non-adherent environments. Nat Commun. 2015;6:6111. doi: 10.1038/ncomms7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Truong Quang BA, Mani M, Markova O, Lecuit T, Lenne PF. Principles of E-cadherin supramolecular organization in vivo. Curr Biol. 2013;23:2197–2207. doi: 10.1016/j.cub.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 108.Gov NS. Traction forces during collective cell motion. HFSP J. 2009;3:223–227. doi: 10.2976/1.3185785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bensamoun SF, Robert L, Leclerc GE, Debernard L, Charleux F. Stiffness imaging of the kidney and adjacent abdominal tissues measured simultaneously using magnetic resonance elastography. Clin Imaging. 2011;35:284–287. doi: 10.1016/j.clinimag.2010.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.