Plasmodium parasites, unicellular alveolates in the Phylum Apicomplexa, are the causative agents of malaria. Their development is a complex interplay among multiple, distinct parasite life stages and host cells in humans and mosquitoes, organisms that are separated by more than 200 million years of evolution. Despite this vast biological divide, malaria parasites have adapted to a life that is dictated by networks of host signaling pathways and mitochondrial physiology that are remarkably conserved in humans and mosquitoes. Among the most important and most well-studied of the malaria parasites affecting humans is Plasmodium falciparum, which causes significant pathology in humans and more modest, although biologically important, pathology in the mosquito host. Rather than a coincidence of convergent host responses, we would suggest that these fundamental malaria parasite-host interactions reflect Apicomplexan radiation and adaptation to parasitism of invertebrate hosts, which preceded the appearance of bloodfeeding and parasitism of vertebrate hosts [1]. In these divergent hosts, the parasite has adapted to patterns of insulin/insulin-like growth factor signaling (IIS), regulation by conserved host protein kinases, and changes in host mitochondrial function that can alter parasite development. Accordingly, we suggest that parasite survival in the invertebrate host depended on the adaptation of parasites to pathways that were similar enough in vertebrate hosts to facilitate survival in these additional hosts over the course of evolutionary time. Further, we argue that a closer examination of malaria parasites within their primeval insect hosts can reveal the most fundamental aspects of host-pathogen interactions in malaria and, hence, provide the key to the development of novel therapeutics that can both cure human disease and block transmission to the mosquito host. To this end, we discuss host-malaria parasite interactions in the context of networked processes of IIS, activation of mitogen-activated protein kinases (MAPKs) and protein kinase C (PKC) isoforms, and bioenergetics.
Insulin/insulin-like growth factor signaling (IIS) in malaria
The highly conserved IIS pathway is comprised of MAPK- and a phosphatidylinositol 3-kinase/Akt-dependent branches that play critical roles in the regulation of growth, longevity, and immunity in vertebrates and invertebrates [2 and 3]. Indeed, the majority of IIS proteins and their interactions are conserved between humans and mosquitoes [4–11, 12•, 13, 14•• and 15••]. In humans, IIS can be induced by members of the insulin superfamily of peptide hormones, which include insulin and insulin-like growth factors (IGF) I and II, and seven relaxin family insulin-like peptides (ILPs) [16]. ILPs have also been identified in Anopheles gambiae 17] and in Anopheles stephensi 7], key mosquito vectors of malaria in sub-Saharan African and in India and parts of Asia, respectively.
IGF-1 is abundant in human blood (0.11–0.13 μM) and its bioavailability is tightly regulated by IGF binding proteins [18] due to its pleiotropic effects on apoptosis, autophagy, and stem cell renewal [19 and 20]. During malaria parasite infection, serum IGF-1 levels decrease significantly in humans and correlate with disease severity and anemia [21]. Ingestion of low serum concentrations of IGF-1 by A. stephensi extends lifespan by inhibiting apoptosis and decreasing damage to the midgut, while simultaneously enhancing mitochondrial function [15••]. This is similar to observations made in mice, where repression of IGF-1 signaling induces resistance to apoptosis by oxidative stress and extends lifespan [19]. Low levels of human IGF-1 in ingested serum also repress the phosphorylation of the MAPK extracellular signal-regulated kinase (ERK) in the mosquito midgut, thereby enhancing midgut synthesis of reactive nitrogen and oxygen species (RNOS) and resistance of A. stephensi to P. falciparum 15••]. In contrast, physiological concentrations of IGF-1 lead to sustained RNOS production and enhanced resistance of A. stephensi to P. falciparum, but also cause damage leading to midgut epithelial dysplasia [15••].
In contrast to IGF-1, insulin levels in healthy humans are significantly lower (17–590 pM), but can rise by as much as 10–35-fold during malaria parasite infection [22 and 23]. This may be due, in part, to the presence of insulin-mimetic P. falciparum-derived glycosylphosphatidylinositols (PfGPIs). PfGPIs tether parasite cell surface proteins, but are produced in vast excess of this need [24], presumably to act as signaling mediators to manipulate host biology. PfGPIs induce hypoglycemia [25] and can reverse much of the pathology associated with type 2 diabetes [26, 27 and 28]. However, PfGPIs synergize with insulin signaling [29], which can also inhibit nuclear factor (NF)-κB-dependent innate immune responses [30, 31 and 32]. The inhibition of innate immunity is responsible, in part, for the increased susceptibility of diabetics to opportunistic infections and malaria [33, 34• and 35]. As in humans, activation of IIS in A. stephensi by insulin results in the inhibition of NF-κB-dependent immunity and increased susceptibility to malaria parasite infection [4, 6 and 12•]. Human insulin and parasite-derived products also induce endogenous A. stephensi ILP production [7], which can further dampen NF-κB-mediated immunity [36]. In sum, these studies highlight the conserved nature of IIS between humans and mosquitoes and suggest that Plasmodium parasites may have evolved to manipulate, and benefit from, this conservation.
Protein kinase-dependent regulation of host-parasite interactions
In addition to IIS activation by PfGPIs, these parasite molecules along with parasite hemozoin act as pathogen-associated molecular patterns (PAMPs) to activate MAPK signaling in both mammalian and mosquito hosts. While activation of IIS by PfGPIs may benefit the parasite through subversion of host cell signaling [25, 26, 27, 28, 37, 38, 39, 40 and 41], activation of Toll-like receptor signaling in mammalian immune cells by PfGPIs also precipitates a protective host response [37 and 38]. In particular, triacylated PfGPIs are recognized by Toll-like receptor 1 (TLR1) and TLR2, while diacylated PfGPIs are recognized by TLR2/TLR6 heterodimers [38]. TLR ligation recruits adapter proteins including myeloid differentiation factor 88 (MyD88), TIR-domain-containing adaptor protein-inducing IFN-β (TRIF), and TRIF-related adaptor molecule (TRAM [42]), which collectively activate NF-κB-dependent activation via ERK, c-Jun N-terminal kinase (JNK), and p38 MAPK [43]. In this context, PfGPIs-mediated TLR-dependent signaling induces proinflammatory cytokine production by macrophages [44] and dendritic cells [45].
In an analogous fashion, PfGPIs function as an early signal of parasite infection in A. gambiae and in A. stephensi. In A. stephensi, PfGPIs induce ERK phosphorylation in the midgut within minutes of ingestion [8]. From studies with A. gambiae, this signaling may be Toll-initiated to activate NF-κB-dependent anti-parasite responses, including synthesis RNOS and antimicrobial peptides [8 and 46]. Hence, in both mammals and mosquitoes innate immunity to parasite infection appears to depend on PAMP-mediated ERK activation of NF-κB-dependent signaling. Hemozoin is a by-product of parasite degradation of hemoglobin that accumulates in the parasite digestive vacuole and induces activation of p38 MAPK-, ERK-, and NF-κB-dependent signaling, but not JNK signaling in murine macrophages and monocytes [47, 48, 49, 50 and 51]. In human monocytes, hemozoin activates p38 MAPK- and NF-κB-dependent signaling [52 and 53]. In contrast to ERK signaling, which is more typically associated with cell survival, both JNK and p38 MAPK signaling induce stress responses that can contribute to host pathology. Consequences of increased p38 MAPK activation in response to P. falciparum include endothelial dysfunction, heightened TLR2 responsiveness, elevated plasma lysozyme levels, and overproduction of inflammatory cytokines [52, 53, 54• and 55]. In A. stephensi, P. falciparum infection rapidly activates p38 MAPK signaling in the mosquito midgut, which precipitates decreased transcription of a variety of NF-κB-dependent innate immune genes [56]. Conversely, delivery of small molecule inhibitors (SMIs) of p38 MAPK via the bloodmeal significantly enhances immune gene expression and reduces P. falciparum development in A. stephensi 56]. While p38 MAPK-dependent signaling increases parasite burden in the mosquito host, resulting pathology from this burden appears to be offset by p38 MAPK-enhanced host survival during infection [56], a situation that may attest to the relatively longer evolutionary relationship of malaria parasites with their invertebrate hosts. Collectively, these observations suggest that therapeutic use of p38 MAPK inhibitors could reduce disease pathology in human hosts and reduce parasite development and transmission by mosquitoes that feed on treated patients1.
In A. stephensi cells, hemozoin activates not only ERK but also atypical PKCζ, which likely regulates the synthesis of RNOS in the mosquito midgut [9]. The genomes of A. stephensi and A. gambiae encode six PKC genes – PKCδ, PKCε, PKCζ, PKD, PKN, and an indeterminate conventional PKC [57]. Pan-inhibition of PKCs in A. stephensi via provision of SMIs in the bloodmeal had no effect on expression of immune genes, but significantly increased midgut barrier integrity and decreased development of P. falciparum 57]. These data suggest that PKC-dependent signaling during infection negatively regulates epithelial barrier function in the mosquito to promote parasite development. Intriguingly, PKC signaling also regulates barrier function in human malaria. In particular, PKCθ- and JNK-dependent signaling are required for the development of microvascular and neuronal pathology, respectively, through disruption of the blood-brain barrier in an experimental murine model of cerebral malaria [58 and 59]. This pathology can be reduced, increasing mouse survivorship, through treatment of parasite-infected mice with SMIs that block p38 MAPK, PKC or JNK signaling [60• and 61]. Together with our data from the mosquito host [56], these observations suggest that protein kinase SMIs could be leveraged for drug treatment to reduce disease pathology in humans and to block parasite transmission in mosquitoes that feed on treated patients.
Mitochondrial physiology during malaria parasite growth and development
Mitochondria reside at the center of cell signaling, immunity and basic intermediary metabolism and control stress responses [62••] as well as the degree of the proinflammatory immune responses fueled by the balance between glycolysis and mitochondria-derived ATP (oxidative phosphorylation or OXPHOS) [63, 64, 65•• and 66]. Most studies of PAMP signaling during infection have focused on the phosphorylation of mitochondria-associated apoptotic proteins [67, 68, 69 and 70]. However, mitochondria are involved in the host response to infection or tissue damage not only via apoptosis, but also through bioenergetics [63, 64, 65••, 71, 72•• and 73] and these latter effects have been ascribed to the translocation and/or activation of critical protein kinases [74, 75, 76 and 77]. For instance, activation and translocation of PKCε to mitochondria (in the presence of redox active cofactors) inhibits the pyruvate dehydrogenase complex (PDHC) and decreases OXPHOS [78]. In addition to PKCε, the MAPKs ERK, JNK, and p38 MAPK can modulate mitochondria function in response to diverse stimuli [79] including infection [80] in a variety of biological models [69, 81, 82 and 83]. Collectively, these data suggest that conserved host protein kinases can regulate parasite development and disease severity in malaria by altering mitochondria-dependent host immunity.
Analogous networking between immunity and mitochondrial biology is evident in the mosquito host from our studies. Following infection with P. falciparum, A. stephensi midgut PKCε and PKCδ exhibited reciprocal expression [57] a pattern similar to that reported for the reciprocal mitochondrial regulation of PHDC by PKCε and PKCδ [78]. Hence, an infection-driven mosquito “signalosome” of PKCε, PKCδ, JNK and p38 MAPK may transduce information between mitochondria and other cellular compartments to modulate not only mitochondrial homeostasis but also host immunity. In A. stephensi, inhibition of p38 MAPK signaling with SMIs significantly enhanced RNOS and an array of anti-parasite immune genes and reduced protein synthesis machinery and OXPHOS [56]. Hence, P. falciparum-induced activation of p38 MAPK signaling in the mosquito midgut appears to facilitate parasite infection through reduced anti-parasite immune defenses and enhanced host protein synthesis and bioenergetics to improve both host and parasite survival, and ultimately, transmission. In contrast, sustained midgut activation of IIS-associated Akt in transgenic A. stephensi resulted in decreased OXPHOS with decreased mitophagy and accumulation of dysfunctional mitochondria – analogous to over-activation of Akt in mammals [84 and 85] – with increased resistance to P. falciparum infection and reduced lifespan [14••]. Given that sustained activation of Akt inhibits autophagy and mitochondrial biogenesis [84 and 85], we predicted that overexpression of phosphatase and tensin homolog (PTEN), which opposes Akt signaling, would upregulate mitochondrial biogenesis to improve both resistance and fitness. Indeed, midgut overexpression of PTEN in transgenic A. stephensi resulted in enhanced resistance to P. falciparum infection with increased midgut barrier integrity and lifespan relative to non-transgenic controls [11]. Similarly, inhibition of PKC-dependent signaling in A. stephensi increased midgut barrier integrity and decreased P. falciparum infection in the absence of any change in NF-κB-dependent anti-parasite defense genes [57], consistent with a role of NF-κB in energy homeostasis [86•]. Notably, PKCs regulate mitochondrial biogenesis via IIS, suggesting that PKC inhibition through IIS leads to increased mitochondrial biogenesis and/or function to enhance the midgut barrier for resistance to P. falciparum infection in A. stephensi.
Conclusions
Collectively, these observations suggest that the relationship between mitochondria and the immune response to Plasmodium infection is conserved in human and mosquito hosts (Figure 1). Hence, targeting conserved protein kinase signaling pathways that regulate the balance between immunity and mitochondrial genes [63, 64, 65•• and 73] may influence host-pathogen interactions with potential to (i) minimize disease severity and/or parasitemia, (ii) decrease gametocytogenesis, and (iii) block malaria parasite transmission to mosquitoes. Furthermore, this same balance can impact genotype by environment interactions. In particular, insecticide resistance in a wide variety of insects, including mosquitoes, has been associated with higher expression of mitochondrial gene products related to mitochondrial respiratory chain and ATP production [87, 88 and 89], mitochondrial NADPH-dependent xenobiotic catabolism [90, 91 and 92], and glutathione S-transferases (GSTs) [93]. GST isoforms can function as activators or inhibitors of JNK and ERK/p38 MAPK/IKK pathways in D. melanogaster 94], suggesting that protein kinase targeting could be leveraged to generate therapeutics for treatment of malaria in the human host that can directly modulate insecticide resistance, immune response, and bioenergetics in mosquitoes that feed on treated patients.
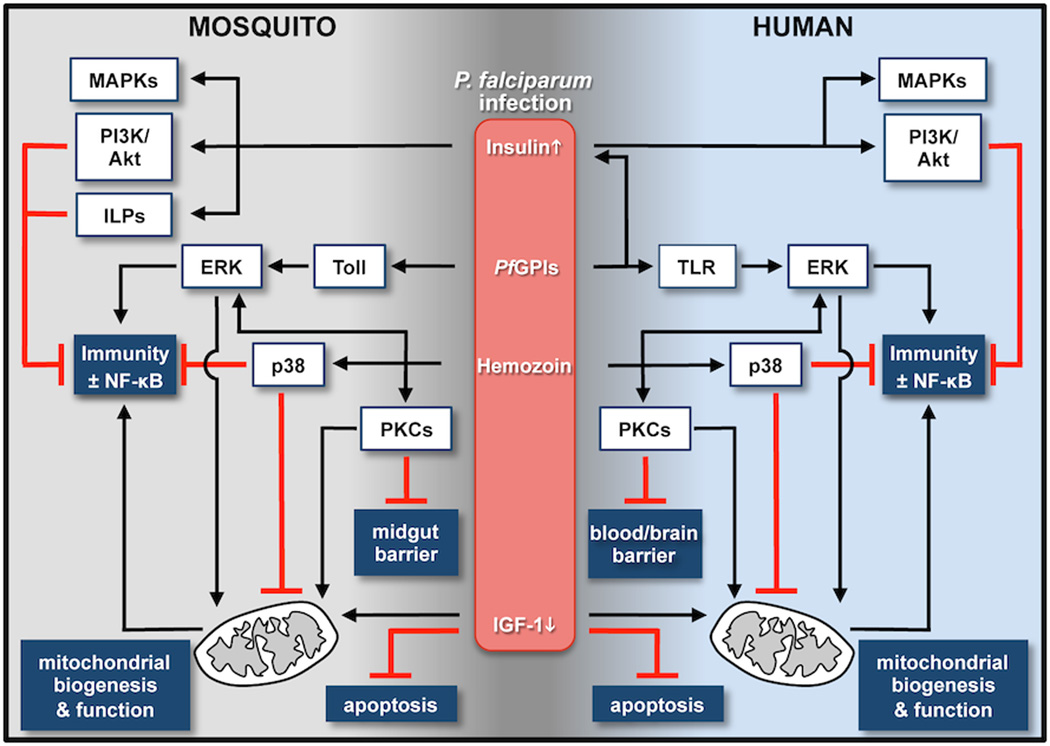
Figure 1.
Host-pathogen interactions in malaria. During infection with P. falciparum, both human and mosquito hosts exhibit responses that reflect physiological changes to infection (insulin/IGF-1) and to parasite PAMPs (PfGPIs, hemozoin). In particular, remarkably conserved protein kinase signaling pathways are networked to regulate epithelial and endothelial barrier function, which can dictate infection success and pathology, as well as mitochondrial biogenesis and function to control immunity through NF-κB-dependent and -independent responses.
Highlights.
Mosquitoes and humans share many responses to malaria parasite infection.
Conserved signaling regulates barrier and mitochondrial function during infection.
Parasite success in both insect and human hosts likely depends on this conservation.
This biology can be translated to novel drugs with transmission blocking activity.
Acknowledgments
Funding was provided by NIH NIAID grants AI080799, AI073745 and Al107263.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
PCT application: THERAPIES FOR DISEASES CAUSED BY ARTHROPOD-BORNE PARASITES International Publication Date: 15 January 2015, Publication number: WO 2015/006753 A2 Inventors: LUCKHART, Shirley; University of California, Davis and GIULIVI, Cecilia; University of California, Davis, Assigned to: THE REGENTS OF THE UNIVERSITY OF CALIFORNIA.
PCT application COMBINATION THERAPIES FOR MALARIA International Publication Date: 15 January 2015, Publication number: WO 2015/006752 Al Inventors: LUCKHART, Shirley; University of California, Davis and GIULIVI, Cecilia; University of California, Davis, Assigned to: THE REGENTS OF THE UNIVERSITY OF CALIFORNIA.
References and recommended reading
• of special interest
•• of outstanding interest
- 1.Kopecna J, Jirku M, Obornik M, Tokarev YS, Lukes J, Modry D. Phylogenetic analysis of coccidian parasites from invertebrates: search for missing links. Protist. 2006;157:173–183. doi: 10.1016/j.protis.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Luckhart S, Riehle MA. The insulin signaling cascade from nematodes to mammals: insights into innate immunity of Anopheles mosquitoes to malaria parasite infection. Dev Comp Immunol. 2007;31:647–656. doi: 10.1016/j.dci.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pakpour N, Akman-Anderson L, Vodovotz Y, Luckhart S. The effects of ingested mammalian blood factors on vector arthropod immunity and physiology. Microbes Infect. 2013;15:243–254. doi: 10.1016/j.micinf.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surachetpong W, Pakpour N, Cheung KW, Luckhart S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid Redox Signal. 2011;14:943–955. doi: 10.1089/ars.2010.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beier MS, Pumpuni CB, Beier JC, Davis JR. Effects of para-aminobenzoic acid, insulin, and gentamicin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae) J Med Entomol. 1994;31:561–565. doi: 10.1093/jmedent/31.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211:741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquez AG, Pietri JE, Smithers HM, Nuss A, Antonova Y, Drexler AL, Riehle MA, Brown MR, Luckhart S. Insulin-like peptides in the mosquito Anopheles stephensi: identification and expression in response to diet and infection with Plasmodium falciparum. Gen Comp Endocrinol. 2011;173:303–312. doi: 10.1016/j.ygcen.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim J, Gowda DC, Krishnegowda G, Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–2789. doi: 10.1128/IAI.73.5.2778-2789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akman-Anderson L, Olivier M, Luckhart S. Induction of nitric oxide synthase and activation of signaling proteins in Anopheles mosquitoes by the malaria pigment, hemozoin. Infect Immun. 2007;75:4012–4019. doi: 10.1128/IAI.00645-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton AA, Wang B, Camp L, Price MS, Arshi A, Nagy M, Nadler SA, Faeder JR, Luckhart S. The mitogen-activated protein kinome from Anopheles gambiae: identification, phylogeny and functional characterization of the ERK, JNK and p38 MAP kinases. BMC Genomics. 2011;12:574. doi: 10.1186/1471-2164-12-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauck ES, Antonova-Koch Y, Drexler A, Pietri J, Pakpour N, Liu D, Blacutt J, Riehle MA, Luckhart S. Overexpression of phosphatase and tensin homolog improves fitness and decreases Plasmodium falciparum development in Anopheles stephensi. Microbes Infect. 2013;15:775–787. doi: 10.1016/j.micinf.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pakpour N, Corby-Harris V, Green GP, Smithers HM, Cheung KW, Riehle MA, Luckhart S. Ingested human insulin inhibits the mosquito NF-kappaB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80:2141–2149. doi: 10.1128/IAI.00024-12. This work demonstrated that human insulin represses NF-κB-dependent immunity in mosquitoes in a manner similar to that observed in humans.
- 13.Corby-Harris V, Drexler A, Watkins de Jong L, Antonova Y, Pakpour N, Ziegler R, Ramberg F, Lewis EE, Brown JM, Luckhart S, et al. Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLoS Pathog. 2010;6:e1001003. doi: 10.1371/journal.ppat.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luckhart S, Giulivi C, Drexler AL, Antonova-Koch Y, Sakaguchi D, Napoli E, Wong S, Price MS, Eigenheer R, Phinney BS, et al. Sustained activation of Akt elicits mitochondrial dysfunction to block Plasmodium falciparum infection in the mosquito host. PLoS Pathog. 2013;9:e1003180. doi: 10.1371/journal.ppat.1003180. These studies showed that IIS-dependent epithelial mitochondrial dynamics controls parasite resistance and insect lifespan, indicating that mitochondrial quality control is key to vector competence.
- 15. Drexler AL, Pietri JE, Pakpour N, Hauck E, Wang B, Glennon EK, Georgis M, Riehle MA, Luckhart S. Human IGF1 regulates midgut oxidative stress and epithelial homeostasis to balance lifespan and Plasmodium falciparum resistance in Anopheles stephensi. PLoS Pathog. 2014;10:e1004231. doi: 10.1371/journal.ppat.1004231. This work connects the metabolic effects of IGF-1 to malaria parasite resistance in a manner that is independent of NF-kB-dependent immunity and dependent on epithelial homeostasis.
- 16.Shabanpoor F, Separovic F, Wade JD. Chapter 1 The Human Insulin Superfamily of Polypeptide Hormones. In: Gerald L, editor. Vitamins & Hormones. Vol. 80. Academic Press; 2009. pp. 1–31. [DOI] [PubMed] [Google Scholar]
- 17.Krieger MJ, Jahan N, Riehle MA, Cao C, Brown MR. Molecular characterization of insulin-like peptide genes and their expression in the African malaria mosquito, Anopheles gambiae. Insect Mol Biol. 2004;13:305–315. doi: 10.1111/j.0962-1075.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- 18.Mohan S, Baylink DJ. IGF-binding proteins are multifunctional and act via IGF-dependent and -independent mechanisms. J Endocrinol. 2002;175:19–31. doi: 10.1677/joe.0.1750019. [DOI] [PubMed] [Google Scholar]
- 19.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18:455–471. doi: 10.1016/j.ghir.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizushima Y, Kato H, Ohmae H, Tanaka T, Bobogare A, Ishii A. Prevalence of malaria and its relationship to anemia, blood glucose levels, and serum somatomedin c (IGF-1) levels in the Solomon Islands. Acta Trop. 1994;58:207–220. doi: 10.1016/0001-706x(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 21.Umbers AJ, Boeuf P, Clapham C, Stanisic DI, Baiwog F, Mueller I, Siba P, King CL, Beeson JG, Glazier J, et al. Placental malaria-associated inflammation disturbs the insulin-like growth factor axis of fetal growth regulation. J Infect Dis. 2011;203:561–569. doi: 10.1093/infdis/jiq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darby SM, Miller ML, Allen RO, LeBeau M. A mass spectrometric method for quantitation of intact insulin in blood samples. J Anal Toxicol. 2001;25:8–14. doi: 10.1093/jat/25.1.8. [DOI] [PubMed] [Google Scholar]
- 23.White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, Turner RC. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309:61–66. doi: 10.1056/NEJM198307143090201. [DOI] [PubMed] [Google Scholar]
- 24.McConville MJ, Ferguson MA. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294(Pt 2):305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schofield L, Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993;177:145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elased KM, Gumaa KA, de Souza JB, Rahmoune H, Playfair JH, Rademacher TW. Reversal of type 2 diabetes in mice by products of malaria parasites. II. Role of inositol phosphoglycans (IPGs) Mol Genet Metab. 2001;73:248–258. doi: 10.1006/mgme.2001.3186. [DOI] [PubMed] [Google Scholar]
- 27.Elased KM, Gumaa KA, de Souza JB, Playfair JH, Rademacher TW. Improvement of glucose homeostasis in obese diabetic db/db mice given Plasmodium yoelii glycosylphosphatidylinositols. Metabolism. 2004;53:1048–1053. doi: 10.1016/j.metabol.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Elased KM, de Souza JB, Playfair JH. Reversal of type 2 diabetes in mice by products of malaria parasites: I. Effect of inactivated parasites. Metabolism. 2000;49:937–941. doi: 10.1053/meta.2000.6756. [DOI] [PubMed] [Google Scholar]
- 29.Taylor K, Carr R, Playfair JH, Saggerson ED. Malarial toxic antigens synergistically enhance insulin signalling. FEBS Lett. 1992;311:231–234. doi: 10.1016/0014-5793(92)81109-y. [DOI] [PubMed] [Google Scholar]
- 30.Martins JO, Ferracini M, Ravanelli N, Landgraf RG, Jancar S. Insulin suppresses LPS-induced iNOS and COX-2 expression and NF-kappaB activation in alveolar macrophages. Cell Physiol Biochem. 2008;22:279–286. doi: 10.1159/000149806. [DOI] [PubMed] [Google Scholar]
- 31.Cuschieri J, Bulger E, Grinsell R, Garcia I, Maier RV. Insulin regulates macrophage activation through activin A. Shock. 2008;29:285–290. doi: 10.1097/SHK.0b013e318123e4d0. [DOI] [PubMed] [Google Scholar]
- 32.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 33.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549–554. doi: 10.1007/s00125-006-0570-3. [DOI] [PubMed] [Google Scholar]
- 34. Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis. 2010;16:1601–1604. doi: 10.3201/eid1610.100399. This work was among the first to document a clinical correlation between Type 2 diabetes and risk of malaria parasite infection in sub-Saharan Africa.
- 35.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 36.Pietri JE, Pietri EJ, Potts R, Riehle MA, Luckhart S. Plasmodium falciparum suppresses the host immune response by inducing the synthesis of insulin-like peptides (ILPs) in the mosquito Anopheles stephensi. Dev Comp Immunol. 2015 doi: 10.1016/j.dci.2015.06.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Krishnegowda G, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: the requirement of extracellular signal-regulated kinase, p38, c-Jun N-terminal kinase and NF-kappaB pathways for the expression of proinflammatory cytokines and nitric oxide. J Biol Chem. 2005;280:8617–8627. doi: 10.1074/jbc.M413539200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnegowda G, Hajjar AM, Zhu J, Douglass EJ, Uematsu S, Akira S, Woods AS, Gowda DC. Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem. 2005;280:8606–8616. doi: 10.1074/jbc.M413541200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferguson MA, Brimacombe JS, Cottaz S, Field RA, Guther LS, Homans SW, McConville MJ, Mehlert A, Milne KG, Ralton JE, et al. Glycosyl-phosphatidylinositol molecules of the parasite and the host. Parasitology. 1994;108(Suppl):S45–S54. doi: 10.1017/s0031182000075715. [DOI] [PubMed] [Google Scholar]
- 40.Gowda DC. Structure and activity of glycosylphosphatidylinositol anchors of Plasmodium falciparum. Microbes Infect. 2002;4:983–990. doi: 10.1016/s1286-4579(02)01619-2. [DOI] [PubMed] [Google Scholar]
- 41.Caro HN, Sheikh NA, Taverne J, Playfair JH, Rademacher TW. Structural similarities among malaria toxins insulin second messengers, and bacterial endotoxin. Infect Immun. 1996;64:3438–3441. doi: 10.1128/iai.64.8.3438-3441.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Krishnegowda G, Li G, Gowda DC. Proinflammatory responses by glycosylphosphatidylinositols (GPIs) of Plasmodium falciparum are mainly mediated through the recognition of TLR2/TLR1. Exp Parasitol. 2011;128:205–211. doi: 10.1016/j.exppara.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar S, Gowda NM, Wu X, Gowda RN, Gowda DC. CD36 modulates proinflammatory cytokine responses to Plasmodium falciparum glycosylphosphatidylinositols and merozoites by dendritic cells. Parasite Immunol. 2012;34:372–382. doi: 10.1111/j.1365-3024.2012.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrighi RB, Debierre-Grockiego F, Schwarz RT, Faye I. The immunogenic properties of protozoan glycosylphosphatidylinositols in the mosquito Anopheles gambiae. Dev Comp Immunol. 2009;33:216–223. doi: 10.1016/j.dci.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Polimeni M, Valente E, Ulliers D, Opdenakker G, Van den Steen PE, Giribaldi G, Prato M. Natural haemozoin induces expression and release of human monocyte tissue inhibitor of metalloproteinase-1. PLoS One. 2013;8:e71468. doi: 10.1371/journal.pone.0071468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cambos M, Bazinet S, Abed E, Sanchez-Dardon J, Bernard C, Moreau R, Olivier M, Scorza T. The IL-12p70/IL-10 interplay is differentially regulated by free heme and hemozoin in murine bone-marrow-derived macrophages. Int J Parasitol. 2010;40:1003–1012. doi: 10.1016/j.ijpara.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Jaramillo M, Gowda DC, Radzioch D, Olivier M. Hemozoin increases IFN-gamma-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase- and NF-kappa B-dependent pathways. J Immunol. 2003;171:4243–4253. doi: 10.4049/jimmunol.171.8.4243. [DOI] [PubMed] [Google Scholar]
- 50.Jaramillo M, Godbout M, Olivier M. Hemozoin induces macrophage chemokine expression through oxidative stress-dependent and -independent mechanisms. J Immunol. 2005;174:475–484. doi: 10.4049/jimmunol.174.1.475. [DOI] [PubMed] [Google Scholar]
- 51.Griffith JW, Sun T, McIntosh MT, Bucala R. Pure hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol. 2009;183:5208–5220. doi: 10.4049/jimmunol.0713552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polimeni M, Valente E, Aldieri E, Khadjavi A, Giribaldi G, Prato M. Haemozoin induces early cytokine-mediated lysozyme release from human monocytes through p38 MAPK- and NF-kappaB-dependent mechanisms. PLoS One. 2012;7:e39497. doi: 10.1371/journal.pone.0039497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khadjavi A, Valente E, Giribaldi G, Prato M. Involvement of p38 MAPK in haemozo-independent MMP-9 enhancement in human monocytes. Cell Biochem Funct. 2014;32:5–15. doi: 10.1002/cbf.2963. [DOI] [PubMed] [Google Scholar]
- 54. Gillrie MR, Lee K, Gowda DC, Davis SP, Monestier M, Cui L, Hien TT, Day NP, Ho M. Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am J Pathol. 2012;180:1028–1039. doi: 10.1016/j.ajpath.2011.11.037. This work implicated parasite histones in pathological changes to host immunity and barrier function that are dependent in part on activation of host p38 MAPK signaling.
- 55.Hartgers FC, Obeng BB, Voskamp A, Larbi IA, Amoah AS, Luty AJ, Boakye D, Yazdanbakhsh M. Enhanced Toll-like receptor responsiveness associated with mitogen-activated protein kinase activation in Plasmodium falciparum -infected children. Infect Immun. 2008;76:5149–5157. doi: 10.1128/IAI.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang BPN, Napoli E, Drexler AL, Glennon E, Surachetpong W, Cheung K, Aguirre A, Eigenheer R, Phinney BS, Giulivi C, Luckhart S. Anopheles stephensi p38 MAPK signaling regulates innate immunity and bioenergetics during Plasmodium falciparum infection. Parasit Vectors. 2015 doi: 10.1186/s13071-015-1016-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pakpour N, Camp L, Smithers HM, Wang B, Tu Z, Nadler SA, Luckhart S. Protein kinase C-dependent signaling controls the midgut epithelial barrier to malaria parasite infection in anopheline mosquitoes. PLoS One. 2013;8:e76535. doi: 10.1371/journal.pone.0076535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fauconnier M, Bourigault ML, Meme S, Szeremeta F, Palomo J, Danneels A, Charron S, Fick L, Jacobs M, Beloeil JC, et al. Protein kinase C-theta is required for development of experimental cerebral malaria. Am J Pathol. 2011;178:212–221. doi: 10.1016/j.ajpath.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anand SS, Babu PP. c-Jun N terminal kinases (JNK) are activated in the brain during the pathology of experimental cerebral malaria. Neurosci Lett. 2011;488:118–122. doi: 10.1016/j.neulet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Millholland MG, Mishra S, Dupont CD, Love MS, Patel B, Shilling D, Kazanietz MG, Foskett JK, Hunter CA, Sinnis P, et al. A host GPCR signaling network required for the cytolysis of infected cells facilitates release of apicomplexan parasites. Cell Host Microbe. 2013;13:15–28. doi: 10.1016/j.chom.2012.12.001. This work demonstrated that orally bioavailable PKC inhibitors could prolong host survival in an experimental cerebral malaria model.
- 61.Anand SS, Maruthi M, Babu PP. The specific, reversible JNK inhibitor SP600125 improves survivability and attenuates neuronal cell death in experimental cerebral malaria (ECM) Parasitol Res. 2013;112:1959–1966. doi: 10.1007/s00436-013-3352-0. [DOI] [PubMed] [Google Scholar]
- 62. Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, Haynes CM. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature. 2014;516:414–417. doi: 10.1038/nature13818. These studies suggest that host cells detect pathogens that target mitochondrial function by inducing an antimicrobial response that also minimizes mitochondrial damage from pathogen exposure.
- 63.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12:901–910. doi: 10.1038/embor.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cloonan SM, Choi AM. Mitochondria: commanders of innate immunity and disease? Curr Opin Immunol. 2012;24:32–40. doi: 10.1016/j.coi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 65. West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. A comprehensive review of the diverse roles of mitochondria in innate immunity.
- 66.Fossati G, Moulding DA, Spiller DG, Moots RJ, White MR, Edwards SW. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J Immunol. 2003;170:1964–1972. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 67.Dey S, Guha M, Alam A, Goyal M, Bindu S, Pal C, Maity P, Mitra K, Bandyopadhyay U. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radical Biol. Med. 2009;46:271–281. doi: 10.1016/j.freeradbiomed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 68.Kang YH, Lee SJ. The role of p38 MAPK and JNK in Arsenic trioxide-induced mitochondrial cell death in human cervical cancer cells. J Cell Physiol. 2008;217:23–33. doi: 10.1002/jcp.21470. [DOI] [PubMed] [Google Scholar]
- 69.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, et al. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 70.Kumar KA, Babu PP. Mitochondrial anomalies are associated with the induction of intrinsic cell death proteins-Bcl2, Bax, cytochrome-c and p53 in mice brain during experimental fatal murine cerebral malaria. Neurosci. Lett. 2002;329:319–323. doi: 10.1016/s0304-3940(02)00470-6. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Song W, Wu J, Zhang Q, He J, Li A, Qian J, Zhai A, Hu Y, Kao W, et al. MAVS-mediated host cell defense is inhibited by Borna disease virus. Int J Biochem Cell Biol. 2013;45:1546–1555. doi: 10.1016/j.biocel.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 72. Liehl P, Zuzarte-Luis V, Chan J, Zillinger T, Baptista F, Carapau D, Konert M, Hanson KK, Carret C, Lassnig C, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nat Med (N. Y., N. Y. U. S.) 2014;20:47–53. doi: 10.1038/nm.3424. These studies identified Plasmodium RNA as a previously unrecognized PAMP that activates cytosolic RNA sensors as well as mitochondrial antiviral-signaling protein (MAVS) for host immunity to liver-stage infection.
- 73.Chen Y, Lu H, Liu Q, Huang G, Lim CP, Zhang L, Hao A, Cao X. Function of GRIM-19, a mitochondrial respiratory chain complex I protein, in innate immunity. J Biol Chem. 2012;287:27227–27235. doi: 10.1074/jbc.M112.340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papa S, Scacco S, De Rasmo D, Signorile A, Papa F, Panelli D, Nicastro A, Scaringi R, Santeramo A, Roca E, et al. cAMP-dependent protein kinase regulates posttranslational processing and expression of complex I subunits in mammalian cells. Biochim Biophys Acta. 2010;1797:649–658. doi: 10.1016/j.bbabio.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 75.Matsuda S, Kitagishi Y, Kobayashi M. Function and characteristics of PINK1 in mitochondria. Oxid Med Cell Longev. 2013;2013:601587. doi: 10.1155/2013/601587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vrailas-Mortimer A, del Rivero T, Mukherjee S, Nag S, Gaitanidis A, Kadas D, Consoulas C, Duttaroy A, Sanyal S. A muscle-specific p38 MAPK/Mef2/MnSOD pathway regulates stress, motor function, and life span in Drosophila. Dev Cell. 2011;21:783–795. doi: 10.1016/j.devcel.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaminski M, Kiessling M, Suss D, Krammer PH, Gulow K. Novel role for mitochondria: protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol Cell Biol. 2007;27:3625–3639. doi: 10.1128/MCB.02295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gong J, Hoyos B, Acin-Perez R, Vinogradov V, Shabrova E, Zhao F, Leitges M, Fischman D, Manfredi G, Hammerling U. Two protein kinase C isoforms, delta and epsilon, regulate energy homeostasis in mitochondria by transmitting opposing signals to the pyruvate dehydrogenase complex. FASEB J. 2012;26:3537–3549. doi: 10.1096/fj.11-197376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horbinski C, Chu CT. Kinase signaling cascades in the mitochondrion: a matter of life or death. Free Radic Biol Med. 2005;38:2–11. doi: 10.1016/j.freeradbiomed.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 80.Aslami H, Pulskens WP, Kuipers MT, Bos AP, van Kuilenburg AB, Wanders RJ, Roelofsen J, Roelofs JJ, Kerindongo RP, Beurskens CJ, et al. Hydrogen sulfide donor NaHS reduces organ injury in a rat model of pneumococcal pneumosepsis, associated with improved bio-energetic status. PLoS One. 2013;8:e63497. doi: 10.1371/journal.pone.0063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 82.Deng X, Ruvolo P, Carr B, May WS., Jr Survival function of ERK1/2 as IL-3-activated, staurosporine-resistant Bcl2 kinases. Proc Natl Acad Sci U S A. 2000;97:1578–1583. doi: 10.1073/pnas.97.4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- 84.Napoli E, Hung C, Wong S, Giulivi C. Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicol Sci. 2013;132:196–210. doi: 10.1093/toxsci/kfs339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D, Angelastro J, Omanska-Klusek A, Schoenfeld R, Giulivi C. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS One. 2012;7:e42504. doi: 10.1371/journal.pone.0042504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mauro C, Leow SC, Anso E, Rocha S, Thotakura AK, Tornatore L, Moretti M, De Smaele E, Beg AA, Tergaonkar V, et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–1279. doi: 10.1038/ncb2324. This work identified a critical and novel function for NF-κB as a physiological regulator of mitochondrial oxidative phosphorylation, tethering cell activation and proliferation to energy sensing and metabolic homeostasis.
- 87.Kabula B, Tungu P, Malima R, Rowland M, Minja J, Wililo R, Ramsan M, McElroy PD, Kafuko J, Kulkarni M, et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med Vet Entomol. 2013 doi: 10.1111/mve.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.David J-P, Coissac E, Melodelima C, Poupardin R, Riaz MA, Chandor-Proust A, Reynaud S. Transcriptome response to pollutants and insecticides in the dengue vector Aedes aegypti using next-generation sequencing technology. BMC Genomics. 2010;11:216–216. doi: 10.1186/1471-2164-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang W, Liu SL, Liu YY, Qiao CL, Chen SL, Cui F. Over-transcription of genes in a parathion-resistant strain of mosquito Culex pipiens quinquefasciatus. Insect Sci. 2015;22:150–156. doi: 10.1111/1744-7917.12106. [DOI] [PubMed] [Google Scholar]
- 90.Lee SH, Kang JS, Min JS, Yoon KS, Strycharz JP, Johnson R, Mittapalli O, Margam VM, Sun W, Li H-M, et al. Decreased detoxification genes and genome size make the human body louse an efficient model to study xenobiotic metabolism. Insect Mol Biol. 2010;19:599–615. doi: 10.1111/j.1365-2583.2010.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feyereisen R. Evolution of insect P450. Biochem Soc Trans. 2006;34:1252–1255. doi: 10.1042/BST0341252. [DOI] [PubMed] [Google Scholar]
- 92.Tijet N, Helvig C, Feyereisen R. The cytochrome P450 gene superfamily in Drosophila melanogaster: annotation, intron-exon organization and phylogeny. Gene. 2001;262:189–198. doi: 10.1016/s0378-1119(00)00533-3. [DOI] [PubMed] [Google Scholar]
- 93.Goto S, Kawakatsu M, Izumi S, Urata Y, Kageyama K, Ihara Y, Koji T, Kondo T. Glutathione S-transferase pi localizes in mitochondria and protects against oxidative stress. Free Radic Biol Med. 2009;46:1392–1403. doi: 10.1016/j.freeradbiomed.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 94.Udomsinprasert R, Bogoyevitch MA, Ketterman AJ. Reciprocal regulation of glutathione S-transferase spliceforms and the Drosophila c-Jun N-terminal kinase pathway components. Biochem J. 2004;383:483–490. doi: 10.1042/BJ20040519. [DOI] [PMC free article] [PubMed] [Google Scholar]