Abstract
Background
The biological actions of various ginseng extracts have been studied for treating obesity and diabetes mellitus. However, few studies have evaluated the effects of fermented Korean Red Ginseng (Panax ginseng Meyer) on metabolic syndrome. The present study evaluated the antiobesity and antidiabetic effects of fermented red ginseng (FRG) on old-aged, obese, leptin-deficient (B6.V-Lepob, “ob/ob”) mice.
Methods
The animals were divided into three groups and given water containing 0%, 0.5%, and 1.0% FRG for 16 wk. The effect of FRG on ob/ob mice was determined by measuring changes in body weight, levels of blood glucose, serum contents of triglycerides, total cholesterol and free fatty acids, messenger RNA (mRNA) expressions of key factors associated with insulin action, such as insulin receptor (IR), lipoprotein lipase (LPL), glucose transporter 1 and 4 (GLUT1 and GLUT4), peroxisome proliferators-activated receptor gamma (PPAR-γ), and phosphoenolpyruvate carboxykinase (PEPCK) in the liver and in muscle, and histology of the liver and pancreas.
Results
FRG-treated mice had decreased body weight and blood glucose levels compared with control ob/ob mice. However, anti-obesity effect of FRG was not evident rather than hypoglycemic effect in old aged ob/ob mice. The hyperlipidemia in control group was attenuated in FRG-treated ob/ob mice. The mRNA expressions of IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK in the liver and in muscle were increased in the FRG-treated groups compared with the control group.
Conclusion
These results suggest that FRG may play a vital role in improving insulin sensitivity relative to reducing body weight in old-aged ob/ob mice.
Keywords: fermented red ginseng, hypoglycemic effect, insulin sensitivity, ob/ob mice, Panax ginseng
1. Introduction
Obesity is excessive fat accumulation that is associated with numerous health risks. A person with a body mass index of ≥30 is generally considered obese [1]. According to data from a previous survey, between 1999–2002 and 2007–2010, the age-adjusted prevalence of obesity among adults aged ≥18 yr increased from 26.5% to 33.0% among men and from 32.4% to 34.9% among women [2]. Obesity, a complex condition influenced by diet, developmental stage, age, physical activity, and genetic factors [3], is a major risk factor for a number of chronic diseases including diabetes mellitus, cardiovascular disease, and cancer [1]. In particular, type 2 diabetes mellitus (noninsulin dependent diabetes mellitus) is closely associated with obesity. Type 2 diabetes mellitus is caused by the ineffective use of insulin resulting from excess body weight and physical inactivity [4]. Along with the increased rates of obesity, there has been a parallel increase in the prevalence of type 2 diabetes mellitus and impaired glucose tolerance [5]. The causes of impaired glucose tolerance include insulin resistance associated with obesity and type 2 diabetes mellitus. The term “insulin resistance” is defined as resistance to the effects of insulin on the uptake, metabolism, or storage of glucose [6]. Pharmacological agents (e.g., oral hypoglycemic agents and insulin) and the use of complementary and alternative approaches for treating diabetes mellitus, such botanicals with antihyperglycemic activities, are being considered more frequently as treatment options by patients and healthcare professionals [7], [8]. Several studies on the use of herbal medicine for treating obesity and diabetes mellitus have been recently conducted [9], [10].
Ginseng has been used in traditional herbal medicine for >2000 yr in Asian countries including Korea, China, and Japan. Commercially available ginseng is broadly categorized into three types; (1) fresh ginseng is <4 yr old and has not been dried; (2) white ginseng is 4∼6 yr old, and has been peeled or either sun dried or oven dried; and (3) red ginseng is older than 6 yr and is steamed before being dried. The specific process for drying red ginseng results in a nonenzymatic color change that gives its characteristic hue. The various methods of preparing ginseng alter the formation and concentration of saponin within the plant [11]. A wide range of biological and biochemical actions of various ginseng extracts have been studied. Clinical studies have shown that ginseng improves psychological functions, immune functions, and conditions associated with diabetes mellitus [8], [12], [13], [14], [15]. Asian ginseng is known to facilitate blood flow, alleviate fatigue, and relieve oxidative stress under diabetic conditions through various mechanisms such as the inhibition of lipid peroxidation [13]. The berry of American ginseng (Panax quinquefolius L.) exerts significant antidiabetic and hypoglycemic effects in C57BL/6J ob/ob and C57BL/Ks db/db mice [12], [14]. Leptin-deficient ob/ob mice are obese, hyperphagic, type 2 diabetic, have decreased body temperature, hypogonadotropic, and suffer from hypogonadism [16]. Oral administration of American ginseng berry juice significantly reduced high blood glucose levels and body weight in ob/ob mice [8]. These studies showed that ginseng may be a suitable therapy for treating diabetes mellitus. Fermented red ginseng (FRG) is treated with microorganisms and enzymes that increase the saponin content for maximum efficacy [15]. FRG reduced the blood glucose level in streptozotocin-induced diabetic rats [17]. Although many studies have examined the properties of ginseng, no research has been conducted to evaluate the effects of FRG on obesity and diabetes mellitus in old-aged (>24-wk-old), obese mice. The purpose of the present study was to examine the effects of FRG on body weight, blood glucose, serum contents of triglycerides, total cholesterol and free fatty acids, changes in hepatic and pancreatic histology, and messenger RNA (mRNA) expression of molecules related to metabolism and insulin resistance in old-aged ob/ob mice.
2. Materials and methods
2.1. Preparation of FRG
Two liters of distilled water was added to 300 g of 6-yr-old white dried Korean ginseng (Panax ginseng Meyer) provided by the Samkwang Company (Daejeon, Korea), and steamed for 24 h. A further 4 L of distilled water was added, and the ginseng solution was steamed at 90°C for 48 h to make the red ginseng (RG) extracts. The RG extracts were used to make FRG extracts by the addition of red yeast rice (Monascus purpureus, CM12002) and 12 h of fermentation at 40°C. The FRG was stored at −20°C until use. The FRG contained 4.8 mg Rb1, 3.4 mg Rb2, 0.8 mg Rg1, 2.9 mg Rg2, 4.9 mg Rg3, 1.8 mg Re, and 1.4 mg compound K per gram. The voucher specimen was deposited in the Veterinary Toxicology Laboratory of Kyungpook National University (Daegu, Korea).
2.2. Animals
Twenty-four-wk-old, female, leptin-deficient (B6.V-Lepob, ‘ob/ob’) mice were obtained from the Korean Research Institute of Bioscience and Biotechnology (Daejeon, Korea). The animals were housed in an environmentally controlled room with a 12-h light/dark cycle at 22°C and relative humidity of 50 ± 5%. The mice were fed a sterilized (2M rad radiation) pellet diet (Purina, Seoul, Korea) and had access to sterilized water ad libitum. All animal experiment protocols were approved by the Institutional Animal Care and Use Committee of Kyungpook National University.
2.3. Experimental design
A total of 18 female mice were used. The animals were divided into three groups (n = 6 mice in each group). Group I animals received drinking water (filtered tap water) and served as the untreated control group. Group II animals received drinking water containing 0.5% FRG and Group III mice received drinking water with 1% FRG for 16 wk. The effect of FRG on ob/ob mice was determined by measuring changes in body weight biweekly. Blood glucose levels were measured using a glucometer (Super Glucocard II, ARKRAY INC., Kyoto, Japan) after 3 h of fasting. Serum triglycerides were measured using the Wako L-Type TG M (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and serum total cholesterol levels were measured using the Wako Cholesterol E (Wako Pure Chemical Industries, Ltd.). Serum free fatty acids levels were measured by an enzymatic colormetric method using Wako NEFA-HR2 reagents (Wako Pure Chemical Industries, Ltd.). Finally, the animals were sacrificed, and the liver and skeletal muscle (quadriceps) were quickly removed for RNA isolation.
2.4. Determination of insulin receptor, lipoprotein lipase, glucose transporter 1/4, peroxisome proliferators-activated receptor gamma, and phosphoenolpyruvate carboxykinase mRNA expression
Total RNA was isolated from fresh liver and skeletal muscle using Easy-BLUE (iNtRON Biotec, Seongnam, Korea) and stored frozen at −70°C. Aliquots of 0.5∼5 μL of total RNA from each sample were reverse-transcribed to complementary DNA (cDNA) using a first-strand cDNA synthesis kit (Fermentas, Vilnius, Lithuania). Polymerase chain reaction (PCR) amplification of 1∼4 μL of cDNA was carried out in a final volume of 20 μL including primer and distilled water, and using a AccuPower PCR Premix (Bioneer, Daejeon, Korea). Sequences of the primers used were as follows: glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: 5ʹ-CCATCAACGACCCCTTCATTGACC-3ʹ and reverse: 5ʹ-TGGTTCACACCCATCACAAACATG-3ʹ), insulin receptor (IR; forward: 5ʹ-ATGGACATCCGGAACAACCT-3ʹ and reverse: 5ʹ-TTGATGACAGTGGCAGGACA-3ʹ), lipoprotein lipase (LPL; forward: 5ʹ-ATGGAGAGCAAAGCCCTGC-3ʹ and reverse: 5ʹ-AGTCCTTCTGCAATCAC-3ʹ), glucose transporter 1 (GLUT1; forward: 5ʹ-AGGCTTGCTTGTAGAGTGAC-3ʹ and reverse: 5ʹ-TAAGGATGCCAACGACGATTCC-3ʹ), glucose transporter 4 (GLUT4; forward: 5ʹ-CAACGTGGCTGGGTAGGCA-3ʹ and reverse: 5ʹ-ACACATCAGCCCAGCCGGT-3ʹ), peroxisome proliferator-activated receptor gamma (PPAR-γ; forward: 5ʹ-GGTGAAACTCTGGGAGATTC-3ʹ and reverse: 5ʹ-CAACCATTGGGTCAGGCTT-3ʹ), and phosphoenolpyruvate carboxykinase (PEPCK; forward: 5ʹ-AGCGGATATGGTGGGAAC-3ʹ and reverse: 5ʹ-GGTCTCCACTCCTTGTTC-3ʹ). The standard amplification program included an initial denaturing step at 94°C for 5 min, 30–40 cycles of denaturing at 94°C for 30 s, annealing at 55∼60°C for 30 s, and extending at 72°C for 45–60 s; and a final extension step at 72°C for 5 min. The PCR products were separated by electrophoresis on a 1–1.5% agarose gel, stained with ethidium bromide, and viewed under UV light using the gel-doc system (WiseDoc, Daihan Scientific Co., Seoul, Korea). The abundance of each type of mRNA was normalization relative to GAPDH mRNA.
2.5. Histological examination
The liver and pancreas were removed, fixed with 4% paraformaldehyde, and embedded in paraffin. Paraffin block samples were cut into 5-μm sections, and stained with hematoxylin and eosin (H&E) stain and anti-insulin antibody for histological observation.
2.6. Statistical analysis
The results are presented as the mean ± standard deviation. Data were analyzed using SAS software (version 9.3; SAS Institute Inc., Cary, NC, USA). Statistically significant differences between the control and FRG-treated groups were analyzed using the Student t test and analysis of variance. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Body weight changes
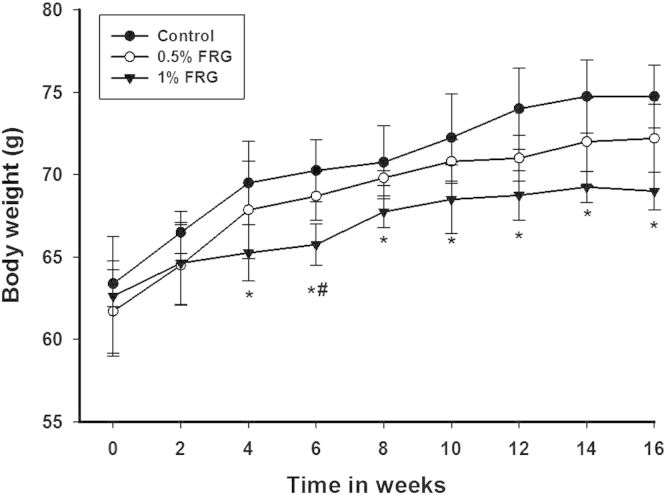
Changes in body weight of the experiment groups during the 16-wk FRG treatment period are shown in Fig. 1. The body weight of the 0.5% FRG-treated groups was time-dependently decreased compared with the control group, but no statistical significance was found. The body weight of animals treated with 1.0% FRG significantly decreased compared with the controls after 4 wk of administration.
Fig. 1.
Effect of fermented red ginseng (FRG) treatment on body weight in ob/ob mice for 16 wk. Values are the mean ± standard deviation from six animals in each group. The animals were divided into three groups: control group, 0.5% FRG group, and 1% FRG group. * Significantly different from control (p < 0.05). ** Significantly different from 0.5% FRG (p < 0.05).
3.2. Blood glucose levels
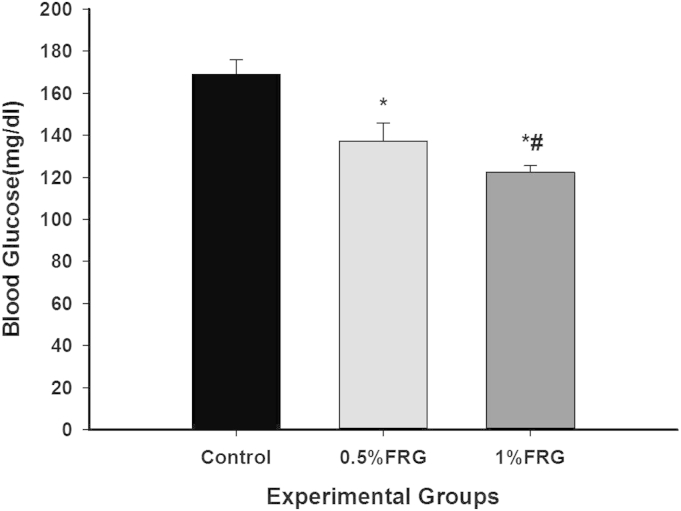
The effects of FRG treatment on blood glucose in the ob/ob mice are presented in Fig. 2. Dose-dependent decreases in blood glucose levels after 3 h of fasting were observed after 16 wk of FRG treatment. However, the blood glucose level of the control group was consistently moderate hyperglycemia.
Fig. 2.
Effect of fermented red ginseng (FRG) treatment on blood glucose level in ob/ob mice for 16 wk. Values are the mean ± standard deviation from six animals in each group. The animals were divided into three groups: control group, 0.5% FRG group, and 1% FRG group. Blood glucose levels were measured using a glucometer (Super Glucocard II, ARKRAY INC., Kyoto, Japan) after 3 h of fasting. * Significantly different from control (p < 0.05). ** Significantly different from 0.5% FRG (p < 0.05).
3.3. Serum contents of triglycerides, total cholesterol, and free fatty acids
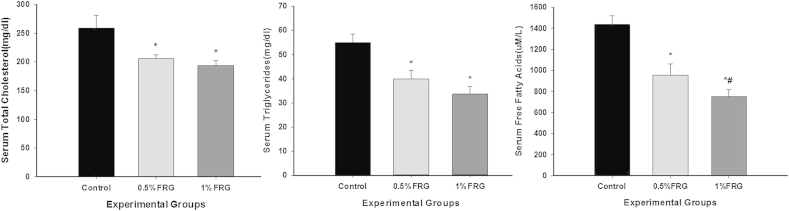
The effects of FRG treatment on the serum contents of triglycerides, total cholesterol, and free fatty acids in the ob/ob mice are presented in Fig. 3. FRG markedly lowered the triglycerides, cholesterol, and free fatty acids in the serum of ob/ob mice after the 16-wk treatment. However, ob/ob mice in the control group had moderate hyperlipidemia.
Fig. 3.
Effects of fermented red ginseng (FRG) treatment on serum levels of triglycerides, total cholesterol, and free fatty acids in ob/ob mice for 16 wk. Values are the mean ± standard deviation from six animals in each group. The animals were divided into three groups: control group, 0.5% FRG group, and 1% FRG group. * Significantly different from control (p < 0.05). ** Significantly different from 0.5% FRG (p < 0.05).
3.4. IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK mRNA expression in skeletal muscle
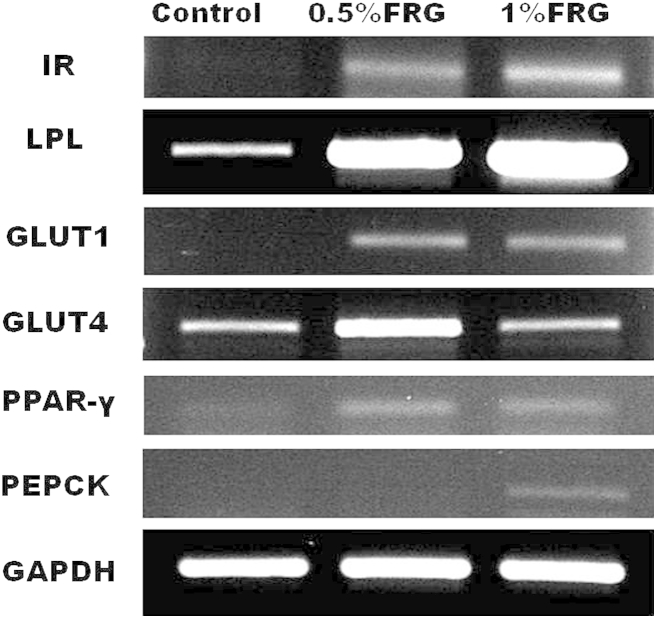
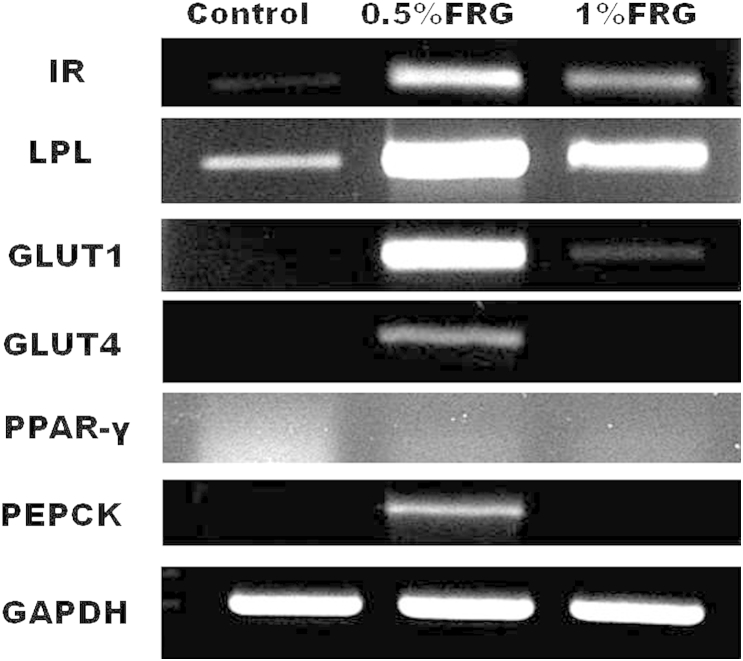
Fig. 4 shows the effect of FRG on IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK mRNA expression in skeletal muscle. The mRNA expressions of IR, LPL, and GLUT1 in muscle increased in a dose-dependent manner in the FRG-treated groups compared with the control group. GLUT4 and PPAR-γ mRNA expression in muscle was remarkably increased in the 0.5% FRG-treated group compared with the control group. In particular, the GLUT4 mRNA expression level of the control group and 1% FRG-treated group were almost the same. PEPCK mRNA expression in muscle increased in the 1% FRG-treated group compared with the control group.
Fig. 4.
Insulin receptor (IR), lipoprotein lipase (LPL), glucose transporter 1 and 4 (GLUT1 and GLUT4), peroxisome proliferators-activated receptor gamma (PPAR-γ), and phosphoenolpyruvate carboxykinase (PEPCK) messenger RNA (mRNA) expression in skeletal muscle. The mRNA expressions of IR, LPL, and GLUT1 in muscle increased in a dose-dependent manner in fermented red ginseng (FRG)-treated groups compared with the control group. GLUT4 and PPAR-γ mRNA expressions in muscle were remarkably increased in the 0.5% FRG-treated group compared with the control group and the 1% FRG-treated group.
3.5. IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK mRNA expression in the liver
Fig. 5 shows the effect of FRG on IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK mRNA expression in the liver. The IR and LPL mRNA expressions in the liver were increased in the FRG-treated groups compared with the control group. However, the levels of mRNA expression of IR and LPL in the 0.5% FRG-treated group were increased compared with those of the 1% FRG-treated group. The hepatic GLUT1, GLUT4, and PEPCK mRNA expressions in the 0.5% FRG-treated group were only increased compared with the control group. GLUT1, GLUT4, and PEPCK mRNA expressions in the liver were not detected in the 1.0% FRG-treated group and the control group. In addition, the hepatic PPAR-γ mRNA expression was not detected in all experimental groups.
Fig. 5.
Insulin receptor (IR), lipoprotein lipase (LPL), glucose transporter 1 and 4 (GLUT1 and GLUT4), peroxisome proliferators-activated receptor gamma (PPAR-γ), and phosphoenolpyruvate carboxykinase (PEPCK) messenger RNA (mRNA) expression in the liver. The IR and LPL mRNA expressions in the liver were increased in the fermented red ginseng (FRG)-treated groups compared with the control group. However, the levels of mRNA expression of IR and LPL in the 0.5% FRG-treated group were increased compared with those of the 1% FRG-treated group. The hepatic GLUT1, GLUT4, and PEPCK mRNA expressions in the 0.5% FRG-treated group were only increased compared with the control group.
3.6. Liver histology
The H&E stained liver sections in FRG treated ob/ob mice are shown in Fig. 6. In the control group, the histology showed fatty changes, swelling, and extensive necrosis of hepatocytes in whole regions. By contrast, FRG treatment showed less severe lobular damage of hepatocytes in a dose-dependent manner compared with the control group.
Fig. 6.
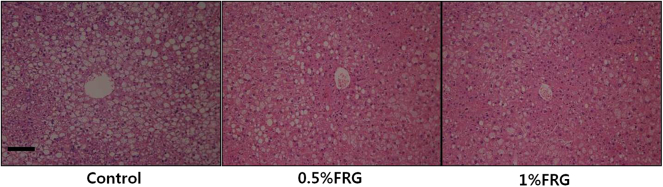
Histology of the liver in ob/ob mice treated with fermented red ginseng (FRG) for 16 wk. Hematoxylin and eosin stain. In the control group, the histology showed fatty changes and swelling of hepatocytes in whole regions. In contrast, FRG treatment showed less severe lobular damage of hepatocytes in a dose-dependent manner compared with the control group. Original magnification, 200×. Scale bar, 100 μm.
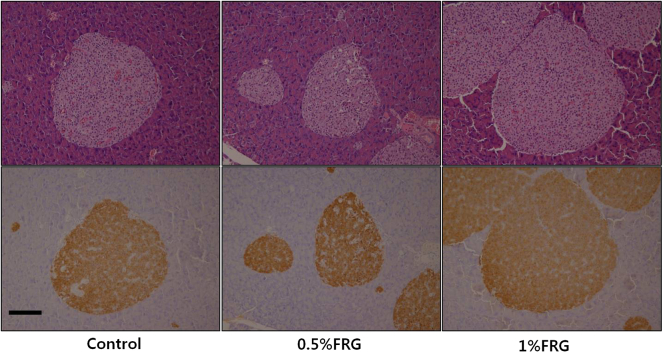
3.7. Histology and immunohistochemical evaluation of the pancreas
H&E staining of the pancreas did not show any differences in pancreatic histology between the FRG-treated groups and control group (Fig. 7, upper panel). Results of immunohistochemical staining of the insulin (Fig. 7, lower panel) were not significantly different between the FRG-treated groups and control group. These findings indicated that FRG did not directly affect insulin production and morphology of β-cells.
Fig. 7.
Histology and immunohistochemical staining for insulin of the pancreas in ob/ob mice treated with fermented red ginseng (FRG) for 16 wk. Hematoxylin and eosin staining of the pancreas did not show any differences in histological appearance between the FRG-treated groups and control group (upper panel). Results of immunohistochemical staining of the insulin were not significantly different between the FRG-treated groups and control group (lower panel). Original magnification, 400×. Scale bar, 100 μm.
4. Discussion
We conducted this study to evaluate the antiobesity and antidiabetic effects of FRG in old-aged, ob/ob mice. We confirmed that treatment with FRG alters body weight, blood glucose levels, mRNA expression of genetic hallmarks of insulin resistance, and histologic changes. Along the age, obesity and metabolic diseases including diabetes mellitus have different pathologic conditions. The risk of developing diabetes mellitus increases with age, particularly because in humans older adults (>50 yr old) have a greater tendency to be obese [18]. Obesity is abnormal or excessive fat accumulation and is associated with many metabolic diseases such as diabetes mellitus, cardiovascular disease, hyperlipidemia, and hypertension. Obesity is frequently accompanied by insulin resistance which leads to the development of type 2 diabetes mellitus. Insulin resistance is characterized by impaired glucose uptake. Both decreasing insulin resistance and increasing insulin sensitivity are important for the treatment of type 2 diabetes mellitus. Currently available drugs for type 2 diabetes mellitus have a number of limitations such as adverse effects and high rates of secondary failure [19]. Medicinal herbs are expected to have a similar degree of efficacy without the side effects associated with conventional drug treatment such as skin irritation and diarrhea [20]. Clinical studies have shown that ginseng improves pathologic conditions associated with diabetes mellitus [12], [14]. FRG has been treated with red yeast rice to enhance the ginseng saponin content [15]. Red yeast rice is a bright, reddish–purple, fermented rice and acquires its color by being cultivated with the mold Monascus purpureus. This type of rice is also used for the production of several Chinese wines, Japanese sake (akaisake), and Korean rice wine (hongju).
Ob/ob mice are a model of obesity and type 2 diabetes mellitus [21]. These mice are insulin resistant due to a mutation in the gene that encodes leptin [19], and obesity in ob/ob mice is directly caused by leptin signaling abnormalities such as uncontrolled appetite, hyperphagia, and reduced energy expenditure mediated by insulin resistance hallmarks [22]. The pathophysiological characteristics of ob/ob mice resemble those of humans with type 2 diabetes mellitus. Because onset and type of obesity and diabetes differs according to age, the management and treatment of these disorders should vary according to the relative age. Most animal studies of obesity and diabetes mellitus were carried out using young or young adult (4-wk-old to 12-wk-old) mice, and to the best of our knowledge, no ginseng-related research using aged ob/ob mice >13 wk old exists, therefore, we used 24-wk-old ob/ob mice for the present study.
In this study, significant decreases in body weight and blood glucose levels were observed after the administration of 1.0% FRG in ob/ob mice. Body weight loss and the hypoglycemic effects of FRG in ob/ob mice were dose-dependent. Given these results, we can confirm that the hypoglycemic effect of FRG was evident rather than the antiobesity effect in old-aged ob/ob mice.
IR mRNA expression in the FRG-treated groups increased, indicating that FRG improved insulin sensitivity. Mice with heterozygotic deletions of both the IR and insulin receptor substrate 1 (IRS-1) show severely impaired insulin action. Furthermore, progeny that are homozygous for both IR and IRS-1 deletions eventually develop diabetes mellitus [23]. The results of the present study showed that FRG may improve insulin sensitivity by increasing IR expression.
LPL mRNA expression in skeletal muscle and in the liver were also increased in the FRG-treated groups compared with the control group. LPL is an enzyme that is synthesized in adipose tissue and muscle. This factor plays a key role in regulating the entry of triglycerides into muscle and adipose tissue [24]. The importance of LPL in fuel partitioning and utilization is underscored by the finding that tissue-specific perturbations in LPL activity result in dramatic shifts in body composition as well as lipid and glucose metabolism, particularly in heart and skeletal muscle [25]. LPL levels in muscle appear to correlate with insulin resistance [26]. LPL depletion in skeletal muscle reduces lipid storage and increases insulin signaling without inducing changes in body composition [27]. Moreover, a lack of LPL expression in skeletal muscle results in insulin resistance in other key metabolic tissues, and ultimately leads to obesity and systemic insulin resistance [27]. By contrast, reduced LPL delivery in skeletal muscle increases insulin sensitivity in skeletal muscle [28]. In the present study, LPL mRNA expression levels in the liver showed that FRG-treated mice had increased insulin sensitivity. However, LPL mRNA levels in muscle were also increased in the FRG-treated groups. Other studies examining the role of LPL in the development of insulin resistance have been performed in vitro, or used different models of diabetes mellitus other than the ob/ob mouse [27], [29]. These differences in experimental methods may have affected LPL mRNA expression in muscle, and should be further evaluated in future studies.
In the present study, FRG treatment increased GLUT1 and GLUT4 mRNA expression in ob/ob mice. One of the most important effects of insulin is the stimulation of GLUT expression in cells. Studies of GLUT1–5 have shown that each has a specific role in the control of sugar homeostasis due to tissue-specific expression, kinetic characteristics, substrate specificity, or mechanisms regulating cell surface expression [6], [30], [31], [32], [33], [34], [35]. GLUT1 is a nucleotide binding protein that, when bound to ATP, has a reduced glucose import capacity but increased affinity for sugars [34]. Sugar transport in astrocytes, vascular smooth muscle cells, basal cardiomyocytes, and cells of the reticuloendothelial system are mediated by GLUT1 [33]. The very high GLUT1 content of higher primate and odontocete erythrocytes may contribute to glucose transfer from the blood to tissue [30]. The role of GLUT4 downregulation in the pathogenesis of insulin resistance and glucose tolerance has been confirmed in mice with muscle-specific ablation of GLUT4 [32]. Conversely, overexpression of GLUT4 in adipose tissue enhances whole body insulin sensitivity and glucose tolerance [31]. The ability of insulin to reduce blood glucose levels results from the suppression of hepatic glucose production and increased glucose uptake in muscle and adipose tissue via GLUT4 [6]. The results of the present study showed that FRG improved insulin sensitivity by increasing the expression of both GLUT1 and GLUT4.
In skeletal muscle, PPAR-γ mRNA expression levels were increased in the FRG-treated groups. PPAR-γ is a member of the nuclear hormone receptor superfamily of ligand-dependent transcription factors and regulates the expression of genes involved in insulin signaling and lipid metabolism [36], [37]. This subtype is known to play a pivotal role in lipid and carbohydrate metabolism, and has been implicated in various metabolic and inflammatory disorders such as dyslipidemia, atherosclerosis, diabetes mellitus, and obesity [38]. PPAR-γ is known to have a specific role in the etiology of insulin resistance [36], [39]. Muscle-specific PPAR-γ downregulation in mice causes insulin resistance [40]. Our results showed that FRG decreased insulin resistance by increasing PPAR-γ expression.
PEPCK mRNA expressions in muscle were increased in mice treated with water containing 1% FRG. PEPCK mRNA expression levels in the liver were increased in animals administered with water containing 0.5% FRG. PEPCK is an enzyme that catalyzes the conversion of oxaloacetate and ATP into phosphoenolpyruvate, carbon dioxide, and ADP. This factor has two forms: cytosolic and mitochondrial. Skeletal muscle has a small but significant level of the cytosolic form of PEPCK (PEPCK-C) activity [41], which plays a role in glyceroneogenesis in skeletal muscle. This tissue contains triglycerides, and glyceroneogenesis is the main contributor to triglyceride glycerol formation [42]. It has the effect of hyperactivity on glucose utilization in skeletal muscle (increased insulin sensitivity), and adipose tissue is reduced (reduced leptin formation) in the PEPCK-Cmus mice (transgenic mice were generated using PEPCK-C cDNA which was linked to the α-skeletal actin gene promoter). The increased concentration of triglycerides in the skeletal muscle of PEPCK-Cmus mice is caused by elevated PEPCK-C activity, which increases the rate of glyceroneogenesis [43]. Deletion of the PEPCK-C gene in mouse liver leads to ablated hepatic gluconeogenesis [44]. Our results showed that FRG improved glyceroneogenesis and increased insulin sensitivity in muscle, improved gluconeogenesis in the liver.
All of the genetic changes observed in the present study indicate that FRG treatment decreased insulin resistance and increased glucose utilization. Some of these changes in gene expression due to FRG were dose-dependent. Although we suppose that differences in efficacy of FRG due to differences in FRG intake volume, but volume of water intake during the experimental period was no notable difference (data not shown). Additional studies with various concentrations of FRG need to be conducted to further elucidate the dose-dependent effects of FRG or its active compound.
Cha et al. [45] reported that the serum contents of total lipid, triglyceride, total cholesterol, HDL-cholesterol, and free fatty acids tended to be lowered in the FRG group compared with high-fat diet-induced hyperlipidemia in rats [45]. This study showed that FRG markedly lowered the triglycerides, total cholesterol, and free fatty acids levels in the serum of ob/ob mice. Liver histology showed that FRG treatment ameliorates hepatic pathological changes such as fatty changes, swelling, and cytoplasmic vacuolization in ob/ob mice. Therefore, FRG has an effect on the attenuation of hyperlipidemia and appears to exert a hepatic regenerative effect on the histological appearance of the liver in obese and diabetes mellitus conditions. Immunohistochemistry of β–cells staining showed that FRG did not change the morphology of β-cells in old-aged ob/ob mice.
Previous studies have found that various types of ginseng reduce body weight in animals with obesity [12], [14], [17], [46]. In this study, however, FRG treatment less affected the body weight of old-aged ob/ob mice. Previous studies mostly used young or young-adult (6∼10-wk-old) animals. However, we used relatively older (24-wk-old) animals in this study. We assume that the age of animals and the onset and duration of obesity are important factors in the antiobesity effect in these studies. Further studies about the antiobesity effect of FRG in young animals are necessary.
In conclusion, we showed that the administration of FRG significantly reduced blood glucose and increased the expression of IR, LPL, GLUT1, GLUT4, PPAR-γ, and PEPCK mRNA in key metabolic tissues of old-aged ob/ob mice, indicating that FRG improved insulin sensitivity. Decreased blood glucose levels and reduced insulin resistance are key characteristics of treatments for diabetes mellitus, hence, FRG could be an effective antidiabetic agent, and may possibly be used as an alternative to supportive treatment for type 2 diabetes mellitus in old-aged individuals.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was supported by Kyungpook National University Research Fund (2012), and in part by a grant from the Medical Cluster R&D Support Project of Daegu Gyeongbuk Medical Innovation Foundation, Republic of Korea (HI13C1035, 2013).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.World Health Organization. Obesity. Available from: http://www.who.int/topics/obesity/en/. [2015.03.08]
- 2.May A.L., Freedman D., Sherry B., Blanck H.M. Obesity—United States, 1999–2010. MMWR Surveill Summ. 2013;62:120–128. [PubMed] [Google Scholar]
- 3.Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Diabetes. Available from: http://www.who.int/topics/diabetes_mellitus/en/. [2015.03.08]
- 5.Rose D.P., Haffner S.M., Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev. 2007;28:763–777. doi: 10.1210/er.2006-0019. [DOI] [PubMed] [Google Scholar]
- 6.Singla P., Bardoloi A., Parkash A.A. Metabolic effects of obesity: a review. World J Diabetes. 2010;1:76–88. doi: 10.4239/wjd.v1.i3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee L. Introducing herbal medicine into conventional health care settings. J Nurse Midwifery. 1999;44:253–266. doi: 10.1016/s0091-2182(99)00042-7. [DOI] [PubMed] [Google Scholar]
- 8.Cicero A.F.G., Derosa G., Gaddi A. What do herbalists suggest to diabetic patients in order to improve glycemic control? Evaluation of scientific evidence and potential risks. Acta Diabetol. 2004;41:91–98. doi: 10.1007/s00592-004-0150-2. [DOI] [PubMed] [Google Scholar]
- 9.Hasani-Ranjbar S., Larijani B., Abdollahi M. A systematic review of Iranian medicinal plants useful in diabetes mellitus. Arch Med Sci. 2008;4:285–292. [Google Scholar]
- 10.Hasani-Ranjbar S., Nayebi N., Larijani B., Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009;15:3073–3085. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.T., Jang J.H., Kwon J.H., Moon K.D. Change in the chemical components of red and white ginseng after puffing. Korean J Food Preserv. 2009;16:355–361. [Google Scholar]
- 12.Xie J.T., Zhou Y.P., Dey L., Attele A.S., Wu J.A., Gu M., Polonsky K.S., Yuan C.S. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine. 2002;9:254–258. doi: 10.1078/0944-7113-00106. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S.J., Li C.Y., Qian Y.C., Luo X.P., Zhang X., Wang X.S., Kang B.Y. Induction of hairy roots of Panax ginseng and studies on suitable culture condition of ginseng hairy roots. Chin J Biotechnol. 2004;20:215–220. [PubMed] [Google Scholar]
- 14.Xie J.T., Wang C.Z., Ni M., Wu J.A., Mehendale S.R., Aung H.H., Foo A., Yuan C.S. American ginseng berry juice intake reduces blood glucose and body weight in ob/ob mice. J Food Sci. 2007;72:S590–S594. doi: 10.1111/j.1750-3841.2007.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung J.W., Kang H.R., Ji G.E., Park M.S., Song W.J., Kim M.H., Kwon J.W., Kim T.W., Park H.W., Cho S.H. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011;3:103–110. doi: 10.4168/aair.2011.3.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T., Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 17.Kim H.J., Chae I.G., Lee S.G., Jeong H.J., Lee E.J., Lee I.S. Effects of fermented red ginseng extracts on hyperglycemia in streptozotocin-induced diabetic rats. J Ginseng Res. 2010;34:104–112. doi: 10.5142/jgr.2011.35.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biggs M.L., Mukamal K.J., Luchsinger J.A., Ix J.H., Carnethon M.R., Newman A.B., de Boer I.H., Strotmeyer E.S., Mozaffarian D., Siscovick D.S. Association between adiposity in midlife and older age and risk of diabetes in older adults. J Am Med Assoc. 2010;303:2504–2512. doi: 10.1001/jama.2010.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan C.S., Attele A.S., Zhang L., Lynch J.P., Xie J.T., Shi J.Q. Leptin reduces body weight gain in neonatal rats. Pediatr Res. 2000;48:380–383. doi: 10.1203/00006450-200009000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Kim S.H., Hyun S.H., Choung S.Y. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 21.Muoio D.M., Lynis D.G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–666. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 22.Lindstrom P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brüning J.C., Winnay J., Bonner-Weir S., Taylor S.I., Accili D., Kahn C.R. Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell. 1997;88:561–572. doi: 10.1016/s0092-8674(00)81896-6. [DOI] [PubMed] [Google Scholar]
- 24.Eckel R.H. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989;320:1060–1068. doi: 10.1056/NEJM198904203201607. [DOI] [PubMed] [Google Scholar]
- 25.Zechner R. The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr Opin Lipidol. 1997;8:77–88. doi: 10.1097/00041433-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Pulawa L.K., Eckel R.H. Overexpression of muscle lipoprotein lipase and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2002;5:569–574. doi: 10.1097/00075197-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Wang H., Knaub L.A., Jensen D.R., Jung D.Y., Hong E.G., Ko H.J., Coates A.M., Goldberg I.J., Becky A., Janssen R.C. Skeletal muscle-specific deletion of lipoprotein lipase enhances insulin signaling in skeletal muscle but causes insulin resistance in liver and other tissues. Diabetes. 2009;58:116–124. doi: 10.2337/db07-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz W.S., Kwon G., Marshall C.A., McDaniel M.L., Semenkovich C.F. Glucose and insulin stimulate heparin-releasable lipoprotein lipase activity in mouse islets and INS-1 cells. J Biol Chem. 2001;276:12162–12168. doi: 10.1074/jbc.M010707200. [DOI] [PubMed] [Google Scholar]
- 29.Lopez V., Saraff K., Medh J.D. Down-regulation of lipoprotein lipase increases glucose uptake in L6 muscle cells. Biochem Biophys Res Commun. 2009;389:34–39. doi: 10.1016/j.bbrc.2009.08.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craik J.D., Young J.D., Cheeseman C.I. GLUT-1 mediation of rapid glucose transport in dolphin (tursiops truncatus) red blood cells. Am J Physiol Regul Integr Comp Physiol. 1998;274:R112–R119. doi: 10.1152/ajpregu.1998.274.1.R112. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd P.R., Kahn B.B. Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 32.Zisman A., Peroni O.D., Abel E.D., Michael M.D., Mauvais-Jarvis F., Lowell B.B., Wojtaszewski J.F.P., Hirshman M.F., Virkamaki A., Goodyear L.J. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000;6:924–928. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- 33.Hruz P.W., Mueckler M.M. Structural analysis of the GLUT1 facilitative glucose transporter. Mol Membr Biol. 2001;18:183–193. doi: 10.1080/09687680110072140. [DOI] [PubMed] [Google Scholar]
- 34.Levine K.B., Cloherty E.K., Hamill S., Carruthers A. Molecular determinants of sugar transport regulation by ATP. Biochemistry. 2002;41:12629–12638. doi: 10.1021/bi0258997. [DOI] [PubMed] [Google Scholar]
- 35.Thorens B., Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2010;298:E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barroso I., Gurnell M., Crowley V.E.F., Agostini M., Schwabe J.W., Soos M.A., Maslen G.L.I., Williams T.D.M., Lewis H., Schafer A.J. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 37.Herzig S., Hedrick S., Morantte I., Koo S.H., Galimi F., Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2003;426:190–193. doi: 10.1038/nature02110. [DOI] [PubMed] [Google Scholar]
- 38.Rangwala S.M., Lazar M.A. Peroxisome proliferator-activated receptor γ in diabetes and metabolism. Trends Pharmacol Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 39.Rangwala S.M., Rhoades B., Shapiro J.S., Rich A.S., Kim J.K., Shulman G.I., Kaestner K.H., Lazar M.A. Genetic modulation of PPAR γ phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 40.Hevener A.L., He W., Barak Y., Le J., Bandyopadhyay G., Olson P., Wilkes J., Evans R.M., Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 41.Newsholme E.A., Williams T. The role of phosphoenolpyruvate carboxykinase in amino acid metabolism in muscle. Biochem J. 1978;176:623–626. doi: 10.1042/bj1760623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nye C.K., Hanson R.W., Kalhan S.C. Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat. J Biol Chem. 2008;283:27565–27574. doi: 10.1074/jbc.M804393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Kalhan S.C., Hanson R.W. What is the metabolic role of phosphoenolpyruvate carboxykinase? J Biol Chem. 2009;284:27025–27029. doi: 10.1074/jbc.R109.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.She P., Burgess S.C., Shiota M., Flakoll P., Donahue E.P., Malloy C.R., Sherry A.D., Magnuson M.A. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes. 2003;52:1649–1654. doi: 10.2337/diabetes.52.7.1649. [DOI] [PubMed] [Google Scholar]
- 45.Cha J.Y., Park J.C., Ahn H.Y., Eom K.E., Park B.K., Jun B.S., Lee C.H., Cho Y.S. Effect of Monascus purpureus-Korean Red Ginseng power on the serum lipid levels and antioxidative activity in rats. J Korean Soc Food Sci Nutr. 2009;38:1153–1160. [Google Scholar]
- 46.Mollah M.L., Kim G.S., Moon H.K., Chung S.K., Cheon Y.P., Kim J.K., Kim K.S. Antiobesity effects of wild ginseng (Panax ginseng CA Meyer) mediated by PPAR-γ, GLUT4 and LPL in ob/ob mice. Phytother Res. 2009;23:220–225. doi: 10.1002/ptr.2593. [DOI] [PubMed] [Google Scholar]