Abstract
Background
Ginseng has been used as a tonic for invigoration of the human body. In a previous report, we identified a novel candidate responsible for the tonic role of ginseng, designated gintonin. Gintonin induces [Ca2+]i transient in animal cells via lysophosphatidic acid receptor activation. Gintonin-mediated [Ca2+]i transient is linked to anti-Alzheimer's activity in transgenic Alzheimer's disease animal model. The previous method for gintonin preparation included multiple steps. The aim of this study is to develop a simple method of gintonin fraction with a high yield.
Methods
We developed a brief method to obtain gintonin using ethanol and water. We extracted ginseng with fermentation ethanol and fractionated the extract with water to obtain water-soluble and water-insoluble fractions. The water-insoluble precipitate, rather than the water-soluble supernatant, induced a large [Ca2+]i transient in primary astrocytes. We designated this fraction as gintonin-enriched fraction (GEF).
Results
The yield of GEF was approximately 6-fold higher than that obtained in the previous gintonin preparation method. The apparent molecular weight of GEF, determined using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, was equivalent to that obtained in the previous gintonin preparation method. GEF induced [Ca2+]i transient in cortical astrocytes. The effective dose (ED50) was 0.3 ± 0.09 μg/mL. GEF used the same signal transduction pathway as gintonin during [Ca2+]i transient induction in mouse cortical astrocytes.
Conclusion
Because GEF can be prepared through water precipitation of ginseng ethanol extract and is easily reproducible with high yield, it could be commercially utilized for the development of gintonin-derived functional health food and natural medicine.
Keywords: ethanol extraction, GEF, ginseng, H2O precipitation
1. Introduction
Ginseng, the root of Panax ginseng C.A. Meyer, has been used as a tonic for human vitality and health [1]. Recent reports have shown that ginseng contains a novel G protein-coupled lysophosphatidic acid (LPA) receptor ligand, gintonin, in addition to ginsenosides. The primary action of gintonin is to induce [Ca2+]i transient through LPA receptor activation, with a high affinity in cells expressing LPA receptors either endogenously or heterologously. Gintonin-mediated LPA receptor activation is coupled to diverse downstream events, including stimulation of phospholipase C, protein kinase C, mitogen-activated protein kinases, and phosphoinositide 4-kinase, through multiple G proteins such as Gαi/o, Gα12/13, and Gαq/11 [2]. The transient elevation of intracellular Ca2+ via LPA receptor activation is a key mediator of diverse gintonin-mediated in vitro and in vivo effects. For example, gintonin regulates various Ca2+-dependent ion channels [3], [4], [5] and modulates N-methyl-d-aspartic acid and P2X1 receptors via Ca2+-dependent signaling pathways [6], [7]. Gintonin-mediated ion channels and receptor regulations are linked to the increase of gastrointestinal contractility by stimulation of pacemaker activity in the gastrointestinal system [8] and enhancement of synaptic transmission in hippocampal slices in the brain [9]. In vivo studies showed that gintonin reduces brain inflammation and amyloid plaque formation in transgenic Alzheimer's disease animal models and shows antimetastatic effect [10], [11].
The previous methods for gintonin preparation included multiple steps using various organic solvents and anion exchange chromatography with a time-consuming separation process [12], [13]. In addition, if gintonin has to be commercially utilized as a ginseng-derived functional health food, these previous processes for gintonin preparation are required for its in vivo safety test. In the present study, we developed a simple method for gintonin-enriched fraction (GEF) preparation using only ethanol and water from ginseng. We report here that this procedure simplified the GEF preparation process and produced a much higher yield of gintonin than the previous method. GEF induces [Ca2+]i transient through the same signal transduction pathways as gintonin via LPA receptor activation in cultured mouse cortical astrocytes. Finally, the present report discusses the possibility of substituting anion exchange chromatography with water for GEF preparation.
2. Materials and methods
2.1. Materials
Four-year-old Korean white ginseng (Korea Ginseng Cooperation, Daejon, Korea) was purchased from a local ginseng market; the other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Preparation of GEF from ginseng root and ginsenoside determination
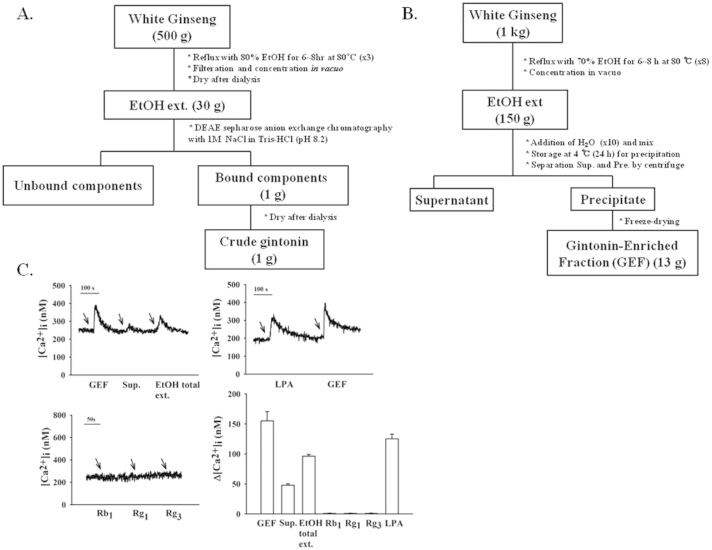
One kilogram of 4-yr-old ginseng was ground into small pieces (> 3 mm) and refluxed with 70% fermentation ethanol eight times for 8 h at 80°C each. The ethanol extracts (150 g) were concentrated as described in Fig. 1B. Ethanol extract was dissolved in distilled cold water in a ratio of 1:10 and stored at 4°C in a cold chamber for 24 h. The supernatant and precipitate produced by water fractionation, after the ethanol extraction of ginseng, was separated by centrifugation (1977 g, 20 min). The precipitate was lyophilized after being centrifuged. This fraction was designated GEF with a yield of 1.3% (Fig. 1C). The representative ginsenosides, such as ginsenoside Rb1 and Rg1, were determined in the ethanol extract, supernatant, and precipitate from water fractionation by the Gyeonggi Bio Center (Suwon, Korea).
Fig. 1.
Methods for gintonin-enriched fraction (GEF) preparation from white ginseng extract. (A) A method for gintonin fraction preparation using ethanol extraction and diethylaminoethyl (DEAE) anion exchange chromatography. Gintonin fraction was prepared from eluate of anion exchange chromatography [11], [12]. (B) A simple method for GEF using only ethanol and water. Ethanol extract is dissolved in water, leading to the formation of a precipitate, and centrifuge is used to separate the supernatant and precipitate. The precipitate from water fractionation is designated GEF with a yield of 1.3%. (C) Effects of three fractions of white ginseng after ethanol extraction, water precipitation, and ginsenosides such as ginsenoside Rb1, Rg1, and Rg3 on cultured mouse cortical astrocytes. Comparison of [Ca2+]i transient in astrocytes by various white ginseng preparations, such as GEF (1 μg/mL), supernatant (1 μg/mL), ethanol total extract (1 μg/mL) after ethanol extraction (left upper panel), ginsenosides (30μM each; left lower panel), or lysophosphatidic acid (LPA) C18:1 (1μM; right upper). Summary histograms show that GEF after water fractionation exhibits more effects than upper layer and total ethanol extract. LPA exhibits [Ca2+]i transient, but ginsenosides tested had no effects in cortical astrocytes (right lower panel).
2.3. Gel filtration chromatography
Gel filtration chromatography of the ginseng ethanol extract, supernatant from water fractionation, or GEF was performed using an Superdex 75 column (10 × 300 mm) equilibrated with Tris–HCl (pH 8.0) on the BioLogic DuoFlow chromatography systems (Bio-Rad, Hercules, CA, USA) according to a previous report [12], [13]. Fractions were collected with a flow rate of 0.5 mL/min and monitored at 280 nm. Each fraction was tested for [Ca2+]i transient in cultured mouse cortical primary astrocytes [14].
2.4. Electrophoresis and Sudan Black staining
Ginseng ethanol extract, the supernatant from water fractionation, and GEF from ginseng were subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) independently [12]. The ethanol extract, supernatant from water fractionation, GEF, and gintonin (200 μg each) were loaded into individual lanes. After electrophoresis, the GEF bands were visualized by Coomassie Brilliant Blue R-250 staining [12]. The gel was stained with Sudan Black B solution producing 500 mg Sudan Black B (Sigma-Aldrich) in 20 mL acetone; 15 mL acetic acid and 85 mL water were added to the solution, stirred for 30 min, and centrifuged to remove the precipitate. The gel was stained in a solution overnight. The gel was destained with three changes in the following solution: 150 mL of acetic acid, 200 mL of acetone, and 650 mL of water [13].
2.5. Gintonin amino acid composition analysis
GEF (300 μg) from ginseng was hydrolyzed in vacuo with 6N HCl for 24 h at 110°C for general amino acid analysis. For the analysis of cysteine, GEF was hydrolyzed with 6N HCl for 24 h at 110°C after peroxidation treatment with formic acid, hydrogen peroxide (10:1). For the analysis of tryptophan, the sample was hydrolyzed with 4M methanesulfonic acid, and 4M KOH was added. Amino acids converted to phenyl isothiocyanate derivatives were analyzed with high-performance liquid chromatography (Hewlett Packard 1100 series; Hewlett Packard, Palo Alto, CA, USA) with a Waters Nova-Pak C18 column (3.9 mm × 300 mm) at the Korea Basic Science Institute (Seoul, Korea). Protein contents were determined using the Bradford method with bovine serum albumin as a standard [12].
2.6. Carbohydrate composition
GEF from ginseng was hydrolyzed in 2M trifluoroacetic acid for 4 h at 100°C for neutral sugar and hydrolyzed in 6N HCl for 4 h at 100°C for amino sugar and acid sugar in glass. Carbohydrate compositions of gintonin were analyzed using a high-performance anion exchange chromatography-pulsed amperometric detection system (HPAEC–PAD system; Dionex, Sunnyvale, CA, USA) with a CarboPac PA1 column at the Carbohydrate Bioproduct Research Center, Sejong University (Seoul, Korea). The molar ratios of monosaccharides were calculated from the peak areas. The carbohydrate contents were also determined using the phenol–sulfuric acid method for neutral sugar [12] and the anthrone method for acid sugar [12].
2.7. Lipid composition analysis
Total lipids in the ethanol extract, supernatant, and precipitate from water fractionation were determined using the procedure described by Folch et al [15]. The lipid composition of GEF was analyzed by hydrolyzing GEF from ginseng with 6N HCl for 4 h at 100°C or by digesting lipoprotein lipase to confirm lipid and hydrophobic moiety. Acid hydrolyzed or digested GEF was partitioned between distilled water and n-butanol (BuOH). The n-BuOH layer, after concentration, was partitioned further between distilled water and n-hexane. The n-hexane layer was prepared for lipid and hydrophobic moiety analysis using a 6890N GC–MS system (Agilent, Santa Rosa, CA, USA) with a DB5-MS capillary column (30 cm × 250 μm × 0.25 μm) at the Korea Basic Science Institute and by gas chromatography using an Agilent 6890N chromatograph equipped with a flame ionization detector and a split injection system fitted with a Supelco SPB-1 capillary column (15 m × 0.32 mm inside diameter, 0.25 mm thickness) [11].
2.8. Mouse cortical primary astrocyte culture
Astrocytes were prepared from cerebral cortices of postnatal Day 1 ICR mice according to Shano et al [14]. Briefly, mice cerebral cortices were dissected and dissociated with 0.25% trypsin/0.1mM EDTA. Cortical cells were plated in T75 or T25 flasks (cells from 1 mouse/25 cm2) and cultured to confluence with Dulbecco's modified Eagle's medium (Wako Pure Chemicals, Osaka, Japan) containing 10% fetal calf serum. Cells were harvested with trypsin/EDTA, replated in 24-well plates, and cultured in 10% fetal calf serum-containing Dulbecco's modified Eagle's medium for 1 day. Cells were then cultured in serum-free Opti-MEM (Invitrogen, Tokyo, Japan) and subjected to assay. Cells (80–90%) were identified as glial fibrillary acidic protein (GFAP)-positive using immunocytochemical staining with anti-GFAP antibodies. The ratios of microglia, oligodendrocytes, and neurons were < 5%, 1%, and 1%, respectively [14].
2.9. Measurement of intracellular calcium concentration
The intracellular free calcium concentration was measured using dual excitation spectrofluorometric analysis of cell suspensions loaded with Fura-2 AM, as previously described [10]. Briefly, cortical astrocytes were harvested with trypsin/EDTA solution and resuspended in N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES)-buffered saline solution (120mM NaCl, 5mM KCl, 1mM MgCl2, 1.5mM CaCl2, 10mM glucose, 25mM HEPES, pH 7.4). The cells were incubated with Fura-2 AM (final concentration 2.5μM) in HEPES-buffered saline solution at 37°C for 30 min. The extracellular Fura-2 AM was removed by centrifugation. Each aliquot of 3 × 106 cells was loaded into a cuvette, and free calcium mobilization was measured using a RF-5301PC spectrofluorophotometer and Supercap software (Ex: 340 nm and 380 nm; Em: 520 nm; Shimadzu, Tokyo, Japan).
2.10. Data analysis
To obtain the concentration–response curve in the presence of GEF, the observed peak amplitudes were normalized, plotted, and fitted to the Hill equation using Origin software (Origin, Northampton, MA, USA). The Hill equation is:
| y/ymax = [A]n/([A]n + [EC50]n), |
where y represents the percent activation at a given concentration of GEF, ymax is the maximal peak current, EC50 is the concentration of GEF producing half-maximum effect of the control response to GEF, A is the concentration of GEF, and n is the interaction coefficient. All values are presented as mean ± standard deviation. The differences between the means of the control and GEF treatment data were analyzed using an unpaired Student t test. A p value of < 0.05 was considered statistically significant.
3. Results
3.1. Simple method for GEF preparation from ginseng
In previous reports, we have shown that ginseng contains a novel G protein-coupled receptor ligand, gintonin [16]. Gintonin induces intracellular Ca2+ mobilization via the phospholipase C–IP3–Ca2+ pathway in mammalian cells expressing endogenous LPA receptors [2]. Further studies demonstrated that gintonin-mediated transient mobilization of [Ca2+]i is coupled with Ca2+-dependent in vitro regulations of ion channels, neurotransmitter release by enhancing synaptic transmission, and in vivo anti-Alzheimer's activity by activating nonamyloidogenic pathways [3], [4], [5], [6], [7]. We extracted ginseng with multiple procedures such as anion exchange chromatography and dialysis, yielding approximately 0.2% in the previous method (Fig. 1A) [13]. However, to use gintonin as a ginseng-derived functional health food, we have sought a new extraction method without harmful organic solvents and chromatography. In the present study, we developed a simple protocol to obtain the active component, gintonin, using only ethanol and water instead of anion exchange chromatography. As shown in Fig. 1B, we first prepared concentrates through 70% fermentation ethanol extraction and then mixed the concentrates with water in a ratio of 1:10 (w/v). Next, we kept the solution at 4°C for 24 h and separated the precipitate from the supernatant by centrifugation; finally, the supernatant and precipitate were lyophilized. As shown in Fig. 1C, treatment of ethanol extract (1 μg/mL) of ginseng induced a slight [Ca2+]i transient in cultured cortical primary astrocytes, which is known to express endogenous LPA receptors [15]. The supernatant from water fractionation (1 μg/mL) had a negligible effect on [Ca2+]i transient, whereas the precipitate (1 μg/mL) induced a larger [Ca2+]i transient than the total extract and supernatant in astrocytes. As a positive control, we used LPA C18:1. LPA C18:1 also induced [Ca2+]i transient in primary astrocytes (Fig. 1C). These results indicate that the main fraction for [Ca2+]i transient induction is the precipitate from water fractionation after ethanol extraction. We designated the precipitate obtained from water fractionation after ethanol extraction as GEF. Thus, we could prepare GEF with a high yield of 1.3%, compared to previous yields of 0.2% gintonin [13]. Next, we determined the representative ginsenoside contents in GEF, because we did not perform anion exchange chromatography. We found that GEF contains 1.2% and 0.2% representative ginsenosides Rb1 and Rg1, respectively. However, ginsenosides such as Rb1, Rg1, and Rg3 (30μM each) had no effect on [Ca2+]i transient. These results indicated that gintonin, but not ginsenosides, from GEF contribute to [Ca2+]i transient in astrocytes.
3.2. Pattern of gel filtration chromatography of GEF and determination of molecular weight of GEF
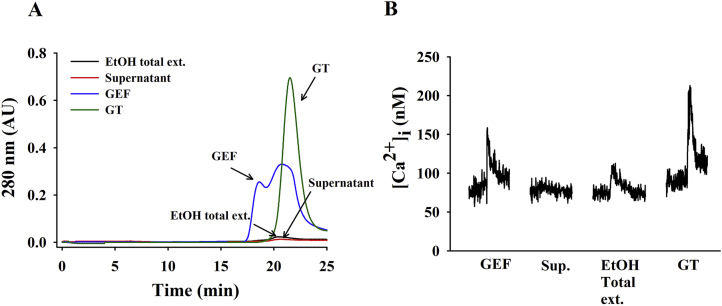
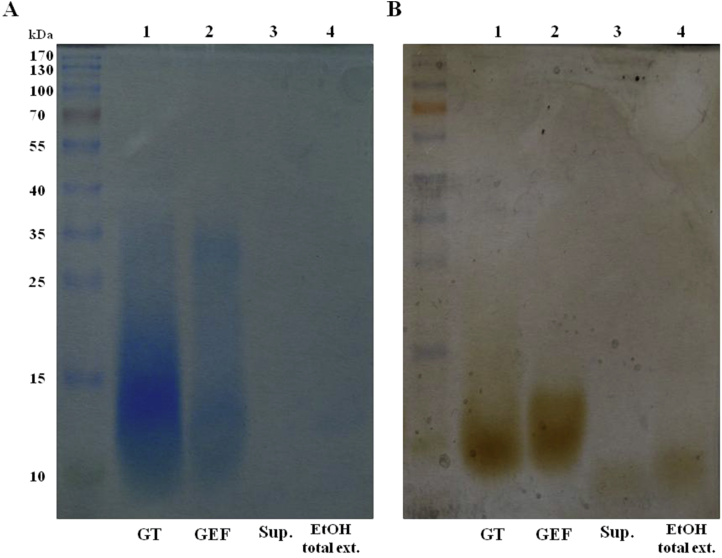
We first compared the gel filtration chromatographic patterns of the GEF with gintonin, ginseng ethanol extract, and supernatant after water precipitation. As shown in Fig. 2A, GEF showed a broad peak at the elution time of 20 min. Similarly, gintonin was eluted for nearly 20 min with a narrower peak than GEF. However, the ginseng ethanol extract and supernatant after water precipitation did not show the peak as much as GEF did. In addition, we found that the main peak of GEF, but not the ginseng ethanol extract or supernatant, exhibited [Ca2+]i transient induction in cultured astrocytes similar to gintonin (Fig. 2B). We estimated the apparent molecular weight of GEF prepared from ginseng. SDS-PAGE and Coomassie blue staining revealed a broad major GEF band similar to gintonin, and its molecular weight was approximately 13 kDa (Fig. 3A). However, the ethanol extract and supernatant from water fractionation almost did not show a band, revealing a molecular weight of GEF similar to the gintonin prepared through anion exchange chromatography; most of the gintonin was found to be present in the precipitate from water fractionation. Because gintonin is a complex of protein, carbohydrate, and lipid, and LPAs are the main biologically active ingredient of gintonin [13], [16], we also compared the degree of lipid staining after SDS-PAGE. As shown in Fig. 3B, gintonin and GEF showed a strong lipid staining with Sudan Black, whereas the ethanol extract and supernatant from water fractionation were almost unstained with Sudan Black, indicating that GEF contains most of the lipid components.
Fig. 2.
Comparison of fast protein liquid chromatography (FPLC) patterns among gintonin (GT), ethanol total extract, supernatant, and gintonin-enriched fraction (GEF). (A) Only GEF, as well as GT, show high absorbance at 280 nm, at about 22 min of elution, compared to the ethanol extract and supernatant from water fractionation, which corresponds to about 67 kDa. FPLC analysis was performed according to the procedures in the previous report [11]. (B) The representative traces of [Ca2+]i transient induced by each peak of FPLC of gintonin, ethanol total extract, supernatant, or GEF. GT and GEF induce a large [Ca2+]i transient in cultured astrocytes. Each peak eluate was collected and dried. Gintonin (0.3 μg/mL), ethanol total extract (1 μg/mL), supernatant (1 μg/mL), or GEF (1 μg/mL) was applied for [Ca2+]i transient induction to astrocytes.
Fig. 3.
Comparison of protein staining. (A) Coomassie Blue. (B) Sudan Black. Comparison is made after 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) among gintonin (GT; lane 1), which is prepared from anion exchange column chromatography, gintonin-enriched fraction (GEF; lane 2), supernatant of water fractionation (lane 3), and ethanol total extract (lane 4). The loaded amount in each lane was 200 μg for SDS-PAGE. GT and GEF, but not total extract and supernatant, were stained by Coomassie Blue (A). Similarly, GT and GEF were strongly stained by Sudan Black (B). These results are consistent with the FPLC pattern in Fig. 2A. Methods for SDS-PAGE, Coomassie Blue, and Sudan Black staining were performed according to previously described procedures [11], [16].
3.3. Amino acid, carbohydrates, and lipid composition of GEF prepared from ginseng
The total proteins in GEF, measured by the Bradford method, were approximately 30.3%. The amino acid composition of individual gintonin is summarized in Table 1. In previous reports, we showed that gintonin contains carbohydrate moieties. We examined the carbohydrate contents and composition of GEF using the HPAEC–PAD system (Table 2). The total carbohydrates in GEF were approximately 30%. Table 2 shows the carbohydrate compositions of GEF. Glucose was a major carbohydrate component of GEF, as shown previously [12]. Finally, these results confirmed that GEF contains carbohydrates and glycoproteins.
Table 1.
Amino acid composition of GEF1)
| Amino acid | Result (%) | Mol (%) | (μg/mg) | (nmol/mg) |
|---|---|---|---|---|
| CYA | 339.91 | 2.82 | 4.24 | 22.66 |
| ASX | 1,342.67 | 11.15 | 11.91 | 89.51 |
| GLX | 1,647.04 | 13.68 | 16.16 | 109.80 |
| SER | 448.52 | 3.73 | 3.14 | 29.90 |
| GLY | 814.16 | 6.76 | 3.91 | 54.28 |
| HIS | 200.65 | 1.67 | 2.08 | 13.38 |
| ARG | 1,686.01 | 14.00 | 19.58 | 112.40 |
| THR | 602.23 | 5.00 | 4.78 | 40.15 |
| ALA | 922.82 | 7.67 | 5.48 | 61.52 |
| PRO | 691.55 | 5.74 | 5.31 | 46.10 |
| TYR | 132.69 | 1.10 | 1.60 | 8.85 |
| VAL | 538.64 | 4.47 | 4.21 | 35.91 |
| MET | 124.64 | 1.04 | 1.24 | 8.31 |
| ILE | 395.03 | 3.28 | 3.45 | 26.34 |
| LEU | 933.45 | 7.75 | 8.16 | 62.23 |
| PHE | 609.15 | 5.06 | 6.71 | 40.61 |
| TRP | 250.62 | 2.08 | 3.41 | 16.71 |
| LYS | 359.21 | 2.98 | 3.50 | 23.95 |
| Total | 12,038.98 | 100.00 | 108.88 | 802.60 |
ASX, sum of asparagine and aspartic acid; CYA, sum of cysteine and cystine; GEF, gintonin-enriched fraction; GLX, sum of glutamine and glutamic acid [10]
The detailed methods for amino acid composition analysis of GEF [10]. The order of amino acid content in GEF is Arg > GLX > ASX > Leu > Ala > Pro
Table 2.
Carbohydrate composition of GEF1)
| Carbohydrate | Result (%) | Amount (mg/mg) |
|---|---|---|
| Fructose | 1.94 | 0.009 |
| Arabinose | 5.75 | 0.019 |
| Galactose | 0.74 | 0.002 |
| Glucose | 91.57 | 0.247 |
GEF, gintonin-enriched fraction
The detailed methods for carbohydrate composition analysis of GEF [10]. Most carbohydrates in GEF are glucose
Because GEF was obtained from water-insoluble precipitates after ethanol extraction (Fig. 3B), we first examined the lipid contents of the ethanol extract of ginseng, supernatant, and GEF from water fractionation. Surprisingly, we found that the lipid content of GEF was 20.2%, whereas that of the ethanol extract and supernatant from water fractionation was 1.4% and 0.9%, respectively. In addition, we examined the lipid composition of GEF. As shown in Table 3, GEF contained fatty acids such as palmitic acid, stearic acid, linoleic acid in ester form, and other minor fatty acids. Interestingly, the portion of palmitic acid in GEF was higher than unsaturated fatty acids such as linoleic acid. These results indicated that GEF prepared using ethanol and water fractionation is a glycolipoprotein, and the lipid content of GEF is higher than that of the ethanol extract or supernatant from water fractionation.
Table 3.
Lipid composition of GEF1)
| Lipids | Result (%) |
|---|---|
| Propanoic acid, 2,3-bis[(trimethylsilyl)oxy]-, trimethylsilyl ester | 0.13 |
| Nonanoic acid, trimethylsilyl ester | 0.01 |
| Butanedioic acid,[(trimethylsilyl)oxy]-, bis(trimethylsilyl)ester | 1.51 |
| Dodecanoic acid, trimethylsilyl ester | 0.11 |
| Palmitoleic acid 1TMS | 0.03 |
| Palmitic acid, trimethylsilyl ester | 8.17 |
| Margaric acid, trimethylsilyl ester | 0.16 |
| Linoleic acid, trimethylsilyl ester | 6.17 |
| 11-cis-Octadecenoic acid 1TMS | 1.35 |
| Oleic acid, trimethylsilyl ester (CAS) | 0.40 |
| Stearic acid, trimethylsilyl ester | 2.74 |
| (E)-3-Hexenedioic acid 2TMS | 0.10 |
Data are presented as percentage of fatty acids in GEF
GEF, gintonin-enriched fraction
The detailed methods for lipid composition analysis of GEF [10]. The order of fatty acid content in GEF is palmitic acid > linoleic acid > stearic acid. Other minor components of GEF are not shown
3.4. Signal transduction pathway of GEF on [Ca2+]i transient induction in astrocytes
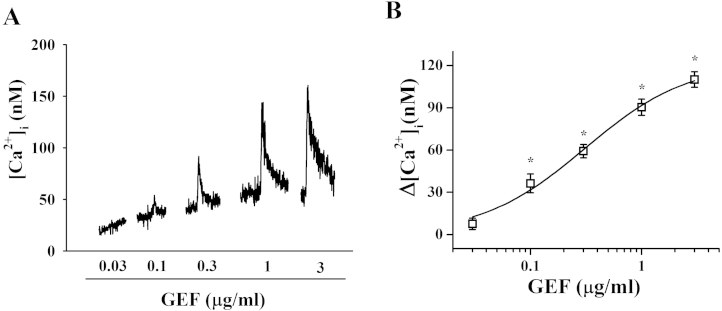
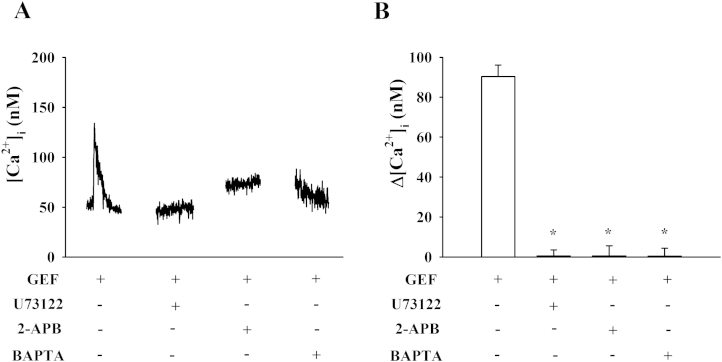
We also examined the signaling pathway of GEF-mediated [Ca2+]i transient induction. As shown in Fig. 4, treatment of GEF induced a large [Ca2+]i transient in a concentration-dependent manner. The effective dose (ED50) was 0.3 ± 0.09 μg/mL in astrocytes. We examined the effects of GEF on [Ca2+]i transient in the absence or presence of the LPA1/3 receptor antagonist, Ki16425. As shown in Fig. 5, the presence of Ki16425 significantly attenuated the GEF-mediated [Ca2+]i transient. The active phospholipase C inhibitor, U73122, inositol 1,4,5-triphosphate receptor antagonist 2-APB, and intracellular Ca2+ chelator (BAPTA-AM) all blocked GEF-mediated [Ca2+]i transients in astrocytes (Fig. 5). This showed that GEF elicits [Ca2+]i transient via the activation of LPA receptor–phospholipase C–intracellular IP3 receptor signaling transduction pathways. Thus, these results indicated that the main component of GEF that elicits [Ca2+]i transient is gintonin, and GEF uses the same signal transduction pathways as gintonin.
Fig. 4.
Concentration-dependent effects of gintonin-enriched fraction (GEF) on [Ca2+]i transient in astrocytes. (A) Representative traces on GEF-mediated [Ca2+]i transient in cortical astrocytes. (B) GEF showed concentration-dependent induction of [Ca2+]i transient in cultured cortical astrocytes. The method for [Ca2+]i transient was performed according to procedures described in a previous report [9]. *p < 0.001, compared to GEF (0.03 μg/mL) treatment. Data are presented as mean ± standard error of the mean (n = 3–4).
Fig. 5.
Effect of signal transduction pathway inhibitors on gintonin-enriched fraction (GEF)-induced [Ca2+]i transients in cultured astrocytes. (A) Representative traces of GEF-mediated [Ca2+]i transients in the absence or presence of various antagonists. The arrows indicate application of GEF (1 μg/mL). LPA1/3 receptor antagonist (Ki16425, 10μM), phospholipase C (PLC) inhibitor (U73122, 5μM), IP3 receptor antagonist (2-APB, 100μM), or Ca2+ chelator (BAPTA-AM, 50μM) were added prior to GEF application. (B) Histograms representing net increases of GEF-mediated [Ca2+]i transients calculated from traces obtained in the absence or presence of various pharmacological agents. *p < 0.01, compared to GEF only treatment. Data are presented as mean ± standard error of the mean (n = 3–4).
4. Discussion
We have sought a brief method of gintonin preparation from ginseng, with a high yield, for commercial applications as a functional health food. We observed that the ethanol extract of ginseng was not completely dissolved in water and that some of the insoluble components of the ethanol extract after water fractionation were spontaneously precipitated and separated from the supernatant by gravity when kept at 4°C over 24 h. When we compared the capability of [Ca2+]i transient induction in cultured mouse cortical astrocytes using the ethanol extract, supernatant, or precipitate from water fractionation, we found that the precipitate, GEF, from water fractionation exhibited the highest [Ca2+]i transient induction activity in mammalian cells (Fig. 1, Fig. 2B).
As we previously reported, gintonin consists of carbohydrate, lipid, and protein portions as a glycolipoprotein complex [12]. Because GEF contains most of the lipid moiety compared to the ethanol extract and supernatant from water fractionation (Fig. 3B), the lipid components of gintonin seem to contribute water insolubility. These results indicated that gintonin could be enriched by collecting water-insoluble components after the ethanol extraction, and that ethanol or other organic solvents such as methanol and butanol, but not water, are necessary for the extraction of gintonin from ginseng [12], [13]. This property might provide the simplified method for GEF preparation and exhibits multiple merits compared to previous methods. First, we showed that GEF can be prepared using only ethanol with a one-step extraction, following water fractionation. Second, GEF prepared using this method could be exempted from safety tests for ginseng-derived functional health food, because this procedure does not use anion exchange column chromatography or dialyses. Third, the procedure for GEF preparation is simple, reproducible, and time-saving compared to previous methods. Fourth, the procedure for GEF preparation is not only cost-effective, but could also increase yield by 6-folds. Finally, because gintonin exhibited in vivo anti-Alzheimer's and antimetastatic activities [2], GEF could be applicable for use as a functional health food.
In previous studies, we demonstrated that gintonin induced endogenous Ca2+-activated Cl– channel activation in Xenopus oocytes through [Ca2+]i mobilization [9], [11]. In the present study, we examined GEF's effects on [Ca2+]i transient in cultured mouse cortical astrocytes, which express endogenous LPA receptors [14]. When we compared the potency of GEF for [Ca2+]i transient induction in astrocytes cultured with gintonin, we found that GEF was less potent for [Ca2+]i transient compared to gintonin, but it still shows high affinity and potent effects with an ED50 of 0.3 ± 0.09 μg/mL. GEF also exhibited cellular effects via LPA receptor activation and signaling pathways similar to gintonin in astrocytes (Fig. 5). Because gintonin-mediated [Ca2+]i transient via LPA receptors is linked to Ca2+-dependent diverse in vitro and in vivo effects [3], [4], [5], [6], [7], [9], [10], the procedure for GEF preparation containing gintonin as an active ingredient could be applicable for mass production with a high yield (Fig. 1).
However, we also observed several similarities and differences between the present GEF and the previous gintonin. The elution time of GEF in fast protein liquid chromatography was similar to that of gintonin, which was obtained after anion exchange column chromatography, although GEF shows a broad peak with less absorbance at 280 nm compared to gintonin. Their molecular weights from SDS-PAGE were almost the same, although the degree of staining with Coomassie Blue was less in GEF compared to gintonin prepared through anion exchange column chromatography (Fig. 3A). However, lipid staining by Sudan Black between gintonin and GEF is similar (Fig. 3B). Interestingly, in SDS-PAGE GEF showed a broad but single major band, indicating that GEF is a complex of carbohydrate, lipid, and proteins (Fig. 3) [12], [13].
In the analysis of amino acid composition, gintonin was shown to contain a high amount of hydrophobic amino acids such as phenylalanine, leucine, isoleucine, alanine, and proline, in agreement with the composition of the previously obtained gintonin (Table 1). In carbohydrate composition analysis, glucose was predominant in both gintonin and GEF obtained using ethanol (Table 2). Palmitic acid (C16:0) was the major lipid component in GEF, followed by linoleic acid (C18:2), whereas linoleic acid was dominant, followed by palmitic acid in previously obtained gintonin [11]. We observed the presence of some minor components (Table 3). The discrepancy in lipid content between present and previous gintonin might be attributable to batch-to-batch variability and from different sources of ginseng cultivation. The previous gintonin was obtained after more purification steps of anion exchange chromatography and dialysis, whereas GEF was prepared from ethanol extraction and water fractionation. Thus, the differences in gintonin preparation procedures might also affect lipid content. However, we could not exclude the possibility that some minor components of lipids in GEF could be derived from ethanol. Further study will be required to elucidate the composition differences of lipids in gintonin and GEF. In addition, we could observe that most of GEF consist of gintonin, because we found that > 90% of GEF are gintonin when we estimated gintonin content in GEF using the enzyme immunoassay method (data not shown). We found that GEF contains small amounts of ginsenosides such as Rb1 and Rg1; these ginsenosides had no effect on [Ca2+]i transient (Fig. 1C), indicating that, although GEF contains small amounts of ginsenosides, these ginsenosides are not related to [Ca2+]i transient induction.
In summary, we developed a simple method for GEF preparation from ginseng with a high yield by collecting water-insoluble fractionation after ethanol extraction. The chemical composition of GEF and signal transduction pathways of GEF for [Ca2+]i transient induction in astrocytes was the same as gintonin prepared using previous methods. This method is particularly useful for mass production of gintonin, as an active ingredient of ginseng, for future development of functional health foods and natural medicine.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the Basic Science Research Program (NRF-2014R1A1A2054538). This paper was also written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2015.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Im D.S., Nah S.Y. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin. 2013;34:1367–1373. doi: 10.1038/aps.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nah S.Y. Gintonin: a novel ginseng-derived ligand that targets G protein-coupled lysophosphatidic acid receptors. Curr Drug Targets. 2012;13:1659–1664. doi: 10.2174/138945012803529947. [DOI] [PubMed] [Google Scholar]
- 3.Choi S.H., Lee B.H., Kim H.J., Jung S.W., Kim H.S., Shin H.C., Lee J.H., Kim H.C., Rhim H., Hwang S.H. Ginseng gintonin activates the human cardiac delayed rectifier K+ channel: involvement of Ca2+/calmodulin binding sites. Mol Cells. 2014;37:656–663. doi: 10.14348/molcells.2014.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.H., Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Rhee J., Chung C., Nah S.Y. Activation of lysophosphatidic acid receptor by gintonin inhibits Kv1.2 channel activity: involvement of tyrosine kinase and receptor protein tyrosine phosphatase α. Neurosci Lett. 2013;548:143–148. doi: 10.1016/j.neulet.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 5.Choi S.H., Lee B.H., Hwang S.H., Kim H.J., Lee S.M., Kim H.C., Rhim H., Nah S.Y. Molecular mechanisms of large-conductance Ca2+-activated potassium channel activation by ginseng gintonin. Evid Based Complement Alternat Med. 2013;2013:323709. doi: 10.1155/2013/323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin T.J., Kim H.J., Kwon B.J., Choi S.H., Kim H.B., Hwang S.H., Lee B.H., Lee S.M., Zukin R.S., Park J.H. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-d-aspartic acid receptor activation: involvement of LPA receptors. Mol Cells. 2012;34:563–572. doi: 10.1007/s10059-012-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi S.H., Kim H.J., Kim B.R., Shin T.J., Hwang S.H., Lee B.H., Lee S.M., Rhim H., Nah S.Y. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, potentiates ATP-gated P2X1 receptor channel currents. Mol Cells. 2013;35:142–150. doi: 10.1007/s10059-013-2293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim B.J., Nam J.H., Kim K.H., Joo M., Ha T.S., Weon K.Y., Choi S., Jun J.Y., Park E.J., Wie J. Characteristics of gintonin-mediated membrane depolarization of pacemaker activity in cultured interstitial cells of Cajal. Cell Physiol Biochem. 2014;34:873–890. doi: 10.1159/000366306. [DOI] [PubMed] [Google Scholar]
- 9.Park H., Kim S., Rhee J., Kim H.J., Han J.S., Nah S.Y., Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in fully developed central synapses. J Neurophysiol. 2015;113:1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 10.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S.H., Lee B.H., Kim H.J., Cho H.J., Shin H.C., Im K.S., Choi S.H., Shin T.J., Lee S.M., Nam S.W. Suppression of metastasis of intravenously-inoculated B16/F10 melanoma cells by the novel ginseng-derived ingredient, gintonin: involvement of autotaxin inhibition. Int J Oncol. 2013;42:317–326. doi: 10.3892/ijo.2012.1709. [DOI] [PubMed] [Google Scholar]
- 12.Pyo M.K., Choi S.H., Hwang S.H., Shin T.J., Lee B.H., Lee S.M., Lim Y.H., Kim D.H., Nah S.Y. Novel glycolipoproteins from ginseng. J Ginseng Res. 2011;35:92–103. [Google Scholar]
- 13.Choi S.H., Shin T.J., Lee B.H., Hwang S.H., Kang J., Kim H.J., Park C.W., Nah S.Y. An edible gintonin preparation from ginseng. J Ginseng Res. 2011;35:471–478. doi: 10.5142/jgr.2011.35.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shano S., Moriyama R., Chun J., Fukushima N. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochem Int. 2008;52:216–220. doi: 10.1016/j.neuint.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folch J., Lees M., Sloane-Stanely G.H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids–protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]