Abstract
Rotavirus is a leading cause of severe acute gastroenteritis in children worldwide, and globally licensed vaccines are available. To expedite the introduction of rotavirus vaccines in the national immunisation programme, a simple, ecological method to monitor changes in the burden of rotavirus disease may be of great help. Here, we report an application of a time-series analysis on a publicly-available dataset in Japan on the weekly number of laboratory-confirmed rotavirus-positive samples over the last 5 year period between the 36th week of 2009 and the 35th week of 2014 during which rotavirus vaccines became marketed in Japan and presumed to reach an uptake rate of at least 39% as a national average. Compared with the expected number of rotavirus detection based on the preceding four rotavirus seasons, the number of rotavirus detection during the 2013–2014 season was 42.9% (95% CI: 38.6, 47.8). This suggests that the use of rotavirus vaccine had a positive impact on reducing the burden of rotavirus diarrhoea in Japan. This method, because of its simplicity and little cost, should be applicable to early detection of the impact of rotavirus vaccine even in resource-poor countries where the World Health Organization funded and implemented the sentinel surveillance programmes of laboratory-confirmed rotavirus cases.
Keywords: rotavirus, gastroenteritis, vaccine, surveillance, impact, time-series analysis
Introduction
Rotavirus is a major etiological agent of severe acute gastroenteritis in infants and young children worldwide, and causes each year an estimated 430,000 deaths that occur primarily among children in developing countries[1, 2]. While death toll is much smaller in high-income countries, rotavirus imposes a large health and economic burden on their health-care systems [3–5]. In countries where rotavirus vaccines were introduced in the national immunisation schedules there have been ecological studies showing the reduction in the detection rates of rotaviruses among diarrheal samples [6–8]. Such studies highlight the impact of rotavirus vaccines on infection rates, and may encourage decision makers to include a rotavirus vaccine into the national immunisation programme.
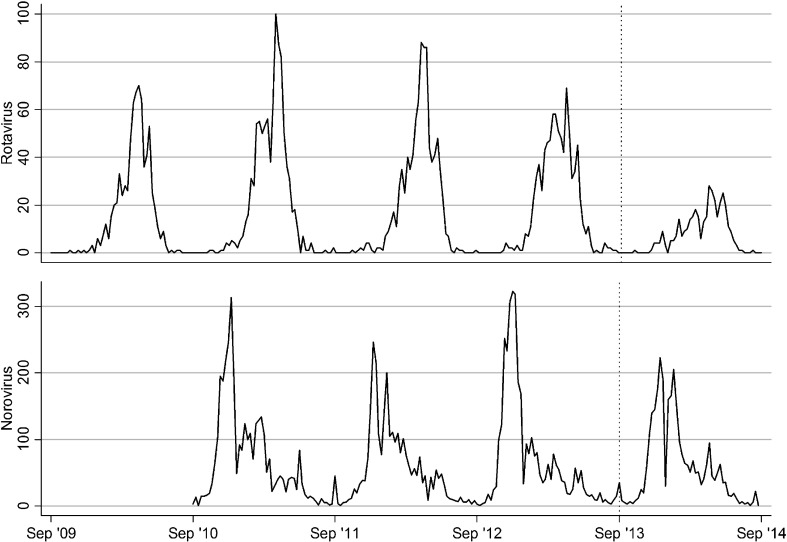
Like other high-income countries rotavirus gastroenteritis imposes a huge disease burden on the healthcare system in Japan [2], where an attenuated human rotavirus vaccine (Rotarix®: GlaxoSmithKline Biologicals, Rixensart, Belgium) and a pentavalent reassortant rotavirus vaccine (RotaTeq®: Merck & Co., Inc., Whitehouse Station, New Jerzy, USA) have been marketed since November 2011 and July 2012, respectively. An interim report by the rotavirus vaccine working group under the Ministry of Health, Labour and Welfare, Japan (www.mhlw.go.jp/file/05-Shingikai-10601000.../0000029637.pdf) estimated that the rotavirus vaccines were taken up by an average of 39% of the age-eligible children in Japan between July 2012 and April 2013. According to the experiences in early adopter countries of rotavirus vaccines, the impact of rotavirus vaccines was detected soon after the vaccine introduction in terms of reductions in the number or proportion of laboratory-confirmed rotavirus infections available in relatively simple surveillance database [6–8]. Thus, in an attempt at detecting an early, preliminary indication of the impact of rotavirus vaccine, we analysed the changes in the number of rotaviruses detected by the prefectural public health laboratories across the country over the last four years. As a control, we also analysed the changes in the number of noroviruses, which should not be affected by the use of rotavirus vaccine, over the last three years.
Methods
Data source
In accordance with the law concerning the prevention of infections and medical care for patients with infectious diseases, there is in Japan an infectious agent surveillance system in which clinical specimens collected at sentinel hospitals were examined for relevant pathogens depending on the nature of the case patients at the prefectural public health laboratories and the information is then sent to the National Institute of Infectious Diseases where the data were collated and published on-line as Infectious Agents Surveillance Report (http://idsc.nih.go.jp/iasr/index-j.htm). From this online database, we extracted the weekly numbers of rotavirus and norovirus detected in prefectural public health laboratories between September 2009 (week 36) and August 2014 (week 35), and between September 2010 (week 36) and August 2014 (week 35), respectively.
Statistical analysis
We defined that the rotavirus vaccine was introduced in September (in the 35th week) 2013, because the uptake rates were expected to be very low in the first two rotavirus seasons since Rotarix and RotaTeq were marketed in November 2011 and July 2012, respectively (Fig. 1). Expected normal numbers of rotavirus positive samples for each week after the rotavirus vaccine was introduced in 2013 were obtained as the season-specific average over the four preceding years (from week 36 in 2009 to week 35 in 2013). This season-specific average was obtained by fitting a Poisson generalized linear model with trigonometric terms (sine and cosine functions) of annual cycle and harmonics up to an order of six to the baseline four years [9]. The ratios of the observed against the expected number of positive samples were calculated. Confidence intervals (CIs) for the ratios were estimated from standard Poisson assumptions augmented by a refinement to take account of variability in the expected number of positive samples from four years data (2009–2013) as well as variability in the positive samples observed in the year after the vaccine introduction (week 36 in 2013–week 35 in 2014). Specifically, 95% CIs for the ratios were calculated using the following formula [9]:
where, O: Observed number of positive samples, and E: Expected number of positive samples.
Fig. 1.
Time series for the number of rotavirus and norovirus positive samples each week between September (week 36) 2009 and August (week 35) 2014 in Japan. The vertical, dotted line indicates week 36 in 2013. The data for norovirus between September (week 36) 2009 and August (week 35) 2010 was missing.
As a comparison, the same procedures were performed for the number of norovirus positive samples to estimate the observed/expected ratios and the 95% CIs. Expected numbers of norovirus positive samples for each week between week 36 in 2013 and week 35 in 2014 were obtained as the season-specific average over the three preceding years (week 36 in 2010 to week 35 in 2013).
All analyses were conducted using the statistical package Stata 12.0 (Stata Corporation, College Station, Texas, USA).
Results and Discussion
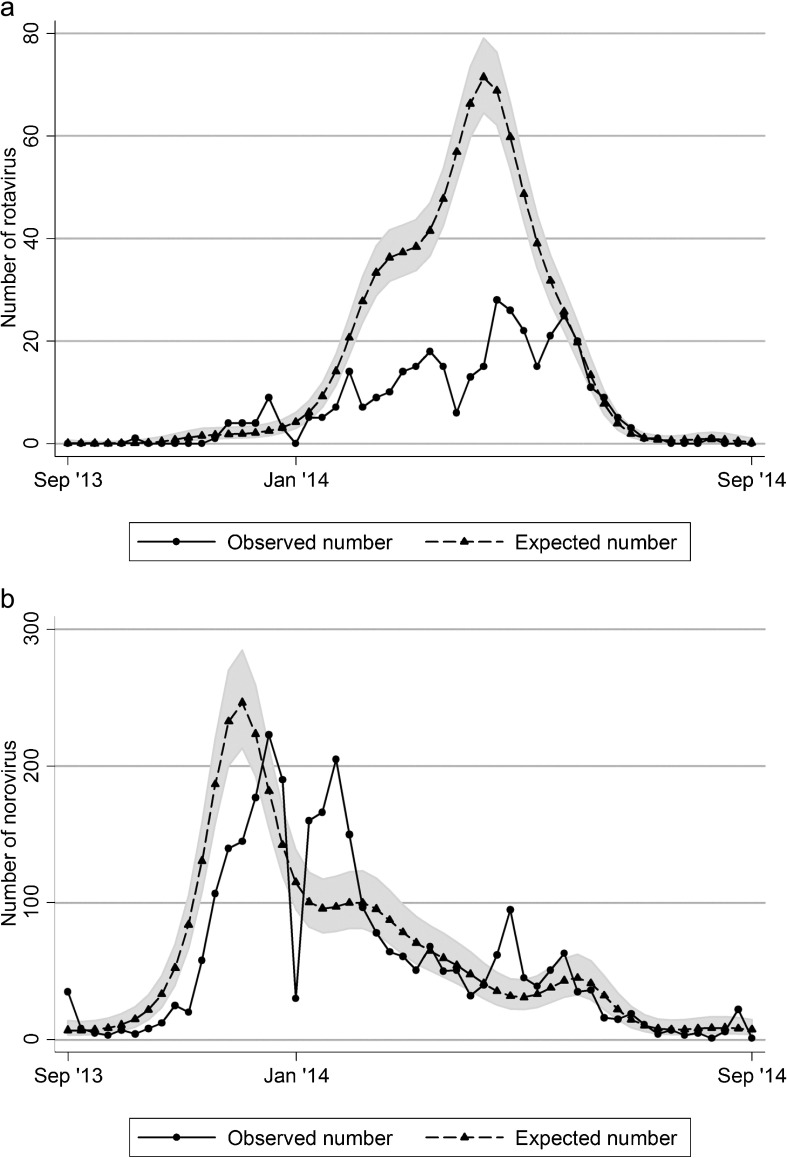
Compared with the expected number of rotavirus detection based on the preceding four years between the 36th week of 2009 and the 35th week of 2013, the number of rotavirus detection during the one-year between the 36th week of 2013 and the 35th week of 2014 was 42.9% (95% CI: 38.6, 47.8) (Table 1 and Fig. 2a). By contrast, there was virtually no change or only a slight decrease at best (92.7%; 95% CI: 89.0, 96.7) observed for the number of norovirus detection in the 2013/2014 season compared with the preceding three seasons (Fig. 2b). Thus, there was a reduction by 57% of the number of laboratory-confirmed rotavirus cases but little of norovirus from the expected number of cases.
Table 1.
Observed and expected number of rotavirus and norovirus positive samples between September (week 36) 2013 and August (week 35) 2014 in Japan.
| Observed (O) | Expected (E) | Ratio (O/E) | 95% CI* | |
|---|---|---|---|---|
| Rotavirus | 367 | 854.5 | 0.429 | 0.386, 0.478 |
| Norovirus | 3006 | 3239.7 | 0.927 | 0.890, 0.967 |
* confidence interval
Fig. 2.
Observed and expected number of a) rotavirus and b) norovirus positive samples in each week between September (week 36) 2013 and August (week 35) 2014 in Japan. The shaded area shows 95% confidence intervals of the expected numbers.
Although observational studies can never prove causality, the closeness of the timing of the decreased case detection to the timing of the vaccine introduction, the failure to explain the decrease in the number of cases by normal seasonality and no simultaneous marked reduction in the number of norovirus cases make causality the most likely explanation.
There are however a few limitations to this study. First, we adjusted only for seasonality when estimating the expected number of rotavirus-positive specimens. In other words, we assumed that the epidemiology of rotavirus would have remained the same if rotavirus vaccines had not been marketed. We also assumed the effect of the rotavirus vaccines was negligible in the first two rotavirus seasons during which Rotarix and RotaTeq were successively marketed and the uptake rates were expected to be very low. A modelling study simulating the rotavirus epidemiology in Japan suggests that even stochastic variation in the annual number of rotavirus cases can be as large as 29% from the average [10], but the 57% reduction we observed in the 2013/2014 season was far beyond the range of stochastic variation. Virtual lack of the reduction in the number of norovirus-positive specimens further assures that the 2013/2014 season was not unusually mild to explain the as large as 57% reduction in rotavirus-positive specimens.
Second, this analysis assumed that the vaccine was introduced and administered to all eligible children in September (in the 35th week) 2013, but the uptake rate of either vaccine was likely to remain approximately 50%. This would have biased the associations towards the null.
Third, as we entirely depended on the publicly available database, we were not able to disaggregate the dataset. Thus, for example, the change in age distribution of rotavirus-positive cases from which clinical specimens were derived was not evaluated. The changes in diagnostic practices or reporting practices, if any, were not known.
Despite all these limitations, this time-series analysis is simple to perform and quantitative to estimate the magnitude of the impact of rotavirus vaccination. The introduction of rotavirus vaccine in the national immunisation programme of Asian countries is far behind that of African countries, despite both continents having a high burden of rotavirus diarrhoea. For example, the Western Pacific Region Office of the World Health Organization funded and implemented the sentinel surveillance programme of laboratory-confirmed rotavirus cases in each country within the region since around 2012 in anticipating the introduction of rotavirus vaccines. Although the programme does not allow for calculation of the incidence rate of rotavirus diarrhoea, the data obtained through this surveillance should allow for a time-series analysis similar to that described in this paper with almost no extra cost. What is more important in this time-series analysis is to secure the dataset that allows sufficiently accurate calculation of the expected number of rotavirus-positive cases which can only be obtained before the vaccine introduction or during the period of limited use of the vaccine in the market.
In conclusion, this time-series analysis of a publicly available dataset on the weekly number of laboratory-confirmed rotavirus-positive cases over the last 5 year period, during which rotavirus vaccines became marketed and presumed to reach an uptake rate of at least 39% as a national average, strongly suggests that the use of rotavirus vaccine had a positive impact on reducing the burden of rotavirus diarrhoea in Japan. Because of its simplicity and little cost, it will be of considerable interest to examine this method for early detection of the impact of rotavirus vaccine in resource-poor countries where the World Health Organization funded and implemented the sentinel surveillance programme of laboratory-confirmed rotavirus cases.
Acknowledgements
This study was in part supported by a grant from the Japan Agency for Medical Research and Development.
Conflict of Interest
The authors have no conflict of interest to declare regarding this study.
References
- 1.Tate JE, Burton AH, Boschi-Pinto C, et al. . WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 136–141. [DOI] [PubMed] [Google Scholar]
- 2.Tate JE, Patel MM, Steele AD, et al. . Global impact of rotavirus vaccines. Expert Rev Vaccines 2010; 9: 395–407. [DOI] [PubMed] [Google Scholar]
- 3.Van Damme P, Giaquinto C, Huet F, et al. . Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis 2007; 195 Suppl 1: S4–S16. [DOI] [PubMed] [Google Scholar]
- 4.Nakagomi T, Kato K, Tsutsumi H, et al. . The Burden of Rotavirus Gastroenteritis among Japanese Children during Its Peak Months: an Internet Survey. Jpn J Infect Dis 2013; 66: 269–275. [DOI] [PubMed] [Google Scholar]
- 5.Nakagomi O, Iturriza-Gomara M, Nakagomi T, et al. . Incorporation of a rotavirus vaccine into the national immunisation schedule in the United Kingdom: a review. Expert Opin Biol Ther 2013; 13: 1613–1621. [DOI] [PubMed] [Google Scholar]
- 6.Aliabadi N, Tate JE, Haynes AK, et al. . Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination - United States, 2000–2014. Morb Mortal Wkly Rep 2015; 64: 337–342. [PMC free article] [PubMed] [Google Scholar]
- 7.Hanquet G, Ducoffre G, Vergison A, et al. . Impact of rotavirus vaccination on laboratory confirmed cases in Belgium. Vaccine 2011; 29: 4698–4703. [DOI] [PubMed] [Google Scholar]
- 8.Atchison C, Collins S, Brown D, et al. . Reduction in rotavirus disease due to the infant immunisation programme in England; evidence from national surveillance. J Infect 2015: doi: 10.1016/j.jinf.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume M, Wagatsuma Y, Faruque AS, et al. . Factors determining vulnerability to diarrhoea during and after severe floods in Bangladesh. J Water Health 2008; 6: 323–332. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Nakagomi T, Naghipour M, et al. . Modeling seasonal variation in rotavirus hospitalisations for use in evaluating the effect of rotavirus vaccine. J Med Virol 2010; 82: 1468–1474. [DOI] [PubMed] [Google Scholar]