Abstract
Hepatitis B virus (HBV) is a noncytopathic, hepatotropic, double-stranded DNA virus that causes acute and chronic hepatitis. Although HBV does not induce a measurable innate immune response in the infected liver, the outcome of infection is determined by the kinetics, breadth, vigor, trafficking, and effector functions of HBV-specific adaptive T cell responses, and the development of neutralizing antibodies. Dysregulation of one or more of these events leads to persistent HBV infection and a variably severe chronic necroinflammatory liver disease that fosters the development of hepatocellular carcinoma. Deeper understanding of the mechanisms responsible for immunological tolerance to HBV is needed in order to devise immunotherapeutic strategies to cure chronic HBV infection and prevent its life-threatening sequellae.
Introduction
Hepatitis B virus (HBV) is a small, circular, hepatotropic dsDNA virus that causes acute and chronic hepatitis and hepatocellular carcinoma [1]. Our understanding of the host-virus interactions that determine the outcome of HBV infection reflects the results of studies conducted in naturally infected humans, experimentally infected chimpanzees, surrogate animal models (e.g. woodchucks and ducks infected by closely related hepadnaviruses and transgenic mice supporting HBV gene expression and replication) and assorted cell culture systems that support some or all aspects of the HBV life cycle in vitro [1].
It is generally acknowledged that hepatocellular HBV infection is noncytopathic, that it does not trigger innate immune responses readily measurable in vivo [2], and that robust, polyclonal and multispecific CD4+ and CD8+ T cell responses and neutralizing antibody responses contribute to the resolution of HBV infection. CD4+ T cells facilitate induction and maintenance of both CD8+ T cells and neutralizing antibodies [3]. CD8+ T cells kill infected cells and secrete antiviral cytokines (mainly interferon [IFN]-γ) that inhibit HBV replication noncytopathically [4,5]. Neutralizing antibodies limit viral spread from residual productively infected hepatocytes that aren’t eliminated by the CD8+ T cells [6]. The virus persists in the majority of neonatal/perinatal infections and a small minority of adult onset infections, reflecting the failure of one or more of these aspects of the immune response and mutational escape from immune recognition; and it secondarily triggers an indolent low grade chronic necroinflammatory liver disease that can progress to hepatocellular carcinoma (HCC) [4,7]. Vaccines that elicit neutralizing antibodies to hepatitis B surface antigen (HBsAg) efficiently prevent de novo HBV infection [8], but have no therapeutic potential for the ~ 240 million people that are persistently infected with this virus worldwide [9]. Therapy for these individuals mainly relies on direct acting antiviral (DAA) drugs that suppress virus production but do not eradicate HBV from the liver, requiring lifelong treatment [10]. Importantly, chronic HBV infection spontaneously resolves in a small fraction of patients, usually concomitant with functional restoration of adaptive immunity to HBV [11,12].
Despite these advances, important aspects of HBV immunobiology and pathogenesis remain ill defined, especially the immunological mechanisms responsible for viral persistence. A more complete understanding of these mechanisms is needed before immunotherapeutic strategies to cure chronic HBV infection can be rationally developed.
Understanding the role of innate immunity in HBV infection
Studies in acutely infected chimpanzees have shown that IFN-α/β, IFN-λ, IFN-γ and IFN-stimulated genes [ISGs] are not measurably induced during the initial spread of HBV throughout the liver (i.e. before the arrival of T cells) [2,13], indicating that HBV replicates noncytopathically and remains largely undetected by the infected hepatocytes or by intrahepatic innate immune cells such as NK and NKT cells.
Recently, Sato et al showed that i) low levels of IFN-λ and selected ISGs are transiently induced in HBV-infected primary human hepatocytes and ii) modest IFN-λ elevation is also observed after transfection of a human hepatoma cell line with a region of pregenomic HBV RNA that is required for viral replication [14]. Other studies in mouse models and human hepatocytes have shown that HBV gene expression can activate NKT cells [15,16] that recognize specific lysophospholipids presented by HBV-positive hepatocytes [17]. It is interesting to note that HBV replication in transgenic mice and hepatocyte cell lines is profoundly suppressed by IFN-α/β [18–20] IFN-λ[21], and IFN-γ produced by activated parenchymal and nonparenchymal liver cells (including NK and NKT cells) [22,23], or in response to toll like receptor (TLR) activation [24]. Although the relevance of these reports to the natural history of authentic HBV infection remains to be determined, they suggest that HBV is exquisitely sensitive to the innate host response, such that therapeutic activation of the innate immune response might contribute to viral clearance in chronically infected patients.
Accordingly, oral administration of a TLR7 agonist capable of stimulating robust IFN responses in plasmacytoid dendritic cells induces HBV suppression in chronically infected chimpanzees [25,26]. Of note, this antiviral activity is associated with activation of intrahepatic NK, NKT, and T cell responses that produce IFN-γ [25]. Pertinent to this, the human liver, unlike the mouse liver, contains relatively few NKT cells [27]. However, the human liver is enriched in NK CD56 bright cells and mucosal-associated invariant T (MAIT) cells [28] that secrete large quantities of IFN-γ in response to specific cytokines (e.g. IL-12 and IL-18) induced by TLR8 agonists [29]. IL-12 has also been shown to promote functional recovery of exhausted HBV-specific CD8+ T cells [30]. Collectively, these results appear to justify the testing of TLR8 agonists in chronically infected patients. There is one important caveat, however, since the same NK cells were shown to kill HBV-infected hepatocytes [31] and they have the unexpected potential to kill HBV-specific CD8+ T cells [32]. These findings suggest that potentially positive effects linked to NK cell activation (e.g., the production of antiviral cytokines such as IFN-γ) could be overwhelmed by excessive liver pathology and suppression of adaptive immunity. Human studies intended to explore the clinical benefit of activating innate immune responses will have to consider these possibilities.
It is also worth mentioning that recent mouse studies showed that systemic administration of a TLR9 agonist induces the formation of hepatic aggregates of myeloid cells (i.e. iMATEs: intrahepatic myeloid-cell aggregates for T cell population expansion) that promote the expansion of antigen experienced intrahepatic CD8+ T cells favoring viral clearance [33].
Understanding the role of adaptive immunity in HBV infection
As mentioned earlier, adaptive immune responses, particularly the HBV-specific CD8+ T cell response, play a key role in viral clearance and disease pathogenesis. Hence the failure to prime, expand or differentiate an effector memory T cell response to HBV or effective evasion of that response could be responsible for viral persistence
Both viral and host factors are thought to contribute to HBV persistence. Viral factors include the possibility that circulating hepatitis B e-antigen (HBeAg) functions as a tolerogenic protein promoting anergy of HBcAg/HBeAg cross-reactive T cells [34,35], although these results were produced in transgenic mice and have not been confirmed in humans. Circulating hepatitis B surface antigen (HBsAg) may also be immune suppressive since defective peripheral HBsAg-specific T cell responses in chronically infected patients correlate with serum HBsAg titers [36]. Unfortunately, the extent to which these results reflect suppressive effects of HBsAg on the T cell response or the consequence of a deficient T cell response has never been resolved. Mutational inactivation of HBV-derived B cell or T cell epitopes and mutational selection of epitope residues that anergize or antagonize TCR-dependent recognition by CD8+ T cells in infected patients have been suggested as additional mechanisms favoring HBV persistence [37,38].
That host factors determine infection outcome is supported by the fact that most neonatal/perinatal HBV infections and some adult onset infections result in immune tolerance and chronicity [4]. Why T cell responses are quantitatively deficient and functionally inadequate to terminate infection in these settings remains ill defined. Numerous observations in surrogate animal models and HBV-infected patients suggest that several nonexclusive mechanisms contribute to viral persistence.
Studies in HBV transgenic mice have demonstrated that naïve HBV-specific CD8+ T cells primed by hepatocellular antigen presentation fail to differentiate into IFN-γ-producing cytolytic effector memory cells, i.e. they display an “exhausted” phenotype [39]. T cell exhaustion is mediated by the activation of programmed cell death protein 1 [PD1]-dependent negative regulatory signals induced by PD-L1-expressing hepatocytes in the absence of positive costimulation [39]. As the inhibitory PD1-PD-L1 axis can be interrupted or overcome by infusion of agonistic anti-CD40 antibodies that presumably activate positive costimulatory signals in intrahepatic nonparenchymal cells [39], therapeutic strategies, like CD40 activation, that overcome the negative impact of checkpoint inhibitors may lead to functional T cell priming and viral clearance in chronically infected patients. In keeping with this, hepatic lymphoid structures consisting of professional antigen presenting cells (e.g. macrophages, DCs and B cells) have been shown to promote functional T cell priming in the mouse liver [40] and liver sinusoidal endothelial cells (LSECs) that cross-present circulating exogenous antigen can also prime naïve CD8+ T cells properly [41]. Whether these events can be activated therapeutically in the liver of chronically infected patients remains to be determined.
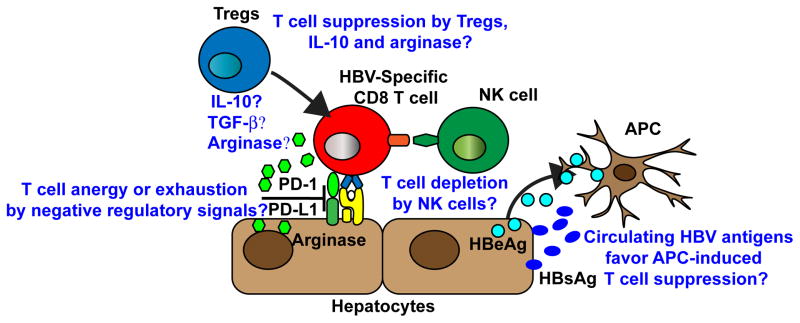
Naïve or antigen experienced HBV-specific T cells could also become anergic or exhausted as a result of i) activation of additional negative regulatory pathways involving cytotoxic T-lymphocyte antigen-4 (CTLA-4) or T-cell immunoglobulin and mucin 3 (Tim-3) [42,43], ii) antigen overload and excessive T cell stimulation [12], iii) action of regulatory T cells [44] and iv) relative intrahepatic abundance of selected cytokines (e.g. IL-10 or TGF-beta) or enzymes (e.g. arginase) with immunosuppressive potential [45,46]. All together, these results indicate that primary and secondary immunological unresponsiveness to HBV apparently contributes to the establishment of persistent infection (see also Figure 1).
Figure 1. Potential mechanisms of immunological unresponsiveness to HBV that promote viral persistence.
A weak T cell response is presumably the main cause for HBV persistence. HBV-specific T cells could be anergized or exhausted by negative signals delivered via PD-1, CTLA-4, and Tim-3. Tregs, IL-10, TGF- β, and arginase could also suppress HBV-specific T cell responses. NK cells may also contribute to viral persistence by depleting HBV-specific T cells. Finally, APCs that acquire secreted subviral particles and proteins may develop immunoregulatory functions.
For viral clearance to ultimately occur, functionally primed and expanded HBV-specific CD8+ T cells must also traffic throughout the liver, recognize antigen and deploy effector functions. Dynamic imaging in HBV-replication competent mice has recently shown that circulating CD8+ T cells initially arrest in liver sinusoids by docking onto sinusoidal endothelial cell-adherent platelets [47]. CD8+ T cells then crawl intrasinusoidally and probe underlying hepatocytes by extending filopodia-like protrusions through sinusoidal fenestrae, whereupon they recognize hepatocellular antigen, produce IFN-γ and kill HBV-replicating hepatocytes from within the intravascular space [47]. Notably, hepatocellular Ag recognition by HBV-specific CD8+ T cells is inhibited by the processes of sinusoidal defenestration (i.e. the reduction in number and size of sinusoidal fenestrae) and sinusoidal capillarization (i.e. the deposition of extracellular matrix underneath the sinusoidal wall) [47], both of which are characteristic of liver fibrosis [48]. This suggests that CD8+ T cells might have impaired antigen recognition capacity in fibrotic patients.
Human studies intended to explore the clinical benefit of endogenously inducing functional CD8+ T cell responses will also have to consider this possibility. Similar concerns should apply to approaches aimed at exogenously infusing autologous CD8+ T cells that are reprogrammed to express HBV-specific TCRs into patients [49,50]. Of note, autologous CD8+ T cells modified to express an HBsAg-specific TCR have been recently infused in a patient suffering from HBsAg-positive extrahepatic HCC metastases [51].
On a final note, it is worth mentioning that - along with viral factors that include the deregulation of procarcinogenic genes due to HBV DNA integration and/or the expression of HBV-derived procarcinogenic polypeptides [7] - the dysfunctional adaptive immune response that fails to eliminate HBV during chronic infection likely plays a relevant role in the pathogenesis of HCC. Indeed, studies in a mouse model of immune-mediated chronic hepatitis indicate that HBV-specific CD8+ T cells can maintain an indolent low grade chronic necroinflammatory liver disease that promotes hepatocellular proliferation and exposes proliferating hepatocytes to inflammatory mutagens, ultimately triggering HCC development [52,53]. Building on the observation that platelets are instrumental to intrahepatic CD8+ T cell homing [47,54], this same mouse model has been recently utilized to demonstrate that low doses of the anti-platelet drugs aspirin and clopidogrel prevent the onset of HCC and greatly improve overall survival [55]. These outcomes are preceded by and associated with reduced accumulation of pathogenic, IFN-γ-non-producing HBV-specific CD8+ T cells in the liver [55], indicating that a functionally inefficient CD8+ T cell response is both necessary and sufficient to induce hepatocellular transformation.
Concluding Remarks
Based on the evidence summarized herein, it is clear that continuing analysis of the host-virus interactions that determine the outcome of HBV infection is needed if we are to develop curative strategies for chronic HBV infection. Relevant to this, it is important to point out that a cure for HBV requires that the covalently closed circular (ccc) DNA, the HBV transcriptional template in the nucleus of infected hepatocytes [1], must be eliminated, which almost never occurs after DAA treatment [10]. By contrast, cccDNA is readily cleared from the liver of patients and chimpanzees that resolve an acute HBV infection in the context of a functionally efficient adaptive immune response [4]. Interestingly, cccDNA clearance in chimpanzees starts to occur prior to the manifestation of liver injury and concomitant with the appearance hepatic IFN-γ expression [2,13,56], suggesting that cytokine-mediated noncytolytic elimination of cccDNA is possible. Future studies aimed at addressing this and other immune-correlates of viral clearance and the means by which such events can be deregulated will not only improve our understanding of HBV immunobiology, but they may also lead to the design of immune-based approaches for the treatment of persistent HBV infection and its life-threatening complications. Immunotherapy - whether by vaccination or cytokine induction with or without checkpoint inhibition - will be challenging, however, because the breadth and magnitude of the HBV-specific T cell repertoire are severely diminished in chronically infected patients and the remaining effector functions are severely impaired [12]. Moreover, since a single infectious HBV particle is sufficient to initiate an infection that can spread to all of the hepatocytes [57], durable viral clearance will require the coordinated induction of (1) CD8+ T cell-mediated destruction of all, or nearly all, of the infected cells without triggering hepatic decompensation, and/or (2) cytokine-mediated noncytolytic elimination of cccDNA from the hepatocyte, the feasibility of which has not yet been convincingly achieved and, thus, may not be possible, plus (3) a robust neutralizing antibody response that prevents viral spread from any remaining infected cell to uninfected or cured cells in the liver. Nonetheless, in the absence of available antivirals that directly target cccDNA, it is imperative to vigorously pursue immunotherapeutic approaches to terminate chronic HBV infection, since a robust, multispecific, and well coordinated B and T cell response to HBV has been abundantly shown to be capable of doing the job [4].
Highlights.
Hepatocellular HBV infection is noncytopathic
HBV does not induce a measurable innate immune response in vivo
Robust, broad adaptive immune responses mediate HBV clearance and acute hepatitis
Weak, narrow adaptive responses mediate HBV persistence and chronic hepatitis
Chronic hepatitis evolves to cirrhosis of the liver and hepatocellular carcinoma
Acknowledgments
This work was supported by grants: 250219 (LGG) from the European Research Council (ERC), KAKENHI 26461015 (MI) from JSPS, and Research Program on Hepatitis (MI) from AMED, and R01-AI-020001 and R01-CA-040489 (FVC) from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480C:672–686. doi: 10.1016/j.virol.2015.02.031. This review summarizes current concepts of HBV replication, immunopathologenesis of acute and chronic hepatitis, hepatocarcinogenesis and therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. doi: 10.1146/annurev.iy.13.040195.000333. [DOI] [PubMed] [Google Scholar]
- 4.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 5.Guidotti LG, Ishikawa T, Hobbs MV, Matzke B, Schreiber R, Chisari FV. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 6.Ciupe SM, Ribeiro RM, Perelson AS. Antibody responses during hepatitis B viral infection. PLoS Comput Biol. 2014;10:e1003730. doi: 10.1371/journal.pcbi.1003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Bisceglie AM. Hepatitis B and hepatocellular carcinoma. Hepatology. 2009;49:S56–60. doi: 10.1002/hep.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, Bell B. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 9.Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4:a024935. doi: 10.1101/cshperspect.a024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoulim F, Durantel D. Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boni C, Laccabue D, Lampertico P, Giuberti T, Vigano M, Schivazappa S, Alfieri A, Pesci M, Gaeta GB, Brancaccio G, et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology. 2012;143:963–973. e969. doi: 10.1053/j.gastro.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 12*.Ferrari C. HBV and the immune response. Liver Int. 2015;35 (Suppl 1):121–128. doi: 10.1111/liv.12749. This review highlights the immunobiology of HBV infection, and offers potential immnunotherapeutic strategies to resolve chronic hepatitis. [DOI] [PubMed] [Google Scholar]
- 13.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123–132. doi: 10.1016/j.immuni.2014.12.016. This manuscript suggests that an element in HBV pregenomic RNA (pgRNA) may both activate the innate response and inhibit an early step in viral replication, but only if the conditions used in this elegant in vitro study are attained in vivo. [DOI] [PubMed] [Google Scholar]
- 15.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 16.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, et al. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidotti LG, Morris A, Mendez H, Koch R, Silverman RH, Williams BR, Chisari FV. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J Virol. 2002;76:2617–2621. doi: 10.1128/JVI.76.6.2617-2621.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClary H, Koch R, Chisari FV, Guidotti LG. Relative sensitivity of hepatitis B virus and other hepatotropic viruses to the antiviral effects of cytokines. J Virol. 2000;74:2255–2264. doi: 10.1128/jvi.74.5.2255-2264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieland SF, Guidotti LG, Chisari FV. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J Virol. 2000;74:4165–4173. doi: 10.1128/jvi.74.9.4165-4173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J Immunol. 2002;169:5188–5195. doi: 10.4049/jimmunol.169.9.5188. [DOI] [PubMed] [Google Scholar]
- 24.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508–1517. 1517 e1501–1510. doi: 10.1053/j.gastro.2013.02.003. This study demonstrates that oral administration of a TLR7 agonist can profoundly activate pDCs, NK, NKT, and T cells and suppress HBV replication in chronically infected chimpanzees. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell RH, London WT, McAuliffe VJ, Palmer AE, Kaplan PM, Gerin JL, Wagner J, Popper H, Lvovsky E, Wong DC, et al. Modification of chronic hepatitis-B virus infection in chimpanzees by administration of an interferon inducer. Lancet. 1976;2:757–761. doi: 10.1016/s0140-6736(76)90598-5. [DOI] [PubMed] [Google Scholar]
- 27.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang XZ, Jo J, Tan AT, Sandalova E, Chia A, Tan KC, Lee KH, Gehring AJ, De Libero G, Bertoletti A. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol. 2013;190:3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- 29.Jo J, Tan AT, Ussher JE, Sandalova E, Tang XZ, Tan-Garcia A, To N, Hong M, Chia A, Gill US, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10:e1004210. doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9:e1003208. doi: 10.1371/journal.ppat.1003208. This study demonstrates that IL-12 stimuation in vitro restores the capacity of functionally exhausted HBV-specific CD8+ T cells isolated from chronic HBV patients to produce antiviral cytokines, a potential antiviral strategy if it can be replicated in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A, Micco L, Nebbia G, Singh HD, Adams DH, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210:99–114. doi: 10.1084/jem.20121172. This study demonstrates that activated NK cells isolated from chronic HBV patients can kill autologous HBV-specific CD8+ T cells in vitro, another candidate immunosuppressive mechanism to explain viral persistence and chronic hepatitis in addition to T cell exhaustion, immune checkpoint activation, neonatal tolerance, mutational escape, regulatory T cell activation, etc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z, Schildberg FA, Odenthal M, Dienes HP, van Rooijen N, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Sallberg M, Hughes J, Jones J, Guidotti LG, Chisari FV, Billaud JN, Milich DR. Immune tolerance split between hepatitis B virus precore and core proteins. J Virol. 2005;79:3016–3027. doi: 10.1128/JVI.79.5.3016-3027.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen MT, Billaud JN, Sallberg M, Guidotti LG, Chisari FV, Jones J, Hughes J, Milich DR. A function of the hepatitis B virus precore protein is to regulate the immune response to the core antigen. Proc Natl Acad Sci U S A. 2004;101:14913–14918. doi: 10.1073/pnas.0406282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reignat S, Webster GJ, Brown D, Ogg GS, King A, Seneviratne SL, Dusheiko G, Williams R, Maini MK, Bertoletti A. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med. 2002;195:1089–1101. doi: 10.1084/jem.20011723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertoletti A, Sette A, Chisari FV, Penna A, Levrero M, De Carli M, Fiaccadori F, Ferrari C. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature. 1994;369:407–410. doi: 10.1038/369407a0. [DOI] [PubMed] [Google Scholar]
- 38.Rehermann B, Pasquinelli C, Mosier SM, Chisari FV. Hepatitis B virus (HBV) sequence variation of cytotoxic T lymphocyte epitopes is not common in patients with chronic HBV infection. J Clin Invest. 1995;96:1527–1534. doi: 10.1172/JCI118191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Isogawa M, Chung J, Murata Y, Kakimi K, Chisari FV. CD40 activation rescues antiviral CD8(+) T cells from PD-1-mediated exhaustion. PLoS Pathog. 2013;9:e1003490. doi: 10.1371/journal.ppat.1003490. This manuscript demonstrates that priming of HBV-specific CD8+ T cells by the hepatoctye in vivo induces PD-1-mediated exhaustion, and that dendritic cell activation by an agonsitic CD40 antibody in vivo overcomes the negative impact of PD-1 signaling, a potential therapeutic strategy to terminate chronic HBV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Publicover J, Gaggar A, Nishimura S, Van Horn CM, Goodsell A, Muench MO, Reinhardt RL, van Rooijen N, Wakil AE, Peters M, et al. Age-dependent hepatic lymphoid organization directs successful immunity to hepatitis B. J Clin Invest. 2013;123:3728–3739. doi: 10.1172/JCI68182. The manuscript demonstrates that intrahepatic lymphoid aggregates can favor the priming of HBV-specific B and T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bottcher JP, Schanz O, Wohlleber D, Abdullah Z, Debey-Pascher S, Staratschek-Jox A, Hochst B, Hegenbarth S, Grell J, Limmer A, et al. Liver-primed memory T cells generated under noninflammatory conditions provide anti-infectious immunity. Cell Rep. 2013;3:779–795. doi: 10.1016/j.celrep.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G, et al. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-Prone CD8 T cells in persistent hepatitis B virus infection. Hepatology. 2011;53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 43.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billerbeck E, Bottler T, Thimme R. Regulatory T cells in viral hepatitis. World J Gastroenterol. 2007;13:4858–4864. doi: 10.3748/wjg.v13.i36.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45**.Pallett LJ, Gill US, Quaglia A, Sinclair LV, Jover-Cobos M, Schurich A, Singh KP, Thomas N, Das A, Chen A, et al. Metabolic regulation of hepatitis B immunopathology by myeloid-derived suppressor cells. Nat Med. 2015;21:591–600. doi: 10.1038/nm.3856. The manuscript shows that myeloid-derived suppressor cells isolated from chronically HBV-infected patients inhibit HBV-specific and non-specific T cell responses in vitro by producing arginase. If these events also occur in vivo, they could contribute to HBV persistence and chronic hepatitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47**.Guidotti LG, Inverso D, Sironi L, Di Lucia P, Fioravanti J, Ganzer L, Fiocchi A, Vacca M, Aiolfi R, Sammicheli S, et al. Immunosurveillance of the liver by intravascular effector CD8(+) T cells. Cell. 2015;161:486–500. doi: 10.1016/j.cell.2015.03.005. This manuscript couples mouse models of HBV infection and dynamic imaging to define the means by which effector HBV-specific CD8+ T cells traffic to and within the liver and deploy their antiviral and pathogenic functions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 49.Krebs K, Bottinger N, Huang LR, Chmielewski M, Arzberger S, Gasteiger G, Jager C, Schmitt E, Bohne F, Aichler M, et al. T cells expressing a chimeric antigen receptor that binds hepatitis B virus envelope proteins control virus replication in mice. Gastroenterology. 2013;145:456–465. doi: 10.1053/j.gastro.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 50.Gehring AJ, Xue SA, Ho ZZ, Teoh D, Ruedl C, Chia A, Koh S, Lim SG, Maini MK, Stauss H, et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J Hepatol. 2011;55:103–110. doi: 10.1016/j.jhep.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 51*.Qasim W, Brunetto M, Gehring AJ, Xue SA, Schurich A, Khakpoor A, Zhan H, Ciccorossi P, Gilmour K, Cavallone D, et al. Immunotherapy of HCC metastases with autologous T cell receptor redirected T cells, targeting HBsAg in a liver transplant patient. J Hepatol. 2015;62:486–491. doi: 10.1016/j.jhep.2014.10.001. Case report indicating that autologous T cells, modified to produce an HBsAg-specific TCR and infused into a patient suffering from HBsAg-expressing extrahepatic HCC-derived metastases, significantly reduce serum HBsAg levels, an important preliminary step that demonstrates the potential feasibility of adoptive immunotherapy of chronic HBV infection. [DOI] [PubMed] [Google Scholar]
- 52.Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakamoto Y, Suda T, Momoi T, Kaneko S. Different procarcinogenic potentials of lymphocyte subsets in a transgenic mouse model of chronic hepatitis B. Cancer Res. 2004;64:3326–3333. doi: 10.1158/0008-5472.can-03-3817. [DOI] [PubMed] [Google Scholar]
- 54.Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11:1167–1169. doi: 10.1038/nm1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, Esposito A, Ruggeri ZM, Chisari FV, Iannacone M, et al. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A. 2012;109:E2165–2172. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guidotti LG, Rochford R, Chung J, Shapiro M, Purcell R, Chisari FV. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284:825–829. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- 57.Asabe S, Wieland SF, Chattopadhyay PK, Roederer M, Engle RE, Purcell RH, Chisari FV. The size of the viral inoculum contributes to the outcome of hepatitis B virus infection. J Virol. 2009;83:9652–9662. doi: 10.1128/JVI.00867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]