INTRODUCTION
Penicillin allergy is reported by 10% of the population and up to 15% of inpatients.1-5 A reported penicillin allergy is associated with increased use of alternative antibiotics, including vancomycin, clindamycin, fluoroquinolones, aminoglycosides, and aztreonam. 2,3,6-8 These antibiotics are often more costly, 9-12 less effective in certain clinical circumstances, 13-15 more toxic, 16 and broader spectrum, which potentially unnecessarily increases patients’ risk of infection with resistant pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistent Enterococcus (VRE), as well as Clostridium difficile colitis.5,17-19
A reported allergy to penicillin infrequently reflects the inability to tolerate penicillins. After formal allergy evaluation, including penicillin skin testing, between 90-99% of patients with reported allergy can tolerate penicillins. 4,6,11,20-22 This large discrepancy between reported penicillin allergy and true allergy is partially due to the original misclassification of the reaction as well as the waning natural history of Type I, IgE-mediated penicillin allergy.23,24 Most patients with reported penicillin allergy are therefore unnecessarily avoiding penicillin and related β-lactams antibiotics, such as the cephalosporins.2,10,25-27 Even in the presence of true, Gell and Coombs 28 type I hypersensitivity reaction (HSR) to penicillin , many β-lactam antibiotics, including later generation cephalosporins, can be safely administered by initiating therapy under observation with a small dose called a graded challenge or test dose procedure.1,25,26,29-32 The test dose, a limited-step graded challenge, is important to the evaluation of adverse drug reactions (ADRs).33-36
Prior studies have evaluated the use of penicillin skin testing in the emergency, perioperative, and intensive care unit settings, with results demonstrating that skin testing can lead to decreased use of vancomycin and fluoroquinolones, and increased use of cephalosporins.6,7,11,20 However, inpatient use of penicillin skin testing may predominantly shift antibiotic use toward the later generation cephalosporins,4 which are safe for use in the absence of preceding skin testing with a test dose.25,26,31 Moreover,while skin testing is a procedure that may be costly or challenging for hospitals to widely implement, the test dose requires fewer inpatient resources.
To address the large discrepancy between reported and true penicillin allergy among inpatients, we developed a clinical guideline for inpatient providers that uses the allergy history to determine which β-lactam antibiotics can be administered to patients with a history of penicillin or cephalosporin allergy with a full dose, a test dose, or only with preceding penicillin skin testing. We then evaluated this guideline's safety, feasibility, and impact on consultations and antibiotic utilization.
METHODS
The Clinical Guideline
The guideline, developed by Allergy/Immunology (AI), Infectious Diseases (ID), and Pharmacy at Massachusetts General Hospital (MGH), assisted providers in taking a drug allergy history and prescribing antibiotics topatients with a history of penicillin or cephalosporin allergy (Figure 1, Supplementary text). The guideline also provided a standardized method for primary teams to perform test doses of β-lactams on the general medical floor and indicated when AI and/or ID consultation was needed. Prior to guideline implementation, AI consultation was a requirement for all test doses at MGH; all test doses were given on the general medical floors by a standardized 2-step procedure since 2008.33 After guideline implementation, test doses of β-lactam antibiotics did not require consultation. The guideline was implemented in all adult inpatient areas on April 1, 2013.
Figure 1. Penicillin allergy pathway in the hospital-wide clinical guideline for antibiotic prescription in patients with penicillin allergy.
The pathway below details which antibiotics can be used for patients with different penicillin allergy histories. Patient reactions are categorized into a type II-IV HSR (serum sickness, SJS/TEN, AIN, DRESS, hemolytic anemia) where cross reactivity data are limited; type I, IgE mediated allergy (anaphylaxis, angioedema, wheezing, bronchospasm, hypotension, urticaria) or unknown reaction; or a mild delayed morbilliform/maculopapular eruption or electronic medical record/medical chart discrepancy with patient interview. The pathway directs the use of a limited-step graded challenge, or test dose, on the general medical floors. When the pathway advises consultation by Allergy/Immunology, penicillin skin testing is recommended prior to use of the specified antibiotics. The complete internal guideline is available in the Supplementary Text.
Abbreviations HSR: hypersensitivity reaction; SJS/TEN: Stevens-Johnson syndrome/toxic epidermal necrolysis; AIN: acute interstitial nephritis; DRESS: Drug Rash Eosinophilia and Systemic Symptoms; IgE: immunologlobulin E.
The Educational Intervention
After training members of AI, ID and pharmacy, brief, targeted educational presentations were delivered to 15 different groups of general inpatient providers throughout the hospital from April 3, 2013 through May 15, 2013. The presentations introduced the clinical guideline, showed providers how navigate to the guideline electronically, and communicated antibiotic stewardship goals and key concepts about penicillin allergy using interactive clinical vignettes. Instruction on taking an allergy history was provided, with emphasis on improved characterization of rashes (urticaria vs. delayed maculopapular rash vs. severe cutaneous adverse reactions). After each presentation, laminated cards with figures from the clinical guideline were distributed to staff and posted in hospital workrooms. The full guideline was available electronically to all staff. After the initial educational roll-out, additional educational sessions included training of the first-year AI and ID fellows (07/2014), noon conference with pediatrics residents (09/2014), and a case-based discussion of penicillin allergy with internal medicine residents (10/2014). Because of housestaff turnover, additional annual education and training have been necessary.
Study Design overview
We conducted a quasi-experimental study evaluating the hospital-wide clinical guideline (Figure 1), comparing 21 months pre-guideline and 12 months post-guideline. Historical pre-guideline data before 21 months were not included because of the variable availability of penicillin skin testing reagents nationally and at MGH at that time. Use of the clinical guideline could result in: (1) use of an alternative antibiotic; (2) use of a β-lactam antibiotic at its full dose;(3) use of a β-lactam antibiotic initiating therapy with a test dose; or (4) a consultation by AI and if appropriate, penicillin skin testing. We assessed patients and providers who used the guideline for a test dose (3 or 4, above). After electronic identification of test doses through the electronic medication administration record (eMAR), manual chart review of the electronic health record (EHR) was performed by a board-certified internist and allergist/immunologist (KGB).
This study was approved by the Institutional Review Board of Partners Human Research Committee. Written informed consent was obtained prior to skin testing.
Demographic and clinical characteristics
Information on the patient's age, gender, and drug allergy history was collected from the EHR. Patient's history of allergy to penicillin or cephalosporin antibiotics and the reported reaction was obtained from the EHR through a centralized allergy repository, Partners Enterprise Allergy Repository.37
Primary outcomes
The primary outcomes werenumber and frequency of test doses of β-lactam antibiotics and resulting ADRs in the two time periods. We identified test doses using eMAR, and performed EHR review for confirmation. Test doses reviewed in both periods included those performed with and without preceding penicillin skin testing. We determined the total doses of β-lactam antibiotics dispensed at MGH in the pre and post-periods, using the pharmacy data system Sunquest (Sunquest Information Systems, Tucson, AZ, USA).
Specific other data on the test included timing of test dose and infectious details. Test dose timing was calculated as an integer value between the date of admission and the date of test dose.Infections treated were identified by review of the admission note and discharge summary. ID consultation notes were also reviewed, when applicable. Culture data were obtained from the microbiology record. Therapy was classified as culture-driven if there were relevant culture data by the date/time of test dose. Otherwise, therapy was classified as empiric.
ADR frequency and severity were determined through EHR review. Patients discharged on the test dose antibiotic were classified as having no ADR. For patients not discharged on the antibiotic for which a test dose was performed, EHR was reviewed for evidence of tolerance or reaction to the drug. When an ADR was suspected, the clinical team was contacted for details regarding reaction timing, symptoms, and treatment. ADR details were reviewed independently and graded by two allergists (KGB, AB) according to previously used grading scales. 33,38
Secondary outcomes
Secondary outcomes included the use of consultation services by AI and ID, change in antimicrobial therapy resulting from post-period test doses, as well as general usability and adherence to the guideline.
Consultation services were determined by the presence or absence of a consultation note by AI or ID in the EHR prior to the date/time of the test dose. AI notes were reviewed for indication and the results of penicillin skin testing, if applicable.
We determined the total number of pre- and post-period AI inpatient consultations from the AI consultation log, which included all non-chemotherapy desensitization consultations and was maintained by one administrator since 11/01/2011. We reviewed the total ID inpatient consultations in the pre- and post-periods using billing data from the Patientkeeper Version 8.1.1 (Object Data Management Group).
Antimicrobial therapy was determined from eMAR considering therapy as a prescribed course of an antibiotic (e.g., drug was not written as “x1,” “STAT,” or “PRN”) on the day before the test dose and the day after the test dose. If more than two antimicrobials were being co-administered, primary and secondary therapy was determined by considering drugs that typically cover gram-positive and gram-negative organisms. Cephalosporins were grouped by generation for analysis. Piperacillin-tazobactam was considered separately.
We documented which inpatient service ordered the test dose and the type of ordering provider (trainee, attending physician, nurse practitioner, or physician's assistant). Guideline adherence was determined by EHR order for the test dose procedure. Test doses were determined not to adhere to the guideline if documentation was not available to support the clinical decision-making.
Statistical analysis
Demographic data were reported as means with standard deviations or medians with interquartile ranges. Students t test was used for comparison of means and Wilcoxon test for comparison of medians. Wilcoxon rank sum or Fisher's Exact test was used to compare independent groups. McNemar's Test was used to compare paired binary data, such as antibiotics before and after a test dose. Two-sided p values <0.05 were considered significant. All statistical analyses were conducted in SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographic and clinical characteristics
Demographic characteristics between patients who received a test dose in the pre and post- guideline periods were similar (Table 1). Among patients with penicillin and cephalosporin allergy, the most commonly reported reactions were rash and hives. More patients in the post-period had allergy history documented as unknown compared to the pre-period (15% vs 2%, p=0.013).
Table 1.
Demographic and allergy histories of patients receiving test doses of β-lactam antibiotics in the pre-guideline and post-guideline periods.
| Variable | Pre-guideline 07/01/2011- 03/31/2013 (n=49) | Post-guideline 04/01/2013-03/31/2014 (n=183) |
|---|---|---|
| Mean age (SD) | 58.7 (16) | 59.9 (20) |
| Female gender (No., %) | 27 (55) | 98 (54) |
| Primary penicillin allergy (No., %) | 43 (88) | 160 (88) |
| Reported reaction (No., %) | ||
| Rash | 17 (35) | 54 (30) |
| Hives | 12 (25) | 41 (22) |
| Unknown | 1 (2) | 28 (15) |
| Angioedema/swelling | 5 (10) | 23 (13) |
| Anaphylaxis | 6 (12) | 8 (4) |
| Bronchospasm/shortness of breath | 2 (4) | 8 (4) |
| Gastrointestinal upset | 0 (0) | 6 (3) |
| Itching | 2 (4) | 4 (2) |
| Patient denies history, listed in medical record | 0 (0) | 4 (2) |
| Prior evidence of tolerated penicillin | 0 (0) | 2 (1) |
| Tested positive | 2 (4) | 1 (0.5) |
| Fever | 0 (0) | 1 (0.5) |
| Flushing | 0 (0) | 1 (0.5) |
| Serum sickness | 1 (2) | 1 (0.5) |
| Myalgia | 1 (2) | 0 (0) |
| Primary cephalosporin allergy * (No., %) | 12 (25) | 38 (21) |
| Specific cephalosporin that caused the reaction (generation) (No., %) | ||
| Cephalexin (1st) | 2 (4) | 12 (7) |
| Cefepime (4th) | 2 (4) | 7 (4) |
| Unknown | 3 (6) | 6 (3) |
| Cefazolin (1st) | 0 (0) | 3 (2) |
| Cefaclor (2nd) | 1 (2) | 3 (2) |
| Ceftriaxone (3rd) | 4 (8) | 2 (1) |
| Cefuroxime (2nd) | 0 (0) | 2 (1) |
| Cefixime (3rd) | 0 (0) | 1 (0.5) |
| Cefadroxil (1st) | 0 (0) | 1 (0.5) |
| Cefoxitin (2nd) | 0 (0) | 1 (0.5) |
| Reported reaction† (No., %) | ||
| Rash | 6 (12) | 18 (10) |
| Hives | 2 (4) | 8 (4) |
| Angioedema/swelling | 0 (0) | 5 (3) |
| Unknown | 3 (6) | 4 (2) |
| Itching | 0 (0) | 4 (2) |
| Anaphylaxis | 1 (2) | 2 (1) |
| Gastrointestinal Upset | 0 (0) | 2 (1) |
| Bronchospasm | 0 (0) | 1 (0.5) |
| Flushing | 0 (0) | 1 (0.5) |
| Mental status change | 0 (0) | 1 (0.5) |
Six patients in the pre-period and 15 patients in the post-period had both penicillin and cephalosporin allergy
Record may contain more than one description or reaction of clinical feature of drug allergy history
Guideline impact on test doses and adverse drug reactions
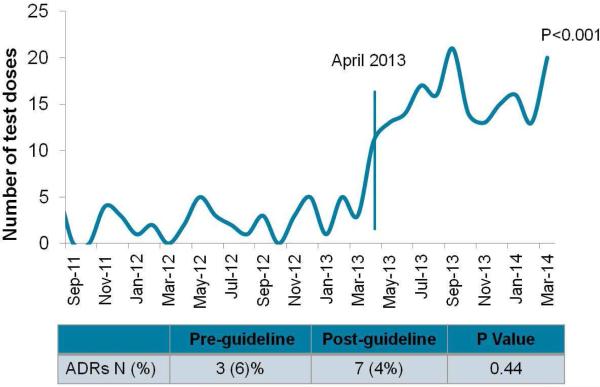
In the pre-period, 49 test doses of β-lactam antibiotics were identified with a median of 2 (IQR 1, 3.25, range 0-5) test doses per month (Figure 2). In the post-period, 183 test doses of β-lactam antibiotics were identified, with a median of 14.5 (IQR 13, 16.25, range 11-20) test doses per month. The adoption of the clinical guideline was associated with a significant increase in the number of test doses of β-lactam antibiotics in the post-period (p<0.001) (Figure 2).
Figure 2. Impact of the clinical guideline on test dose usage and adverse drug reactions to β-lactam antibiotics.
Adoption of the clinical guideline was associated with a significant increase in test doses (p<0.001) without a significant change in the rate of adverse drug reactions (p=0.44).
In the pre-period, test doses were performed a median of four days (IQR 2, 8; range 0-32) into the patient's hospitalization (Table 2). In the post-period, test doses were performed a median of twodays (IQR 1, 5; range 0-44) into the patient's hospitalization, which was significantly earlier in a patient's hospitalization than test doses in the pre-period(p=0.002).
Table 2.
Test doses in the pre-guideline and post-guideline periods.
| Pre-guideline 07/01/2011- 03/31/2013 | Post-guideline 04/01/2013-03/31/2014 | P Value | |
|---|---|---|---|
| Test Doses No. (% of β-lactam doses) | 49 (0.24) | 183 (0.83) | <0.001 |
| Days from admission until test dose | |||
| Median [IQ Range] | 4 [2,8] | 2 [1,5] | 0.002 |
| Test dose antibiotics No. (%) | |||
| Penicillins | 28 (57) | 36 (20) | <0.001 |
| Cephalosporins | 16 (33) | 139 (76) | <0.001 |
| Carbapenems | 5 (10) | 8 (4) | 0.155 |
| Infection history No. (%) | |||
| Empiric treatment | 6 (12) | 93 (51) | <0.001 |
| Culture-driven treatment | 42 (86) | 87 (48) | |
| Unknown | 1 (2) | 3 (2) |
A significantly lower proportion of post-period test doses were to penicillins compared to pre-period test doses (20% vs 57%, p<0.001). A significantly higher proportion of post-period test doses were to cephalosporins compared to pre-period test doses (76% vs 33%, p<0.001). In the post-period, the most common cephalopsorin test doses were cefepime (n=61, 33%), ceftriaxone (n=50, 27%), and cefazolin (n=16, 9%).
Among pre-period test doses (n=49), six (12%) were performed for empiric antimicrobial treatment, and 42 (86%) were performed for culture-driven antimicrobial treatment (Table 2, eTable 1). Among post-period test doses (n=183), 93 (51%) were performed for empiric antimicrobial treatment, and 87 (48%) were performed for culture-driven antimicrobial treatment. Compared to pre-period test doses, post-period test doses were used significantly more for empiric treatment (p<0.001). The most common post-period empiric use was for pneumonia (31%, eTable 1).
There was no significant difference in ADR rate in the post-period compared to the pre-period (4% vs 6%, p=0.44) (Table 3, Figure 2). All three ADRs in the pre-period were determined to be immune-mediated, with two categorized as grade 1 (cutaneous signs only) and one categorized as grade 2 (measurable, but not life-threatening symptoms). Treatment of these ADRs included antihistamines (100%), steroids (67%), and epinephrine (33%). The patient who received epinephrine was a 68 year old female with underlying idiopathic angioedema and history of urticaria to both penicillin and ampicillin. She received a test dose of meropenem and developed immediate lip and tongue swelling with the first full dose.
Table 3.
Adverse drug reactions in the pre-guideline and post-guideline periods.
| Pre-guideline 07/01/2011- 03/31/2013 | Post-guideline 04/01/2013-03/31/2014 | P Value | |
|---|---|---|---|
| Adverse Drug Reactions No. (%) | 3 (6) | 7 (4) | 0.44 |
| Classification of reaction No. (%) | |||
| Immune-mediated | 3 (100) | 5 (71) | >0.5 |
| Not immune mediated | 0 (0) | 1 (14) | |
| Unknown/unclear | 0 (0) | 1 (14) | |
| Timing of the reaction No. (%) | |||
| Immediate | 2 (67) | 6 (86) | >0.5 |
| Delayed | 1 (33) | 1 (14) | |
| Grading of reaction* No. (%) | |||
| Grade 1 | 2 (67) | 6 (86) | >0.5 |
| Grade 2 | 1 (33) | 1 (14) | |
| Grade 3 | 0 (0) | 0 (0) | |
| Grade 4 | 0 (0) | 0 (0) | |
| Treatment No. (%) | |||
| Antihistamines | 3 (100) | 7 (100) | 0.44 |
| Corticosteroids | 2 (67) | 2 (29) | 0.154 |
| Epinephrine | 1 (33) | 0 (0) | 0.21 |
Grade 1: Presence of cutaneous signs; Grade 2: Presence of measurable but non-life-threatening symptoms including angioedema, arterial hypotension, cough or difficulty in mechanical ventilation; Grade 3: Presence of life-threatening reactions: cardiovascular collapse, tachycardia or bradycardia, arrhythmias or severe bronchospasm; Grade 4: Circulatory failure, cardiac arrest or respiratory arrest.33,38
In the post-period, a total of seven ADRs were identified: five (71%) were determined to be immune-mediated, one(14%) not immune-mediated, and one (14%) unknown. Six (86%) of the ADRs were grade 1, and one (14%) was grade 2. Treatment included antihistamines (100%) and steroids (29%). No patients experienced an ADR requiring epinephrine in the post-period. ADR severity and treatment were not significantly different in the pre- or post-periods (Table 3).
Guideline's impact on consultation services
In the pre-period, allpatients were consulted on by AI, as required by the pre-guideline policy, compared to 25/183 (14%) patients in the post-period (p<0.001). When AI was consulted, penicillin skin testing was indicated in a greater proportion of patients in the post-period compared to the pre-period (76% vs 55%, p=0.084). Overall hospital consultations for AI did not significantly change due to guideline implementation (p=0.141), with median pre-period AI consultations of 19 [IQR 18, 20] per month and median post-period AI consultations of 16 [IQR 13, 18.50] per month.
In the pre-period, 41/49 (91%) patients were consulted on by ID compared to 87/183 (49%, p<0.001) in the post-period. Overall median monthly ID consultations had a nonsignificant increase from 354 [IQR 332,396] to 373 [358, 417] following guideline adoption (p=0.21).
Guideline's impact on antimicrobial therapy
Guideline-directed test doses in the post-period resulted in significantly more patients on penicillins (19% vs 2%, p<0.001) and cephalosporins in the 1st (10% vs 0.6 %, p<0.001), 3rd (30% vs 5%, p<0.001), and 4th (32% vs 7%, p<0.001) generations (Table 4). Of patients switched to a penicillin (n=33), 79% had primary penicillin allergy. After the test dose, significantly fewer patients were on vancomycin (37% vs 68%%, p<0.001), aminoglycosides (1% vs 6%, p=0.004), aztreonam (0.6% vs 12%, p<0.001) and fluoroquinolones (3% vs 15%, p<0.001).
Table 4.
Test doses improved antimicrobial therapy in the post-guideline period (n=183)
| Antibiotic or class of antibiotic No.( %) | Before test dose | After test dose | P Value* |
|---|---|---|---|
| Penicillins † | 3 (2) | 35 (19) | <0.001 |
| Piperacillin-tazobactam | 4 (2) | 2 (1) | >0.5 |
| Cephalosporins ‡ | |||
| 1st generation | 1 (0.6) | 18 (10) | <0.001 |
| 3rd generation | 9 (5) | 54 (30) | <0.001 |
| 4th generation | 12 (7) | 58 (32) | <0.001 |
| Aztreonam | 21 (12) | 1 (0.6) | <0.001 |
| Carbapenem | 18 (10) | 11 (6) | 0.21 |
| Clindamycin | 3 (2) | 2 (1) | >0.5 |
| Daptomycin | 2 (1) | 2 (1) | >0.5 |
| Fluoroquinolone | 28 (15) | 6 (3) | <0.001 |
| Gentamycin | 11 (6) | 2 (1) | 0.004 |
| Linezolid | 7 (4) | 4 (2) | 0.45 |
| Vancomycin | 125 (68) | 68 (37) | <0.001 |
McNemar's test
Other than piperacillin-tazobactam, considered separately due to its broad coverage
No patients were treated with 2nd generation cephalosporins
Guideline usage and appropriateness
In the post-period, test doses through the guideline were largely ordered by clinicians in internal medicine and its specialties (n=125, 68%), with the largest groups including general internal medicine (n=99, 54%), oncology (n=19, 10%), and cardiology (n=7, 4%). Surgical specialties performed 43 (24%) test doses, including general surgery (n=38, 21%), oral maxillofacial surgery (n=3, 2%) and urology (n=2, 1%). The provider ordering the test dose was most commonly a house officer (n=131, 72%). Other providers ordering test doses included nurse practitioners (n=24, 13%), attending hospitalist physicians (n=24, 13%), and physician assistants (n=4, 2%).
Overall, 167/183 (91%) test doses were determined to adhere to the clinical guideline; non adherent test doses either did not follow the appropriate recommendations for a given allergy history or the clinician failed to document the allergy history. None of the non adherent test doses resulted in an ADR.
DISCUSSION
Implementation of a novel clinical guideline for prescribing antibiotics to patients with a history of penicillin or cephalosporin allergy that used a standardized 2-step test dose procedure was temporally associated with a significant (over 7-fold) increase in the number of test doses of β-lactam antibiotics, without an increase in ADR frequency, type, or severity. Compared to pre-guideline test doses, post-guideline test doses occurred earlier in the patients’ hospitalization and increasingly for empiric therapy. Furthermore, the guideline-driven test doses were performed by all types of inpatient providers without increasing subspecialty consultations by AI or ID. Test doses in the post-period helped improve antimicrobial management, resulting in less vancomycin, aminoglycoside, aztreonam, and flouroquinolone use. To our knowledge, this report is the first to describe a successful hospital-wide clinical guideline for general inpatient providers to guide β-lactam antibiotic use for infectious treatment among inpatients with a history of penicillin or cephalosporin allergy.
Although there have beenadvances in our understanding of allergy to β-lactam antibiotics, understanding drug and penicillin allergy remains challenging for inpatient providers and allergists alike.29,39-41 General inpatient providers have limited education and knowledge about drug and penicillin allergy, but are interested in tools to help them safely and appropriately care for patients with β-lactam allergies. 40 Existing clinical guides and tools do not account for the specific generation or side chain of the cephalosporin; or they define the need for penicillin skin testing based solely on skin testing availability. 1,30 We used a clinical guideline that advised general inpatient providers on how to take an allergy history and used the type of HSR the patient experienced to prescribe antibiotics to patients with allergies to penicillin or cephalosporins. The guideline, and associated educational sessions, changed prescribing practice , with 183 test doses of β-lactam antibiotics performed on inpatients in one year's time by providers from all specialties and types of training. The test doses performed were as safe as those previously guided by AI specialists in the pre-period, and over 90% of test doses could be verified to have followed the guideline. While the test doses that did not adhere to guideline did not result in an ADR, these were considered “near misses,” and informed future educational sessions to improve patient safety.
Despite the common reporting of penicillin allergy among inpatients and the recognized need to perform antimicrobial stewardship,42-44 few studies have proposed generalizable inpatient solutions to this problem, and those that have used routine penicillin skin testing.4,11,45-46 Past studies found that penicillin skin testing can decrease use of broad spectrum antibiotics and potentially decrease cost of therapy. 4,20,46 However, among inpatients, the shift in antimicrobial therapy realized was predominantly towards use of later generation cephalosporins,4 which are safe for use by test dose without preceding skin testing, even in confirmed IgE-mediated penicillinallergy. 25,26,31,32 We observed a similar increase in later generation cephalosporins, with the guideline often used to treat penicillin-allergic patients with ceftriaxone or cefepime by test dose, without preceding penicillin skin testing or AI consultation. This could reflect the choice of standard antibiotic regimens for common infections, such as later generation cephalosporins in the treatment of pneumonia, which was the most common infection treated empirically in the post-period cohort. Alternatively, this could have been because post-period patients had significantly more “unknown” allergy histories, which presented providers with the option of directly using a 3rd and 4th generation cephalosporin with a test dose.
By implementing a guideline that considers the allergy history, along with the desired antibiotic treatment, we are able to target hospital resources, such as use of procedures and consultations, appropriately. Because the negative predictive value of a penicillin skin test is not 100%, even after a negative penicillin skin test, patients should receive a penicillin or amoxicillin test dose challenge under observation to fully assess the drug allergy.1,22,30 Since our guideline ADR rate was similar to the rate in AI literature after skin testing,4,6,7,21,22 implementation of the guideline may have saved 164 inpatient penicillin skin tests without incurring any additional costs. The cost of 164 skin tests performed by AI, using Medicare reimbursement, would total approximately $60,000 2014 USD (testing $35,793.00; AI consultations $23,914.48). Additionally, post-guideline test doses were performed about 2 hospital days earlier than the pre-guideline test doses, allowing for earlier antibiotic narrowing or delivery of appropriate therapy, which could have affected another cost difference, length of stay. Our analysis, however, was limited to this specific inpatient admission and does not account for the subsequent benefits of a negative penicillin skin test, including improved prescription for all subsequent hospital admissions and outpatient encounters.
Limitations of the study include our inability to control for confounders. Thus, we are unable to establish a causal relationship between the guideline implementation and our reported outcomes. However, to improve our study design, we included multiple measurements from prior to the intervention, and accounted for hospital trends such as overall β-lactam antibiotic usage and hospital-wide AI and ID consultations. Although we evaluated almost 200 post-period test doses, more data are needed to conclusively prove the guideline's safety, particularly with regard to penicillin test doses administered to patients reporting penicillin allergy without preceding skin testing. Another limitation was our ability only to track when the guideline was used for performing a test dose. We were unable to know how often the guideline directed providers to choose an alternative drug or full dose antibiotic. We report on the experience of a single tertiary care referral center in Boston, MA with availability of AI consultation and penicillin skin testing. The experience and resources at this institution may not be similar to other institutions. However, the guideline could be tailored to hospitals with differing resources by using outpatient allergy referrals upon patient discharge.
We report the successful adoption and results of a novel and targeted approach to inpatients with penicillin and cephalosporin allergy. The guideline changed practice through the safe use of test doses. Importantly, in this era of antimicrobial resistance, test doses narrowed therapy in a patient population at risk for healthcare-associated infections.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lynn Simpson, M.P.H and Partners Research Information Services and Computing for assistance with RedCap. We thank Aidan A. Long, M.D, Nesli Basgoz, M.D, Christopher M. Coley, M.D. and Sandra B. Nelson, M.D. for their review and feedback of clinical guideline. We thank Dena Alioto and Kelly Staples for their technical support of the clinical guideline. We thank the fellows and attendings in Allergy/Immunology and Infectious Diseases, as well as all pharmacy staff, for their support of the clinical guideline. We thank Lauren Baino for maintaining the Allergy/Immunology consultation log and Susan Clifford for assistance with assessing consultations by Infectious Diseases.
Funding
This work was supported by the Partners Center of Expertise for Quality and Safety from 2012-2014, departmental funds, and the National Institutes of Health to [NIAID T32 HL116275 to K.G.B.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
KGB, ESS, CAV, DCH, and AB designed the guideline
KGB, ESS, CAV, SH, DCH, and AB designed the study and analysis plan
KGB, ESS, and SH analyzed and interpreted the results.
KGB and ESS drafted the first report.
CAV, SH, DCH, and AB assisted with interpretation and revision of the report.
KGB obtained funding.
References
- 1.Solensky R, Khan D. Drug Allergy: An updated practice parameter. Ann Allergy Asthma Immunol. 2010;105:259–73. doi: 10.1016/j.anai.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Picard M, Begin P, Bouchard H, et al. Treatment of patients with a history of penicillin allergy in a large tertiary care academic hospital. J Allergy Clin Immunol Pract. 2013;1(3):252–7. doi: 10.1016/j.jaip.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implication regarding prescribing patters and emerging bacterial resistance. Arch Intern Med. 2000;160:2819–22. doi: 10.1001/archinte.160.18.2819. [DOI] [PubMed] [Google Scholar]
- 4.Rimawi RH, Cook PP, Gooch M, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. 2013;8:341–5. doi: 10.1002/jhm.2036. [DOI] [PubMed] [Google Scholar]
- 5.Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J Allergy Clin Immunol. 2014;133(3):790–796. doi: 10.1016/j.jaci.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 6.del Real GA, Rose ME, Ramirez-Atamoros MT, et al. Penicillin skin testing in patients with a history of beta-lactam allergy. Ann Allergy Asthma Immunol. 2007;98(4):355–9. doi: 10.1016/S1081-1206(10)60882-4. [DOI] [PubMed] [Google Scholar]
- 7.Frigas E, Park MA, Narr BJ, et al. Preoperative evaluation of patients with history of allergy to penicillin: comparison of 2 models of practice. Mayo Clin Proc. 2008;83:651–7. doi: 10.4065/83.6.651. [DOI] [PubMed] [Google Scholar]
- 8.Lutomski DM, Lafollette JA, Biaglow MA, Haglund LA. Antibiotic allergies in the medical record: effect on drug selection and assessment of validity. Pharmacotherapy. 2008;28(11):1348–53. doi: 10.1592/phco.28.11.1348. [DOI] [PubMed] [Google Scholar]
- 9.Harris AD, Sauberman L, Kabbash L, Greineder DK, Samore MH. Penicillin skin testing: a way to optimize anitbiotic utilization. Am Journal Med. 1999;107:166–8. doi: 10.1016/s0002-9343(99)00190-4. [DOI] [PubMed] [Google Scholar]
- 10.Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33:501–6. doi: 10.1046/j.1365-2222.2003.01638.x. [DOI] [PubMed] [Google Scholar]
- 11.Raja AS, Lindsell CJ, Bernstein JA, Codispoti CD, Moellman JJ. The use of penicillin skin testing to assess the prevalence of penicillin allergy in an emergency department setting. Ann Emerg Med. 2009;54:72–7. doi: 10.1016/j.annemergmed.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLaughlin EJ, Saseen JJ, Malone DC. Costs of beta-lactam allergies: selection and costs of antibiotics for patients with a reported beta-lactam allergy. Arch Fam Med. 2000;9:722–6. doi: 10.1001/archfami.9.8.722. [DOI] [PubMed] [Google Scholar]
- 13.Stryjewski ME, Szcech LA, Benjamin DK, et al. Use of vancomycin of first generation cephalosporins for the treatment of hemodialysis dependent patients with methicillin-susceptibile Staphylococcus aureus bacteremia. Clin Infect Dis. 2006;44:190–6. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 14.Schweizer ML, Furuno J, Harris AD, et al. Comparative effectiveness of nafcillin or cefazolin versus vancomycin in methicillin-susceptible Staphylococcus aureus bacteremia. BMC Infect Dis. 2011;11(279):1–7. doi: 10.1186/1471-2334-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slavin RG, Spector SL, Bernstein IL. The diagnosis and management of sinusitis: a practice parameter update. J Allergy Clin Immunol. 2005;116(S13-47) doi: 10.1016/j.jaci.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 16.Tice AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38:1651–72. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 17.Levin BR, Lipstich M, Perrot V, et al. The population genetics of antibiotic resistance. Clin Infect Dis. 1997;24(Suppl 1):S9–16. doi: 10.1093/clinids/24.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 18.Baxter R, Ray GT, Fireman BH. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect Control Hosp Epidemiol. 2008;29(1):44–50. doi: 10.1086/524320. [DOI] [PubMed] [Google Scholar]
- 19.Yip C, Loeb M, Salama S, Moss L, Olde J. Quinolone use as a risk factor for nosocomial Clostridium difficile-associated diarrhea. Infect Control Hosp Epidemiol. 2001;22(9):572–5. doi: 10.1086/501954. [DOI] [PubMed] [Google Scholar]
- 20.Park M, Markus P, Matesic D, Li JT. Safety and effectiveness of a preoperative allergy clinic in decreasing vancomyin use in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol. 2006;97(5):681–7. doi: 10.1016/S1081-1206(10)61100-3. [DOI] [PubMed] [Google Scholar]
- 21.Sagar PS, Katelaris CH. Utility of penicillin skin testing in patients with a history of penicillin allergy. Asia Pac Allergy. 2013;3(2):115–99. doi: 10.5415/apallergy.2013.3.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macy E, Ngor E. Safely diagnosing clinically significant penicillin allergy using only penicilloylpoly-lysine. J Allergy Clin Immunol Pract. 2013;1:258–63. doi: 10.1016/j.jaip.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan TJ, Wedner HJ, Shatz GS, Yecies LD, Parker CW. Skin testing to detect penicillin allergy. J Allergy Clin Immunol. 1981;68:171–80. doi: 10.1016/0091-6749(81)90180-9. [DOI] [PubMed] [Google Scholar]
- 24.Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103:918–24. doi: 10.1016/s0091-6749(99)70439-2. [DOI] [PubMed] [Google Scholar]
- 25.Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2005;115(4):1048–57. doi: 10.1542/peds.2004-1276. [DOI] [PubMed] [Google Scholar]
- 26.Pichichero ME. Cephalosporins can be prescribed safely for penicillin-allergic patients. J Fam Pract. 2006;55:106–12. [PubMed] [Google Scholar]
- 27.DePestel DD, Benninger MS, Danzinger L, et al. Cephalosporin use in treatment of patients with penicillin allergies. J Am Pharm Assoc. 2008;48:530–40. doi: 10.1331/JAPhA.2008.07006. [DOI] [PubMed] [Google Scholar]
- 28.Gell PGH, Coombs RA. The classification of allergic reactions underlying disease. In: Gel PGH, Coombs RA, editors. Clinical Aspects of Immunology. 1st ed. Blackwell; Oxford, U.K.: 1963. pp. 317–337. [Google Scholar]
- 29.Solensky R, Earl HS, Gruchalla RS. Clinical approach to penicillin-allergy patients: a survey. Ann Allergy Asthma Immunol. 2000;84:329–33. doi: 10.1016/S1081-1206(10)62782-2. [DOI] [PubMed] [Google Scholar]
- 30.Solensky R. Penicillin-allergic patients: Use of cephalosporins, carbapenems, and monobactams. Watham, MA: 2014. [June 23, 2014]. Uptodate 2014. [Google Scholar]
- 31.Pichichero ME. Use of selected cephalosporins in penicillin- allergic patients: A paradigm shift. Diag Microbiol Infect Dis. 2007;57:13S–8S. doi: 10.1016/j.diagmicrobio.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Pichichero ME, Casey JR. Safe use of selected cephalosporins in penicillin-allergic patients: a meta-analysis. Otolaryngol Head Neck Surg. 2007;36(3):340–7. doi: 10.1016/j.otohns.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Iammatteo M, Blumenthal KG, Saff R, Long AA, Banerji A. Safety and outcomes of test doses for the evaluation of adverse drug reactions: A 5-Year retrospective review. J Allergy Clin Immunol Pract. 2014;2(6):768–74. doi: 10.1016/j.jaip.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Bousquet PJ, Pipet A, Bousquet-Rounet L, Demoly P. Oral challenges are needed in the diagnosis of beta lactam hypersensitivity. Clin Exp Allergy. 2008;38:185–90. doi: 10.1111/j.1365-2222.2007.02867.x. [DOI] [PubMed] [Google Scholar]
- 35.Bousquet PJ, Gaeta F, Bousquet-Rouanet L, Lefrant JY, Demoly P, Romano A. Provocation tests in diagnosing drug hypersensitivity. Curr Pharm Des. 2008;14:2792–802. doi: 10.2174/138161208786369731. [DOI] [PubMed] [Google Scholar]
- 36.Kao L, Rajan J, Roy L, Kavosh E, Khan DA. Adverse reactions during drug challenges: a single US institution's experience. Ann Allergy Asthma Immunol. 2013;110:86–91. doi: 10.1016/j.anai.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Kuperman G, Marston E, Paterno M, et al. Creating an enterprise-wide allergy repository at Partners Healthcare System. AMAI Annu Symp Proc. 2003:376–80. [PMC free article] [PubMed] [Google Scholar]
- 38.Ring H, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–9. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 39.Puchner TC, Zacharisen MC. A survey of antibiotic prescribing and knowledge of penicillin allergy. Ann Allergy Asthma Immunol. 2002;88:24–9. doi: 10.1016/S1081-1206(10)63589-2. [DOI] [PubMed] [Google Scholar]
- 40.Blumenthal KG, Shenoy ES, Hurwitz S, Varughese C, Hooper DC, Banerji A. Effect of a drug allergy educational program and antibiotic prescribing guideline on inpatient clinical providers' antibiotic prescribing knowledge. J Allergy Clin Immunol Pract. 2014;2(4):407–13. doi: 10.1016/j.jaip.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturm JM, Temprano J. A survey of physician practice and knowledge of drug allergy at a university medical center. J Allergy Clin Immunol Pract. 2014;2(4):461–4. doi: 10.1016/j.jaip.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Executive Office of the President PCAST . Report to the president on combating antibiotic resistance. Washington, D.C.: 2014. [Mar 13 2015]. Available at: https://www.whitehouse.gov/sites/default/files/microsites/ostp/PCAST/pcast_carb_report_sept2014.pdf. [Google Scholar]
- 43.Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1:264–5. doi: 10.1016/j.jaip.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133:797–8. doi: 10.1016/j.jaci.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Forrest DM, Schellenberg RR, Thien VV, King S, Anis AH, Dodek PM. Introduction of a practice guideline for penicillin skin testing improves the appropriateness of antibiotic therapy. Clin Infect Dis. 2001;32:1685–90. doi: 10.1086/320752. [DOI] [PubMed] [Google Scholar]
- 46.Arroliga ME, Wagner W, Bobek MB, Hoffman-Hogg L, Gordon SM, Arroliga AC. A pilot study of penicillin skin testing in patients with a history of penicillin allergy admitted to the medical ICU. Chest. 2000;118:1106–8. doi: 10.1378/chest.118.4.1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.