Abstract
The Seventh International Workshop on Advances in Electrocorticography (ECoG) convened in Washington, DC, on November 13–14, 2014. Electrocorticography-based research continues to proliferate widely across basic science and clinical disciplines. The 2014 workshop highlighted advances in neurolinguistics, brain-computer interface, functional mapping, and seizure termination facilitated by advances in the recording and analysis of the ECoG signal. The following proceedings document is an attempt at summarizing the content of this past year’s successful multidisciplinary gathering.
Keywords: electrocorticography, brain-computer interface, cortico-cortical evoked potentials, brain mapping, seizure termination, gamma-frequency electroencephalography, neuroprosthetics, subdural grid, functional mapping, subdural grid, neurolinguistics, default mode network
1. Introduction
A. Ritaccio
The Seventh International Workshop on Advances in Electrocorticography (ECoG) took place November 13–14, 2014, in Washington, DC. This past year’s meeting charted advances in established and novel investigatory domains. Poeppel’s keynote address described ECoG-based experiments helping to define hierarchical linguistic structures as fundamental as words, phrases, and sentences. “Functional tractography” via ECoG-based cortico-cortical evoked potentials was summarized by its principle evangelist, Riki Matsumoto. Early observations in the chronic electrocorticography of epilepsy patients was expertly described by Morrell. Manufacturing and implementation of customized electrode sheets fitted to the exact curvature of gyral and sulcal anatomy was outlined by Hirata. Advances in applicability and physician acceptance of functional mapping in neurosurgical decision making were represented in the presentations of Ritaccio and Kamada. State-of-the-art presentations on brain-computer interface and basic ECoG neurophysiology rounded out this dense 2-day curriculum. The course directors thank the expert faculty for their participation and the editor of Epilepsy and Behavior for the privilege of a home for these Proceedings.
2. Keynote Address
2.1 Neural oscillations, speech, and linguistic structure building
D. Poeppel
Three problems in current research continue to capture much attention. The first challenge concerns how to develop a theoretically well-motivated and biologically sophisticated functional anatomy of the language processing system. This “maps problem” is a practical issue. As is true for other domains, language research needs fine-grained maps of the regions that underpin the domain; which techniques can be harnessed to build an articulated model remains difficult. Advances in imaging techniques and ECoG yield regular progress, but what constitutes the appropriate mesoscale remains debated (i.e., single cells are arguably too finegrained a map, cortical fields at the “Brodmann scale” are almost certainly too coarse). Building on a dual-stream architecture for speech processing [1], it is argued that looking at these hypothesized regions and their internal structure constitutes a promising way to study an appropriate scale. The second, related challenge concerns the “parts list,” or the set of primitives for language actually under consideration. What ontological commitments are likely to provide a plausible link to neurobiological infrastructure? This “mapping problem” constitutes a more difficult, principled challenge: what is the appropriate level of analysis and granularity that allows us to map between (or align) the biological hardware and the computational requirements of language processing [2, 3]? The first challenge, the maps problem, addresses how to break down linguistic computation in space. The second challenge, the mapping problem, addresses how to break down language function into computational primitives suitable for biology. The third challenge, the timing problem, illustrates one linking hypothesis, breaking down the temporal structure of speech and language processing [4, 5]. Human language is hierarchically structured, and mental representations of such structure are necessary for successful language processing. In speech, however, hierarchical linguistic structures, such as words, phrases, and sentences, are not clearly defined physically and must therefore be internally constructed during comprehension. How multiple levels of abstract linguistic structure are built and concurrently represented remains unclear. On the basis of magnetoencephalography (MEG) and ECoG experiments we demonstrate that, during listening to connected speech, cortical activity of different time scales is entrained concurrently to track the time course of linguistic structures at different hierarchical levels. Critically, entrainment to hierarchical linguistic structures is dissociated from the neural encoding of acoustic cues and from processing the predictability of incoming words. The results demonstrate syntax-driven, internal construction of hierarchical linguistic structure via entrainment of hierarchical cortical dynamics.
3. Clinical
3.1. Probing functional networks with cortical stimulation
R. Matsumoto
A better understanding of seizure networks as well as the mechanisms involved in human higher cortical functions requires a detailed knowledge of neuronal connectivity. In the last decade, single-pulse stimulation has been highlighted in the field of epilepsy surgery to probe functional and seizure networks as well as to evaluate epileptogenicity. This technique is “old” in a sense that the original attempt to record the direct cortical response (DCR) from the immediate adjacent cortices in animals was performed in the early 20th century by Adrian [6] and “new” in that its clinical application has expanded with multichannel intracranial recording in the last decade owing to the development of digital electroencephalography (EEG) equipment. Although limited to the invasive presurgical evaluations with chronic implantation of intracranial electrodes, single electrical pulses (duration 0.3 ms, frequency 1 Hz, alternating polarity 1–12 mA) are directly applied to the cortex, and evoked cortical potentials (cortico-cortical evoked potentials, CCEPs) are recorded from adjacent and remote cortical regions by averaging the electrocorticogram time-locked to the stimulus onset (20–30 × 2 trials). In contrast to diffusion tractography, the CCEP technique has an advantage of tracking the inter-areal connectivity physiologically, providing directional as well as temporal information. Clinically, CCEP or “functional tractography” is highly practical because it can be done (i) easily with an on-line averaging technique in a short time (less than a minute or two for each stimulus site), (ii) without the cooperation of patients, and (iii) with minimal chance of provoking seizures.
The CCEP method has been introduced in the extraoperative setting to probe the dorsal language network [7], cortical cognitive motor network [8], and lateral parietofrontal network [9] and complemented the findings obtained by the diffusion tractography (e.g., the arcuate fasciculus, superior longitudinal fasciculus, and frontal aslant tract) by providing their detailed cortical destinations. CCEP connectivity investigations have expanded in the last several years, and worldwide collaboration warrants establishment of a comprehensive CCEP connectivity standardized map as a reference for noninvasive connectivity studies. Evaluating the state/task-dependent dynamic change of the connectivity is another interesting topic in the academic field.
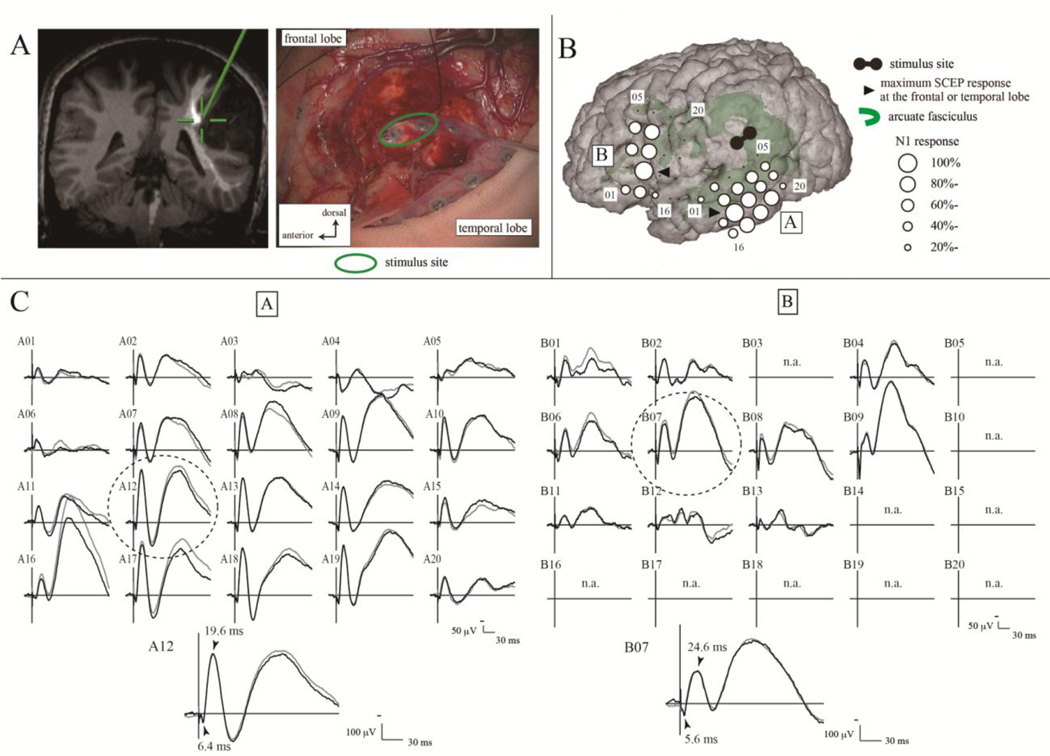
Reflecting its high practicality, intraoperative “system” mapping is one of the most significant clinical applications. CCEP has been recently applied for intraoperative language and cortical motor network mapping [10, 11]. During both general anesthesia and awake craniotomy, single-pulse stimulation has been successfully applied to Broca’s area to probe and monitor the integrity of the dorsal language pathway for brain surgery of the tumors around the arcuate fasciculus. After the language white matter bundle, i.e., the arcuate fasciculus, was defined by 50-Hz stimulation, single-pulse stimulation was applied to trace its connections into the language-related cortices (Fig. 1). Judging from the latencies and distribution of CCEP and subcortico-cortical evoked potential (SCEP) in the anterior and posterior language areas, the eloquent subcortical site was shown to connect directly with the two cortical language areas. Combined 50-Hz and 1-Hz white matter stimulation under awake craniotomy would be a promising method to probe the function and cortical targets of the large white matter bundles involved in higher brain functions such as language for each individual patient.
Fig. 1.
Subcortico-cortical evoked potentials (SCEPs) to probe the target cortices of the arcuate fasciculus. (A) Site of white matter stimulation. A pair of electrodes (green circle) was stimulated at the floor of the tumor removal cavity (right). The site (green cross) was attached to the arcuate fasciculus passing (white line) in the co-registered image on the neuronavigation system (left). (B) Cortical responses evoked by the single-pulse electrical stimulation to the subcortical area in the floor of removal cavity (SCEP) were identified in both the frontal (B plate) and temporal (A plate) areas. (C) Maximum SCEP response was recorded on the A12 electrode in the temporal lobe and the B07 electrode in the frontal lobe (dotted circle). Their N1 onset latencies were 6.4 and 5.6 ms, respectively. Their summation (12.0 ms) was very close to the onset latency of CCEP (12.8 ms) from the anterior to posterior language area in this case. Reprinted with permission from Yamao et al. [11].
3.2. Implantation of intracranial electrodes causes subacute changes in clinical seizures and in the electrocorticogram
M. Morrell
A common clinical observation in patients being evaluated for epilepsy surgery is that seizures may be less frequent immediately after implantation of intracranial electrodes [12, 13]. This “implant effect” has been variously attributed to anesthesia, the craniotomy, or the mechanical effect of electrodes. Two randomized controlled trials (RCTs) of brain stimulation for adjunctive treatment of medically refractory partial seizures reported a seizure reduction after implantation of intracranial electrodes but before stimulation was delivered. The seizure reduction after implant was of similar magnitude (initially about 25%, then waning over time) and duration (at least 3 months) in both studies despite different intracranial targets [14, 15].
One of these RCTs was conducted with a responsive neurostimulator that provides chronic ambulatory electrocorticographic (ECoG) sensing, detection and recording, as well as stimulation in response to detections of specific physician-identified ECoG patterns (RNS System, NeuroPace, Mountain View, CA). Data from 126 patients who were implanted with the RNS System and who had at least 100 scheduled ECoG samples obtained over a minimum of 1 year were analyzed to assess monthly changes in raw and normalized overall power, as well as power in specific frequency bands. Over 100,000 ECoG samples (typical length 90 seconds) were reviewed. There were changes in power over the first 3 months; 56% showed significant changes over the first 2 months after implant and another third over the second and third months. Depth leads stabilized faster (3 months) than cortical strip leads (3–5 months). The timing of these changes is consistent with the subacute impedance changes previously described in patients chronically implanted with leads used with the RNS System [16]. However, the changes observed in normalized frequency bands suggest that the ECoG power changes may also be due to neurophysiological changes. Similar variations in impedance and power have been described with intracranial leads implanted for deep brain stimulation (DBS) treatment of Parkinson’s disease [17]. The practical implications of these findings is that acute changes in impedance and power may impact experiments utilizing ECoG; i.e., ECoG data in the first weeks or months after implant may not be as reliable as chronic data. Additional longitudinal studies are needed to support reliable conclusions.
3.3. Progress in Functional Mapping
A. Ritaccio
Electrocorticographic functional mapping has evolved significantly over the past decade. It has been successfully incorporated as an adjunct to conventional electrocortical stimulation mapping (ESM) in preoperative assessments prior to epilepsy and lesional surgeries, demonstrated in awake operative conditions, and has elucidated spatiotemporal relationships in functional networks.
The ability to detect task-related power modulations in high gamma frequencies (~60–200 Hz) in real time has proved extremely fruitful in the evolution of a passive real-time ECoG mapping alternative to ESM. We have been evaluating a novel detection algorithm called SIGFRIED (SIGnal modeling For Real-time Identification and Event Detection) for approximately a decade [18]. Within seconds and often with single trial accuracy, this novel method identifies cortical locations whose activity changes in response to the task, on a two- or three-dimensional topographical display that is updated in real time as the patient performs different tasks. In our multicenter study, we found that the SIGFRIED procedure identifies motor sites in at least the same contacts or their immediate neighbors compared with electrocortical stimulation (ECS) mapping [19]. Qian et al. [20] recently reported 100% sensitivity and 99.5% specificity for general linear model-based ECoG mapping of motor cortex using the same next-neighbor approach. Somatosensory ECoG mapping in the study of Genetti et al. [21] documented similarly high sensitivity (96%). That study identified several cases that were ESM negative but had gamma functional localization in the post-central gyrus, indicating the dilemma in labeling ECoG positive but ESM negative domains as true false positives. Whereas comparisons are inevitable, they are flawed by distinct differences in physiology and methodology of the two systems. Complimentary ECoG language mapping in Genetti’s series identified multiple cases that were ESM negative, but functional gamma localized to “conventional” language anatomy. In one such ESM negative case, resection of ECoG eloquent sites resulted in severe language deficits. Congruency has also been made with ECS and ECoG in the language domain in the operating room environment, during awake craniotomy [22].
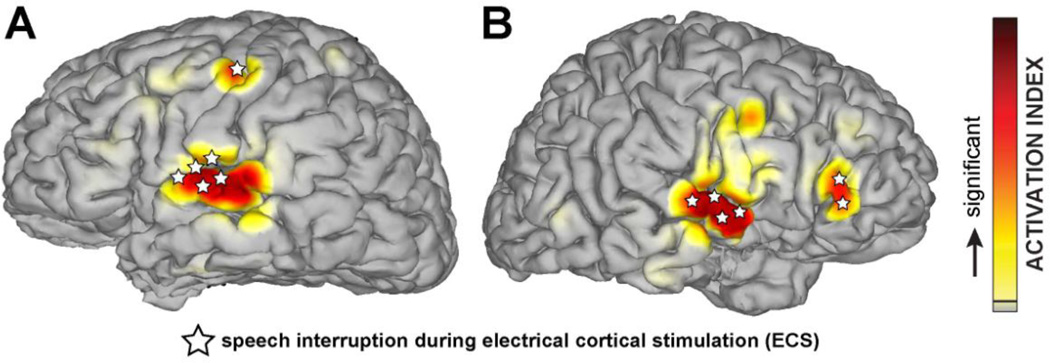
Electrocorticographic mapping of receptive language has clarified that the earliest stage of cortical speech processing involves some form of spectrotemporal analysis, carried out in auditory cortices bilaterally in the superior temporal gyrus (STG). Recording of high-gamma activity (HGA) in human auditory cortex closely tracks the envelope of speech [23]. Stimulus-related ECoG modulations in the high gamma band are seen not only in auditory cortex but also in other perisylvian areas known to be involved in higher-order auditory processing, as well as superior premotor cortex. Detailed analyses using Granger causality have identified causal relationships of HGA between distinct locations in early auditory pathways within STG and posterior STG, between posterior STG and inferior frontal cortex, and between STG and premotor cortex [24]. Furthermore, ECoG has demonstrated clear bilaterality in the speech system, including a homologous Broca’s region in non-language-dominant frontal lobe (Fig. 2). Decoding of the spatial and temporal dynamics of articulatory and phonetic speech [25] has also permitted dramatic demonstrations of transforming raw ECoG signal into corresponding textual representations [26].
Fig. 2.
Cortical representation of receptive language in left (A) and right (B) hemispheres identified through the electrocorticographic (70–170 Hz broadband gamma) response to a passive listening task (red shading) and speech interruption during ECS (white stars). The results from both methods are congruent and identify language-related areas in central superior temporal gyrus, Broca’s area, and pre-motor cortex.
ECoG has become the first clinically relevant brain mapping tool of the 21st century. Its adjunctive use is steadily confirming and expanding its utility. We have showcased our method in several relevant conferences and seven dedicated workshops on ECoG organized by our group. Our prototype is in use in several U.S. and European centers. The technology has been licensed to a corporate partner, and the resulting product is currently being marketed (cortiQ/g.tec medical engineering).
3.4. Minimum invasive passive mapping by real-time analysis of high-gamma activity for awake craniotomy
K. Kamada
Language-related structures require careful functional mapping to preserve expressive function while ensuring maximal lesion resection. However, although several epicenters are known to be involved, the complexity of the human language system has eluded conventional mapping methodologies currently in use. Electrocortical stimulation (ECS) is the gold standard for functional brain mapping during an awake craniotomy. Over recent decades, noninvasive functional magnetic resonance imaging (fMRI) has been developed, which allows blood oxygen level-dependent visualization of activated brain regions. However, it has been shown that fMRI leads to the inclusion of many subsidiary cortical areas not required for specific brain function. High-gamma activity ranging between 80 and 120 Hz on ECoG is assumed to reflect localized cortical processing. We used real-time HGA mapping and functional neuronavigation integrated with fMRI for rapid and reliable identification of motor and language functions.
Four patients with intra-axial tumors in their dominant hemisphere underwent preoperative fMRI and lesion resection with an awake craniotomy. All patients showed significant fMRI activation evoked by motor and language tasks. During the craniotomy, we recorded ECoG activity by placing subdural grids directly on the exposed brain surface.
Each patient performed motor and language tasks and demonstrated real-time HGA dynamics in hand motor areas and parts of the inferior frontal gyrus. Sensitivity and specificity of HGA mapping were 100% compared to ECS mapping in the frontal lobe, which suggested HGA mapping precisely indicated eloquent cortices. We found different HGA dynamics of language tasks in frontal and temporal regions. Specificities of the motor and language fMRI did not reach 85%. The results of HGA mapping were mostly consistent with those of ECS mapping, although fMRI tended to overestimate functional areas.
The novel technique of HGA mapping enables rapid and accurate identification of motor and frontal language areas. Furthermore, real-time HGA mapping sheds light on underlying physiological mechanisms related to human brain functions.
3.5. Electrocorticography and seizure semiology
M. Koubeissi
Detailed study of the seizure semiology is crucial for identification of the seizure onset and propagation zones. Such anatomoclinical correlations are important for clinical purposes in patients with intractable epilepsy, and also for studying functional localization of the cerebral cortex. As a significant subset of individuals with epilepsy do not respond successfully to antiseizure medications, surgical evaluation becomes a necessity, and a number of patients will require invasive monitoring with subdural or intraparenchymal electrodes before respective surgery can be planned. Semiology plays an indispensable role in planning invasive monitoring. This is important in both non-lesional patients in whom, often, implantation of intracranial electrodes is planned an attempt to identify the seizure focus [27], as well as in certain lesional cases, such as those with dual pathology [28], those in whom there is discordance among the presurgical data as regards localization of the seizure focus, or those with extensive lesions with which there may be associated neighboring epileptogenic cortex [29]. Seizure semiology is also crucial for planning post-processing of brain MRIs in subjects with seemingly negative brain imaging [30].
The clinicoanatomical relationships between seizure symptoms or electrically induced phenomena and the particular area of the brain involved must be inferred with caution. There are situations when two different epileptogenic zones may have similar ictal manifestations. For instance, gelastic seizures may result from hypothalamic hamartomas, but alternatively may arise from anterior cingulate, temporal or frontal loci. Similarly, seizures that are clinically and electrographically (with scalp electrodes) indistinguishable from temporal lobe epilepsy may originate in the posterior cingulate gyrus [31]. Conversely, the same anatomic brain region may produce seizures in different individuals or in the same individual that have different manifestations. For example, hippocampal epilepsy may produce an aura of déjà vu in some episodes and cephalic aura in others.
One common way to study clinicoanatomical correlations is to monitor for seizure freedom after resective surgery, and, if sustained and prolonged, one may correlate the resected tissue with the seizure manifestations. However, this method is limited by the duration of follow-up. Recent studies show that seizures can recur after variable postoperative durations of seizure freedom, and upon seizure recurrence, it might not be possible to conclude whether recurring seizures are semiologically novel or identical to the presurgical seizures. Another method to study anatomo-clinical correlations is by implanting intracranial electrodes and not only studying the seizure onset manifestations, but associating any seizure sign with its corresponding ictal discharge [32]. This method has the advantage of not depending on respective surgery but is limited by electrode sampling.
A general consideration in studying semiology is that involvement of the primary cortices results in elemental hallucinations. For example, involvement of Heschl’s gyrus often manifest as engine-like auditory hallucination; primary visual cortex seizures are associated with flashes of light or moving dots in the contralateral hemifield; involvement of the S1 region of the parietal lobe often results in contralateral tingling and numbness. On the other hand, involvement of association cortices results in ictal illusions, as the primary cortices perceive intact signals from the environment, but higher processing gets disrupted by the seizure discharge. Often, involvement of the association cortices is associated with alteration of awareness due to their widely distributed networks. Another general consideration in studying semiology is that seizure phenomena may result from either increased synchronization of activity or decorrelation of electrical activity between brain regions. The oral or manual automatisms, common in temporal lobe epilepsy, are often thought to be release phenomena resulting from decorrelation between brain regions, whereas such psychic experiences as déjà vécu tend to result from increased synchronization between medial and lateral temporal cortices [33].
4. Science
4.1 Looking at both sides of the coin: a few more things we can learn about the default mode network from intracranial EEG
J.-P. Lachaux
For almost 20 years now, ECoG high-frequency activity (usually measured between 50 and 150 Hz: HFA[50–150]) has been used as a proxy of population-level neural activity to map the large-scale dynamics of the human brain at work. But while the spotlight is almost always on cortical regions showing task-induced HFA increases, it should not be forgotten that HFA also decreases in other parts of the cortex when stimuli are processed. This was originally shown in the primary visual cortex, where a foveal stimulus flashed in the center of a screen induced a strong, transient HFA[50–150] decrease in the cortical representation of the peripheral visual field, possibly due to a surround suppression mechanism [34]. Soon after, a similar HFA[50–150] suppression was reported in the left ventral lateral prefrontal cortex in response to written words, as a negative image of the HFA[50–150] increase which occurred simultaneously only centimeters away, in Broca’s area for instance [35]. Interestingly, the suppression was only observed when words were read attentively, as the same stimuli failed to affect HFA[50–150] in that region in any way when seen but unattended. Those two combined observations strongly suggest an active inhibition mechanism to suppress ongoing neural processes which might interfere with the task at hand, as a way to reallocate resources to task-relevant processes. It also shows that HFA-wise, many cortical regions are active during “rest” and that HFA should not be solely considered as the marker of neural responses to novel inputs. This interpretation echoes the fMRI literature on the default mode network (DMN), and not surprisingly, several ECoG studies reported HFA decreases in the DMN when external stimuli were processed attentively, with an anatomical distribution consistent with the famous task-off network [36–39].
Yet, the exquisite time resolution of ECoG allows us to go beyond fMRI in understanding the functions of the DMN in two ways. For instance, it was shown that during a simple visual search task, the duration of HFA[50–150] decreases in the DMN does not exceed search duration: HFA[50–150] goes back to prestimulus baseline level as soon as the target is found, indicating that the DMN might subserve crucial functions, more vital to the individual than “mind-wandering” or “self-monitoring” (both classically associated with the DMN).
ECoG additionally provides the possibility to visualize HFA in the DMN online, while the patient is interacting with her environment, and to use her introspective abilities to draw correlations between her personal subjective experience (what it “feels like” to be the subject) and fast HFA fluctuations. This is particularly useful when investigating the relationship between DMN HFA and the cognitive processes most often associated with that network (mind-wandering, thinking about oneself, etc.), since those processes are spontaneous, with no obvious and immediate behavioral correlate, and are notoriously difficult to trigger with an experimental task. They are, however, immediately experienced by the subject.
Surprisingly however, our first attempts to use that approach (www.braintv.org) drew our interpretations away from endogenous cognitive processes: we found that the best way to trigger transient HFA[50–150] increase in the DMN (at least in several cardinal DMN regions such as the temporoparietal junction or the precuneus) was to use sudden, unexpected, and distracting external stimuli. HFA[50–150] increase in the right temporoparietal junction lasted, for instance, up to 10 seconds after a single phone ring (while that cortical site was strongly deactivated during a visual search task).
In summary, we believe that ECoG research on HFA is still biased toward HFA increase, whereas a human brain at work is the seat of a dynamic balance between HFA increases and decreases, with equivalent functional importance. ECoG HFA is a powerful biomarker of cognition, but it is now time to consider with equal importance both sides of the coin.
4.3. Properties of large-scale brain networks during sensorimotor processing
G. Schalk
Behavior arises from neuronal population activity that occurs across distant areas of the brain. Which physiological principles guide that population activity in its progression across the brain and thereby subserve volitional behavior is of central importance to our understanding of brain function but still largely unclear.
A principal difficulty has been the technical ability to image those aspects of brain function most important to this question across large areas of the brain and with high spatial and temporal resolution. Functional MRI can image metabolic activity across the whole brain volume but cannot readily resolve it on short time scales. It also cannot differentiate modulatory oscillatory activity from population-level cortical activity. Single-neuron recordings can accurately measure the behavior of individual neurons but cannot readily do so across large areas of the brain simultaneously. MEG, and EEG in particular, can only readily detect the simultaneous oscillatory activity produced by large brain areas. ECoG is the only technique that provides ready access to oscillatory activity and population-level cortical activity across large areas of the brain with high spatial resolution.
In a series of studies, we have begun to take advantage of these unique properties of ECoG to study information flow across large-scale brain networks during sensorimotor processing. Results to date suggest that the brain dynamically and predictively alters cortical excitability across the brain in an effort to bias cortical computation to those areas that are most important to the task at hand. Excitability is most directly indexed by the instantaneous amplitude of oscillatory activity [40]. Brain areas that are not relevant to a particular task (e.g., sensorimotor areas during an auditory task, or auditory areas during sensorimotor function) are hyperpolarized (presumably by rhythmic inhibition produced by subcortical areas), whereas task-related areas are depolarized. Thus, in a task that requires communication across large brain areas (e.g., a visuomotor task), connected areas of depolarized populations effectively form a dynamic functionalized pathway that effectively connect visual input to motor output. We recently finalized the development of a novel detection technique that allows us to identify the time of activity onset and offset in neuronal populations in single trials. This technique allows us to study the progression of population-level activity across that functionalized pathway with unprecedented precision.
In summary, our recent methodological advances allow us to begin to identify and understand the most basic and general principles of brain function, and to thereby begin to take comprehensive advantage of the advantages that the ECoG platform has to offer.
5. Engineering
5.1. ECoG of paralyzed patients
T. Yanagisawa
Electrocorticography has been successfully applied to brain-machine interfaces (BMIs), enabling severely paralyzed patients to control external devices such as prosthetic arms. Among the cortical signals available for BMI, ECoG signals are clinically feasible, having superior long-term stability and fewer technical difficulties than other invasive signals. In studies of non-paretic patients with epilepsy, some movements or movement intentions could be inferred from ECoG signals accurately enough to control external devices such as computer cursors. Moreover, recent research demonstrated that patients paralyzed due to stroke or amyotrophic lateral sclerosis could successfully control a prosthetic hand and communication devices through ECoG on the sensorimotor cortices [41]. However, it is unclear whether these findings are applicable to all paralyzed patients (whose sensorimotor cortices may have undergone extensive reorganization after paresis).
Here, we applied an ECoG-based BMI to subjects paralyzed due to stroke or trauma to evaluate how the degree of paresis affects the efficacy of controlling the BMI through ECoG signals and how the signals differed among these paralyzed subjects. We also developed a novel noninvasive BMI system that uses MEG to evaluate the altered cortical activities among the severely paralyzed subjects.
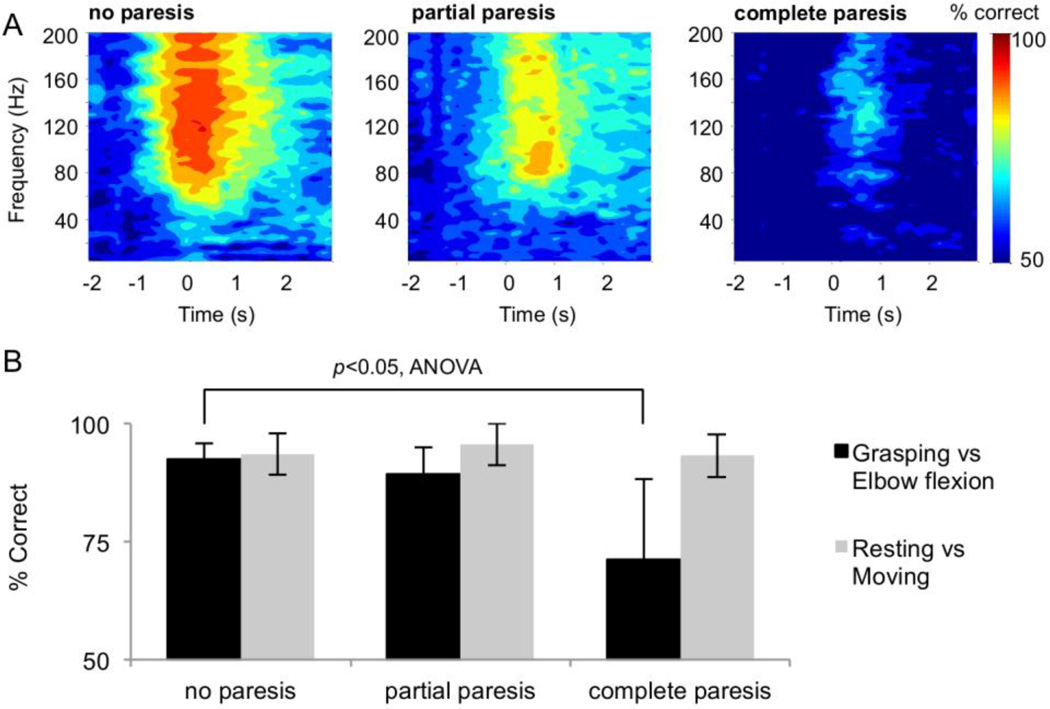
Twelve subjects (4 females, 8 males; age 13–66 years) fitted with subdural electrodes performed two types of upper limb movements while ECoG signals were recorded. The accuracy of classifying movements using the recorded signals was compared among three groups with different degrees of motor dysfunction. Fig. 3A shows the percentage of correct movement classifications averaged over each subject group. Regardless of the level of motor dysfunction, the two movement types were best inferred by using high-gamma power around the movement onset [41]. When movement classifications were performed for all subjects with a high-gamma power around the movement intention, the classification accuracy of severely paralyzed subjects was significantly inferior to that of nonparalyzed subjects (Fig. 3B). In contrast, the classification accuracies of the resting state (before movement intention) and the movement state (around the movement intention) did not differ significantly among the three groups (Fig. 3B). Thus ECoG signals recorded from subjects with chronic motor dysfunctions represented motor information via high-gamma power to a sufficient degree that the signals could be decoded accurately enough to control a prosthetic hand. However, modulation of the representation for different movements was affected by the degree of impairment.
Fig. 3.
Classification accuracy with frequency band power. (A) Classification accuracy at each frequency band power was averaged across subjects in each group and color-coded at the center of each frequency band and time domain. Time 0 corresponds to the onset cue for the movement. (B) The mean and standard error of movement classifications with a high-gamma power (80–150 Hz) among the three groups.
Finally, in severely paralyzed subjects with phantom limb sensations, MEG signals recorded during the hand movement task were used to assess the relationship between neurological symptoms and the altered motor representation. When the cortical currents on the sensorimotor cortex contralateral to the affected hand were used, the subjective movability of phantom limbs was correlated with the classification accuracy. In addition, the MEG-based BMI was successfully controlled by the paralyzed subjects.
We propose that when ECoG-based BMI is applied to severely paralyzed patients in a clinical setting, invasive BMI should be used after evaluating the applicability with the noninvasive BMI and providing some training to improve cortical activities to control the prosthetic devices.
5.2. Individualized cortical electrodes for gyral and sulcal recording
M. Hirata
Noninvasive localization of certain brain functions may be mapped on a millimeter level. However, the inter-electrode spacing of common clinical subdural electrodes still remains around 10 mm, which may not fully utilize the potential of ECoGs. We previously showed that ECoG signals within the central sulcus offer better decoding performance of movements than precentral gyral ECoG signals [42]. In addition, placement of large areas of subdural grid electrodes may compress brain tissue, which can sometimes cause neurological deficits. Here, we developed a custom-designed high-density electrode sheet for not only gyral but also sulcal implantation. This sheet fits to the contour of an individual’s cerebral cortex, resulting in reduced brain tissue compression and the attaining of higher quality ECoG signals for use in functional brain mapping and BMI technologies.
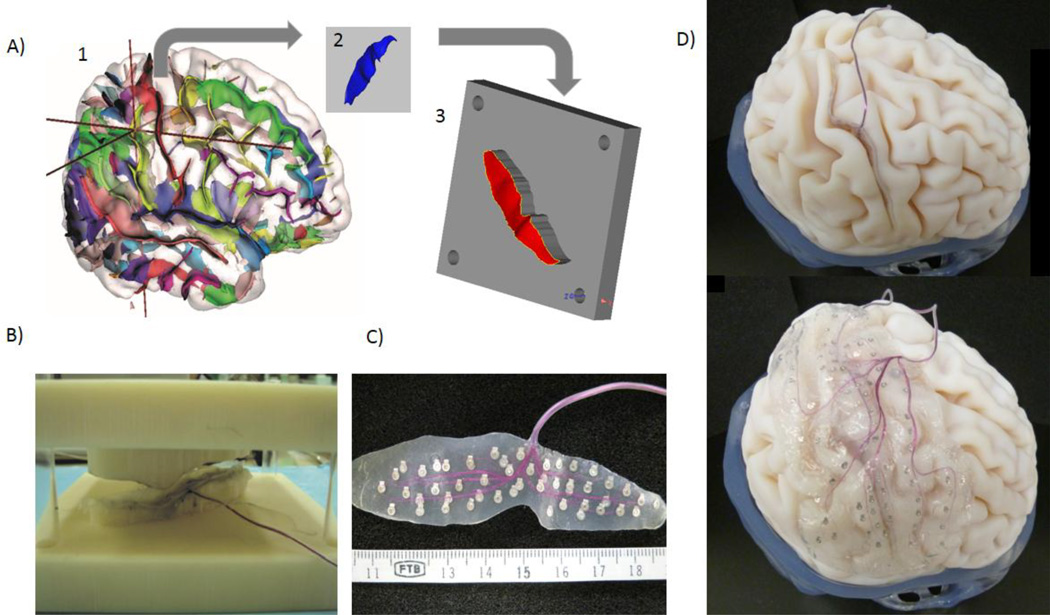
First, the three-dimensional (3D) data of the gyral and sulcal surfaces were extracted using BrainVISA (BrainVISA 4.0.2; http://brainvisa.info/) (Figs. 4A1 and 4A2). We designed individualized male and female molds to press electrode sheets using two 3D computer-aided design (CAD) software programs (Mimics v14.12, 3-matic v5.1; Materialise, Leuven, Belgium) (Fig. 4A3). The molds were then directly manufactured using a 3D printer (PolyJet 3D printer; Objet Geometries, Rehovot, Israel). Silicone sheets were pressed using the male and female molds to make the sheets contour fitting (Fig. 4B). Finally, the electrodes and stainless leads were sandwiched between the personalized sheets. We used platinum plate electrodes of 1-mm diameter. We made a gyral electrode sheet for placement over the central sulcus and a sulcal electrode sheet for insertion within the central sulcus (Fig. 4C).
Fig. 4.
(A) Sulci extraction and mold design. (B) Silicone sheet compression between male and female molds created with a 3D printer. (C) A sulcal electrode sheet for inserting within the central sulcus. (D) Both of the gyral and sulcal electrode sheets fitting with the surfaces of the individual’s brain model. Reprinted with permission from Morris et al. [48].
Both of the gyral and sulcal electrodes sheets fitted well with the curvature of the gyral and sulcal surfaces of the individual’s brain model (Fig. 4D). The minimum inter-electrode distance was 2.5 mm (a density of 16 times that of previous standard types). It took 2 weeks from the time of data extraction to obtain the final products. Cytotoxicity and biocompatibility tests were undertaken in accordance with Japanese regulations for clinical trials (GLP tests) and indicated biocompatibility with no cytotoxicity.
We placed a patient-specific high-density electrode sheet (96 channels, gyral electrode sheet) over the central sulcus in an amyotrophic lateral sclerosis patient with tetraplegia for 3 weeks in clinical BMI research. Clear ECoG signals were recorded from all of the 96-channel electrodes. The electrode sheet clearly detected different spatial distributions of gamma-band activity for different hand and elbow imaginary movements. The patient was able to control a robotic hand in real time using our BMI system [41].
To summarize, we were able to custom design, rapidly manufacture, safely implant, and confirm the efficacy of the personalized electrode sheet. This electrode sheet may contribute to the higher performance of functional brain mapping and fully implantable BMI devices [43].
5.3. ECoG-based brain-computer interfaces
C. Guger
A brain-computer interface (BCI) allows the user to control a device or software with brain activity. Mostly BCIs use motor imagery, the P300 response, or steady-state visual evoked potentials (SSVEP) to achieve control. The ECoG has the advantages of high signal-to-noise ratio and high temporal and spatial resolution. This leads to more dominant visual evoked potentials (VEP), higher signal amplitudes, and a broader spectrum that can be analyzed. A successful ECoG-based P300 BCI for spelling was already shown by Brunner et al. [44], leading to a very high information transfer rate. However, P300 BCIs do not allow continuous control. To realize continuous control, code-based VEPs can be used. In this approach, several control items are presented on a computer screen, each flickering with a certain embedded code, and the user concentrates on one of the items to move, as an example, a robotic device forward, left, or right. Code-based BCI implementations can yield high accuracies and fast response times [45]. Furthermore, the setup can be used with epileptic patients without the risk of inducing seizures, unlike SSVEP-based BCIs stimulating with a constant frequency.
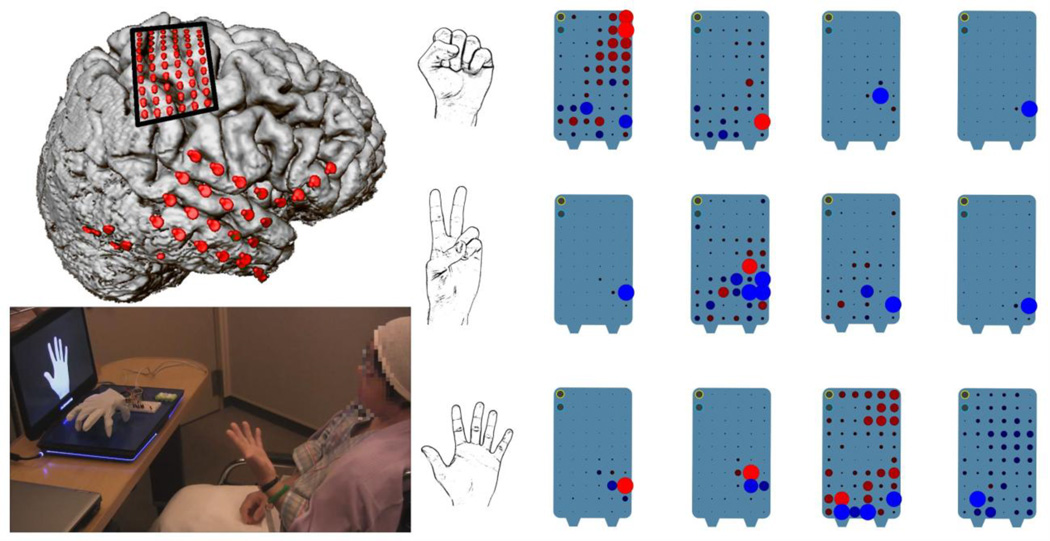
To implement motor imagery-based BCIs with ECoG, it is important to first select reactive frequency components in the alpha, beta, or gamma regions. This can be done with high-gamma mapping systems, where the user is instructed to perform a certain finger movement and the high-gamma activity of the motor cortex is analyzed simultaneously [46]. After only a few seconds, the most important ECoG electrodes for this type of movement can be recognized. Afterward, parameter estimation and classification algorithms are applied to transfer the movement imagination into robotic movements. Fig. 5 represents one subject recorded at Asahikawa Medical University in Japan with a high-density grid over the motor cortex. The user was instructed, via a computer screen, to perform certain movement types: (i) fist, (ii) straight fingers, and (iii) peace sign, 40 times each. The BCI system was then calibrated on these data by using common spatial patterns (CSP) to automatically select the most important electrodes for the discrimination task. Afterward, the user can control a robotic hand in real time by performing that movement. If the experiment is performed with movement imagination, then the BCI system is recalibrated. With the high-density grid, perfect classification accuracy can be achieved and the user reports a strong feeling of embodiment. This is especially interesting because the user does not do any extra tasks to control the robotic hand; it just feels like the user’s own hand. Further research must be done to control a full body human-like robotic device, such as the system realized by Centre National de la Recherche Scientifique [47].
Fig. 5.
Top left: cortex with 2 electrode grids (1 high density, 1 low density) with 3 strips. The high-density grid over the motor cortex is used for robotic hand control. Bottom left: computer screen giving the instruction to the user, who is doing the hand pose and robotic hand controlled via ECoG. Right: weighting of the high-density grid to maximize the difference between the 3 hand poses (fist, peace sign, fingers straight). Experiments were performed with Dr. K. Kamada from Asahikawa Medical University, Japan.
6. Perspectives/conclusion
G. Schalk
Basic and applied ECoG-related research has matured substantially over the past decade. About 10 years ago, only a select number of scientists were engaged in primary ECoG-related research. ECoG-related presence at conferences was sparse and was often met with skepticism. Since then, research output has increased dramatically and has begun to occur in progressively larger areas of cognitive and systems neuroscience. In general, ECoG has become commonly accepted as an important electrophysiological imaging technique.
This recognition of the ECoG platform is appropriate and encouraging. At the same time, it is becoming increasingly clear that the existing conceptual and technical frameworks that guide and implement ECoG-based research protocols are painfully inadequate. With the further necessary and expected improvements in these areas, the value of ECoG for basic and translational neuroscience is likely going to continue to increase substantially.
Highlights.
Basic research suggests that ECoG can elucidate brain function in ways that cannot be readily achieved using other imaging modalities.
Translational research is producing ECoG-based applications that are beginning to be used clinically.
Translational research is producing ECoG-based applications that are beginning to be marketed commercially.
There is great potential for continuing improvements in signal acquisition, signal analysis, and interpretation of ECoG signals.
ECoG is increasingly recognized for its important value for characterizing normal and abnormal brain function.
Acknowledgments
This research was partially supported by a Brain Machine Interface Development and Development of BMI Technologies for Clinical Application grant carried out under the Strategic Research Program for Brain Sciences from the Ministry of Education, Culture, Sports, Science and Technology of Japan (K.K.), by KAKENHI Grant-in-Aid for Scientific Research (26282165) funded by the Japanese Society for the Promotion of Science (K.K.), and through a Health Labour Sciences Research Grant (23100101) from the Ministry of Health, Labour and Welfare of Japan (K.K.).
This research was also partially supported by the NIH [R01-EB00856 (G.S.), R21-EB006356 (G.S.) and P41-EB018783 (G.S.)], the U.S. Army Research Office [W911NF-08-1-0216 (G.S.), W911NF-12-1-0109 (G.S.), W911NF-14-1-0440 (G.S.)], and Fondazione Neurone (G.S. and A.L.R.).
Part of this work was performed within the framework of the LABEX CORTEX (ANR-11-LABX-0042) of Université de Lyon, within the program “Investissements d’Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (J.P.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
We confirm that there are no known conflicts of interest associated with this publication and that there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 2.Embick D, Poeppel D. Towards a computational(ist) neurobiology of language: correlational, integrated, and explanatory neurolinguistics. Lang Cogn Neurosci. 2015;30:357–366. doi: 10.1080/23273798.2014.980750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poeppel D. The maps problem and the mapping problem: two challenges for a cognitive neuroscience of speech and language. Cogn Neuropsychol. 2012;29:34–55. doi: 10.1080/02643294.2012.710600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012;15:511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo H, Poeppel D. Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron. 2007;54:1001–1010. doi: 10.1016/j.neuron.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adrian ED. The spread of activity in the cerebral cortex. J Physiol. 1936;88:127–161. doi: 10.1113/jphysiol.1936.sp003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain. 2007;130:181–197. doi: 10.1093/brain/awl257. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto R, Nair DR, Ikeda A, Fumuro T, Lapresto E, Mikuni N, Bingaman W, Miyamoto S, Fukuyama H, Takahashi R, Najm I, Shibasaki H, Luders HO. Parieto-frontal network in humans studied by cortico-cortical evoked potential. Hum Brain Mapp. 2012;33:2856–2872. doi: 10.1002/hbm.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kikuchi T, Matsumoto R, Mikuni N, Yokoyama Y, Matsumoto A, Ikeda A, Fukuyama H, Miyamoto S, Hashimoto N. Asymmetric bilateral effect of the supplementary motor area proper in the human motor system. Clin Neurophysiol. 2012;123:324–334. doi: 10.1016/j.clinph.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Yamao Y, Matsumoto R, Kunieda T, Arakawa Y, Kobayashi K, Usami K, Shibata S, Kikuchi T, Sawamoto N, Mikuni N, Ikeda A, Fukuyama H, Miyamoto S. Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Hum Brain Mapp. 2014;35:4345–4361. doi: 10.1002/hbm.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katariwala NM, Bakay RA, Pennell PB, Olson LD, Henry TR, Epstein CM. Remission of intractable partial epilepsy following implantation of intracranial electrodes. Neurology. 2001;57:1505–1507. doi: 10.1212/wnl.57.8.1505. [DOI] [PubMed] [Google Scholar]
- 13.Riley TL, Porter RJ, White BG, Penry JK. The hospital experience and seizure control. Neurology. 1981;31:912–915. doi: 10.1212/wnl.31.7.912. [DOI] [PubMed] [Google Scholar]
- 14.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, Group SS. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 15.Morrell MJ, Group RNSSiES. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 16.Sillay KA, Rutecki P, Cicora K, Worrell G, Drazkowski J, Shih JJ, Sharan AD, Morrell MJ, Williams J, Wingeier B. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 2013;6:718–726. doi: 10.1016/j.brs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, Sassi M, Scelzo E, Barbieri S, Priori A. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson’s disease in hyperacute and chronic phases. Neurosignals. 2011;19:151–162. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- 18.Ritaccio A, Brunner P, Crone NE, Gunduz A, Hirsch LJ, Kanwisher N, Litt B, Miller K, Moran D, Parvizi J, Ramsey N, Richner TJ, Tandon N, Williams J, Schalk G. Proceedings of the Fourth International Workshop on Advances in Electrocorticography. Epilepsy Behav. 2013;29:259–268. doi: 10.1016/j.yebeh.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunner P, Ritaccio AL, Lynch TM, Emrich JF, Wilson JA, Williams JC, Aarnoutse EJ, Ramsey NF, Leuthardt EC, Bischof H, Schalk G. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–286. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qian T, Zhou W, Ling Z, Gao S, Liu H, Hong B. Fast presurgical functional mapping using task-related intracranial high gamma activity. J Neurosurg. 2013;119:26–36. doi: 10.3171/2013.2.JNS12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genetti M, Tyrand R, Grouiller F, Lascano AM, Vulliemoz S, Spinelli L, Seeck M, Schaller K, Michel CM. Comparison of high gamma electrocorticography and fMRI with electrocortical stimulation for localization of somatosensory and language cortex. Clin Neurophysiol. 2015;126:121–130. doi: 10.1016/j.clinph.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010;18:123–128. doi: 10.1016/j.yebeh.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Kubanek J, Brunner P, Gunduz A, Poeppel D, Schalk G. The tracking of speech envelope in the human cortex. PLoS One. 2013;8:e53398. doi: 10.1371/journal.pone.0053398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potes C, Brunner P, Gunduz A, Knight RT, Schalk G. Spatial and temporal relationships of electrocorticographic alpha and gamma activity during auditory processing. Neuroimage. 2014;97:188–195. doi: 10.1016/j.neuroimage.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotte F, Brumberg JS, Brunner P, Gunduz A, Ritaccio AL, Guan C, Schalk G. Electrocorticographic representations of segmental features in continuous speech. Front Hum Neurosci. 2015;9:97. doi: 10.3389/fnhum.2015.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herff C, Heger D, de Pesters A, Telaar D, Brunner P, Schalk G, Schultz T. Brain-to-text: Decoding spoken phrases from phone representations in the brain. Frontiers in Neuroscience. 2015:9. doi: 10.3389/fnins.2015.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiruska P, Finnerty GT, Powell AD, Lofti N, Cmejla R, Jefferys JG. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133:1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maciunas JA, Syed TU, Cohen ML, Werz MA, Maciunas RJ, Koubeissi MZ. Triple pathology in epilepsy: coexistence of cavernous angiomas and cortical dysplasias with other lesions. Epilepsy Res. 2010;91:106–110. doi: 10.1016/j.eplepsyres.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Catenoix H, Montavont A, Isnard J, Guenot M, Chatillon CE, Streichenberger N, Ryvlin P, Mauguiere F. Mesio-temporal ictal semiology as an indicator for surgical treatment of epilepsies with large multilobar cerebral lesions. Seizure. 2013;22:378–383. doi: 10.1016/j.seizure.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Hong SJ, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83:48–55. doi: 10.1212/WNL.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koubeissi MZ, Jouny CC, Blakeley JO, Bergey GK. Analysis of dynamics and propagation of parietal cingulate seizures with secondary mesial temporal involvement. Epilepsy Behav. 2009;14:108–112. doi: 10.1016/j.yebeh.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonini F, McGonigal A, Trebuchon A, Gavaret M, Bartolomei F, Giusiano B, Chauvel P. Frontal lobe seizures: from clinical semiology to localization. Epilepsia. 2014;55:264–277. doi: 10.1111/epi.12490. [DOI] [PubMed] [Google Scholar]
- 33.Vignal JP, Maillard L, McGonigal A, Chauvel P. The dreamy state: hallucinations of autobiographic memory evoked by temporal lobe stimulations and seizures. Brain. 2007;130:88–99. doi: 10.1093/brain/awl329. [DOI] [PubMed] [Google Scholar]
- 34.Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, Kahane P, Renault B. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 35.Lachaux JP, Jung J, Mainy N, Dreher JC, Bertrand O, Baciu M, Minotti L, Hoffmann D, Kahane P. Silence is golden: transient neural deactivation in the prefrontal cortex during attentive reading. Cereb Cortex. 2008;18:443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- 36.Ossandon T, Jerbi K, Vidal JR, Bayle DJ, Henaff MA, Jung J, Minotti L, Bertrand O, Kahane P, Lachaux JP. Transient suppression of broadband gamma power in the default-mode network is correlated with task complexity and subject performance. J Neurosci. 2011;31:14521–14530. doi: 10.1523/JNEUROSCI.2483-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramot M, Fisch L, Harel M, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Fried I, Malach R. A widely distributed spectral signature of task-negative electrocorticography responses revealed during a visuomotor task in the human cortex. J Neurosci. 2012;32:10458–10469. doi: 10.1523/JNEUROSCI.0877-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jerbi K, Vidal JR, Ossandon T, Dalal SS, Jung J, Hoffmann D, Minotti L, Bertrand O, Kahane P, Lachaux JP. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Front Syst Neurosci. 2010;4:27. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller KJ, Weaver KE, Ojemann JG. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci U S A. 2009;106:12174–12177. doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schalk G. A general framework for dynamic cortical function: the function-through-biased-oscillations (FBO) hypothesis. Front Hum Neurosci. 2015;9:352. doi: 10.3389/fnhum.2015.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanagisawa T, Hirata M, Saitoh Y, Kishima H, Matsushita K, Goto T, Fukuma R, Yokoi H, Kamitani Y, Yoshimine T. Electrocorticographic control of a prosthetic arm in paralyzed patients. Ann Neurol. 2012;71:353–361. doi: 10.1002/ana.22613. [DOI] [PubMed] [Google Scholar]
- 42.Yanagisawa T, Hirata M, Saitoh Y, Kato A, Shibuya D, Kamitani Y, Yoshimine T. Neural decoding using gyral and intrasulcal electrocorticograms. Neuroimage. 2009;45:1099–1106. doi: 10.1016/j.neuroimage.2008.12.069. [DOI] [PubMed] [Google Scholar]
- 43.Hirata M, Matsushita K, Suzuki T, Yoshida T, Sato F, Morris S, Yanagisawa M, Goto T, Kawato M, Yoshimine T. A fully-implantable wireless system for human brain-machine interfaces using brain surface electrodes: W-HERBS. IEICE Trans Commun. 2011;E94-B:2448–2453. [Google Scholar]
- 44.Brunner P, Ritaccio AL, Emrich JF, Bischof H, Schalk G. Rapid Communication with a “P300” Matrix Speller Using Electrocorticographic Signals (ECoG) Front Neurosci. 2011;5:5. doi: 10.3389/fnins.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kapeller C, Kamada K, Ogawa H, Prueckl R, Scharinger J, Guger C. An electrocorticographic BCI using code-based VEP for control in video applications: a single-subject study. Front Syst Neurosci. 2014;8:139. doi: 10.3389/fnsys.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prueckl R, Kapeller C, Potes C, Korostenskaja M, Schalk G, Lee KH, Guger C. cortiQ -Clinical software for electrocorticographic real-time functional mapping of the eloquent cortex; Engineering in Medicine and Biology Society (EMBC), 2013 35th Annual International Conference of the IEEE; 2013. pp. 6365–6368. [DOI] [PubMed] [Google Scholar]
- 47.Gergondet P, Petit D, Kheddar A. Steering a robot with a brain-computer interface: Impact of video feedback on BCI performance. RO-MAN, 2012 IEEE. 2012:271–276. [Google Scholar]
- 48.Morris S, Hirata M, Sugata H, Goto T, Matsushita K, Yanagisawa T, Saitoh Y, Kishima H, Yoshimine T. Patient-specific cortical electrodes for sulcal and gyral implantation. IEEE Trans Biomed Eng. 2015;62:1034–1041. doi: 10.1109/TBME.2014.2329812. [DOI] [PubMed] [Google Scholar]