Abstract
Adipose tissue resident leukocytes are often cast solely as the effectors of obesity and its attendant pathologies; however, recent observations have demonstrated that these cells support and effect ‘healthy’ physiologic function as well as pathologic dysfunction. Importantly, these two disparate outcomes are underpinned by similarly disparate immune programs; type 2 responses instruct and promote metabolic normalcy, while type 1 responses drive tissue dysfunction. In this Review, we summarize the literature regarding type 2 immunity’s role in adipose tissue physiology and allude to its potential therapeutic implications.
Introduction
From the time of Leeuwenhoek and Hooke, scientists have noted mammalian tissues’ exacting adherence to a defined and reproducible architecture and, over the subsequent three centuries, have layered onto that architecture the twinned significances of function and, later, dysfunction. Despite this intensive study, the ubiquitous and curiously reproducible presence of leukocytes across all tissues was at first overlooked and, more recently, explained away by invoking immune surveillance and host defense. While these explanations are of undoubted validity and importance, they alone fail to explain the suspicious precision with which leukocytes seeded tissues, why this lading was so robustly maintained even in the face of physiologic or experimental perturbation, and why their representation in a given tissue so often seemed disproportionate to potential infectious threat.
Aside from minor evolutions in our understanding of immunity, these questions laid dormant, unanswered, and indeed largely unasked until the relatively recent suggestion that, in many tissues, resident leukocytes actively support, regulate, or even directly effect tissue functionality independent of their host defense role [1,2]. With this framework proposed, examples of such functionalities rapidly accumulated. For example, bone marrow resident macrophages regulate and participate in the production of red blood cells, while splenic macrophages mediate their clearance [3]. Similarly, microglia, the resident macrophages of the central nervous system, play important roles in synapse pruning and neural transmission [4]. Indeed, the number of tissues with described ‘functional’ resident leukocyte populations has grown so that it now defines the rule rather than the exception, strongly suggesting that the basic governing principles of mammalian tissue architecture comprise delegation of at least some aspects of tissue function to resident leukocytes [1,2].
While the specific roles shouldered by resident leukocytes and how those roles are executed vary from tissue to tissue, an intriguing pattern has emerged in the literature: most homeostatic functions undertaken by these cells either directly or indirectly involve type 2 immune programs [5–8]. Conversely, a shift in the timbre of the immune bias fro type 2 to type 1 is almost invariably followed by some degree of homeostatic dysregulation and dysfunction [9–13]. Indeed, the immune program and tissue homeostasis are so intimately linked that experimental or therapeutic perturbation of one may be (and oft has been) used to control the other. In this Review, we will discuss how this paradigm operates in adipose tissue, its most well studied context. Specifically, we will review the functional roles of resident leukocytes and type 2 responses in white, brown, and beige adipose tissue homeostasis and adaptation and briefly discuss the pathologic consequences of their dysfunction.
White adipose tissue
Adipose tissue may be divided developmentally and functionally into two broad categories: brown adipose tissue (BAT) is a highly catabolic tissue dedicated to thermogenesis, while white adipose tissue (WAT) is an anabolic tissue that serves as mammals’ primary long-term nutrient storage depot [14]. WAT may be further subdivided into visceral and subcutaneous depots, each of which subsume distinct but overlapping physiologic functions [15]. In all WAT, however, nutrient storage remains the primary and most well studied physiologic function. During times of nutrient abundance, such as occur immediately after a meal, WAT scavenges lipids from the blood and stores them as triglycerides in the single dominant lipid globule that gives white adipocytes their characteristic ‘signet-ring’ appearance. Without continued food intake, blood lipid concentrations wane, and nutrient flux into adipocytes slows and, eventually, reverses, as adipocytes begin to liberate stored nutrients as free fatty acids, which are delivered into circulation to provide an alternative fuel source [14]. While its regulation is complex and, to a degree, redundant, acute nutrient flux at both its sinks and sources is predominantly orchestrated by two antagonist pancreas-derived peptides—insulin, which drives the anabolic processes, and glucagon, which promotes the catabolic [16,17].
While the insulin-glucagon axis dominates WAT’s storage function, many other regulatory axes also impinge, including the sympathetic nervous system, sex hormones, and gut-, liver-, and muscle-derived endocrine peptides [18]. More recently, immune mediators, leukocytes, and other physiologic modules traditionally considered exclusive to host defense have emerged as another major axis of metabolic regulation [11,12,17]}. Initially, immunity’s involvement in metabolism was understood only in the context of type 1 responses and their ability to induce/exacerbate metabolic dysfunction such as obesity and type 2 diabetes, and indeed, the bulk of the literature continues to accrue to this paradigm. More recent observations, however, have expanded our understanding of the immune regulatory axis beyond pathologic dysfunction to comprise crucial roles in physiologic homeostasis and adaptation. In this expanded paradigm, type 2 immune programs are responsible for maintaining and tuning tissue function, while type 1 responses represent their disruption, with all attendant pathologic sequelae [10,13,17]. In the following sections, we will discuss these type 2 programs, their cellular and molecular determinants, their functional roles, and allude briefly to the pathologic consequences of their disruption. (For more thorough reviews of type 1 immunity’s role in metabolic disease, please see Odegaard et al. and Bresthoff et al.)
Macrophages
Much of the literature surrounding the role of resident leukocytes in WAT physiology centers on the macrophage and for obvious reasons: macrophages are the most populous leukocytes in all adipose tissue depots, are exceedingly sensitive to a wide range of environmental stimuli, and are notoriously effusive in their inflammatory responses there to—indeed, the macrophage is assuredly the favorite bête noire of the literature focused on type 1 responses and their metabolic consequences [19,20]. This characterization is only a half-truth, however; these cells are present and functional in WAT under all physiologic conditions and are more properly cast as highly plastic integrators and effectors across these states [17]. In the absence of metabolic dysfunction, for example, they support adipocytes’ nutrient storage function by promoting insulin sensitivity directly, through the secretion of IL-10 [21], and indirectly by restraining type 1 responses in other leukocyte populations, sequestering and processing excess iron, safely disposing of dead cells and debris, promoting vascularization and tissue matrix remodeling, et cetera [22,23]. Moreover, macrophages also promote and regulate other adipocyte functionalities such as nutrient release (id est lipolysis, see brown and beige adipose tissue sections below) and adipokine-mediated endocrine signaling [24,25]. Importantly, all of these functionalities are associated with variations of the type 2, immunoregulatory phenotype—often experimentally defined as CD206+CD301+IL-10+—any disruption of which abrogates the cells’ functional roles.
ILC2s and eosinophils
Despite initial suggestions, type 2 macrophage activation does not simply represent a default phenotype enacted in the absence of input; rather, it is highly regulated and maintained by input from upstream innate immune sensors and effectors, most notably type 2 innate-like lymphoid cells (ILC2s) and eosinophils [6]. ILC2s are a recently described innate immune cell lineage related to lymphocytes and natural killer cells but possessed of a distinct ontogeny, phenotype, and function [26,27]. In their canonical host defense role, these cells reside in tissues, poised immediately beneath epithelial barriers, and occupy an important link in the early activation of both innate and adaptive immunity [6,28]. In WAT, in contrast, the role of these cells is quite distinct; they function to promote WAT homeostasis through several defined mechanisms (and likely more yet to be defined). Currently, the most well studied mechanism involves their regulation of the resident macrophage phenotype; this is accomplished both directly, through the production of IL-13, and indirectly, through the IL-5-dependent maintenance of tissue resident eosinophils, which support macrophage type 2 activation in turn by producing IL-4 [29,30]. Indeed, disruption of ILC2s, eosinophils, or their associated effectors cripples the homeostatic macrophage phenotype and renders the tissue vulnerable to metabolic derangement, while their supplementation, in contrast, is protective [30,31].
In addition to its role in regulating mature adipocyte function, the type 2 immune axis also exerts potent control over general tissue architecture. While this is effected through multiple pathways, the most important mechanism appears to be the regulation of white adipogenesis, where eosinophil- and ILC2-derived IL-4 and IL-13, respectively, drive adipocyte precursor proliferation, differentiation, and fate to control WAT architecture through both development and adulthood [32]. Indeed, these data suggest that this axis controls the balance of hyperplasia and hypertrophy that has been correlated with such dramatically dissimilar metabolic consequences in the context of excess dietary intake.
Interestingly, factors known to regulate the ILC2-eosinophil axis in barrier immunity are also present in the adipose tissue and appear to play active roles in its maintenance [6,28]. IL-33, for example, is a potent mitogen for ILC2s and, to a lesser extent, other type 2 immune cells. Importantly, endogenous IL-33 levels inversely correlate with both rodent and human obesity [33] and animals lacking IL-33 or its receptor, ST2, are more susceptible to metabolic dysfunction [34,35], while exogenous administration of IL-33 is protective [35]. Further complicating this picture is IL-33’s unique biology; it is constitutively inactive and localized to the nucleus with no known mechanism of release other than mechanical stress and cellular necrosis/necroptosis [36,37]. How exactly this cytokine then regulates WAT physiology in the absence of demonstrable cell death is unclear.
Regulatory T cells and other immune lineages
Despite their numeric and functional dominance, macrophages are far from the only effector cell types important in WAT homeostasis. Indeed, invariant natural killer, regulatory, and Th2 T cells have all been implicated in the maintenance of the type 2 immune microenvironment, as have regulatory B cells [10–12]. Interestingly, the IL-33-ILC2 signaling axis has recently been implicated in the maintenance of both regulatory and Th2 T cell subsets in models focused on host defense [38,39]. While the latter is relatively rare and of unclear significance in WAT homeostasis, regulatory T cells (Tregs) are critical to the maintenance of lean WAT’s tolerogenic type 2 microenvironment and are important sources of IL-10 in their own right [40–42]. As such, any link between the IL-33-ILC2 axis and Tregs is potentially of considerable import to WAT homeostasis.
Brown adipose tissue
While the functional significance of WAT is anchored in nutrient storage, brown adipose tissue’s raison d’ être is thermogenesis. To this end, brown adipocytes comprise relatively little stored lipid and instead pack their cytoplasm with mitochondria laden with uncoupling proteins, which allows these cells to generate heat by dissipating the mitochondrial proton gradients produced by oxidative metabolism [14,43]. In contrast to WAT, BAT is relatively insensitive to nutrient status and endocrine cues and is hardwired by sympathetic nerves for rapid and facile regulation by the central nervous system. As such, the immune regulatory axis has not been shown to significantly influence brown adipocytes directly; however, type 2 immune responses are nonetheless crucial for BAT thermogenesis. Brown adipocytes are highly catabolic and require far more free fatty acids than they contain to fuel their function; these excess fatty acids are supplied by local WAT depots in response to sympathetic stimulation. In contrast to BAT, however, the surrounding WAT is poorly innervated and, thus, unable to respond sufficiently to neural input alone to meet the fatty acid demand [44–46]. To bridge this gap, resident type 2 macrophages respond to sympathetic stimulation by synthesizing and releasing additional catecholamines, thus amplifying and propagating the signal to surrounding white adipocytes, which, in turn, activate lipolysis and release the requisite free fatty acids [25]. Congruently, animals lacking type 2 macrophages or the ability to synthesize catecholamines in macrophages are thus unable to support an effective thermogenic response to a severe cold challenge [25, 47].
Beige adipose tissue
While BAT is mammals’ primary adaptation to acute cold exposure, long-term cold exposure activates other programs to recruit additional heat generating capacity to defend core body temperature. Principal among these is the development of true thermogenic capacity within subcutaneous WAT depots, a process defined by the appearance of clusters of histologically and functionally brown-like adipocytes—variably known as ‘beige’ or ‘brite’ (‘brown-in-white’) adipocytes—within the surrounding tissue [48,49]. While a great diversity of factors may influence this process, the dominant physiologic stimulus for both the evolution of beige fat and the activation of its thermogenic function is sympathetic stimulation. Unsurprisingly, type 2 macrophages play a similar role in the activation of WAT thermogenesis as described above in BAT; however, here, type 2 immune responses also participate in beige adipogenesis itself in at least two additional, distinct ways. First, production of IL-4 and IL-13 by eosinophils and ILC2s, respectively, drives adipocyte precursor proliferation, expanding the precursor pool and making cells available for differentiation [32], as described in the WAT section above; however, it also primes these cells for beige differentiation in response to sympathetic stimulation, an effect apparent only in the context of cold-induced sympathetic stimulation [32,47]. Second, IL-33 activated ILC2s produce methionine-enkephalin peptides (MetEnk), which act directly on adipocytes to stimulate beige adipogenesis [34]. Given the similarity between these mechanisms, it is tempting to speculate that type 2 immunity thus regulates beige adipogenesis by first generating and priming adipocyte precursors for beige differentiation via IL-4/-13 stimulation, then promoting that differentiation through MetEnk signaling, and finally supporting thermogenic activation and provisioning through macrophage-derived catecholamines.
Intriguingly, exercise-induced beiging, a well described though mechanistically enigmatic phenomenon, has also been shown to act through this pathway. Here, exercise causes skeletal muscle to release meteorin-like, an endocrine signaling peptide, which induces beige adipogenesis in subcutaneous WAT depots through an eosinophil-, IL-4-, and macrophage-derived catecholamine-dependent mechanism, congruent with other reports of type 2 immunity’s involvement in this process [50].
Therapeutic significance
While this Review has focused on adipose tissue homeostasis, much of the interest in this topic is undoubtedly due to the catastrophic consequences of the current obesity epidemic—indeed, healthy WAT has largely been relegated to a role as foil for obese WAT. As a result, progress towards immune-directed therapies for metabolic dysfunction has been stunted and circumscribed largely by the search for clinically feasible anti-inflammatory agents with acceptable therapeutic indices. Unsurprisingly, the efficacy of this search has been disappointing. By studying and defining immunity’s functional roles in health and homeostasis, we hope that a more balanced—and hopefully more effective—approach to anti-obesity therapy might be achieved in which interventions promote and defend adaptive homeostasis rather than simply inhibiting maladaptive dysfunction. Indeed, the material we present above suggests two general approaches that have shown early promise—immunomodulation and thermogenesis.
In general terms, immunomodulation intends to restore the type 2 immune microenvironment in metabolic tissues by promoting type 2 responses rather than inhibiting type 1. Extant approaches to this goal are many and varied, but examples that have shown promise in preclinical studies (and in early clinical studies, in some cases) include the administration of helminths or helminth-derived products [51,52], which potently stimulate type 2 immunity; direct restoration of Treg populations, either by T cell ablation and repopulation or IL-2-mediated Treg promotion [53]; and restoration of healthy gut flora through either ablation and reconstitution or supplementation and competition [54], both of which are associated with promotion of systemic type 2 immune tone [55].
The second treatment paradigm, thermogenesis, involves a simple thermodynamic argument—thermogenic adipose tissue, be it brown or beige, robustly burns fat in cold temperatures and, to a lesser degree, at thermoneutrality [43]. This idea has gained particular traction since the relatively recent description of functional brown and beige fat in adult humans (it was previously thought to be lost early in development) and demonstration of its ability to be activated with relative ease without (importantly) resorting to strenuous exercise (an intervention that, unfortunately, is as unpopular as it is effective) [56–59]. Strategies for increasing the mass and activation status of oxidative adipose tissue are currently being hotly investigated [48,49,60].
Regardless of the outcomes of individual therapeutic investigations or even entire strategies, any approach that effectively combats obesity and its associated metabolic ills is likely to rely on multifactorial invention, including behavioral modification and possibly anti-inflammatory agents in addition to type 2-promoting strategies. Indeed, the only clear and unambiguous lesson so far is that any one of the above is destined to failure.
Figure 1. Tissue-resident leukocytes augment beige adipose tissue development and function.
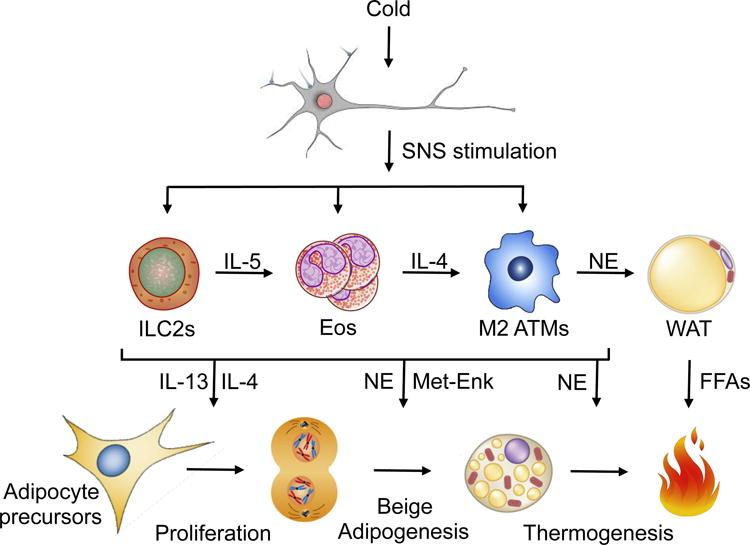
Mammals respond to sustained cold stress by increasing SNS input to subcutaneous white adipose tissue (scWAT), which results in commitment of adipocyte precursors to and their subsequent differentiation into beige adipocytes, activation of thermogenic metabolism in those beige adipocytes, and lipolysis in neighboring white adipocytes to fuel metabolic respiration. Due to scWAT’s scant sympathetic innervation, however, SNS input alone is insufficient to drive this program. Instead, SNS stimulation requires augmentation by tissue resident leukocytes in 4 primary ways: 1) ILC2-derived IL-13 and eosinophil-derived IL-4 directly stimulate adipocyte precursor proliferation, 2) ILC2-derived Met-Enk and adipose tissue macrophage (ATM)-derived catecholamines promote beige differentiation, and finally, ATM-derived catecholamines activate 3) beige adipocyte thermogenesis, and 4) white adipocyte lipolysis. Abbreviations not used elsewhere: Eos, eosinophils; NE, catecholamines; FFAs, free fatty acids; Met-Enk, met-enkephalin.
Box 1. Types of immunity.
Immune functions may be broadly divided into two categories: Type 1 responses are canonically acute, highly inflammatory programs intended to destroy their targets and often associated with bystander tissue damage. The type 1 response archetype is an acute bacterial infection, which triggers bactericidal neutrophils and macrophages (M1) and, if persistent or recurrent, lymphocytes including CD4+ TH1 and CD8+ T cells. Type 2 responses, in contrast, are generally more long-lived programs intended to deal with persistent or ineradicable threats—the archetype here being large parasites—and focus on barrier defenses (e.g. mucus barriers, intestinal motility) and the restoration of tissue integrity (e.g. wound healing, fibrosis). These physiologic contexts are generally dominated by type 2 macrophages (M2), eosinophils, and regulatory lymphocytes (e.g. Tregs).
Highlights.
Type 2 immunity plays a crucial role in white, brown, and beige adipose tissue.
Type 2 immunity acts through ILC2s, eosinophils, Tregs, and macrophages.
Type 2 immunity supports white adipose tissue’s nutrient storage function
Type 2 immunity provisions nutrients for brown adipose tissue thermogenesis.
Type 2 immunity drives precursor proliferation and beiging in white adipose tissue
Acknowledgments
The authors’ work was supported by grants from NIH (HL076746, DK094641, DK101064), American Heart Association Innovative Science Award (14ISA20850001), and an NIH Director’s Pioneer Award (DP1AR064158) to A.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing financial interests.
References
- 1.Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 3.de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R. Of macrophages and red blood cells; a complex love story. Front Physiol. 2014;5:9. doi: 10.3389/fphys.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilimoria PM, Stevens B. Microglia function during brain development: New insights from animal models. Brain Res. 2014 doi: 10.1016/j.brainres.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 5•.Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13:607–614. doi: 10.1038/nri3476. These reviews suggest that allergic inflammation and type 2 immunity might have coevolved to provide a defense against environmental challenges and for the maintenance of tissue repair and tolerance (see also referecnes 6, 7, and 8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng LE, Locksley RM. Allergic inflammation–innately homeostatic. Cold Spring Harb Perspect Biol. 2015;7:a016352. doi: 10.1101/cshperspect.a016352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 10•.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. Reviews the literature regarding how immunity influences metabolic homeostasis in the context of obesity, diabetes, and other pathology not explictly discussed here (see also references 11, 12 and 13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- 12.Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brestoff JR, Artis D. Immune Regulation of Metabolic Homeostasis in Health and Disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38:644–654. doi: 10.1016/j.immuni.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz MW, Seeley RJ, Tschop MH, Woods SC, Morton GJ, Myers MG, D’Alessio D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 27.Diefenbach A, Colonna M, Koyasu S. Development, differentiation, and diversity of innate lymphoid cells. Immunity. 2014;41:354–365. doi: 10.1016/j.immuni.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie AN, Spits H, Eberl G. Innate Lymphoid Cells in Inflammation and Immunity. Immunity. 2014;41:366–374. doi: 10.1016/j.immuni.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 29.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. Cold or exogenous IL-33 stimulate ILC2 and eosinophil production of IL-13 and IL-4, respectively, which drive the proliferation and commitment of adipocyte precursors to the beige fat lineage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan A, Al-Ghimlas F, Warsame S, Al-Hubail A, Ahmad R, Bennakhi A, Al-Arouj M, Behbehani K, Dehbi M, Dermime S. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014;15:19. doi: 10.1186/1471-2172-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34••.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2014 doi: 10.1038/nature14115. Exogenous IL-33 causes ILC2s to produce Met-Enk, which directly drives beige adipogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB, Liew FY. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res. 2010;107:650–658. doi: 10.1161/CIRCRESAHA.110.218867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31C:31–37. doi: 10.1016/j.coi.2014.09.004. Reviews the literature regarding IL-33 and its basic immune role. [DOI] [PubMed] [Google Scholar]
- 37.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–329. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 38•.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. IL-33 is required for regulatory T cell function in the intestine, establishing a paradigm for adipose tissue (see reference 39). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. IL-33 is required for the development and maintenance of adipose tissue-resident regulatory T cells. [DOI] [PubMed] [Google Scholar]
- 40.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 44.Daniel H, Derry DM. Criteria for differentiation of brown and white fat in the rat. Can J Physiol Pharmacol. 1969;47:941–945. doi: 10.1139/y69-154. [DOI] [PubMed] [Google Scholar]
- 45.Slavin BG, Ballard KW. Morphological studies on the adrenergic innervation of white adipose tissue. Anat Rec. 1978;191:377–389. doi: 10.1002/ar.1091910310. [DOI] [PubMed] [Google Scholar]
- 46.Trayhurn P, Ashwell M. Control of white and brown adipose tissues by the autonomic nervous system. Proc Nutr Soc. 1987;46:135–142. doi: 10.1079/pns19870017. [DOI] [PubMed] [Google Scholar]
- 47••.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 20157:14. 1292–1308. doi: 10.1016/j.cell.2014.03.066. Cold stimulates adipose tissue macrophages to locally produce catecholamines, which drive beige adipogenesis in an eosinophil and IL-4-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. This is a good review of beige and brown fat development and function in mice and humans. [DOI] [PubMed] [Google Scholar]
- 49.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. Exercised skeletal muscle induces beige adipogenesis via production of meteorin-like in an eosinophil- and IL-4Rα-dependent manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhargava P, Li C, Stanya KJ, Jacobi D, Dai L, Liu S, Gangl MR, Harn DA, Lee CH. Immunomodulatory glycan LNFPIII alleviates hepatosteatosis and insulin resistance through direct and indirect control of metabolic pathways. Nat Med. 2012;18:1665–1672. doi: 10.1038/nm.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tahapary DL, de Ruiter K, Martin I, van Lieshout L, Guigas B, Soewondo P, Djuardi Y, Wiria AE, Mayboroda OA, Houwing-Duistermaat JJ, et al. Helminth infections and type 2 diabetes: a cluster-randomized placebo controlled SUGARSPIN trial in Nangapanda, Flores, Indonesia. BMC Infect Dis. 2015;15:133. doi: 10.1186/s12879-015-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 55.Nishio J, Honda K. Immunoregulation by the gut microbiota. Cell Mol Life Sci. 2012;69:3635–3650. doi: 10.1007/s00018-012-0993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 59.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 60.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]


