Abstract
In many bacteria and archaea, an adaptive immune system (CRISPR-Cas) provides immunity against foreign genetic elements. This system uses CRISPR RNAs (crRNAs) derived from the CRISPR array, along with CRISPR-associated (Cas) proteins, to target foreign nucleic acids. In most CRISPR systems, endonucleolytic processing of crRNA presursors (pre-crRNAs) is essential for the pathway. Here we study the Cas6 endonuclease responsible for crRNA processing in the Type III-A CRISPR-Cas system from Staphylococcus epidermidis RP62a, a model for Type III-A CRISPR-Cas systems, and define substrate requirements for SeCas6 activity. We find that SeCas6 is necessary and sufficient for full-length crRNA biogenesis in vitro, and that it relies on both sequence and stem-loop structure in the 3′ half of the CRISPR repeat for recognition and processing.
Keywords: Clustered regularly interspaced short palindromic repeats, CRISPR, RNA processing, CRISPR-associated, Cas6
1. INTRODUCTION
All genomes are susceptible to attack by foreign DNA, necessitating mechanisms of protection against invasion. Most bacteria and archaea encode various innate defense systems, but because of ongoing phage evasion of host defense systems, cells benefit from access to a more sophisticated, adaptive immune system. In recent years, CRISPR (clustered, regularly interspaced, short palindromic repeat) systems have been shown to specify an adaptive immune system that is present in most archaea and ~40% of bacteria [1–4]. CRISPR loci are made up of identical repeat sequences interspaced by unique spacer sequences that are derived from invasive genomes. Multiple protein-coding genes called cas (CRISPR-associated) genes can usually be found adjacent to the CRISPR locus. CRISPR systems are divided into five major types (I–V) and further classified into 16 subtypes (I-A through I-F and I-U, II-A through II-C, III-A through III-D, as well as IV and V) based upon CRISPR locus architecture, cas gene repertoire and interference mechanism [5]. These classifications reflect the diversity with which CRISPR pathways confer immunity [1, 3–5].
The proteins encoded by the cas genes, together with small RNAs that are encoded by the CRISPR array, mediate CRISPR immunity in three main stages: adaptation, crRNA biogenesis, and targeting [1, 3, 4]. During the adaptation stage, a bacterial or archaeal host acquires resistance against an invasive element by integrating a “protospacer” fragment of that element into its CRISPR locus [6], thereby forming a new spacer that confers sequence-based immunity against subsequent invasion by the same element [7]. CrRNA biogenesis involves the transcription of the CRISPR locus to generate a crRNA precursor (pre-crRNA) that is then processed into mature crRNAs [8, 9], each of which harbors a single spacer flanked by partial repeat sequences [8–11]. In most Type I and Type III CRISPR-Cas systems, crRNA biogenesis depends on a member of the Cas6 protein family, which recognizes and cleaves the pre-crRNA within the repeat elements [11]. In the targeting stage of CRISPR interference, cRNAs are used as guide sequences that form ribonucleoprotein effector complexes with one or more Cas proteins [1–4]. The crRNA subunit is used to recognize target nucleic acids (usually within invasive sequences) by Watson-Crick base pairing. A Cas protein within the complex then cleaves the identified target, driving the sequence-specific targeting of invaders.
The Type III-A machinery is emerging as a particularly versatile CRISPR-Cas system. The prototypical Type III-A CRISPR-Cas system (from Staphylococcus epidermidis RP62a) was the first to have its target defined as DNA rather than RNA, [12], and that activity has been shown recently to depend upon transcription across the targeted locus [13, 14]. Recent biochemical analyses have also revealed an RNA-cleaving activity [14] that is shared by other Type III-A systems [15, 16]. Both activities depend strictly on a processed crRNA guide. Further study of the S. epidermidis Type III-A system’s mechanistic features, from crRNA biogenesis, Csm complex assembly, and RNA/DNA target cleavage, will be important for understanding the basis for its unusual versatility.
Here we study crRNA biogenesis in the S. epidermidis RP62a Type III-A pathway. In this system, crRNA biogenesis occurs in two stages. During primary processing, S. epidermidis Cas6 (SeCas6) cleaves the pre-crRNA to generate a 71-nt intermediate crRNA [17, 18]. SeCas6 is an endoribonuclease belonging to the “repeat-associated mysterious protein” (RAMP) family [19], members of which adopt a unique ferridoxin-like fold. Genetic analyses indicate that no other S. epidermidis Cas proteins are required for this step in vivo [17, 18, 20]. Following primary processing, one or more nucleases trims the intermediate’s 3′ end in 6-nucleotide (nt) increments to generate mature crRNAs that range in size from 31–67 nt [17, 18]. Within cells, mature crRNA accumulation requires the Cas proteins Csm2, Csm3 and Csm5 [17], which form the Csm ribonucleoprotein complex along with Csm4, Cas10 and crRNA [14, 18, 20]. SeCas6 appears to function independently during processing, and not as a stable component of its cognate effector complex [14, 18, 20], unlike the Cas6 orthologs from most Type I systems [8, 21]. Although these aspects of crRNA maturation have been examined in cells [17, 20], the biochemical basis for S. epidermidis crRNA processing [18], in preparation for Csm effector complex loading, is less well understood. Here we define the pre-crRNA repeat features that are necessary for SeCas6 recognition and primary processing.
2. Materials and methods
2.1. Plasmid construction, growth conditions and cell extract preparation
For pre-crRNA processing assays using cell extracts, we used S. epidermidis strains RP62a, LAM104 (an RP62a derivative carrying a precise deletion of the CRISPR repeats and spacers) [12], or strain LM1680 and its derivatives harboring the pcrispr plasmid (all generously provided by Luciano Marraffini, Rockefeller University, New York, NY) [18]. LM1680 and its derivatives are described in Table 1 of Hatoum-Aslan et al. [18]. Cell extract was prepared by growing 0.5 liters of S. epidermidis cells in Brain Heart Infusion media (BHI; Difco) at 37°C until the culture reached an OD600 ~ 2. The medium was supplemented with antibiotics as follows: neomycin (15 μg/ml) for selection of S. epidermidis LM1680 and chloramphenicol (10 μg/ml) for selection of pcrispr. Cells were harvested via centrifugation and washed successively with 50 ml of cold S30 buffer A [10 mM Tris-acetate (pH 8.0), 14 mM magnesium acetate, 1 mM KCl, and 1 mM DTT] and then 25 ml of buffer A [10 mM Tris-acetate (pH 8.0), 14 mM magnesium acetate, 50 mM KCl, and 1 mM DTT]. Cells were re-suspended to a final volume of 8.25 ml in buffer B [10 mM Tris-acetate (pH 8.0), 20 mM magnesium acetate, 50 mM KCl, and 1 mM DTT] and were lysed by adding 0.1 mg lysostaphin (Sigma-Aldrich) followed by incubation at 37°C for 60 minutes. Cellular debris was removed via centrifugation in an SS34 rotor (Sorvall) at 30,000xg for 15 minutes. 0.25 volumes of pre-incubation buffer [670 mM Tris-acetate (pH 8.0), 20 mM magnesium acetate, and 7 mM DTT) was added to the supernatant and incubated at 37°C for 30 minutes. The preincubated supernatant was collected and the buffer was exchanged to storage buffer [10 mM Tris-acetate (pH 8.0), 14 mM magnesium acetate, 60 mM potassium acetate, 1 mM DTT, 5% glycerol], and split into 100 μl aliquots, flash-frozen in liquid nitrogen, and stored at −80°C.
2.2. Preparation of RNA substrates
For preparation of RNA substrates containing repeat 1, spacer 1, repeat 2, and truncated spacer 2 (see Fig. 1, A and B), we generated a DNA template by PCR amplification from S. epidermidis RP62a CRISPR sequences using a forward primer containing a T7 promoter (5′-TAATACGACTCACTATAGGGACAGCAAAAATGATGCTTG-3′) and a reverse primer corresponding to a portion of the second spacer (5′-GTAACGTATGCAAATGACAATTA-3′). The amplified DNA was gel-purified, cloned into the pCR2.1 TOPO vector (Life Technologies), and confirmed by DNA sequencing. The RNA substrate generated by EcoRI digestion and T7 RNA polymerase transcription includes two 5′-terminal G residues (to facilitate in vitro transcription) followed by: 53 nts of CRISPR leader sequence; the 36-nt CRISPR repeat 1; the 35-nt spacer 1; the 36-nt repeat 2; 29 nts of spacer 2; and 11 nts of plasmid-derived sequence (203 nts total). Substrate mutants were generated using the QuikChange Site-Directed Mutagenesis Kit (Agilent) and confirmed by DNA sequencing. RNAs were generated by in vitro transcription using T7 RNA Polymerase (New England Biolabs) and uniformly labeled with 20 μCi [α-32P] CTP (3,000 Ci/mmol; Perkin-Elmer), 500 μM ATP, 500 μM GTP and 500 μM UTP, 12 μM unlabeled CTP (New England Biolabs), 1x RNA polymerase buffer (New England Biolabs), and 20 U T7 RNA polymerase in a total volume of 20 μl. The RNAs were separated by electrophoresis in a denaturing (8 M urea) 10% polyacrylamide gel, and the appropriate RNA species were excised from the gel with a sterile razor blade after autoradiographic exposure. The RNA was eluted from the gel slices by end-over-end rotation in 400 μl of RNA elution buffer [0.3 M sodium acetate, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 0.5% SDS, 5mM Tris (pH 7.5)] for 6 hours at room temperature. The RNA was precipitated with 2.5 volumes of 100% ethanol after incubation for 2 hours at −20° C. For RNA substrates consisting of repeat sequences only (and repeat truncations), we annealed 100 μm forward and reverse synthetic DNA oligos (IDT) harboring a T7 promoter, and cloned them into a modified pUC57 vector. The vector was linearized with BbsI restriction enzyme and RNAs were generated using the MEGAscript T7 transcription kit (Life technologies). RNAs were run on an 8 M urea, 12% polyacrylamide gel and excised from the gel following UV shadowing by a 254 nm lamp. RNA was eluted from the gel slice and precipitated as described above. These RNAs were 5′ end-labeled with T4 polynucleotide kinase (New England Biolabs) in a 20 μl reaction containing 20 pmol RNA, 500 μCi of [γ32P] ATP (7000 Ci/mmol; MP Biomedical) and 20 U of T4 polynucleotide kinase. Labeled RNAs were separated on an 8 M urea, 12% polyacrylamide gel and purified according to the protocol outlined above.
Fig. 1.
Cas6 is responsible for primary processing of pre-crRNA transcripts in S. epidermidis. (A) Organization of the S. epidermidis RP62a CRISPR locus. Leader (black box), repeats (grey diamonds), spacers (colored rectangles), and cas/csm genes (grey arrows) are indicated. Individual elements are not to scale. The 36-nt sequence of each CRISPR repeat is given above, with the proposed stem-loop and the cleavage site (between nts 28 and 29) indicated. (B) Schematic representation of a model pre-crRNA substrate, and its primary processing in vitro. Using in vitro transcription, we generated a body-labeled, truncated pre-crRNA that includes two spacers [red (Spc1) and blue (Spc2)] and two repeats (grey). The transcribed pre-crRNA is subject to primary processing events, one within each repeat (sites indicated by a dotted line), that liberate an intermediate crRNA (71 nt) or two crRNA fragments (84 and 48 nt), as indicated. (C) In vitro crRNA processing using extracts from wildtype, Δcrispr/cas, Δcrispr, and individual Δcas/csm gene in-frame deletion strains. The reaction products [see the schematic in (B)] are indicated to the right. M: DNA size markers (sizes indicated on the left); Input: unprocessed substrate.
2.3. Expression and purification of recombinant SeCas6
Cultures of E. coli Rosetta (DE3) cells (EMD Millipore) containing a pET21a vector encoding an N-terminally His6-tagged SeCas6 (gift from Alfonso Mondragón) were grown at 37 °C in Terrific Broth (TB) containing 100 μg/ml of ampicillin and 34 μg/ml chloramphenicol until the culture reached an OD600 of ~0.8. Recombinant His6-SeCas6 expression was induced by 0.15 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 16 hrs at 16°C with constant shaking. Cells were harvested via centrifugation and all subsequent protein purification steps were performed at 4°C.
For purification of His6-SeCas6, cells were resuspended in 20 ml of Buffer A [50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 5 mM imidazole (pH 8.0), 5% glycerol) containing 0.75 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM Benzamidine, and 1 μg/ml Pepstatin. Harvested cells were disrupted by sonication. Insoluble material was cleared by centrifugation for 35 minutes at 38,000 rpm in a Beckman Coulter centrifuge using a Ti-60 rotor. The resulting supernatant was passed through a 0.2 μm filter and loaded onto a Talon metal affinity Co2+ resin (Takara) column equilibrated with Buffer A. The column was washed with 300 ml of buffer A and the protein was subsequently eluted step-wise with 100 ml of Elution buffer [50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 5% glycerol) containing 50, 100, and 500 mM imidazole, and collected in 10 ml aliqouts. His6-SeCas6 was analyzed using SDS–PAGE and staining with Coomassie Blue. The 100 mM imidazole eluates containing purified His6-SeCas6 were pooled and the buffer was exchanged to storage buffer [50 mM Tris-HCl (pH 8.0), 500 mM NaCl, 250 mM imidazole (pH 8.0), 5% glycerol) using Spectra/Por molecular porous membrane tubing (Spectrum Labs).
2.4. RNA Cleavage Reactions
Recombinant SeCas6 protein (rSeCas6; 500 nM) was incubated with trace amounts of end-labeled or body-labeled RNA in nuclease assay buffer [880 mM potassium acetate, 20 mM Tris acetate (pH 8.0), 2 mM EDTA] and dialysis buffer [10 mM Tris acetate (pH 8.0), 150 mM potassium acetate, 1 mM DTT, 5% glycerol) at 37°C for 5 minutes in a 20 μl reaction. 10ul samples were removed from the reaction and quenched in 10 ul of quenching buffer (1 μg/μl of Proteinase K and 1% SDS) and incubated at 37°C for 15 minutes. RNAs were recovered by phenol/chloroform extraction and ethanol precipitation and then separated by electrophoresis in a denaturing (8M urea), 10–12% polyacrylamide gel. The gels were dried and radiolabeled RNAs were visualized by phosphorimaging.
3. Results
3.1. SeCas6 is necessary and sufficient for crRNA primary processing in S. epidermidis
S. epidermidis RP62a harbors a Type III-A CRISPR-cas locus that contains nine cas/csm genes, as well as four CRISPR repeats and three spacers (spc1, spc2 and spc3) (Fig. 1A) [12]. CrRNAs generated from spc1 direct interference against conjugation by targeting the nickase (nes) gene that is carried on conjugative plasmids [12, 17, 18, 20, 22]. Pre-crRNA processing is essential for this and other forms of CRISPR interference in this system [17, 18, 20], and depends upon cas6 [17, 18, 20]. Primary processing by Cas6 occurs between nts 28 and 29 of the crRNA repeat, immediately following a proposed four-bp stem (Fig. 1A). To investigate further the roles of the Cas/Csm machinery in pre-crRNA processing, we used an in vitro system that tracks site-specific cleavage of a body-labeled S. epidermidis pre-crRNA substrate, encompassing 55 5′-flanking nts, repeat 1, spacer 1, repeat 2, and most of spacer 2 (Fig. 1B, top). When this model pre-crRNA substrate is fully cleaved, three products are expected: an 84-nt 5′-terminal byproduct that includes nts 1-28 of repeat 1 along with its upstream flank; the spc1 intermediate crRNA (71 nts); and a truncated spc2 crRNA (48 nts) (Fig. 1B, bottom). We prepared crude extracts from S. epidermidis cells, incubated them with radiolabelled model pre-crRNA substrate, and then analyzed the resulting RNAs by denaturing gel electrophoresis and autoradiography (Fig. 1C). For extract preparation we used the RP62a-derived mutant strain LM1680 that harbors a large deletion that removes the entire crispr/cas locus (Δcrispr/cas) [18]. We also used LM1680 complemented with a plasmid that includes the entire CRISPR-cas locus (pcrispr), or with pcrispr derivatives that remove the CRISPR repeats and spacers (Δcrispr) or that carry in-frame deletions of each individual cas/csm gene that is essential for plasmid interference (Δcas10, Δcsm2, Δcsm3, Δcsm4, Δcsm5, Δcsm6, or Δcas6) [17, 18, 20]. We observed the intact 71-nt intermediate crRNA (as well as the 84-nt 5′-terminal byproduct) in all pcrispr complementation strains except for the Δcas6 derivative. The limited accumulation of the other partially processed products likely reflects poor stability of those RNAs in the crude extract. These results provide biochemical confirmation that cas6, alone among the cas/csm genes, is required for primary pre-crRNA processing in S. epidermidis RP62a [17].
To test whether SeCas6 is sufficient for primary pre-crRNA processing in this Type III-A system, we expressed recombinant SeCas6 (rSeCas6) protein in E. coli and purified it to homogeneity (Fig. S1). Incubation of the model pre-crRNA substrate (Fig. 1B) with rSeCas6 led to the rapid, efficient appearance of processing products and byproducts (Fig. 2). All products and byproducts were readily detectable, consistent with a lack of nonspecific RNA degradation in this purified system. For comparison, we concurrently tested processing efficiency in crude cellular extract [in this case from the RP62a-derived strain LAM104, which lacks the CRISPR locus (and therefore lacks endogenous crRNAs) but includes all chromosomal cas/csm genes] [12]. We used this CRISPR-deleted strain to avoid potential competitive effects (if any) from endogenous pre-crRNAs. Although cellular extract was again successful at generating the 71-nt intermediate crRNA, accumulation was more modest, and most other reaction products were absent, consistent with RNA degradation (Fig. 2). Our results confirm that SeCas6 is sufficient for pre-crRNA processing in the S. epidermidis CRISPR-Cas system [20]. Because rSeCas6 processing is more efficient than crude extract and is also less prone to nonspecific RNA degradation, we used this purified system for our subsequent experiments.
Fig. 2.
Purified, recombinant SeCas6 reconstitutes pre-crRNA processing in vitro. The model pre-crRNA depicted in Fig. 1B was uniformly labeled and incubated for 2.5 and 5 minutes in the presence (+) or absence (−) of 500 nM rSeCas6 protein, as indicated at the top of each lane. For comparison, processing reactions were also done in crude extract from S. epidermidis cells (strain LAM104) that express native SeCas6. The reaction products (see the schematic in Fig. 1B) are indicated to the right. M: DNA size markers (sizes indicated on the left); Input: unprocessed substrate.
3.2. In vitro pre-crRNA processing by rSeCas6 requires specific sequence elements within the CRISPR repeat
Mutagenic analyses in S. epidermidis cells have identified CRISPR repeat nts that are important for processed crRNA accumulation and interference, and identified the three nts on either side of the SeCas6 cleavage site (between G28 and A29; Fig. 1A) as being particularly crucial [17]. To test whether these nts function during the pre-crRNA processing itself (rather than, for example, post-processing RNA stability), we mutagenized the first repeat in the pre-crRNA that we had previously developed for in vitro processing. Initially we mutated four contiguous nts at a time (changing each nt to its Watson-Crick complementary nt) to identify sequence blocks that include potential SeCas6 recognition elements. All mutations were introduced into Repeat 1, whereas the wild-type Repeat 2 sequence was retained in all cases. Accordingly, cleavage within Repeat 2 served as an internal positive control for SeCas6 activity. A series of mutated pre-crRNAs was used in in vitro processing reactions with purified rSeCas6.
The results of this analysis are shown in Fig. 3. Two block substitutions – within nts 25-28 (the four G residues on the 3′ side of the predicted stem, immediately upstream of the cleavage site; Fig. 3A) and nts 29-32 (immediately downstream of the cleavage site) – resulted in strong processing defects (Fig. 3B). These defects were reflected in the loss of the 71-nt intermediate crRNA, and in the accumulation of the partially processed substrate (including 5′ flank, Repeat 1, Spacer 1, and most of Repeat 2) that had been cleaved within Repeat 2 only. These tetranucleotide substitutions include the nts that were previously shown to be important for crRNA accumulation in cells [17]. Mutation of repeat nts 13-16 (the four C residues on the 5′ side of the proposed stem) also yielded a partial defect, with diminished intermediate crRNA accumulation and moderately increased partially-processed substrate (Fig. 3C). Although none of the nts from positions 13-16 were required individually for crRNA accumulation in cells [17], the quadruple mutation would be expected to abolish stem formation, and the in vivo mutagenesic analyses did provide some support for a requirement for base pairing within the stem [17]. All other four-nt substitutions (beyond those in nts 13-16, 25-28, and 29-32) had little or no effect on pre-crRNA processing (Fig. 3B and 3C).
Fig. 3.
In vitro processing analysis of pre-crRNA carrying block mutations within the first CRISPR repeat. (A) the sequence of Repeat 1, with the predicted hairpin-forming nucleotides underlined. Nt numbering is shown. (B, C) rSeCas6-catalyzed pre-crRNA cleavage reactions with a series of pre-crRNAs carrying quadruple or quintuple mutations (with the indicated repeat nts replaced by their respective Watson-Crick complementary nts). In each case the mutations were in repeat 1 only, while the wildtype sequence of repeat 2 was retained to provide an internal positive control for rSeCas6 processing activity. Cleavage assays were performed using uniformly radiolabeled RNAs in the presence (+) or absence (−) of rSeCas6. The reaction products (see the schematic in Fig. 1B) are indicated to the right. In: input (unprocessed substrate); M: DNA size markers (sizes indicated on the left). Nts that exhibit a substantial processing defect when mutated are highlighted in red above each panel, and in the sequence at the top of the figure.
For the 13-16 and 25-28 substitutions, all mutations were upstream of the Repeat 1 cleavage site, and the resulting 71-nt intermediate crRNA therefore had a fully wildtype RNA sequence. Accordingly, the diminished or abolished 71-nt crRNA accumulation in those mutants (Fig. 3B and 3C) is likely to be caused solely by a primary processing defect rather than from increased susceptibility to post-processing RNA degradation.
To deconvolute the effects of the four mutations within each mutagenically sensitive block (repeat nts 13-16, 25-28, and 29-32), we constructed the corresponding single mutations and tested them in the rSeCas6 processing assay (Fig. 4A). Only four nts – C30 (two nts downstream of the cleavage site) (Fig. 4B) and G26, G27, and G28 (the three nts upstream of the cleavage site) (Fig. 4C) – yielded strong processing defects that abolished crRNA generation. Mutation of several other nts [A29, immediately downstream of the cleavage site (Fig. 4B); G25, at the top of the 3′ side of the proposed stem (Fig. 4C); and C13, C14, C15, and C16, on the 5′ side of the proposed stem (Fig. 4D)] – did not abolish 71-nt intermediate crRNA generation, but nonetheless induced some accumulation of partially processed substrate cleaved within Repeat 2 only. This suggests partial processing defects in those mutants. Furthermore, our observation that the wild-type Repeat 2 is efficiently cleaved in all of the Repeat 1 mutant substrates (Figs. 3 and 4) confirms that rSeCas6 pre-crRNA processing is unit-independent, i.e. involves no discernable interaction or cooperativity from one pre-crRNA repeat processing unit to another. This conclusion is also consistent with previous in vivo results [17].
Fig. 4.
In vitro processing analysis of pre-crRNA carrying single-nt mutations within the first CRISPR repeat. (A) the sequence of Repeat 1, with the predicted hairpin-forming nucleotides underlined. Nt numbering is shown. (B, C) rSeCas6-catalyzed pre-crRNA cleavage reactions with a series of pre-crRNAs carrying single mutations (with each indicated repeat nt replaced by its Watson-Crick complementary nt). In each case the mutation was in repeat 1 only, while the wildtype sequence of repeat 2 was retained to provide an internal positive control for rSeCas6 processing activity. Cleavage assays were performed using uniformly radiolabeled RNAs in the presence (+) or absence (−) of rSeCas6. The reaction products (see the schematic in Fig. 1B) are indicated to the right. In: input (unprocessed substrate); M: DNA size markers (sizes indicated on the left). Individual nts that exhibit a substantial processing defect when mutated are highlighted in red above each panel, and in the sequence at the top of the figure.
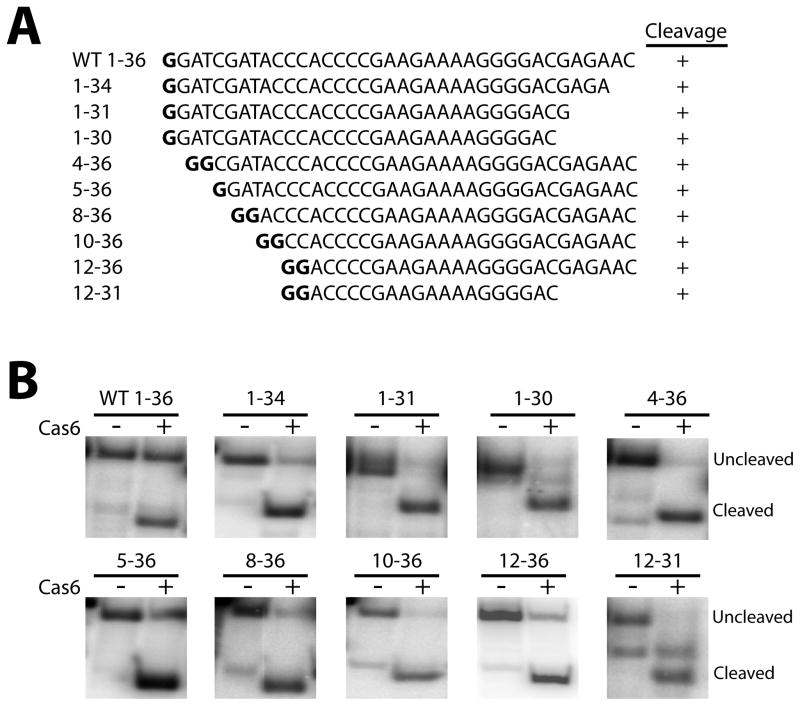
To corroborate the results of the substitution mutations, and to define the regions of the pre-crRNA repeat that are completely dispensable for processing, we tested the ability of rSeCas6 to cleave short RNAs consisting of a single repeat only, or truncation derivatives thereof (Fig. 5A). Truncation of the 5′ end of the repeat (the 5′-terminal 4, 5, 8, 10, or 12 nts) or the 3′ end of the repeat (the 3′-terminal 2, 5, or 6 nts) all retained the important nts identified above, and were all cleavaed by rSeCas6 (Fig. 5B). Simultaneous truncation from both ends (substrate 12-31, Fig. 5A), consisting of the stem-loop and only one additional nt upstream and two additional nts downstream, was also accurately cleaved, indicating that the stem-loop and the 1-2 nts that immediately flank it include all sequences that are necessary for rSeCas6 recognition and cleavage.
Fig. 5.
An individual CRISPR repeat RNA, and truncation derivatives thereof, are accurately cleaved by rSeCas6 in vitro. (A) Sequences of the wild-type and mutant repeat RNAs. In some cases, one or two G residues (bold, and not included in the numbering scheme) were added to the 5′ end to facilitate in vitro transcription. (B) Detailed analysis of cleavage with a series of crRNAs and mutant derivatives. Cleavage assays were performed using 5′-end-radiolabeled RNAs in the presence (+) or absence (−) of purified rSeCas6.
3.3. The role of the repeat stem-loop structure in SeCas6 pre-crRNA processing
The processing defects observed in mutants involving C13, C14, C15, and C16 (the nts on the 5′ side of the proposed stem; Fig. 6A), or in mutants involving G25, G26, G27, or G28 (the nts on the 3′ side of the proposed stem), could result from the loss of base-specific SeCas6-RNA contacts, or from the loss of secondary structural elements that are important for SeCas6 recognition, or both. To distinguish between these alternatives, we tested whether compensatory mutations in the stem could reverse the defects of individual mutations. We were particularly interested in the G26C, G27C, and G28C mutants because they exhibited the strongest defects in rSeCas6 cleavage (Fig. 4C). In all three cases, the compensatory double mutant (C15G/G26C, C14G/G27C, and C13G/G28C) failed to revert the processing defect observed in the G→C mutation alone (Fig. 6A). This suggests that base pairing interactions at those positions are not sufficient for rSeCas6 processing. The limited processing defect of the G25C mutant (Fig. 4C) could be partially overcome in the compensatory double mutant (C16G/G25C), indicating that base pairing at this position (or its contribution to overall stem stability) plays a role in SeCas6 processing.
Fig. 6.
Compensatory mutational analyses of CRISPR repeat stem-loop base pairs. (A) rSeCas6-catalyzed pre-crRNA cleavage reactions with pre-crRNAs carrying single G→C mutations on the 3′ side of the stem (as in Fig. 4C), but also carrying the compensatory C→G mutation on the 5′ side of the stem. Repeat 1, with the predicted hairpin structure, is depicted above. Stem nt numbering and the site of SeCas6 cleavage are indicated. In each case the mutations were in repeat 1 only, while the wildtype sequence of repeat 2 was retained to provide an internal positive control for rSeCas6 processing activity. Cleavage assays were performed using uniformly radiolabeled RNAs in the presence (+) or absence (−) of rSeCas6. The reaction products (see the schematic in Fig. 1B) are indicated to the right. In: input (unprocessed substrate); M: DNA size markers (sizes indicated on the left). Substrates that exhibit a substantial processing defect when mutated are highlighted in red above the panel. (B) rSeCas6-catalyzed repeat cleavage assays (similar to those in Fig. 5) with substrates carrying compensatory transition rather than transversion mutations in the proposed stem. Cleavage assays were performed using internally radiolabeled RNAs in the presence (+) or absence (−) of rSeCas6. In: input (unprocessed substrate). In this experiment, the wildtype substrate is identical to that depicted in the top row of Fig. 5A, except that it has one fewer nt on each end. The mutant RNAs are as shown on the top row of Fig. 5A, except for the specified base substitutions indicated at the top of each lane. Substrates that exhibit a substantial processing defect when mutated are highlighted in red above the panel.
In all of the substitution mutants described above, the wildtype nt was changed to its Watson-Crick complement, meaning that all mutations were purine→pyrimidine or pyrimidine→purine transversions. It remained possible that purine→purine or pyrimidine→pyrimidine transition mutations that nonetheless maintain base pairing could support SeCas6 processing, even in cases where transversion mutations could not. To address this, we constructed compensatory double mutants involving C→T and G→A transition mutations (C15T/G26A, C14T/G27A, and C13T/G28A), in the context of a single-repeat processing substrate. We also constructed an octanucleotide substitution substrate in which all four C residues (C13-16) were changed to T residies, and all four G residues (G25-28) were changed to A residues. No processing was observed for the octanucleotide substitution (Fig. 6B), indicating that the potential for stem formation (while retaining purine and pyrimidine identity at each position) does not suffice for SeCas6 recognition and processing. For the less drastic mutations, two of the individually mutated base pairs that had failed to tolerate compensatory transversion mutations (C13G/G28C and C14G/G27C; Fig. 6A) likewise failed to tolerate compensatory transition mutations (C13T/G28A and C14T/G27A; Fig. 6B). In contrast, the third base pair that had failed to tolerate a compensatory transversion mutation (C15G/G26C; Fig. 6A) could be cleaved when compensatory transition mutations were used instead (C15T/G26A; Fig. 6B). Taken together, our compensatory analyses provide some support for a role for pairing in the top two base pairs of the proposed stem (C15/G26 and C16/G25). For the bottom two base pairs (C13/G28 and C14/G27), the strong (G27C and G28C) and moderate (C13G and C14G) defects observed in individual mutants does not rule out a partial role for base pairing at these positions. Nonetheless, the failure of all of these sites’ compensatory mutations (both transitions and transversions) to restore processing indicates that base pairing is certainly not sufficient, and suggests that SeCas6 requires base-specific recognition elements at these sites. Accordingly, SeCas6 appears to use a combination of sequence- and structure-specific recognition to identify its processing substrates.
4. Discussion
Universal to all CRISPR systems described thus far is the transcription of repeat-spacer units into long precursors that are cleaved endonucleolytically within each repeat sequence. With few exceptions [23], the generation of mature crRNA is a critical step in CRISPR immunity [8, 11]. These short crRNAs, each harboring a unique invader-derived spacer sequence, can help assemble Cas protein-containing effector complexes, and guide these complexes to recognize and destroy invader sequences. In Type I and Type III systems, primary pre-crRNA processing occurs by way of the Cas6 family of nucleases [8, 9], with the exception of Type I-C systems, in which Cas5d fulfills this role [24, 25]. Although Cas6 sequences are highly divergent, they share the ability to adopt single or double ferridoxin-like “RNA Recognition Motif” (RRM) folds that employ metal-independent mechanisms to cleave their substrates [26–28].
In S. epidermidis, pre-crRNA maturation occurs in two distinct steps, first liberating an intermediate crRNA that is flanked by repeat sequence on either end, followed by 3′ trimming in the second “maturation” stage of biogenesis [17, 18]. Neither intermediate nor mature crRNAs accumulate in the absence of Cas6, Cas10, or Csm4, whereas intermediate but not mature crRNAs accumulate in the absence of Csm2, Csm3, and Csm5 [17]. The relative roles of the different S. epidermidis Cas/Csm proteins in primary processing itself, rather than in postprocessing stabilization of the intermediate crRNA, are only partially understood [18]. Here we have used a biochemical approach to characterize further the biogenesis of intermediate crRNAs in this system. As with most Type III CRISPR-Cas systems, we confirm that SeCas6 is both necessary and sufficient for the processing of S. epidermidis pre-crRNAs (Fig. 1 and Fig. 2) [20]. This observation indicates that the roles of Cas10 and Csm4 in crRNA accumulation in cells [17] most likely involve processed crRNA stabilization. Cas10 and Csm4 are both components of the Csm effector complex [14, 18, 20]; however, given that Csm3 is essential for complex formation [18] yet dispensable for intermediate crRNA generation [17], the Csm complex is clearly not strictly required for primary pre-crRNA processing. Intriguingly, the requirement for Cas10 and Csm4 for post-processing crRNA stability in cells [17] is not recapitulated in cellular extacts in vitro (Fig. 1C). The roles of Cas10 and Csm4 in crRNA stabilization therefore remain unclear.
Scanning mutagenesis across an S. epidermidis CRISPR repeat identified six contiguous nts (G26 through G31; see Fig. 1A and Fig. 6A) that, when changed individually to their respective Watson-Crick complementary nts, abolished the accumulation of processed crRNAs in vivo [17]. Our use of the purified rSeCas6 system allowed us to test whether these nts are important for primary processing itself, as opposed to (for example) post-processing RNA stability. We found that four of these sites (G26, G27, G28, and C30) were critical for SeCas6 primary processing. Mutations at the other two sites (A29 and G31) had limited effects on rSeCas6 cleavage efficiency, suggesting that their role in Spc1 crRNA accumulation involves post-processing steps, such as stability. This is especially plausible because these nts are downstream of the Repeat 1 cleavage site, i.e. within the “5′ handle” of the processed Spc1 crRNA itself.
Some Cas6 substrates form hairpin structures that are important for recognition [28–32], whereas others [9, 33] do not [11]. When hairpin structures are present, the Cas6 cleavage site is immediately 3′ of the stem. Each repeat within the S. epidermidis pre-crRNA has the potential to form a four-base-pair G/C-rich stem with an 8-nt loop immediately upstream of the cleavage site (Fig. 6A), and limited mutational evidence in vivo supports a role for stem formation [17]. Our biochemical analyses likewise support a role for stem formation, especially between the C15/G26 and C16/G25 pairs (Fig. 6). In vivo, a G28A mutation blocks crRNA accumulation, and that phenotype can be partially reverted by a compensatory C13T mutation, suggesting that the 13–28 base pair forms and plays a role in processing. We did not observe such an effect in our in vitro analyses, for reasons that are not clear. Overall, the picture that emerges from our work, combined with earlier results [17], is of an RNA-protein interaction that involves the recognition of a hairpin structure, but in a manner that also involves base-specific contacts that require specific sequences (and not just a base-paired configuration) at specific positions, especially within the G-rich side of the stem. C30 also appears to be a critical recognition element for SeCas6. Our results are in contrast to those with a different, well characterized Type III Cas6 (from P. furiosus), which recognizes single-stranded sequences at the 5′ end of its cognate repeat, based upon both mutational and crystallographic analyses [9]. A full understanding of SeCas6/pre-crRNA interaction awaits a similarly high-resolution co-crystal structure. In the meantime, our work clarifies the requirements for primary pre-crRNA processing step for the S. epidermidis RP62a CRISPR-Cas pathway, which has emerged as a prototype for mechanistic investigation of Type III-A CRISPR interference [12–14, 17, 18, 20, 22, 34].
Supplementary Material
Highlights.
Staphylococcus epidermidis Cas6 (SeCas6) is necessary and sufficient for CRISPR RNA (crRNA) biogenesis in vitro
SeCas6 recognizes the 3′-terminal region of the CRISPR repeat sequence for processing
Both primary sequence and stem-loop structure are important for SeCas6 processing
Acknowledgments
We thank Yan Huaru (Northwestern University) for technical support and contributions to this project, and Alfonso Mondragón (Northwestern University) for help and advice. We also thank Luciano Marraffini (Rockefeller University) for providing pCRISPR constructs. This work was supported by an NSF predoctoral fellowship to N.W., an American Heart Association postdoctoral fellowship to R.R., and NIH grant R01 GM093769 to E.J.S.
Abbreviations
- CRISPR
clustered regularly interspaced short palindromic repeats
- Cas
CRISPR-associated
- crRNA
CRISPR RNA
- SeCas6
Staphylococcus epidermidis Cas6
- nt
nucleotide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barrangou R, Marraffini LA. CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity. Mol Cell. 2014;54:234–244. doi: 10.1016/j.molcel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plagens A, Richter H, Charpentier E, Randau L. DNA and RNA interference mechanisms by CRISPR-Cas surveillance complexes. FEMS Microbiol Rev. 2015;39:442–463. doi: 10.1093/femsre/fuv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sontheimer EJ, Barrangou R. The bacterial origins of the CRISPR genome-editing revolution. Hum Gene Ther. 2015;26:413–424. doi: 10.1089/hum.2015.091. [DOI] [PubMed] [Google Scholar]
- 4.van der Oost J, Westra ER, Jackson RN, Wiedenheft B. Unravelling the structural and mechanistic basis of CRISPR-Cas systems. Nat Rev Microbiol. 2014;12:479–492. doi: 10.1038/nrmicro3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJJ, Charpentier E, Haft DH, Horvath P, Moineau S, Mojica FJM, Terns RM, Terns MA, White MF, Yakunin AF, Garrett RA, van der Oost J, Backofen R, Koonin EV. An updated evolutionary classification scheme for CRISPR-Cas systems. Nat Rev Microbiol. 2015 doi: 10.1038/nrmicro3569. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- 7.Heler R, Marraffini LA, Bikard D. Adapting to new threats: the generation of memory by CRISPR-Cas immune systems. Mol Microbiol. 2014;93:1–9. doi: 10.1111/mmi.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carte J, Wang R, Li H, Terns RM, Terns MP. Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 2008;22:3489–3496. doi: 10.1101/gad.1742908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charpentier E, Richter H, van der Oost J, White MF. Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity. FEMS Microbiol Rev. 2015;39:428–441. doi: 10.1093/femsre/fuv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg GW, Jiang W, Bikard D, Marraffini LA. Conditional tolerance of temperate phages via transcription-dependent CRISPR-Cas targeting. Nature. 2014;514:633–637. doi: 10.1038/nature13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samai P, Pyenson N, Jiang W, Goldberg GW, Hatoum-Aslan A, Marraffini LA. Co-transcriptional DNA and RNA cleavage during Type III CRISPR-Cas immunity. Cell. 2015;161:1164–1174. doi: 10.1016/j.cell.2015.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staals RH, Zhu Y, Taylor DW, Kornfeld JE, Sharma K, Barendregt A, Koehorst JJ, Vlot M, Neupane N, Varossieau K, Sakamoto K, Suzuki T, Dohmae N, Yokoyama S, Schaap PJ, Urlaub H, Heck AJ, Nogales E, Doudna JA, Shinkai A, van der Oost J. RNA targeting by the Type III-A CRISPR-Cas Csm complex of Thermus thermophilus. Mol Cell. 2014;56:518–530. doi: 10.1016/j.molcel.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamulaitis G, Kazlauskiene M, Manakova E, Venclovas C, Nwokeoji AO, Dickman MJ, Horvath P, Siksnys V. Programmable RNA shredding by the Type III-A CRISPR-Cas system of Streptococcus thermophilus. Mol Cell. 2014;56:506–517. doi: 10.1016/j.molcel.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Hatoum-Aslan A, Maniv I, Marraffini LA. Mature clustered, regularly interspaced, short palindromic repeats RNA (crRNA) length is measured by a ruler mechanism anchored at the precursor processing site. Proc Natl Acad Sci USA. 2011;108:21218–21222. doi: 10.1073/pnas.1112832108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatoum-Aslan A, Samai P, Maniv I, Jiang W, Marraffini LA. A ruler protein in a complex for antiviral defense determines the length of small interfering CRISPR RNAs. J Biol Chem. 2013;288:27888–27897. doi: 10.1074/jbc.M113.499244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarova KS, Aravind L, Wolf YI, Koonin EV. Unification of Cas protein families and a simple scenario for the origin and evolution of CRISPR-Cas systems. Biol Direct. 2011;6:38. doi: 10.1186/1745-6150-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatoum-Aslan A, Maniv I, Samai P, Marraffini LA. Genetic characterization of antiplasmid immunity through a type III-A CRISPR-Cas system. J Bacteriol. 2014;196:310–317. doi: 10.1128/JB.01130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haurwitz RE, Sternberg SH, Doudna JA. Csy4 relies on an unusual catalytic dyad to position and cleave CRISPR RNA. EMBO J. 2012;31:2824–2832. doi: 10.1038/emboj.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marraffini LA, Sontheimer EJ. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature. 2010;463:568–571. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Heidrich N, Ampattu BJ, Gunderson CW, Seifert HS, Schoen C, Vogel J, Sontheimer EJ. Processing-independent CRISPR RNAs limit natural transformation in Neisseria meningitidis. Mol Cell. 2013;50:488–503. doi: 10.1016/j.molcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garside EL, Schellenberg MJ, Gesner EM, Bonanno JB, Sauder JM, Burley SK, Almo SC, Mehta G, MacMillan AM. Cas5d processes pre-crRNA and is a member of a larger family of CRISPR RNA endonucleases. RNA. 2012;18:2020–2028. doi: 10.1261/rna.033100.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam KH, Haitjema C, Liu X, Ding F, Wang H, DeLisa MP, Ke A. Cas5d protein processes pre-crRNA and assembles into a cascade-like interference complex in subtype I-C/Dvulg CRISPR-Cas system. Structure. 2012;20:1574–1584. doi: 10.1016/j.str.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carte J, Pfister NT, Compton MM, Terns RM, Terns MP. Binding and cleavage of CRISPR RNA by Cas6. RNA. 2010;16:2181–2188. doi: 10.1261/rna.2230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebihara A, Yao M, Masui R, Tanaka I, Yokoyama S, Kuramitsu S. Crystal structure of hypothetical protein TTHB192 from Thermus thermophilus HB8 reveals a new protein family with an RNA recognition motif-like domain. Protein Sci. 2006;15:1494–1499. doi: 10.1110/ps.062131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–1358. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gesner EM, Schellenberg MJ, Garside EL, George MM, Macmillan AM. Recognition and maturation of effector RNAs in a CRISPR interference pathway. Nat Struct Mol Biol. 2011;18:688–692. doi: 10.1038/nsmb.2042. [DOI] [PubMed] [Google Scholar]
- 30.Niewoehner O, Jinek M, Doudna JA. Evolution of CRISPR RNA recognition and processing by Cas6 endonucleases. Nucleic Acids Res. 2014;42:1341–1353. doi: 10.1093/nar/gkt922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sashital DG, Jinek M, Doudna JA. An RNA-induced conformational change required for CRISPR RNA cleavage by the endoribonuclease Cse3. Nat Struct Mol Biol. 2011;18:680–687. doi: 10.1038/nsmb.2043. [DOI] [PubMed] [Google Scholar]
- 32.Shao Y, Li H. Recognition and cleavage of a nonstructured CRISPR RNA by its processing endoribonuclease Cas6. Structure. 2013;21:385–393. doi: 10.1016/j.str.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Preamplume G, Terns MP, Terns RM, Li H. Interaction of the Cas6 riboendonuclease with CRISPR RNAs: recognition and cleavage. Structure. 2011;19:257–264. doi: 10.1016/j.str.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramia NF, Tang L, Cocozaki AI, Li H. Staphylococcus epidermidis Csm1 is a 3′-5′ exonuclease. Nucleic Acids Res. 2014;42:1129–1138. doi: 10.1093/nar/gkt914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.