Abstract
Objective
Benign epilepsy with centrotemporal spikes (BECTS), the most common focal childhood epilepsy, is associated with subtle abnormalities in cognition and possible developmental alterations in brain structure when compared to healthy participants as indicated by previous cross-sectional studies. To examine the natural history of BECTS, we investigated cognition, cortical thickness, and subcortical volumes in children with new/recent onset BECTS and healthy controls (HC).
Methods
Participants were 8–15 years of age, including 24 children with new onset BECTS and 41 age- and gender-matched HC. At baseline and two years later, all participants completed a cognitive assessment and a subset (13 BECTS, 24 HC) underwent T1 volumetric MRI scans focusing on cortical thickness and subcortical volumes.
Results
Baseline cognitive abnormalities associated with BECTS (object naming, verbal learning, arithmetic computation, psychomotor speed/dexterity) persisted over two years, with the rate of cognitive development paralleling that of HC. Baseline neuroimaging revealed thinner cortex in BECTS compared to controls in frontal, temporal, and occipital regions. Longitudinally, HC showed widespread cortical thinning in both hemispheres, while BECTS participants showed sparse regions of both cortical thinning and thickening. Analyses of subcortical volumes showed larger left and right putamens persisting over two years in BECTS compared to HC.
Significance
Cognitive and structural brain abnormalities associated with BECTS are present at onset and persist (cognition) and/or evolve (brain structure) over time. Atypical maturation of cortical thickness antecedent to BECTS onset results in early-identified abnormalities that further continue to abnormally develop over time. However, cognition appears more resistant to further change over time compared to anatomical development.
Keywords: Benign epilepsy with centrotemporal spikes (BECTS), cortical thickness, pediatric development, neuropsychological assessment
INTRODUCTION
Benign epilepsy with centrotemporal spikes (BECTS) is the most common localization-related epilepsy syndrome in children1. While the name of this epilepsy syndrome suggests a benign course, there is now substantial evidence documenting that cognitive and academic problems complicate the lives of children with BECTS2–6, challenging the “benign” nature of the syndrome.
Subtle MRI cortical abnormalities in the frontal, temporal, perisylvian and parietal regions have been demonstrated in children with established BECTS7–8, although studies in new-onset BECTS have not been performed. Anomalies in the volume and shape of subcortical structures including the putamen have also been reported9. Similarly, cognitive disturbances including abnormalities in language, verbal learning, attention, and executive function have been reported in BECTS. How these neuroanatomical and cognitive abnormalities arise and progress from the time of epilepsy onset have not been investigated and is the focus of the present study. Specifically, we aimed to uncover the changes imposed by epilepsy on normal brain and cognitive development during the first two years after epilepsy diagnosis.
This study is a prospective examination of the trajectory of cognitive and brain development in a cohort of children with new-onset BECTS over the first two years after diagnosis. First, we investigate the cognitive trajectories in language, academic, verbal learning, motor, and psychomotor processing speed and determine whether cognitive impairment is static or progressive over the first two years after diagnosis. Second, we compare brain structural development in children with new-onset BECTS to age- and gender-matched typically developing children. Specifically, we investigate potential differences in the developmental trajectory of cortical thickness and putaminal volumes in children with BECTS compared to healthy controls
METHODS
Participants
Study participants include 24 children with new-/recent-onset BECTS and 41 healthy first-degree cousin controls matched for age and gender while blinded to MRIs. All participants completed comprehensive cognitive assessment (see below) while a subset (13 BECTS, 24 matched HC) also underwent T1 volumetric MRI scans. MRI scans for other participants could not be used because of excessive motion, susceptibility artifacts caused by orthodontic braces, claustrophobia, and other reasons. Children were recruited from pediatric neurology clinics at three medical centers (University of Wisconsin, Madison, WI; Marshfield Clinic, Marchsfield, WI; Dean Clinic, Madison, WI) and met the following inclusion criteria: (i) diagnosis of epilepsy within the past 12 months; (ii) no other developmental disabilities (e.g. intellectual impairment, autism); (iii) no other neurological disorder, and (iv) normal clinical MRI. A pediatric neurologist certified by the American Board of Psychiatry and Neurology diagnosed individuals with BECTS according to the ILAE international classification of epilepsy10. Specifically, all children with BECTS demonstrated: 1) the presence of tonic–clonic nocturnal seizures or simple partial seizures during waking hours, 2) centrotemporal spikes on EEG occurring independently in the right and/or left hemispheres, and 3) normal EEG background. Participants demonstrating epileptiform activity outside the rolandic area were not included in the study. We did not exclude children on the basis of psychiatric comorbidities (including ADHD) or learning disabilities. We did however exclude children with intellectual disabilities, autism, or other neurological disorders. All children with epilepsy were attending regular schools at the time of baseline evaluations and IQ was within normal levels.
The children with epilepsy were recruited from the practices of 8 child neurologists across three institutions. Each child’s epilepsy syndrome was reviewed and confirmed in a research consensus meeting by the research pediatric neurologist who reviewed all available clinical data (e.g., seizure description and phenomenology, EEG, clinical imaging, neurodevelopmental history) while blinded to all research cognitive, behavioral, and neuroimaging data.
Criteria for inclusion of control participants include no neurological disorders, no history of seizures, no history of any classic precipitating injury (e.g., febrile seizures), no previous loss of consciousness for more than 5 minutes, and no other family history of a first-degree relative with epilepsy or febrile convulsions. Further details and rationale for selection criteria for participants can be found in previous publications11. Details regarding demographic and clinical characteristics of the study participants are provided in Table 1. All patients and controls were seen at baseline and two years later and all procedures were conducted by research staff.
Table 1.
Demographic characteristics of participants
| All participants | BECTS (n=24) | HC (n=41) |
|---|---|---|
| Age at baseline (mean±SD)a,c | 10.5 ± 1.7 | 11.2 ± 1.8 |
| Sex (F/M)a,c | 11/13 | 19/22 |
| Grade level (mean±SD)a,c | 4.3 ± 1.7 | 5.1 ± 1.8 |
| Parent education (mean±SD)a,c,f | 4.2 ± 1.4 | 4.4 ± 1.5 |
| IQ (mean±SD)b,c,f | 103.3 ± 14.3 | 110.0 ± 9.7 |
| AED (yes/no)c | 15/9 | - |
| Duration of BECTS (months: mean±SD)c,d | 7.8 ± 3.7 | - |
| Seizure-free at follow-up (yes/no)c | 10/14 | - |
| Participants with imaging data | BECTS (n=13) | HC (n=24) |
| Age at baseline (mean±SD)a | 10.3 ± 1.9 | 11.3 ± 2.0 |
| Sex (F/M)a | 6/7 | 13/11 |
| Grade level (mean±SD)a | 4.2 ± 2.0 | 5.0 ± 1.9 |
| Parent education (mean±SD)a,c,g | 4.2 ± 1.3 | 4.4 ± 1.6 |
| IQ (mean±SD)a,c,g | 104.5 ± 15.1 | 107.8 ± 12.2 |
| AED (yes/no)c | 8/5 | - |
| Duration of BECTS (months: mean±SD) c,e | 6.5 ± 3.4 | - |
| Seizure free at follow-up (yes/no)c | 5/8 | - |
AED: antiepileptic drug; F: female; M: male; SD: standard deviation.
No significant differences between BECTS and Controls;
Significant differences between BECTS and Controls;
No significant differences between 24 BECTS and BECTS with imaging data;
No significant differences between 24 seizure-free and non-seizure-free BECTS;
No significant differences between 13 seizure-free and non-seizure-free BECTS;
Significant differences between 24 seizure-free and non-seizure-free BECTS;
Significant differences between 13 seizure-free and non-seizure-free BECTS.
Parent education: 1= <high school, 2=GED/HSED, 3=high school, 4=associates degree/trade school, 5=some college, 6=college graduate, 7=master’s degree, 8=master’s +
The project protocol was reviewed and approved by the institutional review board of the University of Wisconsin School of Medicine and Public Health. Families and children gave written informed consent or assent, respectively, on the day of the study.
Clinical assessments
In children with BECTS, the attending child neurologist recorded their seizure frequency and antiepileptic medication use over the course of two years from diagnosis. If changes in seizure frequency or antiepileptic medication were discovered during the research interview, this information was related to the treating neurologist and the record was adjusted. Seizure remission or seizure-free status was defined as a cessation of seizures for 12 months or more and termination of AED use. Seizure severity was measured with the Liverpool Seizure Severity Scale for Children in those participants with active seizures (at least 1 seizure in past year). The scale is based on the adult version12 and consists of 10 questions with good internal consistency (r= 0.71–0.84) and test–retest correlation (r= 0.67–0.84)13.
Neuropsychological assessment
At baseline and two-year follow-up, all participants were administered a comprehensive neuropsychological test battery that included measures of intelligence, academic achievement, language, verbal memory, motor function, attention and executive function. We previously reported14 that, at baseline, children with BECTS exhibited significantly poorer performance compared to controls on measures of immediate and delayed verbal memory (Children’s Memory Scale [CMS])15, motor function (Grooved Pegboard--dominant hand16; and Wechsler Intelligence Scale for Children-III [WISC-III] Digit Symbol—Coding)17, confrontation naming (Boston Naming Test [BNT])18 and arithmetic ability (Wide Range Achievement Test-3 [WRAT-3])19. In the current study, we focused on change in age and gender adjusted raw scores over time for each of these 6 measures using a 2 (Group: BECTS vs HC) × 2 (Time: Baseline vs Follow-up) mixed repeated measure ANCOVA design, with age and sex as covariates.
MRI acquisition
MR images were obtained on a 1.5-T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, USA). T1-weighted images were acquired using a three-dimensional (3D) spoiled gradient recall (SPGR). The imaging parameters were: TE = 5 ms, TR = 24 ms, flip angle = 40°, NEX = 1, slices = 124, slice thickness = 1.5 mm, plane = coronal, field of view (FOV) = 200 mm, matrix = 256 × 256.
MRI processing and cortical thickness mapping
MR Images were processed with the FreeSurfer image analysis suite (version 5.1). The T1-weighted MR images were used for cortical reconstruction and volumetric segmentation. The technical details of these procedures include motion correction and averaging, removal of non-brain tissue20, automated Tailarach transformation21, segmentation of the subcortical white matter and deep gray matter volumetric structures22 (e.g. hippocampus, amygdala, caudate, putamen, ventricles), intensity normalization, tessellation of the gray matter white matter boundary, automated topology correction23, and surface deformation following intensity gradients to effectively place the boundaries between brain tissue (CSF, WM and GM)24. This software calculates thickness as the closest distance from the gray matter–white matter boundary to the gray matter–CSF boundary at each vertex on the tessellated surface24. All data were processed with the longitudinal stream in FreeSurfer25. This stream is designed to be unbiased with respect to any time point by creating a within-subject template volume (base image). In this way, reliability and statistical power are significantly increased25. All data were visually inspected for quality assurance.
Cortical thickness analysis
Cross-sectional analysis of cortical thickness differences at baseline as well as longitudinal change in cortical thickness was investigated between groups. These analyses were undertaken using FreeSurfer’s statistical tool, Qdec. Data were smoothed with a 15-mm full-width half-maximum (FWHM) to improve inter-subject variability. To correct for multiple comparisons, a Qdec Monte Carlo simulation was implemented with cluster-forming threshold set to p<0.05. In order to evaluate prospective neuroanatomical changes in a more accurate way, baseline differences in cortical thickness were controlled for in the analysis. All analyses were corrected for age and gender.
Volumetric analysis
Volume of the putamen was obtained from the FreeSurfer automated segmentation processing stream was analyzed with the software package SPSS. A multivariate analysis of covariance (MANCOVA) was performed examining group differences with age, gender and intracranial volume (ICV) included as covariates. Differences were considered significant at p<0.05.
RESULTS
Cognition
Table 1 shows the clinical characteristics of the BECTS group, including use of anti-epilepsy medications, duration of epilepsy, age of onset, and seizure free status at follow-up. Ten of the 24 children with BECTS were seizure free and not taking anti-seizure medications (42%) at follow-up assessment. Seizure-free BECTS were not significantly different than non-seizure-free BECTS in age, gender, education, number of AEDs, and syndrome duration. However, seizure-free children with BECTS had higher baseline IQ scores and socioeconomic status (reflected in parental education) compared to non-seizure free children with BECTS. Cognitive test scores at both time points showed no differences as a function of remission status; thus, all BECTS participants were used in the following analyses.
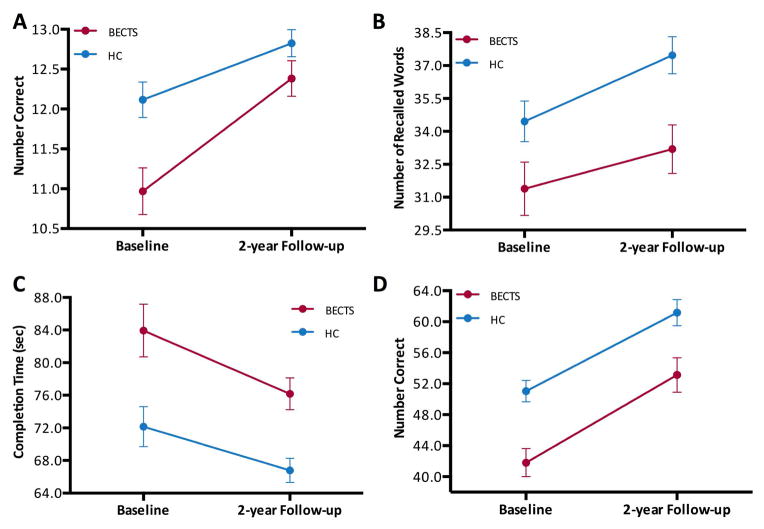
After correcting for multiple comparisons (six tests), main effects for Time (Baseline, Follow-up) was found for arithmetic (WRAT-3: F[1,61]=60.088, p<0.001) where performance was better at two-year follow-up compared to baseline. Main effects for Group (BECTS, HC) were found for immediate verbal memory (CMS: F[1,61]=8.458, p=0.005), confrontation naming (BNT: F[1,61]=8.215, p=0.006), and both motor function tasks (Grooved Pegboard: F[1,61]=14.015, p<0.001; WISC-III Coding: F[1,77]=13.379, p=0.001). In all cases, control participants performed better than children with BECTS at both baseline and follow-up (Figure 1) with prospective improvement present in both BECTS and control groups although not significant (no main effects for Time). The lack of significant effects for Time could be related to the small number of participants in the study. There was no Group x Time interaction for any of the measures.
Figure 1. Prospective change in neuropsychological assessment in BECTS and control participants.
Cognitive results for children with BECTS compared to control participants (HC) in (A) confrontation naming (BNT), (B) immediate verbal memory (CMS), and motor function: (C) Grooved Pegboard and (D) WISC-III Coding. These four tests showed main effects for Group in which, evidently, the group of BECTS were lower than HC in all of them. None of these tests showed main effects for Time. Analyses were corrected for age and gender.
To investigate whether seizure severity has an impact on cognition at the baseline evaluation, the association between seizure severity and baseline scores for each of the six neuropsychological tests was evaluated. The arithmetic test was the only variable associated with seizure severity (R=−0.458, p=0.024, uncorrected for multiple comparisons).
Baseline and prospective cortical thickness
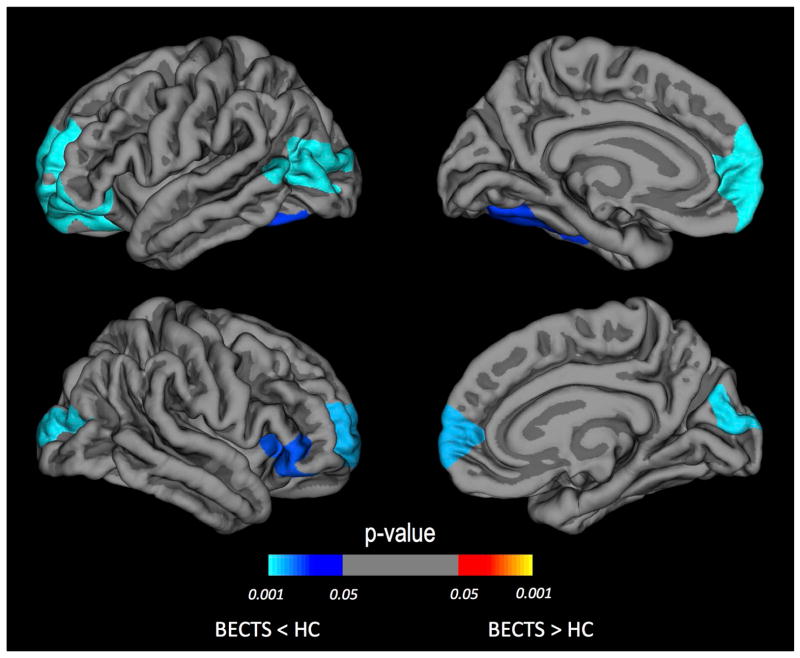
Whole brain vertex-wise analyses of cortical thickness and thickness rate of change were obtained for both groups at baseline (near the time of epilepsy diagnosis) and prospectively two years later. Compared to control participants, children with BECTS presented regions of thinner cortex at baseline (Figure 2) including bilateral rostral middle frontal gyrus (p<0.005), right inferior frontal gyrus (p<0.01), left inferior temporal gyrus (p<0.05), left lateral occipital (p<0.001), and right cuneus (p<0.005). In terms of millimeter changes the average of these differences was 0.13mm lower in BECTS. Note that these p-values are corrected for multiple comparison errors.
Figure 2. Thickness differences at baseline between BECTS and controls.
Areas of thinner cortex identified at baseline by a whole brain vertex-wise analysis. Regions of thinner cortex in BECTS compared to healthy controls (HC) are shown for the left and right hemispheres. The color of the scale bar indicates the statistical significance of the clusters corrected for multiple comparisons. Blue-cyan represents thinner cortex in the BECTS group compared to HC. Cluster-wise corrected at p<0.05. Corrected for age and gender.
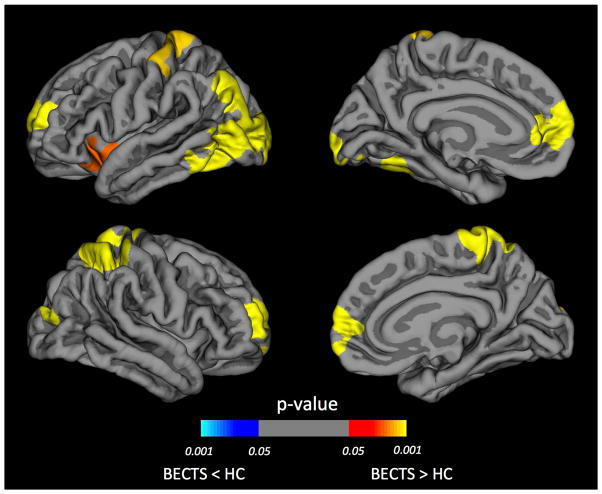
There is considerable evidence that the baseline anatomical characteristics may influence the rate of brain developmental changes26. Given that anatomical differences at baseline exist between BECTS and controls (Figure 2), subsequent analyses of prospective neurodevelopmental changes were controlled for baseline difference in cortical thickness. Figure 3 showed changes in cortical thickness over the two years after the diagnosis of BECTS and demonstrated a small region of cortical thinning in the left isthmus cingulate (p=0.020) and a region of cortical thickening in the right precentral gyrus (p=0.025) (Figure 3A), while control participants showed widespread cortical thinning in both hemispheres (p<0.001) (Figure 3B). Over the two years following epilepsy diagnosis, children with BECTS displayed less cortical thinning bilaterally, relative to controls (Figure 4). Specifically, less cortical thinning was observed in the left rostral middle frontal gyrus (p<0.001), left insula (p=0.01), bilateral occipital gyrus (p<0.001), bilateral postcentral gyrus (p<0.002), and right superior frontal gyrus (p<0.001). These p-values are cluster-corrected for multiple comparisons. To examine the possible effects of seizure frequency status at follow-up, we compared prospective cortical thickness developmental differences between children seizure free for at least one year versus individuals who continued to have seizures. Only one significant cluster was found - right pars opercularis (Talairach coordinates: 40.9, 20.3, 8.2) - in which seizure-free participants had greater thinning compared to the non-remitted subgroup (see figure 1S in supplemental document). Note that this cluster does not overlap with any of the clusters found in the prospective analyses presented above.
Figure 3. Prospective change in cortical thickness for each group.
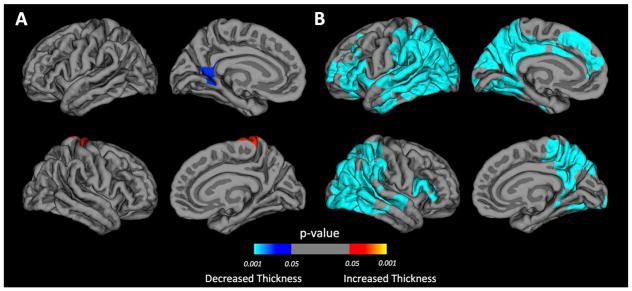
Areas of cortical thickness change identified by whole brain vertex-wise analysis in BECTS (A) and healthy controls (HC) (B). The color of the scale bar indicates the statistical significance of the clusters. Blue-cyan represents cortical thinning; red represents cortical thickening. Cluster-wise corrected for multiple comparisons at p<0.05. Corrected for baseline differences in cortical thickness, age and gender.
Figure 4. Comparison of prospective cortical thickness between BECTS and controls.
Areas of reduced cortical thinning in the BECTS group compared to controls (HC) identified by whole brain vertex-wise analysis in left and right hemispheres. The color of the scale bar indicates the statistical significance of the clusters. Cluster-wise corrected for multiple comparisons at p<0.05. Corrected for baseline differences in cortical thickness, age and gender.
Putamen volume
A main effect of Time (Baseline, Follow-up) was found for right putamen (F[1,32]=5.335, p=0.028), which represented a reduction in volume over time. Main effects of Group (BECTS, HC) were found for left putamen (F[1,32]=7.574, p=0.010) and right putamen (F[1,32]=6.279, p=0.017). In both cases, children with BECTS had increased putaminal volumes compared to HC. No significant Group x Time interactions were found for either left or right putamen. Putamen size at both time points (mm3) can be found in table S1 in supplemental document.
To determine whether differences in putamen volume could be attributed to seizure-remission status in the group of BECTS, the same analysis was performed between remitted and non-remitted BECTS, with no significant main effects found for either Time or Group; likewise, there were no significant Group x Time interactions.
DISCUSSION
This study investigated both baseline and prospective changes in cognition and brain development (cortical thickness and subcortical volumes) in children with new/recent-onset BECTS, providing a comprehensive and dynamic characterization of cognitive and brain maturation early in the course of the disease. Moreover, we demonstrate that brain development is differentially modulated by age in BECTS and controls. Previous cross-sectional neuroimaging studies have demonstrated both thinner7 and thicker8 regions of the perisylvian cortex in BECTS compared to controls. As the dynamics of brain development are better estimated from changes over time rather than at a single time point, the timing of imaging in the context of the developing brain may contribute to these discrepant findings in cross-sectional studies.
The core findings can be summarized as follows. First, children with BECTS showed cognitive abnormalities at baseline that persisted in an unchanged fashion over the two years following epilepsy diagnosis. Both the HC and BECTS groups exhibited cognitive development over the two-year period, but the BECTS group continued to perform below controls in a static fashion. Second, at the time of diagnosis, children with BECTS exhibited regions of thinner cortex in the frontal, temporal, and occipital lobes. Third, over the two years after epilepsy diagnosis, children with BECTS demonstrated restricted regions of both cortical thinning and thickening, while the control group showed the expected pattern of widespread cortical thinning. When contrasting developmental trajectory between the groups, the attenuation of age-related thinning in BECTS was evident in the frontal, parietal, temporal, occipital and insular cortices. Finally, children with BECTS demonstrated atypical baseline and prospective volumetric changes in the putamen.
Cognition
This study demonstrates how baseline cognitive deficits in children with BECTS evolve over the first two years after epilepsy diagnosis. At baseline, children with BECTS perform significantly worse than controls across tasks of expressive language, verbal learning efficiency, motor and psychomotor speed and dexterity (Figure 1). These cognitive deficits show good consistency with existing cross-sectional studies, with abnormalities spanning multiple cognitive domains27–28. Importantly, over time, both groups showed parallel age-related cognitive development (i.e., increased raw scores over time), but the children with BECTS remained significantly below controls across most of the tested domains at the two-year follow-up point. Thus, like controls, children with BECTS exhibited developmental improvement over time but retained the baseline performance deficits compared to controls, representing fixed cognitive differences.
This cognitive trajectory has been observed in different childhood epilepsies. Utilizing both broad classification of localization-related and idiopathic generalized epilepsies29 as well as syndrome-based diagnoses of juvenile myoclonic epilepsy (JME)30 and BECTS, we found that cognitive differences were evident at or near the time of diagnosis with generally stable performance over a 2-year period of follow-up. Thus cognitive abilities are adversely affected by diverse childhood epilepsies, which is surprising given their unique pathophysiologies. Further, control of seizures during the two-year interval did not influence the two-year cognitive course. Collectively, these findings question the longstanding view that cognitive complications associated with childhood onset epilepsies are largely due to chronicity of the seizures and medication effects. Indeed, our findings provide support for the persistence of an antecedent contribution to their cognitive deficits with little modifying impact from on-going disease during the first two years after diagnosis. How cognition will develop further with increasing age remains to be determined. The possibilities include static differences maintained over time going well beyond the presence of active seizures or improvement of cognition upon remission of the active phase of BECTS. Further prospective investigation will help to sort out these and other possibilities (e.g., normalization of some but not all abilities).
Brain Development
Our findings illustrate the dynamics of cortical development in BECTS, highlighting both baseline abnormalities and altered neurodevelopment over the first two years of this epilepsy syndrome. At baseline, thinner bilateral superior frontal, right inferior frontal, left inferior temporal, and bilateral occipital cortices were evident in brains of children with BECTS. Over two years, the brain developmental trajectory of BECTS diverged mostly in opposing directions from the control group. Remarkably, children with BECTS showed only a small region of age-related cortical thinning and another region of greater thickness over time (Figure 3A), indicating an abnormal trajectory of brain structural development.
In addition to cortical development, we also uncovered the persistence of abnormal subcortical development. In our previous cross-sectional study, we compared caudate, putamen, pallidum, and thalamus volumes between BECTS and controls. Among the basal ganglia structures, putamen was selectively hypertrophied in BECTS9. A recent study using FreeSurfer to examine cortical and subcortical structures in newly diagnosed BECTS also reported selective putamen hypertrophy31. Given the growing evidence for putamen anomaly in newly BECTS and in an effort to reduce the number of comparisons, we elected to focus on putamen and examine its prospective change in this study. Thus, we extend these cross-sectional findings by demonstrating that putamen volumes remain greater than controls over the first two years of the disease.
The functional implication of these brain structural changes in children with BECTS is unknown. Abnormal neurodevelopment in the dorsolateral prefrontal cortex, insular, precentral and parietal regions are highlighted in our study. We speculate that these cortical regions might be important for the development of language and executive function in children. For example, the left insula and bilateral middle/superior frontal gyrus have been implicated in language function in typically developing children and children with BECTS2, 32–35. The essential role of prefrontal cortex in attention and executive function is well established36 and executive function deficit, a common comorbidity in BECTS37–38, has been related to abnormal fronto-occipital white matter connectivity39. We note that these structural-functional associations remain tentative and future studies are needed to target this question.
Limitations of the study
There are limitations in our study. First we only assessed gray matter changes in our cohort and future studies using diffusion tensor imaging will provide a more comprehensive analysis of brain maturation in BECTS. Second, we did not perform quantitative EEG analysis in children with BECTS and the burden of EEG abnormalities may contribute to cognitive deficits in this group of children (for a review see 40). However, it is unlikely that disease severity influence our findings, as seizure remission status did not drive our results.
CONCLUSION
Children with BECTS display a complex pattern of abnormal maturational processes. Cognitive and brain alterations are present at or near onset of seizures, suggesting that dysmaturational changes occurred temporally prior to the diagnosis of epilepsy. Furthermore, cognitive development proceeds in both patients with BECTS and controls, but the differences between BECTS and control groups appear static over the first two years after diagnosis. In contrast, brain developmental changes are heterogeneous and include slowing of normal neurodevelopmental processes (thinning) and alterations of subcortical structures. Though cortical and subcortical development in children with BECTS is quite different from controls, their ultimate course remains to be determined. Also, it should be noticed that seizure-remission status did not drive our findings; therefore, differences between BECTS patients and controls are unlikely due to the seizure-related factors examined here. Whether neurodevelopmental processes normalize and “catch up” after seizure remission and termination of AEDs, or whether participants with BECTS recover from their syndrome with static and stable cognitive and neuroanatomical differences, are questions for future prospective studies.
Supplementary Material
KEY POINTS.
Both groups exhibited cognitive development over the two-year period, but BECTS continued to perform below controls in a static fashion.
Close to the time of diagnosis, children with BECTS exhibited regions of thinner cortex in the frontal, temporal, and occipital lobes.
Over the two years after epilepsy diagnosis, children with BECTS demonstrated restricted regions of both cortical thinning and thickening.
Children with BECTS demonstrated atypical baseline and prospective volumetric changes in the putamen.
Acknowledgments
All phases of this study were supported by NINDS 3RO1-44351 and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. We thank Raj Sheth MD, Monica Koehn MD, and Jason Dozier MD for study participation and recruitment of participants. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management.
Footnotes
Competing interests
Dr. Lin serves on the Editorial Board of Epilepsy and Behavior and on the speakers’ bureaus of UCB Pharma and Sunovion Pharmaceuticals. Dr. Stafstrom serves as chief editor of Epilepsy Currents and is past associate editor of Epilepsia. He receives royalties for the publication of two books: Epilepsy and the Ketogenic Diet (Humana 2004) and Epilepsy: Mechanisms, Models, and Translational Perspectives (CRC Press 2010). He also receives royalties for publication of an article, Pathophysiology of seizures and epilepsy, in the on-line resource UpToDate. Dr. Prabhakaran is funded by the Foundation of ASNR. The rest of the authors report no disclosures.
References
- 1.Kramer U. Atypical presentations of benign childhood epilepsy with centrotemporal spikes: a review. J Child Neurol. 2008;23:785–790. doi: 10.1177/0883073808316363. [DOI] [PubMed] [Google Scholar]
- 2.Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54:487–494. doi: 10.1111/epi.12067. [DOI] [PubMed] [Google Scholar]
- 3.Overvliet GM, Besseling RM, van der Kruijs SJ, et al. Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4. Eur J Paediatr Neurol. 2013;17:390–396. doi: 10.1016/j.ejpn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Overvliet GM, Aldenkamp AP, Klinkenberg S, et al. Impaired language performance as a precursor or consequence of Rolandic epilepsy? J Neurol Sci. 2011;304:71–74. doi: 10.1016/j.jns.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Danielsson J, Petermann F. Cognitive deficits in children with benign rolandic epilepsy of childhood or rolandic discharges: A study of children between 4 and 7 years of age with and without seizures compared with healthy controls. Epilepsy Behav. 2009;16:646–651. doi: 10.1016/j.yebeh.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Papavasiliou A, Mattheou D, Bazigou H, et al. Written language skills in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2005;6:50–58. doi: 10.1016/j.yebeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Overvliet GM, Besseling RM, Jansen JF, et al. Early onset of cortical thinning in children with rolandic epilepsy. Neuroimage Clin. 2013;2:434–439. doi: 10.1016/j.nicl.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardoe HR, Berg AT, Archer JS, et al. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res. 2013;105:133–139. doi: 10.1016/j.eplepsyres.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JJ, Riley JD, Hsu DA, et al. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia. 2012;53:677–685. doi: 10.1111/j.1528-1167.2012.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy 1989 Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 11.Hermann B, Jones J, Sheth R, et al. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 12.Baker GA, Smith DF, Dewey M, et al. The development of a seizure severity scale as an outcome measure in epilepsy. Epilepsy Res. 1991;8:245–251. doi: 10.1016/0920-1211(91)90071-m. [DOI] [PubMed] [Google Scholar]
- 13.Baker G, Jacoby A, Berney T, et al. Development of an instrument to assess quality of life in children with epilepsy and learning disability. European Congress of Epileptology; Oporto, Portugal Epilepsia. 1994;35:47. [Google Scholar]
- 14.Jackson DC, Dabbs K, Walker NM, et al. The neuropsychological and academic substrate of new/recent-onset epilepsies. J Pediatr. 2013;162:1047–1053. doi: 10.1016/j.jpeds.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen M. Children’s memory scale. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 16.Trites RL. Neuropsychological Test Manual. Ottawa, Ontario, Canada: Royal Ottawa Hospital; 1977. [Google Scholar]
- 17.Wechsler D. The Wechsler intelligence scale for children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- 18.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 19.Wilkinson GS. The Wide Range Achievement Test: Manual. 3. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- 20.Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- 22.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 23.Fischl B, Liu A, Dale AM. Automated Manifold Surgery: Constructing Geometrically Accurate and Topologically Correct Models of the Human Cerebral Cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 24.Fischl B, Dale A. Measuring the Thickness of the Human Cerebral Cortex from Magnetic Resonance Images. Proc Natl Acad Sci U S A. 2000;97:11044–11049. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 27.Monjauze C, Tuller L, Hommet C, et al. Language in benign childhood epilepsy with centro-temporal spikes abbreviated form: rolandic epilepsy and language. Brain Lang. 2005;92:300–308. doi: 10.1016/j.bandl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Kavros PM, Clarke T, Strug LJ, et al. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008;49:1570–1580. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- 29.Rathouz PJ, Zhao Q, Jones JE, Jackson DC, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP. Cognitive development in children with new onset epilepsy. Dev Med Child Neurol. 2014;56:635–641. doi: 10.1111/dmcn.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JJ, Dabbs K, Riley JD, et al. Neurodevelopment in new-onset juvenile myoclonic epilepsy over the first 2 years. Ann Neurol. 2014;76:660–668. doi: 10.1002/ana.24240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EH, Yum MS, Shim WH, Yoon HK, Lee YJ, Ko TS. Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS) Seizure. 2015;27:40–46. doi: 10.1016/j.seizure.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 32.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 33.Oh A, Duerden EG, Pang EW. The role of the insula in speech and language processing. Brain Lang. 2014;135C:96–103. doi: 10.1016/j.bandl.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skeide MA, Brauer J, Friederici AD. Syntax gradually segregates from semantics in the developing brain. Neuroimage. 2014;100C:106–111. doi: 10.1016/j.neuroimage.2014.05.080. [DOI] [PubMed] [Google Scholar]
- 35.Oser N, Hubacher M, Specht K, et al. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS) Epilepsy Behav. 2014;33:12–17. doi: 10.1016/j.yebeh.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Szczepanski SM, Knight RT. Insights into human behavior from lesions to the prefrontal cortex. Neuron. 2014;83:1002–1018. doi: 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK. Attention impairment in rolandic epilepsy: systematic review. Epilepsia. 2008;49:1570–1580. doi: 10.1111/j.1528-1167.2008.01610.x. [DOI] [PubMed] [Google Scholar]
- 38.Neri ML, Guimarães CA, Oliveira EP, Duran MH, Medeiros LL, Montenegro MA, Boscariol M, Guerreiro MM. Neuropsychological assessment of children with rolandic epilepsy: executive functions. Epilepsy Behav. 2012;24:403–407. doi: 10.1016/j.yebeh.2012.04.131. [DOI] [PubMed] [Google Scholar]
- 39.Kim SE, Lee JH, Chung HK, Lim SM, Lee HW. Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur J Neurol. 2014;21:708–717. doi: 10.1111/ene.12301. [DOI] [PubMed] [Google Scholar]
- 40.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380:1180–1192. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.