Abstract
The pathogenesis of food allergy remains poorly understood. Recent advances in the use of murine models have led to discoveries that mast cells and IgE receptor signaling not only drive immediate hypersensitivity reactions but also exert an immunoregulatory function, promoting the development of allergic sensitivity to foods. We review the evidence that IgE, IgE receptors, key signaling kinases and mast cells impair oral tolerance to ingested foods, preventing the induction of regulatory T cells (Treg) and promoting the acquisition of pro-allergic T helper (Th) 2 responses. We discuss innovative strategies that that could be implemented to counteract these immunoregulatory effects of IgE-mediated mast cell activation, and potentially reverse established sensitization, curing food allergy.
Introduction
Food allergy is an immunological disorder characterized by dysregulated responses and the development of immediate hypersensitivity reactions to ingested foods. Rates of occurrence have soared over the last two decades in developed nations, affecting children disproportionately. The current standard of practice is to advise allergen avoidance, and a lack of mechanistic understanding of the processes underlying the induction of tolerance vs. sensitization to food allergens frustrates our ability to develop actual treatments. In this review, we discuss recent advances in our understanding of the pathology of this disorder stemming largely from novel approaches with animal models of disease. These studies have elucidated a previously unappreciated role for IgE receptor signaling in mast cells as an amplifier of immune sensitization and a disrupter of immunological tolerance to ingested foods.
IgE, its receptors and their signaling pathways
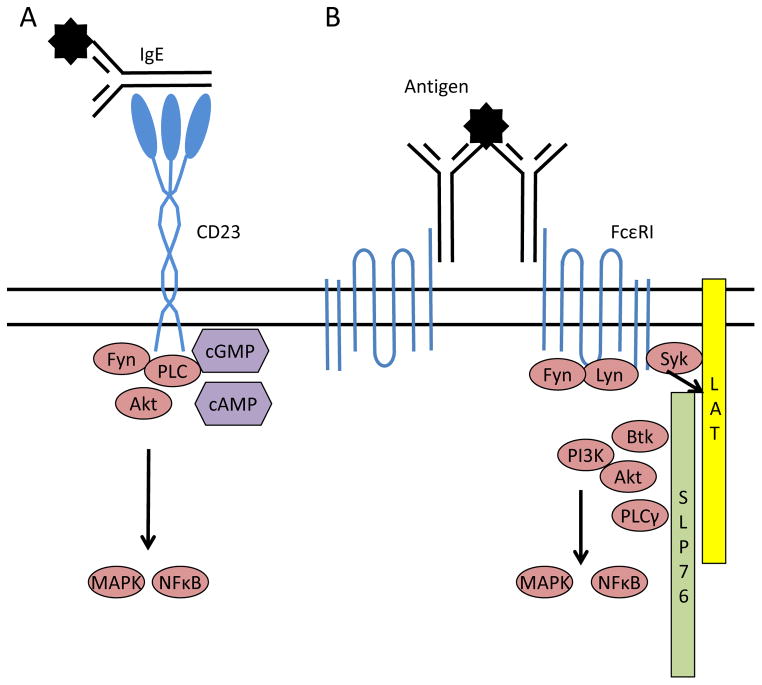
IgE binds to two main receptors, the high affinity FcεRI (Kd ~1nm) and the low affinity FcεRII or CD23 (Kd 0.1–1μm). A handful of other receptors, including galectin-3, FcγRIIb, FcγRIV, have been documented to interact with IgE, but these will not be discussed here [1]. These receptors have set expression patterns, with FcεRI primarily on mast cells, basophils and dendritic cells and CD23 on B cells, a variety of innate cell types and epithelial cells. Crosslinking of FcεRI by IgE and allergen results in initiation of signaling via the immunoreceptor tyrosine-based activation motifs (ITAMs) in the cytoplasmic tail of the receptor, triggering the recruitment and activation of several key kinases including Syk, Lyn and Fyn. Activation of Syk is essential for propagation of signaling as Syk phosphorylates LAT, which serves as a scaffold for the assembly of the signaling complex that leads to both degranulation and cytokine gene transcription (Figure 1). FcεRI signaling has recently been reviewed in depth by Sibilano and colleagues [2]*. CD23 signaling is less well elucidated, and cytoplasmic signaling is thought to be inessential for some of this molecule’s functions. It is known that binding of IgE and antigen to CD23 results in the formation of nucleotide messengers (cAMP) and nuclear translocation of the transcription factor NFκB [3]. Differences in splicing and expression patterns for CD23 may alter signaling between cell types, with B cells but not monocytes reported to activate Fyn and Akt [4].
Figure 1.
IgE receptor signaling pathways. A) CD23 ligation promotes activation of the src kinase Fyn and cytosolic nucleotide intermediates, resulting in MAP kinase cascades and nuclear translocation of NFκB.B) Crosslinking of the high affinity IgE receptor, FcεRI, by IgE and allergen causes Syk-dependent formation of the LAT/SLP76 signaling complex. This scaffold facilitates the activation of multiple kinase pathways, leading to degranulation and cytokine transcription.
Facilitated antigen binding and presentation
IgE interacting with its receptors is considered to have a significant role in maintaining a sensitized state in food allergic patients by focusing and stimulating memory T and B cell responses. Specific IgE binds to allergens with high affinity, creating IgE:allergen complexes that can then be endocytosed though cellular receptors much more efficiently than might normally occur via random sampling by phagocytes. The effect of the IgE in this setting is to target the allergen to antigen-presenting cells, facilitating binding and presentation of the allergen to adaptive immune cells. This effect can occur through both CD23 and FcεRI. Facilitated antigen presentation by IgE has been shown to enhance T cell proliferative responses in peanut-allergic subjects [5].
FcεRI: The hair-trigger response
FcεRI-bearing cells, predominantly mast cells but also basophils to a lesser extent, are responsible for the immediate hypersensitivity reactions in allergy. IgE can trigger FcεRI signaling and degranulatory release of vasoactive mediators in response to minute concentrations of antigen, creating a highly amplified system for allergen detection and response. Studies in murine models have established obligate roles for IgE, FcεRI, Syk, and mast cells in the onset of immediate hypersensitivity reactions and anaphylaxis following acute food allergen challenge in sensitized animals [6–13]6**, 7**, 10*.
In addition to eliciting immediate hypersensitivity, mast cell activation by IgE and FcεRI leads to a secondary, late-phase reaction fueled by cytokine production and leukocyte recruitment and a recurrence of symptoms 8–12 hours after initial allergen exposure. Mast cell-derived cytokines, acting on both innate and adaptive immune cell types, favor Th2 responses that incite chronic allergic inflammation, and have demonstrated roles in the development of pathology in models of allergic disease [14–16]. Most notably with respect to the polarization of the T cell response, mast cell activation by IgE drives the synthesis of the Th2-inducing cytokine interleukin (IL)-4 in quantities that are capable of increasing systemic levels of this cytokine approximately ten-fold (Oettgen and Burton, Advances in Immunology, in press). The relatively high frequency of mast cells at environmental interfaces where allergens are first encountered, including the skin, airways and gastrointestinal tract, suggests that these cells may promote the development of allergic sensitization.
IgE receptor signaling promotes sensitization
We recently set out to test the hypothesis that IgE receptor signaling in mast cells contributed to the development of allergic sensitization and pathology in food allergy. A previous study by Wang et al. had shown a contribution of mast cells, FcεRI and mast cell-derived IL-13 to the development of intestinal inflammation using an adjuvant driven-system [13]. Other studies investigating the effects of mast cells and IgE in sensitization to antigens applied to the skin or airways indicated that the mast cell effect was most evident in the absence of adjuvant [17,18]. Testing the roles of mast cells and IgE in food allergy required the use of an animal model of the disease. The prevailing murine models have relied on the use of parenteral immunization and/or adjuvant to break oral tolerance, and elicit rather weak responses to allergen challenge unless the allergen is injected systemically. Because they circumvent the normal pathways of enteral sensitization, such models are not well suited to providing accurate information regarding the mechanisms of immune sensitization to food allergens. In collaboration with Dr. Talal Chatila, we used associations between susceptibility to allergic disease and IL-4 receptor alpha chain (IL-4Rα) polymorphisms in the human population to inform the development of a new genetic atopic mouse model of food allergy based upon an IL-4Rα F709 mutation [8,19]. In this model, repeated feedings of allergen (peanut or the model allergen OVA) in the absence of adjuvant evoke Th2 responses, IgE production, mast cell expansion and IgE-dependent anaphylaxis upon enteral allergen challenge [6,8]6**. Anaphylactic shock was abrogated in mice lacking FcεRIα, mast cells (either Cre-driven deletion or Kit-deficiency-mediated absence), the FcεRI-proximal kinase Syk specifically in mast cells, or in mice treated with anti-IgE or a small molecule antagonist of Syk [6]**.
Interestingly, we found that F709 mice lacking IgE, FcεRI or mast cells not only failed to manifest allergic reactions, but developed radically different adaptive immune responses to the allergen. Unlike their wild-type counterparts, atopic F709 mice exhibited strong Th2-biased immune responses to allergen and failed to generate effective, stable regulatory T cell (Treg) that could control the allergy [7]**. In the absence of mast cells or IgE signaling, however, F709 mice regained the ability to induce Treg, resulting in reduced Th2 responses in allergen-specific CD4+ T cells and a lack of specific IgE production [6,7]**. Studies employing selective reconstitution of the mast cell compartment in mast cell-deficient mice with normal or cytokine-deficient bone marrow cultured mast cells demonstrated that mast cell-derived IL-4 was central to this process [6]**. While mast cell activation may directly promote Th2 induction, B cell switching to IgE and other aspects of the allergic response, our studies suggest that the ability to disrupt Treg induction and function may be the most critical mast cell effect (Figure 2) [7]**. Treg control of inappropriate Th2 responses to ingested foods is impaired in many food allergic patients, and reappears in individuals who outgrow their allergies [20–22]. In both F709 mice and allergic humans, allergen-specific Treg exhibit reprogramming into Th2-like effector cells, and actively contribute to the worsening of allergic pathology in the mice. In the absence of FcεRI, F709 mice developed stable, effective Treg that suppressed Th2 responses [7]**.
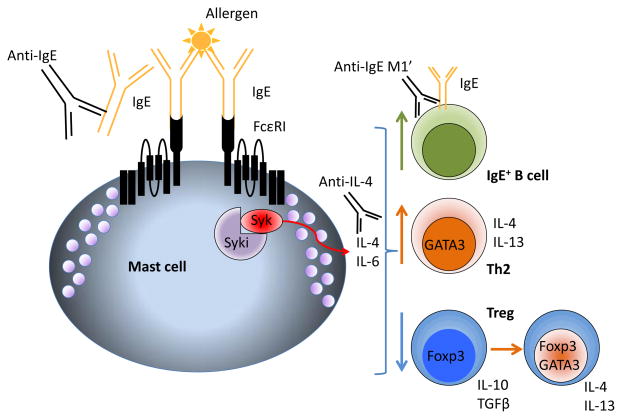
Figure 2.
Immunoregulatory effects of IgE and mast cells, and potential therapeutic interventions. Activation of mast cells through FcεRI and Syk drives the synthesis of IL-4, favoring Th2 polarization in T cells and IgE switching in B cells. Mast cell-derived IL-4 acts via STAT6 in Foxp3+ Treg to induce the expression of GATA3, reprogramming the Treg into a Th2-like pathogenic cell. Molecules that target Syk (Syki), IgE (anti-IgE omalizumab or anti-IgE M1′ quilizumab) or IL-4 (dupilumab, pitrakinra) may have the ability to counteract these effects.
Targeting IgE signaling to reverse sensitization
Current treatment strategies for food allergy are limited. Clinical trials have demonstrated that regulated incrementally increasing ingestion of allergen, called oral immunotherapy (OIT) can lead to a desensitized state in many patients and in some instances, long-term tolerance [23–26]. The process, however, is slow, only partially effective and fraught with side effects due to allergic reactions. Recently, a humanized monoclonal anti-IgE, omalizumab, has been employed as adjunct therapy to OIT, and has permitted rapid allergen dose escalation while reducing untoward reactions [27,28]. We reasoned that neutralization of IgE or inhibition of mast cells might have the added, unanticipated benefit of favoring the restoration of tolerance and Treg-dominated immune responses. We modeled this in F709 mice, placing them on a program of daily allergen ingestion mimicking human OIT, and additionally treated some of the mice with a neutralizing anti-IgE antibody. Mice treated with anti-IgE during desensitization therapy regained Treg-dominated responses to allergen and showed reductions in Th2 cells [6]**. Unlike available anti-mouse IgE antibodies, omalizumab has been engineered such that it does not interact with FcεRI-bound IgE; thus it cannot activate mast cells by crosslinking IgE, but also fails to remove receptor-bound IgE and so leaves cells still able to activate upon exposure to allergen. In the interest of developing a therapeutic approach that could block IgE receptor signaling in food allergy, we employed a novel, highly specific Syk inhibitor developed by Merck. This small molecule kinase inhibitor abrogated anaphylaxis when given prophylactically to mice 15 minutes prior to allergen challenge. When administered during the oral desensitization regimen, Syk inhibitor led to the reversal of sensitization in allergic F709 mice, reducing IgE levels, mast cell burden and Th2 bias while favoring the generation of new Treg-dominated responses, none of which was seen in vehicle control-treated mice. Importantly, anaphylactic responses to allergen challenge were greatly reduced in mice that received OIT with Syk inhibitor long after the therapy was discontinued, indicating that immunological tolerance was restored [6]**. Treg taken from Syk inhibitor-treated mice were able to suppress the development of allergic pathology, an ability that is critically lacking in Treg taken from mice with severe food allergy (Figure 2) [6,7]**.
Conclusions
Clinical studies on the effects of omalizumab during OIT for peanut allergy are underway. Initial studies on the effects of OIT or omalizumab-assisted OIT on the induction of Treg have been inconsistent [21,29–31], but with improved techniques for identifying and analyzing allergen-specific Treg, Syed and colleagues demonstrated that Treg populations are clearly enhanced in peanut-allergic patients undergoing OIT [32]**. While omalizumab cannot neutralize receptor-bound IgE and may be insufficient to block even all soluble IgE in severely allergic individuals, we anticipate it will have a beneficial effect. Omalizumab treatment has been shown to completely inhibit IgE:CD23-mediated facilitated allergen binding to B cells [33], and decrease FcεRI+ DC-driven proliferation of allergen specific Th2 cells [34]. Furthermore, long-term neutralization of IgE by omalizumab leads to reduced expression of FcεRI and loss of receptor-bound IgE on mast cells, basophils and dendritic cells [35–37]. This effect may be instrumental in facilitating successful tolerance induction. Incrementally increasing exposure to allergen within a short time frame (rush desensitization) is an effective means of internalizing IgE and FcεRI, and renders mast cells unresponsive to that allergen [38,39]*. It is our hope that omalizumab is simply the first of many rational approaches to targeting the IgE:mast cell axis, and that the development of other drugs (e.g., quilizumab, Syk inhibitor, anti-FcεRI) [40]* will accelerate our acquisition of knowledge regarding the functions of IgE receptor signaling in allergic pathology and permit effective treatment of these intractable diseases.
Highlights.
IgE, IgE receptor signaling and mast cells promote sensitization to food allergens
IgE and mast cells disrupt Treg induction, favoring Th2 responses
Treg that are generated exhibit Th2 reprogramming and are defective
Silencing the IgE:mast cell axis reverses allergic sensitization
Acknowledgments
Dr. Oliver Burton received NIH funding through an NRSA T32 training grant, number 5T32AI007512-28, and is currently funded by a K01 career development grant, number 1K01DK106303-01. Research in the Oettgen laboratory is funded by the NIH, grant number 1R01AI119918-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hans C. Oettgen, Associate Chief, Division of Immunology, Boston Children’s Hospital, Professor of Pediatrics, Harvard Medical School, Boston, MA 02115, (617) 919-2488.
Oliver T. Burton, Email: Oliver.burton@childrens.harvard.edu, Instructor of Pediatrics, Boston Children’s Hospital and Harvard Medical School, Boston, MA 02115, (617) 919-2452
References
We draw the reader’s attention to particular recent papers, noted as
* (of special interest) and
** (of outstanding interest).
- 1.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011;242:128–143. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sibilano R, Frossi B, Pucillo CE. Mast cell activation: a complex interplay of positive and negative signaling pathways. Eur J Immunol. 2014;44:2558–2566. doi: 10.1002/eji.201444546. [DOI] [PubMed] [Google Scholar]
- 3.Ten RM, McKinstry MJ, Trushin SA, Asin S, Paya CV. The signal transduction pathway of CD23 (Fc epsilon RIIb) targets I kappa B kinase. J Immunol. 1999;163:3851–3857. [PubMed] [Google Scholar]
- 4.Chan MA, Gigliotti NM, Matangkasombut P, Gauld SB, Cambier JC, Rosenwasser LJ. CD23-mediated cell signaling in human B cells differs from signaling in cells of the monocytic lineage. Clin Immunol. 2010;137:330–336. doi: 10.1016/j.clim.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Turcanu V, Stephens AC, Chan SM, Rance F, Lack G. IgE-mediated facilitated antigen presentation underlies higher immune responses in peanut allergy. Allergy. 2010;65:1274–1281. doi: 10.1111/j.1398-9995.2010.02367.x. [DOI] [PubMed] [Google Scholar]
- 6**.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41:141–151. doi: 10.1016/j.immuni.2014.05.017. Burton et al. develop an adjuvant-free ingestion model of peanut allergy in genetically altered mice with a predisposition to atopy. IgE- and mast cell-deficient mice are used to demonstrate that IgE receptor signaling impairs Treg induction while favoring Th2 responses to ingested allergens. Successful reversal of sensitization is obtained in combination with neutralization of IgE or inhibition of Syk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7**.Noval Rivas M, Burton OT, Wise P, Charbonnier LM, Georgiev P, Oettgen HC, Rachid R, Chatila TA. Regulatory T Cell Reprogramming toward a Th2-Cell-like Lineage Impairs Oral Tolerance and Promotes Food Allergy. Immunity. 2015;42:512–523. doi: 10.1016/j.immuni.2015.02.004. Noval Rivas et al. use enteral sensitization to allergen in a genetic model of food allergy to show that regulatory T cell deficits are a critical factor in the development of sensitization. IL-4 signaling via STAT6 and Shp-1 in Foxp3+ T cells reprograms the Treg into pathogenic GATA3+Foxp3+ T cells, whose production of Th2 cytokines exacerbates allergic sensitization. Treg reprogramming is shown to occur in mice and men, and depends on FcεRI signaling in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127:795–805. e791–796. doi: 10.1016/j.jaci.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartnikas LM, Gurish MF, Burton OT, Leisten S, Janssen E, Oettgen HC, Beaupre J, Lewis CN, Austen KF, Schulte S, et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. 2013;131:451–460. e451–456. doi: 10.1016/j.jaci.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, Hartmann K, Karasuyama H, Nadeau KC, Tsai M, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:881–888. e881–811. doi: 10.1016/j.jaci.2013.06.008. Reber et al. employ transgenic mice and antibody-mediated depletion to tease out the cellular contributions to peanut-induced anaphylaxis in food allergic mice. This study clearly demonstrates mast cell involvement, and provides the first evidence for a role for basophils. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, Kim B, Waserman S, Reed J, Coyle AJ, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179:6696–6703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 12.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Joetham A, Gelfand EW. Peanut-induced intestinal allergy is mediated through a mast cell-IgE-FcepsilonRI-IL-13 pathway. J Allergy Clin Immunol. 2010;126:306–316. 316 e301–312. doi: 10.1016/j.jaci.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitawaki T, Kadowaki N, Sugimoto N, Kambe N, Hori T, Miyachi Y, Nakahata T, Uchiyama T. IgE-activated mast cells in combination with pro-inflammatory factors induce Th2-promoting dendritic cells. Int Immunol. 2006;18:1789–1799. doi: 10.1093/intimm/dxl113. [DOI] [PubMed] [Google Scholar]
- 15.Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J Immunol. 2006;177:3577–3581. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- 16.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 17.Bryce PJ, Miller ML, Miyajima I, Tsai M, Galli SJ, Oettgen HC. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 18.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tachdjian R, Mathias C, Al Khatib S, Bryce PJ, Kim HS, Blaeser F, O’Connor BD, Rzymkiewicz D, Chen A, Holtzman MJ, et al. Pathogenicity of a disease-associated human IL-4 receptor allele in experimental asthma. J Exp Med. 2009;206:2191–2204. doi: 10.1084/jem.20091480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199:1679–1688. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shreffler WG, Wanich N, Moloney M, Nowak-Wegrzyn A, Sampson HA. Association of allergen-specific regulatory T cells with the onset of clinical tolerance to milk protein. J Allergy Clin Immunol. 2009;123:43–52. e47. doi: 10.1016/j.jaci.2008.09.051. [DOI] [PubMed] [Google Scholar]
- 22.Torgerson TR, Linane A, Moes N, Anover S, Mateo V, Rieux-Laucat F, Hermine O, Vijay S, Gambineri E, Cerf-Bensussan N, et al. Severe food allergy as a variant of IPEX syndrome caused by a deletion in a noncoding region of the FOXP3 gene. Gastroenterology. 2007;132:1705–1717. doi: 10.1053/j.gastro.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 23.Brozek JL, Terracciano L, Hsu J, Kreis J, Compalati E, Santesso N, Fiocchi A, Schunemann HJ. Oral immunotherapy for IgE-mediated cow’s milk allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2012;42:363–374. doi: 10.1111/j.1365-2222.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 24.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, Stablein D, Henning AK, Vickery BP, Liu AH, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, Sicherer SH, Liu AH, Stablein D, Henning AK, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–127. e111–117. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vickery BP, Lin J, Kulis M, Fu Z, Steele PH, Jones SM, Scurlock AM, Gimenez G, Bardina L, Sampson HA, et al. Peanut oral immunotherapy modifies IgE and IgG4 responses to major peanut allergens. J Allergy Clin Immunol. 2013;131:128–134. e121–123. doi: 10.1016/j.jaci.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, Wong DA. A phase II, randomized, doubleblind, parallelgroup, placebocontrolled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127:1309–1310. e1301. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 28.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127:1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, DeKruyff RH, Schneider LC, Nadeau KC, Umetsu DT. Changes in antigen-specific T-cell number and function during oral desensitization in cow’s milk allergy enabled with omalizumab. Mucosal Immunol. 2012;5:267–276. doi: 10.1038/mi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes-Aparicio V, Alonso-Lebrero E, Zapatero L, Infante S, Lorente R, Munoz-Fernandez MA, Correa-Rocha R. Induction of Treg cells after oral immunotherapy in hen’s egg-allergic children. Pediatr Allergy Immunol. 2014;25:103–106. doi: 10.1111/pai.12137. [DOI] [PubMed] [Google Scholar]
- 31.Mori F, Bianchi L, Pucci N, Azzari C, De Martino M, Novembre E. CD4+CD25+Foxp3+ T regulatory cells are not involved in oral desensitization. Int J Immunopathol Pharmacol. 2010;23:359–361. doi: 10.1177/039463201002300136. [DOI] [PubMed] [Google Scholar]
- 32**.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, Berglund JP, Tsai M, Maecker H, O’Riordan G, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–510. doi: 10.1016/j.jaci.2013.12.1037. Syed et al. assess peanut-specific Foxp3+ Treg in peanut-allergic patients either undergoing OIT or avoiding peanut. Following OIT, Foxp3 expression increases, Foxp3+ numbers are elevated, the Foxp3 locus is demethylated (and indicator of stability), and the Treg exhibit enhanced suppressive activity against effector T cells. This study provides a conclusive demonstration that the onset of clinical tolerance in OIT is associated with increases in Treg and Treg function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klunker S, Saggar LR, Seyfert-Margolis V, Asare AL, Casale TB, Durham SR, Francis JN Immune Tolerance Network G. Combination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: Inhibition of IgE-facilitated allergen binding. J Allergy Clin Immunol. 2007;120:688–695. doi: 10.1016/j.jaci.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder JT, Bieneman AP, Chichester KL, Hamilton RG, Xiao H, Saini SS, Liu MC. Decreases in human dendritic cell-dependent T(H)2-like responses after acute in vivo IgE neutralization. J Allergy Clin Immunol. 2010;125:896–901. e896. doi: 10.1016/j.jaci.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Eggel A, Baravalle G, Hobi G, Kim B, Buschor P, Forrer P, Shin JS, Vogel M, Stadler BM, Dahinden CA, et al. Accelerated dissociation of IgE-FcepsilonRI complexes by disruptive inhibitors actively desensitizes allergic effector cells. J Allergy Clin Immunol. 2014;133:1709–1719. e1708. doi: 10.1016/j.jaci.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–530. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 38*.Khodoun MV, Kucuk ZY, Strait RT, Krishnamurthy D, Janek K, Lewkowich I, Morris SC, Finkelman FD. Rapid polyclonal desensitization with antibodies to IgE and FcepsilonRIalpha. J Allergy Clin Immunol. 2013;131:1555–1564. doi: 10.1016/j.jaci.2013.02.043. Khodoun et al. use antibodies targeting IgE or FcεRI to develop rush desensitization techniques that leave mice resistant to IgE-mediated anaphylaxis. The treatments clear away IgE or IgE receptor, permitting safe exposure to allergens that would otherwise cause mast cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol. 2013;132:922–932. e921–916. doi: 10.1016/j.jaci.2013.05.004. Oka et al. investigate the mechanisms of rush desensitization, a procedure wherein exposure to incrementally increasing amounts of antigen renders mast cells unresponsive in vitro and prevents anaphylaxis. The desensitization results in the internalization of antigen-specific IgE, and does not affect responses to an unrelated antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Gauvreau GM, Harris JM, Boulet LP, Scheerens H, Fitzgerald JM, Putnam WS, Cockcroft DW, Davis BE, Leigh R, Zheng Y, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci Transl Med. 2014;6:243ra285. doi: 10.1126/scitranslmed.3008961. Gauvreau et al. report results from clinical trials investigating the ability of anti-IgE M1′ (quilizumab) to alter IgE production in vivo. Unlike omalizumab, which only binds soluble IgE, quilizumab interacts with the membrane-anchored segment of IgE in IgE-expressing B cells, inhibiting IgE production. Quilizumab reduced serum IgE levels in subjects with allergic rhinitis and allergic asthma. [DOI] [PubMed] [Google Scholar]