Abstract
Several enveloped RNA viruses of the arenavirus, bunyavirus, filovirus and flavivirus families are associated with a syndrome known as viral hemorrhagic fever (VHF). VHF is characterized by fever, vascular leakage, coagulation defects and multi organ system failure. VHF is currently viewed as a disease precipitated by viral suppression of innate immunity, which promotes systemic virus replication and excessive proinflammatory cytokine responses that trigger the manifestations of severe disease. However, the mechanisms by which immune dysregulation contributes to disease remain poorly understood. Infection of nonhuman primates closely recapitulates human VHF, notably Ebola and yellow fever, thereby providing excellent models to better define the immunological basis for this syndrome. Here we review the current state of our knowledge and suggest future directions that will better define the immunological mechanisms underlying VHF.
Introduction
Among the more well-known causes of VHF are the filoviruses Ebola virus (EBOV) and Marburg virus (MARV), the arenavirus Lassa virus, and the flaviviruses yellow fever (YFV) and dengue (DENV) virus. How diverse virus families with different replication strategies cause a similar clinical syndrome is incompletely understood, but several features typify these infections. The viruses effectively suppress innate antiviral defenses and replicate systemically to high titers; monocytes, macrophages and dendritic cells are targets of infection; a systemic cytokine storm occurs; and vascular leakage and hemorrhage may be seen. Nevertheless, specific details of the immunological underpinnings of VHF are lacking, and a unified view as to how the virus, the innate immune response and the adaptive immune response interact in the setting of VHF is also absent. Here, we argue that the availability of well-established nonhuman primate models of EBOV and YFV disease provide the necessary tools to define the immunological features common to VHFs, leading to a greater understanding of the syndrome and the suggestion of novel therapeutic approaches.
Detailed look at EBOV
The ebolaviruses and marburgviruses are enveloped viruses with non-segmented negative-sense single-stranded RNA genomes that belong to the family Filoviridae. There are 5 species of ebolavirus: Zaire ebolavirus (EBOV), Sudan ebolavirus (SUDV), Bundibugyo ebolavirus (BDBV), Tai Forrest ebolavirus (TAFV) and Reston ebolavirus (RESTV) and a single species of marburgvirus, Marburg marburgvirus (MARV). Only EBOV, SUDV, BDBV and MARV have been associated with outbreaks of severe disease and high mortality in humans. The most detailed descriptions of filovirus disease come from studies of EBOV and will form the main basis for our discussion of filovirus hemorrhagic fever.
Filovirus genomes possess 7 genes that encode: nucleoprotein (NP), viral protein of 35 kDa (VP35), VP40, glycoprotein (GP; mediates viral attachment and entry), VP30, VP24 and Large protein (L; the enzymatic component of the viral RNA-dependent RNA polymerase). The EBOV replication cycle takes place in the cytoplasm. Virus release occurs by budding from the plasma membrane in a process directed by the matrix protein VP40 and enhanced by other viral proteins, including GP.
Pathophysiology
Infections occur due to direct contact with infectious material, such as bodily fluids containing infectious virus. Airborne transmission is not thought to be a significant route of human infection, but aerosolized virus does cause rapidly lethal disease in experimentally-infected non-human primates (reviewed in [1]). Following exposure, an incubation period of 2–21 days is followed by an abrupt but non-specific viral syndrome characterized by fever, chills and myalgia. As infection progresses, prostration, nausea, vomiting, abdominal pain and diarrhea appear. The final stages of disease are characterized by coagulopathy and vascular leakage resulting in hemorrhage and shock as reviewed in [2].
Many of the details of EBOV pathogenesis are derived from nonhuman primate studies, as they closely parallel severe human infections and are considered the “gold-standard” model of EBOV disease (EVD). The hallmarks of EVD are high levels of systemic virus replication, cytokine production, liver damage, coagulopathy and lymphopenia [2]. Although filoviruses productively infect a variety of cell types, dendritic cells (DCs), macrophages and monocytes appear to be the preferential targets [2–5]. This may be due to 1) viral GP interaction with lectins, such as dendritic-cell-specific ICAM-3-grabbing non-integrin (DC-SIGN) on the surface of these cells [6– 10], or 2) phosphatidylserine on the surface of virus particles interacting, either directly or through an intermediate, with molecules such as TIM-1, TAM or αVβ3 and αVβ5 integrins [11– 18]. Because these immune cells support productive viral infection and are capable of trafficking in vivo, their infection likely facilitates dissemination of the virus to lymph nodes and systemically [2,4,5,19].
The dissemination of EBOV to hepatocytes, adrenal cortical cells and endothelial cells likely contributes to coagulopathy, which can result in hemorrhage and shock [4]. Virus-induced liver damage reduces production of coagulation factors, while infection of the adrenal gland reduces production of hormones that regulate blood pressure [4]. In addition, infected monocytes and macrophages produce proinflammatory mediators (IL-1β, IL-6, IL-8, IL-10, MIP-1β and TNFα), reactive oxygen species, nitric oxide, and tissue factor (TF) [20–27], which promote endothelial leakage and hypovolemia [28–31]. The cellular sensors and signaling pathways by which EBOV infection promotes production of cytokines and chemokines by monocytes are incompletely defined. In vitro studies demonstrate that extensive EBOV replication is not required to elicit cytokine production, but likely sustains the cytokine response.
Immune evasion
In contrast to monocytes/macrophages, EBOV infection of DCs is characterized by an inhibition of IFN-α/β and cytokine production, down-regulation of co-stimulatory molecules, and reduced ability to activate T cells [32–36]. The VP35 proteins target multiple innate immune signaling pathways to suppress IFN-α/β production and its antiviral effects [37–47]. X-ray crystal structures demonstrate that the EBOV and MARV VP35s bind the phosphodiester backbone of dsRNA and that EBOV VP35 also “caps” the ends of dsRNAs in a manner that could mask 5’-triphosphates [38,40–43,48,49]. EBOV VP35 can also interact with cellular protein PACT to prevent PACT-mediated activation of RIG-I. Mutations in VP35 that disrupt interactions with dsRNA and PACT abrogate VP35 inhibition of IFN responses [36,38,40–43,48–54]. Furthermore, mutations in VP35 impair virus replication in IFN-α/β competent cells and attenuate the virus in vivo, demonstrating a critical role for innate immune suppression for pathogenesis [52–54].
In addition, EBOV and MARV block the Jak-STAT signaling pathways triggered when IFNs are added to cells, thereby disrupting the antiviral effects of these cytokines. EBOV VP24 blocks the nuclear accumulation of tyrosine phosphorylated STAT1 by binding to the NPI-1 subfamily of karyopherin alpha (KPNA) proteins [55–58], whereas MARV VP40 blocks signaling by tyrosine kinase Jak1, preventing all the tyrosine phosphorylation events that typically occur after IFN addition to cells [59,60].
The impact of DC suppression on the adaptive immunity in vivo remains to be determined, as virus-specific T cell responses develop in both EBOV-infected mice and people who survive infection [61,62]. Moreover, lymphopenia is another common feature of EBOV infection, with loss of CD4 T cells, CD8 T cells and NK cells in mouse and nonhuman primate models [63,64] as well as human patients [65]. Cell loss occurs primarily via apoptosis and although the basis for this phenomenon is not yet clear, it is believed to be mediated by pro-inflammatory cytokines, NO and soluble FAS ligand produced by monocytes/macrophages [22,25,66–68].
Detailed look at YFV
Virus epidemiology, genetics and replication
YFV is endemic in central Africa and South America where it results in approximately 200,000 cases and 30,000 deaths annually [69]. YFV is an arbovirus that is spread via mosquitoes belonging to the genera Haemagogus and Aedes. YFV is maintained through two life cycles: in the urban cycle, YFV is transmitted between humans via Aedes aegypti; and in the jungle cycle, YFV transmission occurs between non-human primates via Hemagogus mosquitos in South America and Aedes africanus in Africa while humans can be infected by mosquitos that previously fed on an infected monkey [70,71].
Like other members of the Flaviviridae family, YFV is a single positive stranded RNA virus with an 11Kb genome composed of a 5’ non-coding region, a single open-reading frame (ORF), and a 3’ non-coding region. The ORF encodes 3 structural proteins (capsid (C), membrane (prM), and envelope (E)) and 7 nonstructural proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5) [72]. Virus proteins are processed after translation of the entire polyprotein within the rough endoplasmic reticulum (ER). The main structural protein is envelope, which is anchored in the lipid bilayer of the viral envelope and plays an important role in viral entry [73]. Nonstructural proteins are mainly involved in RNA replication and post-translational cleavage of the virus polyprotein [74].
YFV cell entry is mediated by the surface E protein and is internalized via clathrin-dependent endocytosis. The low-pH environment of endosomes induces un-coating of the virus, and the viral RNA genome is released into the cytoplasm, where replication can occur [73,75]. The positive sense RNA is translated to either synthesize complementary negative RNA strands, which serve as templates for progeny positive strands, or encode structural proteins for virion assembly and viral enzymes required for replication and post-translational processing [72].
Pathophysiology
YFV elicits two patterns of injury, viscerotropism and neurotropism. YFV primarily causes a viscerotropic disease in humans and nonhuman primates with lesions observed in multiple organs such as liver, spleen, heart and kidneys [76–78]. Golden hamsters and immunodeficient mice (AG129 mice) have been developed to study YFV infection [79–82]. However, there are limitations to these models. The hamster model requires a hamster-adapted strain of YFV. Infection of immunodeficient mice with the vaccine strain YFV-17D results in encephalitis but not viscerotropic disease as observed in nonhuman primates and humans and hampers studies of host immune response to YFV [82]. In contrast, non-human primates are a robust model for studying YFV since they are a natural reservoir during the jungle cycle of transmission and the clinical manifestations following YFV challenge of rhesus macaques mimic human viscerotropic disease [83].
Approximately 103 YFV is first introduced into the epidermis via saliva from a blood-feeding mosquito [84]. Previous studies suggest that dendritic cells residing in the epidermis are important early targets for flavivirus replication [85–87]. The virus then spreads via infected DCs through lymphatic channels to draining lymph nodes and subsequently into the bloodstream, eventually disseminating to the liver, spleen, additional lymph nodes, heart, and kidneys [84]. Yellow fever presents in three distinct stages: infection, remission and intoxication. Infection lasts 3–6 days after the initial mosquito bite and is characterized with the onset of fever, headache, malaise, photophobia, backache, myalgia, irritability and nausea with viremia peaking on 3 days after onset of symptoms [74,88]. During remission, which lasts between 12 to 48 hours, fever and symptoms subside [74]. Most patients recover, but approximately 15% of patients will become severely ill and enter the period of intoxication in which patients develop jaundice, oliguria or anuria, cardiovascular instability, hemorrhagic fever and multi-organ dysfunction [74,89]. Case-fatality rates of patients that develop visceral disease with jaundice range from 20% to 50% [89].
Hepatic dysfunction is the hallmark of YFV and is characterized by eosinophilic degeneration of hepatocytes (known as Councilman bodies [90]) and Kupffer cells, Fas mediated midzonal hepatocellular apoptosis, absence of inflammation, and steatosis [91–94]. The predominance of apoptotic versus necrotic liver injury may explain the minimal inflammation and infiltration [91,92,94]. Renal pathology is characterized by eosinophilic degeneration and fatty change of the renal tubular epithelium without inflammation [83].
Similar to Ebola hemorrhagic fever, cytokine dysregulation is thought to mediate endothelial damage, disseminated intravascular coagulation and circulatory shock observed in the terminal stage of YFV. Thrombocytopenia, prolonged clotting and prothrombin times have been observed in human patients and nonhuman primates due to diminished liver production of fibrinogen and clotting factors [74,95].
Immune evasion
Yellow Fever Virus employs strategies to evade host innate immunity by inhibiting type I interferon response. NS4B, whose function is conserved among flaviviruses, can block STAT1 activation and interferon stimulated gene expression in Vero cells after addition of IFNβ [96]. A recent study that characterized gene expression within peripheral blood mononuclear cells from rhesus macaques 3 days post YFV infection reported the down-regulation of 43 genes associated with innate immunity, including interferon gamma receptor (IFNGR1), CD83 (a marker of DC maturation), and TNFSF11 (hypothesized to induce DCs to stimulate naïve T cell proliferation) [97,98]. The importance of evasion of innate immunity to the pathogenesis of YFV is highlighted by the reduced mortality in rhesus macaques treated with polyriboinosinicpolyribocytidylic acid, poly-L-lysine and carboxymethylcellulose, which are inducers of IFNα [99]. Similarly, administration of IFNγ reduced viremia and hepatitis severity in squirrel monkeys while prolonging survival time in rhesus macaques [100].
Similar to EBOV, YFV infection also results in profound lymphopenia. Depletion of lymphocytes in germinal centers of spleen, lymph nodes, tonsils and Peyer’s patches are observed [83,101,102]. In rhesus macaques infected with YFV strain DakH1279, circulating lymphocytes declined by 71% in animals that required euthanasia compared to a 23% decrease in animals that survived challenge, with a significant negative correlation between viral load and extent of lymphocyte loss [97]. The loss of lymphocytes is most likely due to the cytokine storm that accompanies YFV infection. Levels of pro-inflammatory modulators such as IL-6, MCP-1, IP-10, TNFα and anti-inflammatory cytokine IL-1RA were significantly higher in patients with fatal Yellow Fever compared to patients who survived [103]. Similarly, levels of IL-6, IFNy, MCP-1 and IL-15 were elevated in rhesus macaques infected with Yellow Fever strain DakH1279 [97].
Remaining questions and future directions
The host response to infection plays prominent roles in EVD and YFV viscerotropic disease, but a direct demonstration of how specific interactions between virus and host immune response contribute to VHF in vivo is largely lacking. The availability of well-developed animal models provides the opportunity to address these gaps in knowledge and to use this information to develop new therapeutic approaches.
How does viral suppression of the IFN response influence viral pathogenesis?
Although we know a great deal about how filoviral VP35s regulate viral replication and suppress IFN response in vitro, the contribution of these functions to the pathogenesis of VHF in vivo remains poorly defined [32–36]. Recombinant EBOVs with point mutations in VP35 are attenuated in rodent models [52,53], however these models do not fully recapitulate human VHF [104]. Therefore to address the role of VP35 in VHF, primates must be infected with VP35 mutant viruses. Because the role of VP35 in innate immune evasion and viral replication are not easily separable, very early time points should be examined to uncover changes in DC and monocyte activation in draining lymph nodes.
A VP24 mutant EBOV was found to modestly impact DC maturation phenotypes in vitro [33]. However, this mutation may not completely inactivate VP24 IFN-antagonist function [105]. Recent structural and functional studies have defined new amino acid residues on EBOV VP24 critical for suppression of IFN signaling that should be investigated in vivo [58]. Finally, additional studies are required before we can define the contribution of MARV VP40 and to identify additional YFV proteins that contribute to suppression of innate immunity and impact pathogenesis in vivo.
What inflammatory pathways are activated by infection and in which cell types?
Excessive pro-inflammatory cytokine production is thought to be a major factor in pathogenesis of VHF [20,22]. Although the responses of infected monocytes and macrophages in vitro suggest these cells as a source of inflammatory cytokines, the cell types most relevant to the inflammatory response in vivo, the signaling pathways that contribute to this response, and the role of virus infection in triggering these responses remain to be defined. Depletion of specific immune cell subsets together with transcriptome and phenotype analysis of monocytes isolated from infected humans and nonhuman primates during acute infection would help address these questions.
What is the contribution of the immune responses in the vascular leakage and coagulopathy?
In vitro studies attribute EBOV-induced endothelial leakage to cytokines, TF and soluble GP, and in vivo studies suggest mechanisms other than destruction of the endothelium by virus replication [5,28,30]. Although complicated to address in NHPs, the use of cytokine-neutralizing antibodies or specific inhibitors in EBOV or YFV-infected animals, could clarify disease mechanisms and suggest therapeutic approaches. Further, the contribution of liver damage or damage to the adrenal gland to coagulopathy or low blood pressure also need further examination [2].
What is the status of the adaptive immune response?
The suppression of DC maturation in vitro suggests that EBOV may impair development of T cell responses. Fatal infections are associated with the absence of specific antibody responses and with the apoptotic loss of lymphocytes [25,63,64,106]. These findings suggest defects in adaptive immunity during the course of infection; however, survivors develop specific T cell responses [61,62]. Therefore, it is important to further characterize the status of the adaptive immune response in vivo. Important questions to be answered include mechanisms of lymphocyte apoptosis and dysregulation of lymphocyte activation and proliferation using careful examination of lymphocyte transcriptome and phenotype ex vivo using clinical samples and nonhuman primate models.
Tools to address these questions
The availability of well-developed animal models for both EBOV and YFV affords the opportunity to address these gaps in knowledge and to use this information to develop new therapeutic approaches. It should be possible to take advantage of different viruses from within the same family with different degrees of virulence. For example, whereas intramuscular injection of Zaire EBOV is nearly 100 percent lethal in macaques, a comparable injection of Bundibugyo EBOV is only 66 percent lethal [107]. Examination of host responses in survivors versus lethal infections with either virus could highlight those features that most determine the outcome of infection. Moreover, the similar pathogenic mechanisms employed by EBOV and YFV, suggest that YFV may serve as a model for Ebola hemorrhagic fever. One major advantage of YFV is the ability to study it under biosafety level 3 (BSL-3) conditions. In contrast, study of EBOV and other filoviruses requires biosafety level 4 (BSL-4) containment facilities, which are available to only a handful of researchers worldwide.
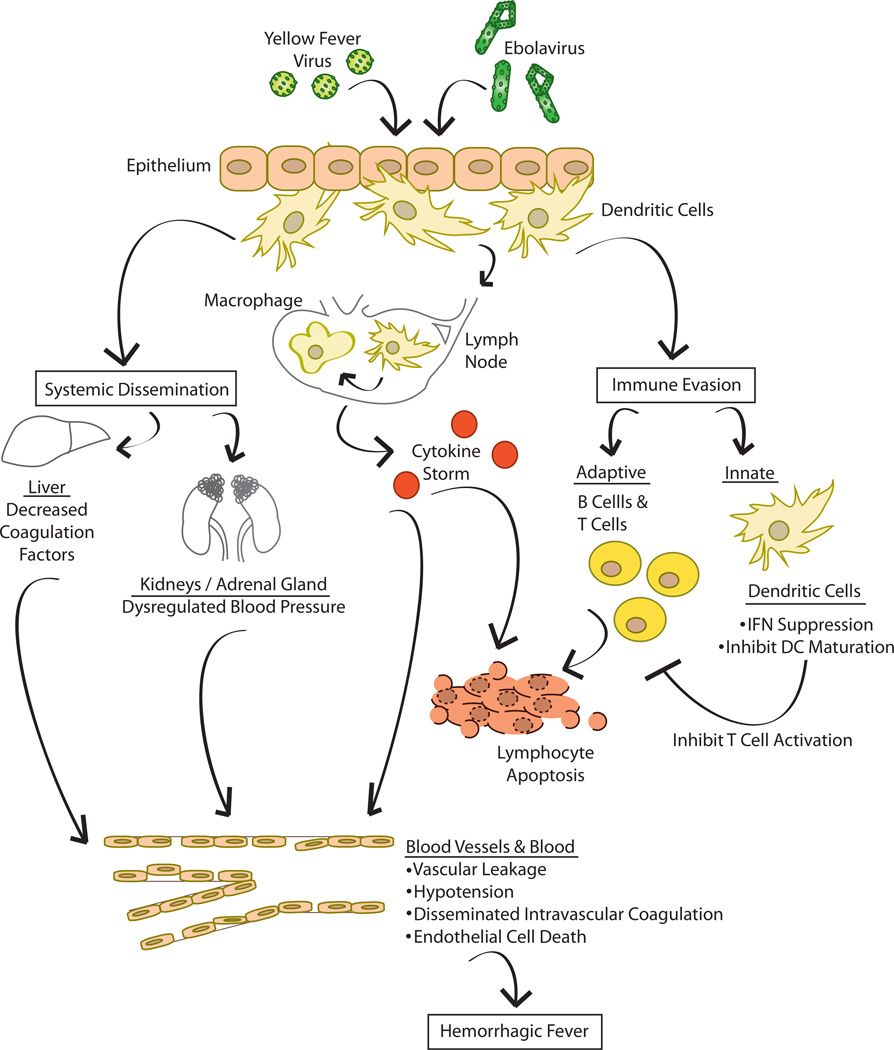
Figure.
Infection with EBOV or YFV occurs when virus breaches epithelial/mucosal barriers. This may occur following exposure of EBOV to breaks in skin or to the mucosal epithelium. For YFV, this occurs via mosquito bite. Macrophages and dendritic cells are important early targets of infection. These cells not only support productive replication but can also traffic to local lymph nodes and to other tissues and organs, thereby promoting systemic dissemination. Infection of and damage to different organs promotes the indicated pathologic processes. Infection of macrophages also results in prolific production of cytokines, commonly referred to as cytokine storm. This can promote vascular leakage and hypotension and can activate coagulation pathways that ultimately lead to disseminated intravascular coagulation. Also, cytokines likely contribute to apoptosis of lymphocytes. Infection of dendritic cells leads to a dysregulated phenotype where interferon (IFN) responses are suppressed and maturation of the dendritic cells is impaired. This likely inhibits activation of T cells, further preventing control of the infection.
Highlights.
Several families of RNA viruses cause viral hemorrhagic fever in humans
Viral hemorrhagic fever (VHF) is characterized by fever, vascular leak and bleeding
Immunological mechanisms are thought to underlie the symptoms of VHF
Ebola virus and Yellow Fever virus provide two excellent models to study VHF
Acknowledgments
The authors thank Christine Schwall for critical reading of the manuscript. We thank the National Institutes of Health for grants U19AI109945 (Basler) to I.M. and C.F.B. and R01AI05953, and U19AI109664 to C.F.B. and the Department of the Defense, Defense Threat Reduction Agency for grants HDTRA1-12-1-0051 and HDTRA1-14-1-0013 to C.F.B. The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osterholm MT, Moore KA, Kelley NS, Brosseau LM, Wong G, Murphy FA, Peters CJ, LeDuc JW, Russell PK, Van Herp M, et al. Transmission of Ebola viruses: what we know and what we do not know. MBio. 2015;6:e00137. doi: 10.1128/mBio.00137-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2010;377:849–862. doi: 10.1016/S0140-6736(10)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M, Geisbert TW. Ebola virus: the role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. Int J Biochem Cell Biol. 2005;37:1560–1566. doi: 10.1016/j.biocel.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Geisbert TW, Hensley LE, Larsen T, Young HA, Reed DS, Geisbert JB, Scott DP, Kagan E, Jahrling PB, Davis KJ. Pathogenesis of Ebola Hemorrhagic Fever in Cynomolgus Macaques: Evidence that Dendritic Cells Are Early and Sustained Targets of Infection. Am J Pathol. 2003;163:2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Larsen T, Kagan E, Hensley LE. Pathogenesis of Ebola hemorrhagic fever in primate models: evidence that hemorrhage is not a direct effect of virus-induced cytolysis of endothelial cells. Am J Pathol. 2003;163:2371–2382. doi: 10.1016/S0002-9440(10)63592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–6844. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- 8.Marzi A, Moller P, Hanna SL, Harrer T, Eisemann J, Steinkasserer A, Becker S, Baribaud F, Pohlmann S. Analysis of the interaction of Ebola virus glycoprotein with DC-SIGN (dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin) and its homologue DC-SIGNR. J Infect Dis. 2007;196(Suppl 2):S237–S246. doi: 10.1086/520607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gramberg T, Hofmann H, Moller P, Lalor PF, Marzi A, Geier M, Krumbiegel M, Winkler T, Kirchhoff F, Adams DH, et al. LSECtin interacts with filovirus glycoproteins and the spike protein of SARS coronavirus. Virology. 2005;340:224–236. doi: 10.1016/j.virol.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaletsky RL, Simmons G, Bates P. Proteolysis of the Ebola virus glycoproteins enhances virus binding and infectivity. J Virol. 2007;81:13378–13384. doi: 10.1128/JVI.01170-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A. 108:8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimojima M, Takada A, Ebihara H, Neumann G, Fujioka K, Irimura T, Jones S, Feldmann H, Kawaoka Y. Tyro3 family-mediated cell entry of Ebola and Marburg viruses. J Virol. 2006;80:10109–10116. doi: 10.1128/JVI.01157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takada A, Watanabe S, Ito H, Okazaki K, Kida H, Kawaoka Y. Downregulation of beta1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology. 2000;278:20–26. doi: 10.1006/viro.2000.0601. [DOI] [PubMed] [Google Scholar]
- 14.Schornberg KL, Shoemaker CJ, Dube D, Abshire MY, Delos SE, Bouton AH, White JM. Alpha5beta1-integrin controls ebolavirus entry by regulating endosomal cathepsins. Proc Natl Acad Sci U S A. 2009;106:8003–8008. doi: 10.1073/pnas.0807578106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt CL, Kolokoltsov AA, Davey RA, Maury W. The Tyro3 receptor kinase Axl enhances macropinocytosis of Zaire ebolavirus. J Virol. 85:334–347. doi: 10.1128/JVI.01278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimojima M, Ikeda Y, Kawaoka Y. The mechanism of Axl-mediated Ebola virus infection. J Infect Dis. 2007;196(Suppl 2):S259–S263. doi: 10.1086/520594. [DOI] [PubMed] [Google Scholar]
- 17.Brindley MA, Hunt CL, Kondratowicz AS, Bowman J, Sinn PL, McCray PB, Jr, Quinn K, Weller ML, Chiorini JA, Maury W. Tyrosine kinase receptor Axl enhances entry of Zaire ebolavirus without direct interactions with the viral glycoprotein. Virology. 415:83–94. doi: 10.1016/j.virol.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahlmann F, Biedenkopf N, Babler A, Jahnen-Dechent W, Karsten CB, Gnirss K, Schneider H, Wrensch F, O'Callaghan CA, Bertram S, et al. Analysis of Ebola Virus Entry Into Macrophages. J Infect Dis. 2015 Apr 14; doi: 10.1093/infdis/jiv140. pii: jiv140. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Twenhafel NA, Mattix ME, Johnson JC, Robinson CG, Pratt WD, Cashman KA, Wahl-Jensen V, Terry C, Olinger GG, Hensley LE, et al. Pathology of experimental aerosol Zaire ebolavirus infection in rhesus macaques. Vet Pathol. 2013;50:514–529. doi: 10.1177/0300985812469636. The authors demonstrate extensive infection of respiratory lymphoid tissues early after aerosol infection of macaques with Ebola virus. This is the first detailed report of pathogenesis via the aerosol route.
- 20.Baize S, Leroy EM, Georges AJ, Georges-Courbot MC, Capron M, Bedjabaga I, Lansoud-Soukate J, Mavoungou E. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–168. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebihara H, Rockx B, Marzi A, Feldmann F, Haddock E, Brining D, LaCasse RA, Gardner D, Feldmann H. Host response dynamics following lethal infection of rhesus macaques with Zaire ebolavirus. J Infect Dis. 2011;204(Suppl 3):S991–S999. doi: 10.1093/infdis/jir336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett. 2002;80:169–179. doi: 10.1016/s0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez A, Lukwiya M, Bausch D, Mahanty S, Sanchez AJ, Wagoner KD, Rollin PE. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–10377. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villinger F, Rollin PE, Brar SS, Chikkala NF, Winter J, Sundstrom JB, Zaki SR, Swanepoel R, Ansari AA, Peters CJ. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–S191. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 25.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;4 doi: 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta M, Mahanty S, Ahmed R, Rollin PE. Monocyte-derived human macrophages and peripheral blood mononuclear cells infected with ebola virus secrete MIP-1alpha and TNF-alpha and inhibit poly-IC-induced IFN-alpha in vitro. Virology. 2001;284:20–25. doi: 10.1006/viro.2001.0836. [DOI] [PubMed] [Google Scholar]
- 27.Stroher U, West E, Bugany H, Klenk HD, Schnittler HJ, Feldmann H. Infection and activation of monocytes by Marburg and Ebola viruses. J Virol. 2001;75:11025–11033. doi: 10.1128/JVI.75.22.11025-11033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmann H, Bugany H, Mahner F, Klenk HD, Drenckhahn D, Schnittler HJ. Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol. 1996;70:2208–2214. doi: 10.1128/jvi.70.4.2208-2214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnittler HJ, Feldmann H. Molecular pathogenesis of filovirus infections: role of macrophages and endothelial cells. Curr Top Microbiol Immunol. 1999;235:175–204. doi: 10.1007/978-3-642-59949-1_10. [DOI] [PubMed] [Google Scholar]
- 30.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618–1629. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 31.Neumann FJ, Ott I, Marx N, Luther T, Kenngott S, Gawaz M, Kotzsch M, Schomig A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arterioscler Thromb Vasc Biol. 1997;17:3399–3405. doi: 10.1161/01.atv.17.12.3399. [DOI] [PubMed] [Google Scholar]
- 32.Mahanty S, Hutchinson K, Agarwal S, McRae M, Rollin PE, Pulendran B. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J Immunol. 2003;170:2797–2801. doi: 10.4049/jimmunol.170.6.2797. [DOI] [PubMed] [Google Scholar]
- 33. Lubaki NM, Ilinykh P, Pietzsch C, Tigabu B, Freiberg AN, Koup RA, Bukreyev A. The lack of maturation of Ebola virus-infected dendritic cells results from the cooperative effect of at least two viral domains. J Virol. 2013;87:7471–7485. doi: 10.1128/JVI.03316-12. The authors implicate previously identified Ebola virus-encoded innate immune antagonist proteins, particuarly the VP35 protein, as critical for Ebola virus suppression of dendritic cell maturation.
- 34.Bosio CM, Aman MJ, Grogan C, Hogan R, Ruthel G, Negley D, Mohamadzadeh M, Bavari S, Schmaljohn A. Ebola and Marburg viruses replicate in monocyte-derived dendritic cells without inducing the production of cytokines and full maturation. J Infect Dis. 2003;188:1630–1638. doi: 10.1086/379199. [DOI] [PubMed] [Google Scholar]
- 35.Jin H, Yan Z, Prabhakar BS, Feng Z, Ma Y, Verpooten D, Ganesh B, He B. The VP35 Protein of Ebola Virus Impairs Dendritic Cell Maturation Induced by Virus and Lipopolysaccharide. J Gen Virol. 2010;91:352–361. doi: 10.1099/vir.0.017343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yen B, Mulder LC, Martinez O, Basler CF. Molecular Basis for Ebola Virus VP35 Suppression of Human Dendritic Cell Maturation. J Virol. 2014;88:12500–12510. doi: 10.1128/JVI.02163-14. This study demonstrates that the Ebola virus VP35 protein is sufficient to block dendritic cell maturation when the inducer of maturation signals through RIG-I-like receptors.
- 37.Bale S, Julien JP, Bornholdt ZA, Kimberlin CR, Halfmann P, Zandonatti MA, Kunert J, Kroon GJ, Kawaoka Y, MacRae IJ, et al. Marburg virus VP35 can both fully coat the backbone and cap the ends of dsRNA for interferon antagonism. PLoS Pathog. 2012;8:e1002916. doi: 10.1371/journal.ppat.1002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bale S, Julien JP, Bornholdt ZA, Krois AS, Wilson IA, Saphire EO. Ebolavirus VP35 coats the backbone of double-stranded RNA for interferon antagonism. J Virol. 2013;87:10385–10388. doi: 10.1128/JVI.01452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Basler CF, Mikulasova A, Martinez-Sobrido L, Paragas J, Muhlberger E, Bray M, Klenk HD, Palese P, Garcia-Sastre A. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J Virol. 2003;77:7945–7956. doi: 10.1128/JVI.77.14.7945-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimberlin CR, Bornholdt ZA, Li S, Woods VL, Jr, MacRae IJ, Saphire EO. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc Natl Acad Sci U S A. 2009;107:314–319. doi: 10.1073/pnas.0910547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung DW, Prins KC, Borek DM, Farahbakhsh M, Tufariello JM, Ramanan P, Nix JC, Helgeson LA, Otwinowski Z, Honzatko RB, et al. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol. 2010;17:165–172. doi: 10.1038/nsmb.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luthra P, Ramanan P, Mire CE, Weisend C, Tsuda Y, Yen B, Liu G, Leung DW, Geisbert TW, Ebihara H, et al. Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe. 2013;14:74–84. doi: 10.1016/j.chom.2013.06.010. This study demonstrates that the interaction of Ebola virus VP35 with cellular protein PACT plays a critical role in VP35 suppression of interferon-α/β responses.
- 44.Ramanan P, Edwards MR, Shabman RS, Leung DW, Endlich-Frazier AC, Borek DM, Otwinowski Z, Liu G, Huh J, Basler CF, et al. Structural basis for Marburg virus VP35-mediated immune evasion mechanisms. Proc Natl Acad Sci U S A. 2012;109:20661–20666. doi: 10.1073/pnas.1213559109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schumann M, Gantke T, Muhlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol. 2009;83:8993–8997. doi: 10.1128/JVI.00523-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prins KC, Cardenas WB, Basler CF. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol. 2009;83:3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog. 2009;5:e1000493. doi: 10.1371/journal.ppat.1000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartman AL, Towner JS, Nichol ST. A C-terminal basic amino acid motif of Zaire ebolavirus VP35 is essential for type I interferon antagonism and displays high identity with the RNA-binding domain of another interferon antagonist, the NS1 protein of influenza A virus. Virology. 2004;328:177–184. doi: 10.1016/j.virol.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Leung DW, Ginder ND, Fulton DB, Nix J, Basler CF, Honzatko RB, Amarasinghe GK. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci U S A. 2009;106:411–416. doi: 10.1073/pnas.0807854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feng Z, Cerveny M, Yan Z, He B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol. 2007;81:182–192. doi: 10.1128/JVI.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leung LW, Park MS, Martinez O, Valmas C, Lopez CB, Basler CF. Ebolavirus VP35 suppresses IFN production from conventional but not plasmacytoid dendritic cells. Immunol Cell Biol. 2011 doi: 10.1038/icb.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartman AL, Bird BH, Towner JS, Antoniadou ZA, Zaki SR, Nichol ST. Inhibition of IRF-3 activation by VP35 is critical for the high level of virulence of ebola virus. J Virol. 2008;82:2699–2704. doi: 10.1128/JVI.02344-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prins KC, Delpeut S, Leung DW, Reynard O, Volchkova VA, Reid SP, Ramanan P, Cardenas WB, Amarasinghe GK, Volchkov VE, et al. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol. 2010;84:3004–3015. doi: 10.1128/JVI.02459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Albarino CG, Wiggleton Guerrero L, Spengler JR, Uebelhoer LS, Chakrabarti AK, Nichol ST, Towner JS. Recombinant Marburg viruses containing mutations in the IID region of VP35 prevent inhibition of Host immune responses. Virology. 2015;476:85–91. doi: 10.1016/j.virol.2014.12.002. This study describes the first VP35 mutated recombinant Marburg viruses. The study demonstrates that, as with Ebola virus, the Marburg virus VP35 modulates innate immune responses to infection.
- 55.Mateo M, Carbonnelle C, Reynard O, Kolesnikova L, Nemirov K, Page A, Volchkova VA, Volchkov VE. VP24 is a molecular determinant of Ebola virus virulence in guinea pigs. J Infect Dis. 2011;204(Suppl 3):S1011–S1020. doi: 10.1093/infdis/jir338. [DOI] [PubMed] [Google Scholar]
- 56.Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J Virol. 2006;80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J Virol. 2007;81:13469–13477. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, et al. Ebola Virus VP24 Targets a Unique NLS Binding Site on Karyopherin Alpha 5 to Selectively Compete with Nuclear Import of Phosphorylated STAT1. Cell Host Microbe. 2014;16:187–200. doi: 10.1016/j.chom.2014.07.008. This study presents the X-ray crystal structure of the Ebola virus VP24 protein in complex with host protein karyopherin alpha 5. Using the structural information, the study demonstrates that VP24 interaction with karyopherin alpha 5 prevents karyopherin alpha 5 interaction with tyrosine phosphorylated STAT1 and that this blocks cellular responses to interferons.
- 59.Valmas C, Grosch MN, Schumann M, Olejnik J, Martinez O, Best SM, Krahling V, Basler CF, Muhlberger E. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010;6:e1000721. doi: 10.1371/journal.ppat.1000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valmas C, Basler CF. Marburg virus VP40 antagonizes interferon signaling in a species-specific manner. J Virol. 2011;85:4309–4317. doi: 10.1128/JVI.02575-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bradfute SB, Warfield KL, Bavari S. Functional CD8+ T cell responses in lethal Ebola virus infection. J Immunol. 2008;180:4058–4066. doi: 10.4049/jimmunol.180.6.4058. [DOI] [PubMed] [Google Scholar]
- 62. McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, Lyon GM, Ribner BS, Varkey J, Sidney J, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. This study characterizes adaptive immune responses in Ebola virus-infected people. The subjects, who go on to survive infection, are demonstrated to mount robust immune responses to the virus.
- 63.Bradfute SB, Braun DR, Shamblin JD, Geisbert JB, Paragas J, Garrison A, Hensley LE, Geisbert TW. Lymphocyte death in a mouse model of ebola virus infection. J Infect Dis. 2007;196(Suppl 2):S296–S304. doi: 10.1086/520602. [DOI] [PubMed] [Google Scholar]
- 64.Reed DS, Hensley LE, Geisbert JB, Jahrling PB, Geisbert TW. Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic Fever in cynomolgus macaques. Viral Immunol. 2004;17:390–400. doi: 10.1089/vim.2004.17.390. [DOI] [PubMed] [Google Scholar]
- 65.Gupta M, Spiropoulou C, Rollin PE. Ebola virus infection of human PBMCs causes massive death of macrophages, CD4 and CD8 T cell sub-populations in vitro. Virology. 2007;364:45–54. doi: 10.1016/j.virol.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 66.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 67.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 68.Snyder CM, Shroff EH, Liu J, Chandel NS. Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS One. 2009;4:e7059. doi: 10.1371/journal.pone.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 70.Barrett AD, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 71.Monath TP, Vasconcelos PF. Yellow fever. J Clin Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 72.Lindenbach BDRC. Flaviviridae: the viruses and their replication. I. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 991. [Google Scholar]
- 73.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 74.Monath TP, Barrett AD. Pathogenesis and pathophysiology of yellow fever. Adv Virus Res. 2003;60:343–395. doi: 10.1016/s0065-3527(03)60009-6. [DOI] [PubMed] [Google Scholar]
- 75.Kaufmann B, Rossmann MG. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect. 2011;13:1–9. doi: 10.1016/j.micinf.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hudson NP. The Pathology of Experimental Yellow Fever in the Macacus Rhesus: I. Gross Pathology. Am J Pathol. 1928;4:395–406. 391. [PMC free article] [PubMed] [Google Scholar]
- 77.Bugher . The mammalian host in yellow fever. New York: McGraw-Hill; 1951. [Google Scholar]
- 78.TP M. Yellow Fever. V. Boca Raton: CRC PRess; 1988. [Google Scholar]
- 79.Sbrana E, Xiao SY, Popov VL, Newman PC, Tesh RB. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus) III. Clinical laboratory values. Am J Trop Med Hyg. 2006;74:1084–1089. [PubMed] [Google Scholar]
- 80.Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J Infect Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- 81.Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J Infect Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]
- 82.Thibodeaux BA, Garbino NC, Liss NM, Piper J, Blair CD, Roehrig JT. A small animal peripheral challenge model of yellow fever using interferon-receptor deficient mice and the 17D-204 vaccine strain. Vaccine. 2012;30:3180–3187. doi: 10.1016/j.vaccine.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monath TP, Brinker KR, Chandler FW, Kemp GE, Cropp CB. Pathophysiologic correlations in a rhesus monkey model of yellow fever with special observations on the acute necrosis of B cell areas of lymphoid tissues. Am J Trop Med Hyg. 1981;30:431–443. doi: 10.4269/ajtmh.1981.30.431. [DOI] [PubMed] [Google Scholar]
- 84.Turell . Horizontal and vertical transmission of viruses by insect and tick vectors. I. Boca Raton: CRC Press; 1988. [Google Scholar]
- 85.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–829. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marovich M, Grouard-Vogel G, Louder M, Eller M, Sun W, Wu SJ, Putvatana R, Murphy G, Tassaneetrithep B, Burgess T, et al. Human dendritic cells as targets of dengue virus infection. J Investig Dermatol Symp Proc. 2001;6:219–224. doi: 10.1046/j.0022-202x.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 87.Barba-Spaeth G, Longman RS, Albert ML, Rice CM. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med. 2005;202:1179–1184. doi: 10.1084/jem.20051352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berry GP KS. Yellow fever accidentally contracted in the laboratory. A study of seven cases. Am J Trop Med Hyg. 1931;11 [Google Scholar]
- 89.Monath TP. Treatment of yellow fever. Antiviral Res. 2008;78:116–124. doi: 10.1016/j.antiviral.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 90.Vieira WT, Gayotto LC, de Lima CP, de Brito T. Histopathology of the human liver in yellow fever with special emphasis on the diagnostic role of the Councilman body. Histopathology. 1983;7:195–208. doi: 10.1111/j.1365-2559.1983.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 91.Quaresma JA, Barros VL, Fernandes ER, Pagliari C, Guedes F, da Costa Vasconcelos PF, de Andrade Junior HF, Duarte MI. Immunohistochemical examination of the role of Fas ligand and lymphocytes in the pathogenesis of human liver yellow fever. Virus Res. 2006;116:91–97. doi: 10.1016/j.virusres.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 92.Quaresma JA, Barros VL, Fernandes ER, Pagliari C, Takakura C, da Costa Vasconcelos PF, de Andrade HF, Jr, Duarte MI. Reconsideration of histopathology and ultrastructural aspects of the human liver in yellow fever. Acta Trop. 2005;94:116–127. doi: 10.1016/j.actatropica.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Andrade HF, Jr, Vasconcelos PF, Duarte MI. Hepatocyte lesions and cellular immune response in yellow fever infection. Trans R Soc Trop Med Hyg. 2007;101:161–168. doi: 10.1016/j.trstmh.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 94.Quaresma JA, Barros VL, Pagliari C, Fernandes ER, Guedes F, Takakura CF, Andrade HF, Jr, Vasconcelos PF, Duarte MI. Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology. 2006;345:22–30. doi: 10.1016/j.virol.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 95.Dennis LH, Reisberg BE, Crosbie J, Crozier D, Conrad ME. The original haemorrhagic fever: yellow fever. Br J Haematol. 1969;17:455–462. doi: 10.1111/j.1365-2141.1969.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 96.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Engelmann F, Josset L, Girke T, Park B, Barron A, Dewane J, Hammarlund E, Lewis A, Axthelm MK, Slifka MK, et al. Pathophysiologic and transcriptomic analyses of viscerotropic yellow fever in a rhesus macaque model. PLoS Negl Trop Dis. 2014;8:e3295. doi: 10.1371/journal.pntd.0003295. This study describes the pathophysiology of severe yellow fever virus in a non-human primate model. This model of viral hemorrhagic fever shares a number of features with hemorrhagic fever caused by Ebola virus, including dysregulated immune responses.
- 98.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 99.Stephen EL, Sammons ML, Pannier WL, Baron S, Spertzel RO, Levy HB. Effect of a nuclease-resistant derivative of polyriboinosinic-polyribocytidylic acid complex on yellow fever in rhesus monkeys (Macaca mulatta) J Infect Dis. 1977;136:122–126. doi: 10.1093/infdis/136.1.122. [DOI] [PubMed] [Google Scholar]
- 100.Arroyo JI, Apperson SA, Cropp CB, Marafino BJ, Jr, Monath TP, Tesh RB, Shope RE, Garcia-Blanco MA. Effect of human gamma interferon on yellow fever virus infection. Am J Trop Med Hyg. 1988;38:647–650. [PubMed] [Google Scholar]
- 101.Klotz O, Belt TH. The Pathology of the Spleen in Yellow Fever. Am J Pathol. 1930;6:655–662. 653. [PMC free article] [PubMed] [Google Scholar]
- 102.Klotz O, Belt TH. The Pathology of the Liver in Yellow Fiver. Am J Pathol. 1930;6:663–688. 661. [PMC free article] [PubMed] [Google Scholar]
- 103.ter Meulen J, Sakho M, Koulemou K, Magassouba N, Bah A, Preiser W, Daffis S, Klewitz C, Bae HG, Niedrig M, et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis. 2004;190:1821–1827. doi: 10.1086/425016. [DOI] [PubMed] [Google Scholar]
- 104.Bente D, Gren J, Strong JE, Feldmann H. Disease modeling for Ebola and Marburg viruses. Dis Model Mech. 2009;2:12–17. doi: 10.1242/dmm.000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mateo M, Reid SP, Leung LW, Basler CF, Volchkov VE. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J Virol. 2010;84:1169–1175. doi: 10.1128/JVI.01372-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debre P, Fisher-Hoch SP, McCormick JB, Georges AJ. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 107. Mire CE, Geisbert JB, Marzi A, Agans KN, Feldmann H, Geisbert TW. Vesicular stomatitis virus-based vaccines protect nonhuman primates against Bundibugyo ebolavirus. PLoS Negl Trop Dis. 2013;7:e2600. doi: 10.1371/journal.pntd.0002600. This study characterizes the efficacy of a vaccine against Bundibugyo ebolavirus and also demonstrates that Bundibugyo ebolavirus is less pathogenic in this model than is Ebola virus (Zaire ebolavirus).