Abstract
The innate immune system mediates protection against neurotropic viruses that replicate in the central nervous system (CNS). Virus infection within specific cells of the CNS triggers activation of several families of pattern recognition receptors including Toll-like receptors, retinoic acid-inducible gene 1 like receptors, nucleotide-binding oligomerization domain-like receptors, and cytosolic DNA sensors. In this review, we highlight recent advances in our understanding of how cell-intrinsic host defenses within the CNS modulate infection of different DNA and RNA viruses.
INTRODUCTION
The central nervous system (CNS) coordinates autonomic functions, cognition, and higher-order learning, and is essential for propagation and survival. Accordingly, the CNS must be protected against invasion by microorganisms including viruses. However, the host carefully regulates access of immune cells to the brain and spinal cord to prevent excessive inflammation, swelling, and damage to specialized cells with limited capacity for regeneration. Beyond infiltration of immune cells from peripheral tissues, the CNS utilizes cell-intrinsic and cell-extrinsic innate immune responses to defend against viral infections.
Members of at least 11 virus families including DNA viruses, retroviruses and RNA viruses can infect cells in the CNS [1], and cause direct injury, bystander injury, or trigger immune-mediated encephalitis, all of which results in morbidity and mortality. In this review, we discuss recent discoveries of the intrinsic antiviral pathways in the CNS against different neurotropic viruses that are mediated by type I interferon (IFN), the inflammasome, microRNA, and autophagy signaling pathways.
Virus entry into the CNS is modulated by innate immunity
One of the major paths of viral entry into the CNS is hematogenous transport across the blood-brain barrier (BBB). The BBB is a multicellular structure that protects the CNS from toxic solutes or pathogens that accumulate or become present in circulation. The BBB is composed of specialized endothelial cells attached to each other via tight and adherens junctions. Astrocytes, microglia, and pericytes provide structural and molecular support to form a functional neurovascular unit. Among viruses that enter the CNS across the BBB, three routes have been described: (a) “Trojan horse” model; intracellular transport within infected myeloid cells [2,3]; (b) paracellular entry due to loss of integrity of the BBB [4–6]; (c) infection of endothelial cells with basolateral virus spread [4]. Viruses also can gain access to the CNS by axonal retrograde transport along peripheral neurons into the spinal cord [7–9] and infection of olfactory neurons adjacent to the cribriform plate [10–12] or choroid plexus epithelial cells [10,13].
BBB permeability can be altered by cytokines that accumulate in the bloodstream as a result of systemic inflammation or as a consequence of matrix metalloproteinases that disrupt tight junctions and basement membranes. As an example, tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β increase BBB permeability to viruses [14,15]. However, other inflammatory stimuli tighten the BBB. Endothelial cells can respond to type I and type III IFNs to stabilize the barrier [15–17] and limit the flux of viruses into the brain. A complete breakdown of the BBB was observed in mice lacking the type I IFN receptor (Ifnar−/−) that were challenged with different encephalitic flaviviruses [15,18].
Viral recognition in the CNS
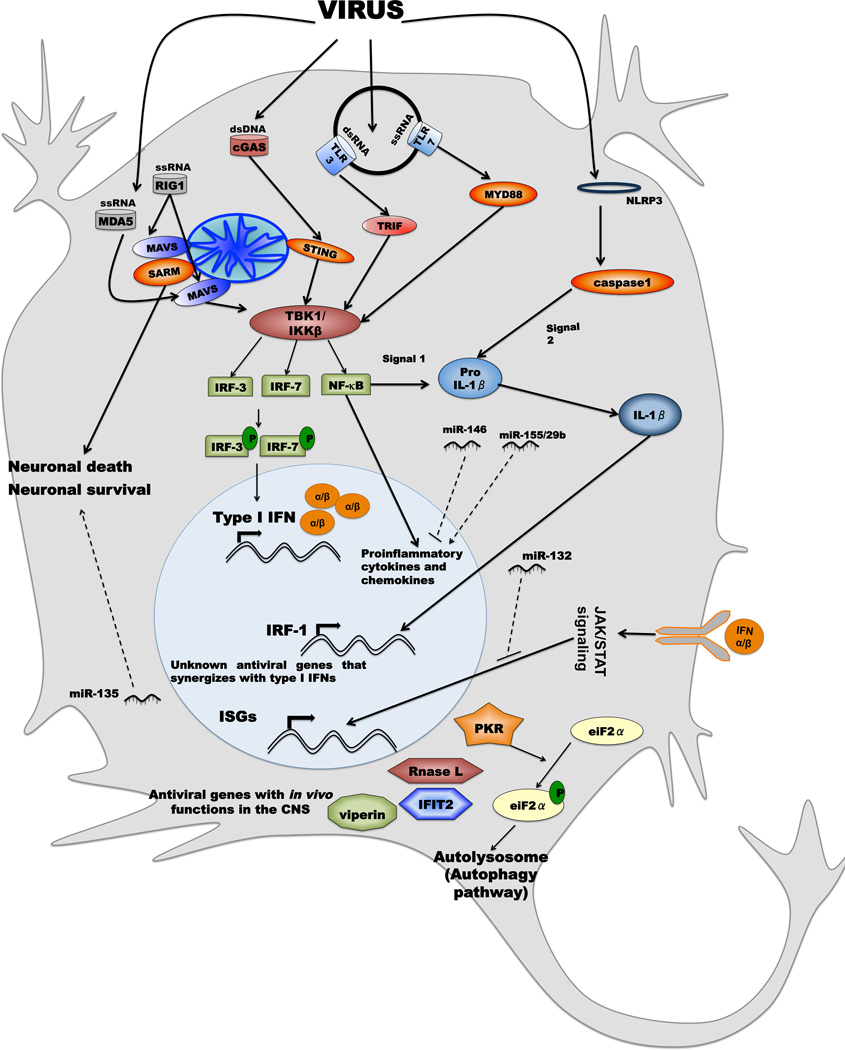
Our understanding of innate antiviral mechanisms that function in the CNS has expanded greatly (Fig 1). This includes insight as to how viruses are recognized by specific cells in the CNS, the differences in responsiveness among neuronal cell subtypes, the particular pattern recognition pathways that are activated, and the antiviral genes that inhibit infection of individual or groups of viruses.
Figure 1. Antiviral signaling pathways in the CNS.
Viruses can be detected by one of four classes of pathogen recognition receptors in cytoplasm or endosome of CNS cells: TLRs (e.g., TLR3 and TLR7), RLRs (e.g., RIG-I and MDA5), NLRs (e.g., NLRP3), or DNA sensors (e.g., cGAS). Engagement of dsRNA and ssRNA by TLR3 or TLR7 results in the respective activation of TRIF and MyD88 dependent signaling pathways to induce expression of proinflammatory cytokines and type I IFNs. RLR activation by viruses triggers MAVS-dependent signaling to activate the transcription factors IRF-3, IRF-7, and NF-κB to trigger expression of type I IFNs and immunomodulatory cytokines. Type I IFN binds to its receptor in an autocrine and paracrine manner and induces expression of hundreds of IFN-stimulated genes (ISGs) that inhibit different viral infections by a variety of mechanisms. As an example, PKR, RNase L, IFIT2, and viperin inhibit WNV infection in the CNS. PKR also confers antiviral effects via the autophagy pathway. SARM-1, which is activated by MAVS signaling, can induce neuronal apoptosis. cGAS detects DNA viruses and activates STING signaling pathways that lead to induction of IRF-3-dependent genes. Several miRNA can augment (miR-155/29b) or inhibit antiviral/inflammatory signaling (miR-132/146) or promote neuronal survival (miR-135). Finally, virus infection can activate the NLRP3 inflammasome through undefined pathogen associated molecular patterns, to trigger caspase-1 activation. This protease cleaves pro-IL-β generated by NF-κB signaling to its mature form, which is then secreted. Some of the IL-β antiviral signaling may be mediated by an IRF-1 transcriptional response.
The innate immune system is defined in part by a network of pattern recognition receptors (e.g., Toll-like receptor (TLR), Retinoic acid-inducible gene (RIG)-I-like receptors, and DNA sensors) that detect conserved pathogen-associated molecular patterns on microbes. Pattern recognition receptors activate signaling cascades that promote nuclear translocation of latent transcription factors (e.g., IFN regulatory factor (IRF)-3 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)) and induce transcriptional activation of genes that direct and mediate cell immunity against viruses, including secretion of antiviral cytokines (IFN-α and IFN-β) and expression of antiviral IFN-stimulated genes. Recent studies have elucidated specific innate immune signaling and effector pathways that restrict or contribute to pathogenesis of different viruses in the CNS:
(a) Toll-like receptors
TLR signaling can have protective or pathogenic effects in the CNS. Infection of poliovirus, a picornavirus, was restricted in the brain and spinal cord by the TLR3–TIR-domain-containing adapter-inducing IFN-β (TRIF) signaling pathway [19]. TLR signaling against Rhabdoviridae family members showed distinct effects. Mice lacking TLR3 had reduced viral load and improved survival after rabies virus infection, suggesting that TLR3 contributes to pathogenesis. The role of TLR3 in response to West Nile virus (WNV) infection also is complex. One study showed that TLR3 signaling restricted WNV infection in neurons [20] whereas as second showed it altered BBB permeability and enhanced viral neuroinvasion [14].
Analysis of humans with primary immunodeficiencies has revealed protective effects of the TLR3 signaling pathway against encephalitic herpesviruses. Single-gene mutations in the TLR3-TRIF signaling pathway (TLR3, TRIF, TNF receptor-associated factor (TRAF)3), and TANK-binding kinase (TBK)1) exist in children with susceptibility to herpes simplex virus (HSV) encephalitis [21–24]. Correspondingly, mice lacking TLR3 or TRIF had increased viral burden in the CNS [25]. Although HSV replication in the CNS primarily targets neurons, mice lacking TLR3 acquired a broader tropism, with infection observed in astrocytes [25].
The TLR7-Myeloid differentiation primary response gene 88 (MyD88) signaling axis protected against vesicular stomatitis virus (VSV) infection as mice lacking MyD88 mice exhibited reduced survival with increased viral burden in the CNS [26]. Again for WNV, the net function of TLR7 varied: one study demonstrated a protective role [27], whereas a second suggested that TLR7 contributed to neuroinvasion [28].
(b) RIG-I-like receptors
Several neurotropic RNA viruses engage RLRs to generate antiviral responses. Type I IFN production in response to rabies virus is mediated preferentially by RLR signaling as mice lacking the downstream adaptor molecule mitochondrial antiviral signaling protein (MAVS) developed worse limb paralysis upon infection [29]. RLR signaling also limits WNV replication in the brain and spinal cord, particularly in neurons [30]. Similarly, other positive stranded RNA viruses (e.g., Japanese encephalitis (JEV) and Sindbis (SINV) viruses) are restricted by MAVS signaling in the CNS [31,32]. Nonetheless, RLR signaling in the CNS has been implicated in neuronal pathology. La Crosse virus (LACV), an Orthobunyavirus, triggered RIG-I-MAVS signaling to upregulate the sterile alpha and TIR-containing motif 1 (SARM1), an adaptor molecule involved in oxidative stress, mitochondrial damage, and cell death in neurons [33].
(c) DNA sensors
Most studies describing DNA sensor mediated antiviral effects with neurotropic viruses have been performed with HSV. One cytoplasmic viral DNA sensor is cGAMP synthase (cGAS), which signals through an adaptor molecule, stimulator of IFN genes (STING). STING recruits TBK1, which results in the activation and nuclear translocation of IRF-3 and induction of type I IFN and proinflammatory cytokines. STING-deficient mice sustained higher HSV-1 infection in the brain and greater death [34]. Consistent with this, mice lacking cGAS mice were more vulnerable to HSV-1 and failed to produce type I IFN [35].
(d) IRF transcription factors
Induction of type I IFN and ISGs are regulated by IRF family transcription factors that are activated by pathogen recognition receptor signaling. Although the role of IRF-3 and IRF-7 in mediating antiviral responses in myeloid cells is established, their function in resident cells of the CNS is less well characterized. IRF-3−/− neurons from the cerebral cortex showed blunted type I IFN responses and were more susceptible to WNV infection [36]. Analogously, IFN-α gene expression was reduced and WNV titers were increased in IRF-7−/− neurons [37]. In studies with lymphocytic chlorimeningitis virus (LCMV), type I IFN responses in the brain were regulated by the concerted actions of IRF-3 and IRF-7 [38]. Similarly, IRF-3 and IRF-7 signaling in the CNS protected against HSV infections; increased viral replication and inflammatory cytokine production were observed in brains of IRF-3−/− or IRF-7−/− mice with a concomitant deficit in production of type I IFN [39,40].
Type I IFN responses in the brain
Neurons and other CNS cells actively respond to virus infection by producing and responding to type I IFN [41]. Intranasal infection of mice with cell type-specific IFNAR deletion in neuroectodermal cells (IFNARfl/fl NesCre+) with VSV identified the olfactory bulb as a primary site of type I IFN production in the CNS [42]. IFN produced from the olfactory bulb primed antiviral responses in other regions of the brain [43]. The use of cell type-specific IFN-β reporter mice revealed that astrocytes and neurons both produce IFN-β in the olfactory bulb in response to viral infection [11]. In comparison, in vitro studies describe neurons as poor producers yet good responders of type I IFN to VSV and HSV [44,45]. Some of the disparity in results may reflect the specific neuronal subtypes studied, as a differential innate immune response program in distinct neuronal subtypes was identified by microarray analysis that determined susceptibility to infection within specific regions of the brain by different positive-stranded RNA viruses [46]. Thus, neurons from distinct regions of the brain respond uniquely to antiviral cytokines and infectious challenges.
Antiviral actions of Interferon-stimulated genes in the CNS
Binding of type I IFN to its receptor (IFNAR) results in a Janus kinase (JAK)-Signal transducer and activator of transcription (STAT) signaling cascade that induces expression hundreds of IFN-stimulated genes, subsets of which inhibit replication and infection of different families of viruses. IFN-stimulated genes can inhibit viruses at different stages of their lifecycles including entry, translation, replication, and egress [47]. Although inhibitory IFN-stimulated genes have been identified in cell culture, few have confirmed activities in vivo in the CNS. Among those known to contribute to restricting virus infection in the CNS, the mechanism of action remains uncertain. In vivo studies in Ifit2−/− mice revealed that Ifit2 protects neurons from infection by VSV and WNV [43,48]. Viperin, protein kinase R (PKR), and RNAse L-deficient mice also showed reduced survival after WNV infection and increased viral burden specifically in the CNS [49,50]. Other IFN-stimulated genes (Irg1 and Ifi27l2a) reportedly have antiviral activity in neurons against multiple viruses including WNV, JEV, and mouse hepatitis viruses [46], although these have not yet been validated in vivo.
Antiviral actions of the inflammasome in the CNS
Inflammasomes are multiprotein complexes that respond to pathogens and induce expression and processing of proinflammatory cytokine, including IL-1β and IL-18. The inflammasome-signaling complex is comprised of NOD like receptors (NLRs, e.g., Nlrp3 or AIM2), adaptor molecules (Apoptosis-associated speck-like protein containing a CARD (ASC)) and an effector caspase protein. A pathogen associated stimulus (signal 1) transcriptionally upregulates expression of pro-IL-1β and pro-IL-18. In a second step (signal 2), pro-IL-1β/IL-18 is processed to its mature form by caspase 1 or caspase 11 within the cytosol. Mice lacking Nlrp3, ASC, caspase 1, or the IL-1 receptor showed reduced survival after WNV infection [51–54]. Inflammasome activation and IL-1β signaling appear to mediate antiviral responses in the CNS directly against flaviviruses [51,55], perhaps as a second wave after type I IFN signaling. Analogously, control of VSV infection in the CNS also occurs in two waves: the first by type I IFN and the second mediated by IRF-1 signaling [56]. Since IL-1β can induce expression of IRF-1 [57] and IRF-1 can mediate antiviral actions in the CNS by type I IFN independent pathways [56], it will be interesting to assess whether the protective IL-1β response is mediated in part by a set of IRF-1 regulated genes.
MicroRNA in the CNS
MicroRNAs (miRs) are ~22 nucleotide-long RNA that target sequences in mRNA to regulate their expression. Several groups have identified miRs that regulate viral infection or pro-inflammatory responses in cells of the CNS. Neuron specific miR-138 promoted HSV latency and neuronal survival by repressing expression of the viral lytic gene, ICP0 [58]. Other miRs indirectly impact viral pathogenesis in the CNS by regulating immune responses. miR-155 and miR-29b promoted the neuroinflammation that occurs downstream of NF-κB signaling during JEV infection [59,60]. Viruses also exploit cellular miRs to create more permissive environments. JEV infection induces miR-146a in microglia, which then targets proteins (TRAF6, interleukin-1 receptor-associated kinase (IRAK)1, IRAK2, and STAT1) required for NF-κB activation and JAK-STAT antiviral signaling. Differences in antiviral programs between different neuronal subsets may be explained in part, by miR expression. miR-132 targets the p300 co-activator of STAT1, which regulated basal IFN-stimulated gene expression level within different neuronal subsets [46].
Autophagy as a defense mechanism in neurons
Autophagy targets foreign molecules for degradation in autophagosomes and has emerged as an independent antiviral defense mechanism in neurons. As neurons are post-mitotic cells with limited regenerative capacity, apoptosis of infected neurons is not a desirable antiviral defense as it is in dividing cells, and thus the upregulation of classical autophagy genes may be a particularly important means of protecting neurons from viral infections.
Over-expression of the autophagy related genes Atg6 or Beclin-1 resulted in decreased cell death and increased survival of neonatal mice injected with SINV [61]. Administration of the peptide tat-beclin-1, a potent inducer of the autophagy pathway in CNS, resulted in increased survival of mice in the context of WNV infection [62]. Analogously, deletion of Atg genes in the CNS resulted in a failure to clear SINV infection and increased virus-induced cell death [63]. PKR, a previously described ISG also promotes autophagy by phosphorylating eiF2α, which leads to the development of autophagosomes that target viruses for degradation [64]. However, viruses have developed strategies to evade autophagy-mediated immune responses. For example, the ability of HSV-1 ICP34.5 protein to target beclin-1 and PKR, both of which stimulate autophagy, determines its neurovirulence [64,65]. Atg5 deficient neurons infected with ICP34.5 mutant HSV-1 sustained higher viral replication, suggesting that autophagy directly or indirectly mediates an antiviral response in neurons [44].
Concluding remarks
Resident cells of the CNS have unique innate immune antiviral strategies to defend against neurotropic viruses. Neurons are primary targets of replication for many viruses and can use different mechanisms, from IFN-stimulated gene mediated antiviral action to autophagy-mediated pathways, as defense strategies. However, more insight is needed as to the role of other CNS resident cells (e.g., astrocytes, microglia, oligodendrocytes) in generating immune responses via cellular communication events in response to viral infections. For example, the mechanism by which glial cells contribute to immune responses such as type I IFN and proinflammatory cytokine production, despite not being targets of replication remains poorly characterized. Use of genetic tools such as Cre-flox mice to generate cell specific deletions of immune signaling pathways in the CNS may begin to elucidate how individual cell types can orchestrate a rapidly protective antiviral response.
HIGHLIGHTS.
CNS cells defend against viruses by several different innate immune mechanisms
Type I IFN, inflammasome signaling, and autophagy have antiviral roles in neurons
Micro-RNA expression in neurons can inhibit virus infection or promote pathogenesis
ACKNOWLEDGEMENTS
This work was supported by NIH grants U19 AI083019, R01 AI074972 and R01 AI104002 (to M.S.D). The DFG Research Fellowship supports S.N.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Knipe DM, Howley MP, editors. Fields Virology. Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Schweighardt B, Atwood WJ. Virus receptors in the human central nervous system. J Neurovirol. 2001;7:187–195. doi: 10.1080/13550280152403236. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Welte T, McGargill M, Town T, Thompson J, Anderson JF, Flavell RA, Fikrig E, Hedrick SM, Wang T. Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis. J Immunol. 2008;181:2084–2091. doi: 10.4049/jimmunol.181.3.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma S, Kumar M, Gurjav U, Lum S, Nerurkar VR. Reversal of West Nile virus-induced blood-brain barrier disruption and tight junction proteins degradation by matrix metalloproteinases inhibitor. Virology. 2010;397:130–138. doi: 10.1016/j.virol.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Waeckerlin R, Urbanowski MD, van Marle G, Hobman TC. West Nile virus infection causes endocytosis of a specific subset of tight junction membrane proteins. PLoS One. 2012;7:e37886. doi: 10.1371/journal.pone.0037886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afonso PV, Ozden S, Cumont MC, Seilhean D, Cartier L, Rezaie P, Mason S, Lambert S, Huerre M, Gessain A, et al. Alteration of blood-brain barrier integrity by retroviral infection. PLoS Pathog. 2008;4:e1000205. doi: 10.1371/journal.ppat.1000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ugolini G. Rabies virus as a transneuronal tracer of neuronal connections. Adv Virus Res. 2011;79:165–202. doi: 10.1016/B978-0-12-387040-7.00010-X. [DOI] [PubMed] [Google Scholar]
- 8.Smith G. Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol. 2012;66:153–176. doi: 10.1146/annurev-micro-092611-150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samuel MA, Wang H, Siddharthan V, Morrey JD, Diamond MS. Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc Natl Acad Sci U S A. 2007;104:17140–17145. doi: 10.1073/pnas.0705837104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudd PA, Cattaneo R, von Messling V. Canine distemper virus uses both the anterograde and the hematogenous pathway for neuroinvasion. J Virol. 2006;80:9361–9370. doi: 10.1128/JVI.01034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detje CN, Lienenklaus S, Chhatbar C, Spanier J, Prajeeth CK, Soldner C, Tovey MG, Schluter D, Weiss S, Stangel M, et al. Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis. J Virol. 2015;89:2731–2738. doi: 10.1128/JVI.02044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles PC, Walters E, Margolis F, Johnston RE. Mechanism of neuroinvasion of Venezuelan equine encephalitis virus in the mouse. Virology. 1995;208:662–671. doi: 10.1006/viro.1995.1197. [DOI] [PubMed] [Google Scholar]
- 13.Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111:194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 15. Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio. 2014;5:e01476–e01414. doi: 10.1128/mBio.01476-14. This paper shows a novel role of type I IFN in tightening the BBB.
- 16.Sorgeloos F, Kreit M, Hermant P, Lardinois C, Michiels T. Antiviral type I and type III interferon responses in the central nervous system. Viruses. 2013;5:834–857. doi: 10.3390/v5030834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M, Jr, Klein RS, Diamond MS. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7:284ra259. doi: 10.1126/scitranslmed.aaa4304. This study demonstrates that type III IFN also can tighten the BBB and prevent neuroinvasion of WNV.
- 18.Weber E, Finsterbusch K, Lindquist R, Nair S, Lienenklaus S, Gekara NO, Janik D, Weiss S, Kalinke U, Overby AK, et al. Type I interferon protects mice from fatal neurotropic infection with Langat virus by systemic and local antiviral responses. J Virol. 2014;88:12202–12212. doi: 10.1128/JVI.01215-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oshiumi H, Okamoto M, Fujii K, Kawanishi T, Matsumoto M, Koike S, Seya T. The TLR3/TICAM-1 pathway is mandatory for innate immune responses to poliovirus infection. J Immunol. 2011;187:5320–5327. doi: 10.4049/jimmunol.1101503. [DOI] [PubMed] [Google Scholar]
- 20.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 22.Sancho-Shimizu V, Zhang SY, Abel L, Tardieu M, Rozenberg F, Jouanguy E, Casanova JL. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- 23.Herman M, Ciancanelli M, Ou YH, Lorenzo L, Klaudel-Dreszler M, Pauwels E, Sancho-Shimizu V, Perez de Diego R, Abhyankar A, Israelsson E, et al. Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J Exp Med. 2012;209:1567–1582. doi: 10.1084/jem.20111316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, Herman M, Cardon A, Durandy A, Bustamante J, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010;33:400–411. doi: 10.1016/j.immuni.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, Dagnaes-Hansen F, Ulhoi BP, Holm TH, Mogensen TH, Owens T, et al. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest. 2012;122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang KS, Navarini AA, Recher M, Lang PA, Heikenwalder M, Stecher B, Bergthaler A, Odermatt B, Akira S, Honda K, et al. MyD88 protects from lethal encephalitis during infection with vesicular stomatitis virus. Eur J Immunol. 2007;37:2434–2440. doi: 10.1002/eji.200737310. [DOI] [PubMed] [Google Scholar]
- 27.Town T, Bai F, Wang T, Kaplan AT, Qian F, Montgomery RR, Anderson JF, Flavell RA, Fikrig E. Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity. 2009;30:242–253. doi: 10.1016/j.immuni.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welte T, Reagan K, Fang H, Machain-Williams C, Zheng X, Mendell N, Chang GJ, Wu P, Blair CD, Wang T. Toll-like receptor 7-induced immune response to cutaneous West Nile virus infection. J Gen Virol. 2009;90:2660–2668. doi: 10.1099/vir.0.011783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faul EJ, Wanjalla CN, Suthar MS, Gale M, Wirblich C, Schnell MJ. Rabies virus infection induces type I interferon production in an IPS-1 dependent manner while dendritic cell activation relies on IFNAR signaling. PLoS Pathog. 2010;6:e1001016. doi: 10.1371/journal.ppat.1001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredericksen BL, Keller BC, Fornek J, Katze MG, Gale M., Jr Establishment and maintenance of the innate antiviral response to West Nile Virus involves both RIG-I and MDA5 signaling through IPS-1. J Virol. 2008;82:609–616. doi: 10.1128/JVI.01305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollish AC, Ferris MT, Blevins LK, Loo YM, Gale M, Jr, Heise MT. An attenuating mutation in a neurovirulent Sindbis virus strain interacts with the IPS-1 signaling pathway in vivo. Virology. 2013;435:269–280. doi: 10.1016/j.virol.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazmi A, Dutta K, Basu A. RIG-I mediates innate immune response in mouse neurons following Japanese encephalitis virus infection. PLoS One. 2011;6:e21761. doi: 10.1371/journal.pone.0021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee P, Woods TA, Moore RA, Peterson KE. Activation of the innate signaling molecule MAVS by bunyavirus infection upregulates the adaptor protein SARM1, leading to neuronal death. Immunity. 2013;38:705–716. doi: 10.1016/j.immuni.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science. 2013;341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daffis S, Samuel MA, Keller BC, Gale M, Jr, Diamond MS. Cell-specific IRF-3 responses protect against West Nile virus infection by interferondependent and -independent mechanisms. PLoS Pathog. 2007;3:e106. doi: 10.1371/journal.ppat.0030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daffis S, Samuel MA, Suthar MS, Keller BC, Gale M, Jr, Diamond MS. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J Virol. 2008;82:8465–8475. doi: 10.1128/JVI.00918-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen JE, Fenger C, Issazadeh-Navikas S, Krug A, Liljestrom P, Goriely S, Paludan SR, Finsen B, Christensen JP, Thomsen AR. Differential impact of interferon regulatory factor 7 in initiation of the type I interferon response in the lymphocytic choriomeningitis virus-infected central nervous system versus the periphery. J Virol. 2012;86:7384–7392. doi: 10.1128/JVI.07090-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menachery VD, Pasieka TJ, Leib DA. Interferon regulatory factor 3-dependent pathways are critical for control of herpes simplex virus type 1 central nervous system infection. J Virol. 2010;84:9685–9694. doi: 10.1128/JVI.00706-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy AA, Rosato PC, Parker ZM, Khalenkov A, Leib DA. Synergistic control of herpes simplex virus pathogenesis by IRF-3, and IRF-7 revealed through non-invasive bioluminescence imaging. Virology. 2013;444:71–79. doi: 10.1016/j.virol.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103:7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol. 2009;182:2297–2304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- 43.Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, Bergmann CC, Diamond MS, Virgin HW, Sen GC. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 2012;8:e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yordy B, Iijima N, Huttner A, Leib D, Iwasaki A. A neuron-specific role for autophagy in antiviral defense against herpes simplex virus. Cell Host Microbe. 2012;12:334–345. doi: 10.1016/j.chom.2012.07.013. This report describes a difference in the innate antiviral strategies employed by neurons and mitotic cells to control HSV-1 infection.
- 45.Rosato PC, Leib DA. Intrinsic innate immunity fails to control herpes simplex virus and vesicular stomatitis virus replication in sensory neurons and fibroblasts. J Virol. 2014;88:9991–10001. doi: 10.1128/JVI.01462-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cho H, Proll SC, Szretter KJ, Katze MG, Gale M, Jr, Diamond MS. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat Med. 2013;19:458–464. doi: 10.1038/nm.3108. This study showed that neurons from distict regions of the brain have unique antiviral signatures and respond differently to infection by neurotropic viruses.
- 47.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–545. doi: 10.1146/annurev-immunol-032713-120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho H, Shrestha B, Sen GC, Diamond MS. A role for Ifit2 in restricting West Nile virus infection in the brain. J Virol. 2013;87:8363–8371. doi: 10.1128/JVI.01097-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szretter KJ, Brien JD, Thackray LB, Virgin HW, Cresswell P, Diamond MS. The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J Virol. 2011;85:11557–11566. doi: 10.1128/JVI.05519-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BR, Silverman RH, Gale M, Jr, Diamond MS. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ramos HJ, Lanteri MC, Blahnik G, Negash A, Suthar MS, Brassil MM, Sodhi K, Treuting PM, Busch MP, Norris PJ, et al. IL-1beta signaling promotes CNS-intrinsic immune control of West Nile virus infection. PLoS Pathog. 2012;8:e1003039. doi: 10.1371/journal.ppat.1003039. This paper reports inflammasome mediated antiviral effects act at later stages of viral replication by type I IFN-independent pathways.
- 52.Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M, Jr, Verma S. Inflammasome adaptor protein Apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. J Virol. 2013;87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durrant DM, Daniels BP, Klein RS. IL-1R1 signaling regulates CXCL12-mediated T cell localization and fate within the central nervous system during West Nile Virus encephalitis. J Immunol. 2014;193:4095–4106. doi: 10.4049/jimmunol.1401192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durrant DM, Robinette ML, Klein RS. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J Exp Med. 2013;210:503–516. doi: 10.1084/jem.20121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaushik DK, Gupta M, Kumawat KL, Basu A. NLRP3 inflammasome: key mediator of neuroinflammation in murine Japanese encephalitis. PLoS One. 2012;7:e32270. doi: 10.1371/journal.pone.0032270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nair S, Michaelsen-Preusse K, Finsterbusch K, Stegemann-Koniszewski S, Bruder D, Grashoff M, Korte M, Koster M, Kalinke U, Hauser H, et al. Interferon regulatory factor-1 protects from fatal neurotropic infection with vesicular stomatitis virus by specific inhibition of viral replication in neurons. PLoS Pathog. 2014;10:e1003999. doi: 10.1371/journal.ppat.1003999. This paper describes IRF-1 mediated antiviral effects at later stages of viral replication in neurons by IFN-independent mechanisms.
- 57.Harikumar KB, Yester JW, Surace MJ, Oyeniran C, Price MM, Huang WC, Hait NC, Allegood JC, Yamada A, Kong X, et al. K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5. Nat Immunol. 2014;15:231–238. doi: 10.1038/ni.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pan D, Flores O, Umbach JL, Pesola JM, Bentley P, Rosato PC, Leib DA, Cullen BR, Coen DM. A neuron-specific host microRNA targets herpes simplex virus-1 ICP0 expression and promotes latency. Cell Host Microbe. 2014;15:446–456. doi: 10.1016/j.chom.2014.03.004. This study shows that neuron specific miR-138 promotes cell survival by repressing HSV lytic gene ICP0.
- 59.Thounaojam MC, Kaushik DK, Basu A. MicroRNAs in the brain: it's regulatory role in neuroinflammation. Mol Neurobiol. 2013;47:1034–1044. doi: 10.1007/s12035-013-8400-3. [DOI] [PubMed] [Google Scholar]
- 60.Thounaojam MC, Kaushik DK, Kundu K, Basu A. MicroRNA-29b modulates Japanese encephalitis virus-induced microglia activation by targeting tumor necrosis factor alpha-induced protein 3. J Neurochem. 2014;129:143–154. doi: 10.1111/jnc.12609. [DOI] [PubMed] [Google Scholar]
- 61.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, et al. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. This paper describes the therapeutic activity of an autophagy-inducing peptide against viruses in the CNS.
- 63.Orvedahl A, MacPherson S, Sumpter R, Jr, Talloczy Z, Zou Z, Levine B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe. 2010;7:115–127. doi: 10.1016/j.chom.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talloczy Z, Jiang W, Virgin HWt, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. Regulation of starvation- and virus-induced autophagy by the eIF2alpha kinase signaling pathway. Proc Natl Acad Sci U S A. 2002;99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]