Abstract
Panax ginseng is a well-known economic medical plant that is widely used in Chinese traditional medicine. This species contains a unique class of natural products—ginsenosides. Recent clinical and experimental studies have presented numerous lines of evidence on the promising role of ginsenosides on different diseases including neurodegenerative diseases, cardiovascular diseases, and certain types of cancer. Nowadays, most of the attention has focused on ginsenoside Rd as a neuroprotective agent to attenuate ischemic stroke damages. Some of the evidence showed that ginsenoside Rd ameliorates ischemic stroke-induced damages through the suppression of oxidative stress and inflammation. Ginsenoside Rd can prolong neural cells' survival through the upregulation of the endogenous antioxidant system, phosphoinositide-3-kinase/AKT and extracellular signal-regulated protein kinase 1/2 pathways, preservation of mitochondrial membrane potential, suppression of the nuclear factor-kappa B, transient receptor potential melastatin, acid sensing ion channels 1a, poly(ADP-ribose) polymerase-1, protein tyrosine kinase activation, as well as reduction of cytochrome c-releasing and apoptosis-inducing factor. In the current work, we review the available reports on the promising role of ginsenoside Rd on ischemic stroke. We also discuss its chemistry, source, and the molecular mechanism underlying this effect.
Keywords: ginsenoside Rd, Panax ginseng, signal transduction
1. Introduction
Stroke, which statistically ranks as the third leading cause of death and as the most important cause of permanent disability worldwide, is classified into ischemic and hemorrhagic stroke [1], [2], [3]. Nearly 80% of stroke patients suffer from ischemic stroke, which results from artery and/or vascular occlusion inducing a transient or permanent reduction in blood supply to the different regions of the brain [4], [5]. Old age, smoking, and concomitant diseases such as obesity, diabetes, and hypertension are risk factors of stroke [6], [7], [8], [9], [10]. Statistical data also show that the occurrence of stroke events is asymmetrical in different sexes and ethnicities [11], [12].
In addition, stroke is associated with several complications including depression, insomnia, or cognitive impairment, which have detrimental effects on therapeutic outcomes, increasing medical costs and mortality rate [13], [14], [15], [16]. Experimental and clinical evidence also showed that depending on the ischemic/reperfusion duration, infarct volume and site, patient age, and the presence of concomitant diseases, the stroke-induced complications and mortality rate are different [14], [17], [18], [19], [20]. Oxidative stress and systemic inflammation appear to have a key role in the pathophysiology of ischemic stroke and stroke-induced complications [21], [22], [23]. A growing body of evidence obtained from epidemiological studies shows that diets containing large amounts of vegetables and fruits decrease the risk of ischemic stroke [24], [25], [26]. This reality is supported by extensive experimental studies on isolated phytochemicals [27], [28], [29], [30]. A plethora of evidence also shows the antioxidant and anti-inflammatory effects of these phytochemicals [31], [32], [33], [34], which seem to be promising compounds against ischemic stroke [35], [36]. During the past decades, much attention has been paid to health promotion related to the activity of phytochemicals and a great revolution occurred in the use of medical plant-derived phytochemicals as effective strategy for the treatment of different diseases [37], [38], [39], [40], [41], [42], [43], [44], [45].
Panax ginseng Meyer (Korean red ginseng) is a well-known medicinal plant from eastern Asian countries, especially Korea, China, and Japan [46], [47], and its root has been used since ancient times for the treatment of numerous diseases and increasing physical strength [48], [49]. The word Panax originates from the word “panacea,” which means “cure all diseases,” and refers to a traditional belief about its promising effects on longevity and health promotion [46]. The therapeutic role of Panax ginseng on several diseases such as cancer and cardiovascular diseases, has been reported by recent experimental and clinical studies on ginseng and/or its bioactive constituents [50], [51], [52], [53], [54]. The first attempts to isolate the active constituents of ginseng began many years ago, leading to the isolation of ginsenosides in 1963 [55]. To date, > 40 different ginsenosides have been found [56]. Extensive studies evaluated the different pharmacological activities of ginsenosides, such as their ability to suppress inflammation and oxidative stress as well as their vasorelaxation effect [57], [58], [59], [60], [61]. Many lines of evidence also showed the promising effects of ginseng and its purified ginsenoside constituents on cardiovascular disease and its risk factors [54], [62], [63], [64], [65], [66], [67], [68]. The promising role of ginsenosides Rd on ischemic stroke is widely publicized [69], [70], [71]. The aim of this paper is to review the available reports on the therapeutic role of ginsenosides Rd on ischemic stroke. We also discuss the molecular mechanism underlying this effect, as well as the chemistry and safety of ginsenoside Rd.
2. Source and chemistry
Ginseng has been used for thousands of years in folk medicine in Eastern Asia. The most popular ginseng comes from the root of P. ginseng Meyer, a slow-growing perennial plant belonging to the family Araliaceae. The plant is native to China and Korea but is now widely cultivated in other countries including Japan, Russia, United States, and Canada. The vast array of medicinal uses of P. ginseng includes its use as a remedy against aging, diabetes, sleep disorders, and sexual dysfunction [72], [73]. In addition to ginseng from P. ginseng—commonly known as Asian, Chinese, or Korean ginseng—American ginseng (from Panax quinquefolius) is also available.
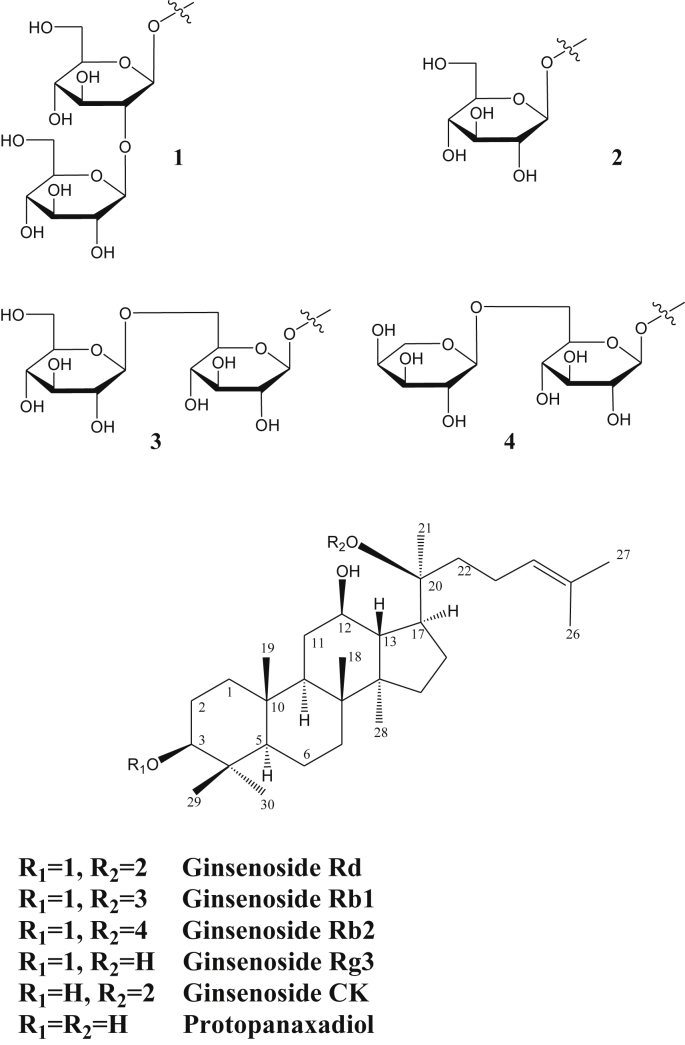
The active principles responsible for the pharmacological activities of ginseng are a group of unique triterpene glycosides or saponins called ginsenosides. The chemical structure of one of the best-known active ingredients, ginseng Rd, along with some related ginsenosides, is shown in Fig. 1. These compounds are based on the dammarane triterpene skeleton that is hydroxylated at three positions (C-3, C-12, and C-20 positions) to produce protopanaxadiol (Fig. 1). Based on the variability in the type of sugars and the degree of glycosylation at the C-3 and C-20 positions, several ginsenosides of protopanaxadiol skeleton have been identified from ginseng (Fig. 1). Remarkably, hydroxylation at the C-6 position of protopanaxadiol is also possible, leading to further structural diversities via glycosylation.
Fig. 1.
Structure of ginsenoside Rd and related ginsenosides.
Today, the quality control of ginseng is a difficult task owing to the diversity of plant sources, which include P. ginseng, Panax notoginseng, P. quinquefolius, and Panax japonicas, and the variability in the composition (> 150 ginsenosides are known in the genus, and > 35 are found in the roots) [56], [62], [74] based on plant age, harvesting conditions, and growing media such as soil, temperature, light intensity, and water content [75], [76], [77], [78], [79]. One alternative approach is to use the most active ginsenoside, such as ginsenoside Rd; however, the low yield (as low as 0.4%) coupled with the difficulty and high cost of isolating the compound from the complex mixture pose a great barrier. Fortunately, the major component of ginsenosides is Rb1, which differs from Rd in that it has two glucose units at C-20 position instead of only one (Fig. 1). To date, a number of enzyme and/or microbial-based biotransformation [80], [81], [82], [83], [84], [85], [86], [87], [88] methodologies have been proposed to obtain ginsenoside Rd. The exploitation of ginsenosides from plant cell cultures is also an active topic of current research [89], [90]. Further developments in these research areas are therefore likely to make the concept of using ginsenoside Rd as a single chemical entity very feasible.
3. Ginsenoside Rd and ischemic stroke
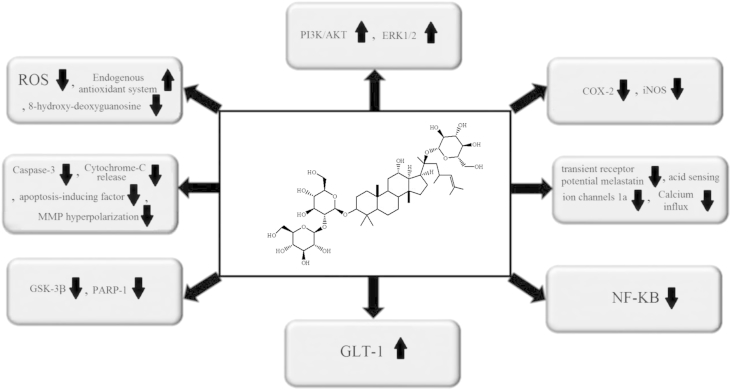
The promising effect of pre- and/or posttreatment of ginsenoside Rd on ischemic stroke-induced neural damages has been studied in different experimental models including transient middle cerebral artery occlusion (tMCAO) in rat and mice [91], [92], [93], oxygen glucose deprivation-induced damages to hippocampal neuron [70], or glutamate-induced damages in rat cortical neurons [94]. Evidence obtained from in vitro and in vivo studies shows that ginsenoside Rd treatment prior to and/or following an ischemic stroke can reduce infarct volume [69], [91], [92], increase neuronal survival (Fig. 2) [70], [94], [95], [96], and enhance cognitive and neurological functions [91], [92], [97]. Ginsenoside Rd administration to a Sprague–Dawley rat has been shown to downregulate ischemic stroke-induced tau protein phosphorylation at Ser199/202 and PHF-1 sites through the downregulation of glycogen synthase kinase-3β and to enhance ischemia-induced cognitive impairment [97]. Ginsenoside Rd administration has also been shown to upregulate the protein kinase B/AKT pathway and, from this, suppress glycogen synthase kinase-3β activity [97]. Ginsenoside Rd administration after tMCAO upregulates glial glutamate transporter-1 (GLT-1) expression and promotes glutamate clearance in rats [95]. Similarly, exposing ginsenoside Rd to astrocyte cells increases glutamate uptake after oxygen-glucose deprivation. It is also reported that phosphorylated protein kinase B and phospho-extracellular signal-regulated protein kinase (ERK) 1/2 pathways have a crucial role in the promising effect of ginsenoside Rd on glutamate uptake and GLT-1 expression [95]. Ginsenoside Rd is also a selective and competitive Ca2+ receptor antagonist and, therefore, can suppress calcium influx after cytotoxic injuries [93]. A previous study showed that pretreatment with ginsenoside Rd (10 mg/kg) increases acid sensing ion channels 2a and suppresses transient receptor potential melastatin, and acid sensing ion channels 1a expression in tMCAO-induced ischemic rats and, through this effect, prolongs neuronal survival [93]. An in vitro study on rat cortical neurons reported that ginsenoside Rd administration dose-dependently suppresses glutamate-induced DNA laddering and apoptosis through the suppression of glutamate-induced caspase-3 activation and Ca2+ entry [94]. Another study showed that pretreatment with ginsenoside Rd (10 mg/kg) inhibits poly(ADP-ribose) polymerase-1 and consequently downregulates apoptosis-inducing factor translocation and nuclear factor-kappa B p65 subunit nuclear accumulation in Dawley rats suffering from right middle cerebral artery occlusion [96]. This finding supported the concept that both the antiapoptotic and anti-inflammatory effects of ginsenoside Rd may be responsible for its promising effects on ischemic stroke [96].
Fig. 2.
Molecular mechanisms underlying the promising effects of ginsenoside Rd on ischemic stroke. COX-2, cyclooxygenase-2; ERK ½, extracellular signal-regulated protein kinases 1 and 2; GLT-1, glial glutamate transporter-1; GSK-3β, glycogen synthase kinase-3β; iNOS, inducible nitric oxide synthase; MMP, mitochondrial membrane potential; NF-κB, nuclear factor-kappa B; PARP-1, poly(ADP-ribose) polymerase 1; PI3K/AKT, phosphatidylinositol 3-kinase/AKT; ROS, reactive oxygen species.
Another study on 16- to 18-month-old mice suffering from tMCAO-induced ischemic stroke shows that pretreatment with ginsenoside Rd (10–50 mg/kg) significantly decreases cortical and striatal infarct size and oxidative stress (DNA damage, protein carbonyl formation, and lipid peroxidation) [91]. Ginsenoside Rd also upregulates the endogenous antioxidant system, sustains the mitochondrial respiratory chain complex and aconitase activities, downregulates mitochondrial hydrogen peroxide leakage, and stabilizes mitochondrial membrane potential [91]. In addition to these effects, another similar report also showed that ginsenoside Rd administration (50 mg/kg) to rats prior to tMCAO significantly decreases the infarcted area (52.8%), and decreases the lactate/pyruvate ratio, the reactive oxygen species production, cytochrome releasing, and apoptosis-inducing factor [92]. Similarly, an in vitro study showed that exposure to ginsenoside Rd can also protect mitochondria against calcium-induced damages through downregulation of reactive oxygen species generation, suppression of mitochondrial membrane potential hyperpolarization, and amelioration of mitochondrial swelling [92]. In another in vitro study, a 2-hour oxygen-glucose deprivation followed by a 24-hour reoxygenation in cultured hippocampal neurons showed that ginsenoside Rd administration upregulates the endogenous antioxidant system including glutathione and antioxidant enzymes, suppresses reactive oxygen species production and lipid peroxidation, preserves mitochondrial membrane potential, and eventually, decreases apoptotic death [70]. The treatment with ginsenoside Rd at doses of 10–50 mg/kg decreases the infarct size and enhances neurological function in both tMCAO-induced ischemic mice and rats [69], [91]. Ginsenoside Rd also upregulates the endogenous antioxidant system in ischemic penumbra, and decreases 8-hydroxy-deoxyguanosine (marker of DNA damage), hydroxyl radicals formation, protein carbonyl, malondialdehyde, and 4-hydroxynonenal (markers of lipid peroxidation), as well as advanced glycosylation end product levels [69]. It also decreases inflammation through the downregulation of inducible nitric oxide synthase, cyclooxygenase-2, and microglial activation [69].
A randomized, double-blind, placebo-controlled, Phase II multicenter trial study on 199 patients suffering from ischemic stroke showed that ginsenoside Rd infusion at doses of 10 and 20 mg/kg for 2 weeks significantly enhanced the National Institutes of Health Stroke Scale when compared with the placebo group [98]. However, ginsenoside Rd does not improve stroke disability and daily activity in Barthel index and the modified Rankin scales [98]. The authors of the study concluded that ginsenoside Rd may be beneficial for ischemic patients [98]. Another randomized, double-blind, placebo-controlled, multicenter trial study on 390 patients suffering from acute ischemic stroke, showed that a 2-week intravenous infusion of ginsenoside Rd significantly enhanced the primary outcome and improved the National Institutes of Health Stroke Scale and modified Rankin scale at 90 days [71]. The study suggested that ginsenoside Rd can have a promising neuroprotective role as adjuvant therapy in acute ischemic patients.
4. Conclusion and recommendation
In conclusion, the available data suggest that the antiapoptotic, antioxidant, and anti-inflammatory activities of ginsenoside Rd as well as its ability to suppress calcium influx, may play a key role in the neuroprotective action of this compound against ischemic stroke. Ginsenoside Rd's ability to upregulate GLT-1, phosphatidylinositol 3-kinase/AKT, and ERK1/2 signaling pathways, and to inhibit poly(ADP-ribose) polymerase 1, glycogen synthase kinase-3β, nuclear factor kappa B, apoptosis-inducing factor, cytochrome c release, and caspase activation plays crucial role in prolonging neuronal cell survival during an ischemic stroke and in the recovery of cognitive function. However, owing to the lack of clinical studies on ginsenoside Rd, it is difficult to make a clear decision. We recommend that future studies on ginsenoside Rd should focus on the following areas: (1) molecular mechanisms underlying the beneficial role of ginsenoside Rd on ischemic stroke; (2) pharmacokinetic studies on ginsenoside Rd and finding methods to increase its bioavailability using different delivery systems; (3) pharmacodynamic studies on ginsenoside Rd and its possible interaction with well-known ischemic stroke drugs; and (5) clinical studies to ascertain the best effective doses.
Conflicts of interest
There is no conflict of interest.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Mukherjee D., Patil C.G. Epidemiology and the global burden of stroke. World Neurosurg. 2011;76:S85–S90. doi: 10.1016/j.wneu.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Grysiewicz R.A., Thomas K., Pandey D.K. Epidemiology of ischemic and hemorrhagic stroke: incidence, prevalence, mortality, and risk factors. Neurol Clin. 2008;26:871–895. doi: 10.1016/j.ncl.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Kleinig T.J., Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22:294–301. doi: 10.1097/wco.0b013e32832b4db3. [DOI] [PubMed] [Google Scholar]
- 4.Nabavi S.F., Li H., Daglia M., Nabavi S.M. Resveratrol and stroke: from chemistry to medicine. Curr Neurovasc Res. 2014;11:390–397. doi: 10.2174/1567202611666140912114833. [DOI] [PubMed] [Google Scholar]
- 5.Hossmann K.-A. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32:1310–1316. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonita R. Epidemiology of stroke. Lancet. 1992;339:342–344. doi: 10.1016/0140-6736(92)91658-u. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz S.W., Carlucci C., Chambless L.E., Rosamond W.D. Synergism between smoking and vital exhaustion in the risk of ischemic stroke: evidence from the ARIC study. Ann Epidemiol. 2004;14:416–424. doi: 10.1016/j.annepidem.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Winter Y., Rohrmann S., Linseisen J., Lanczik O., Ringleb P.A., Hebebrand J., Back T. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39:3145–3151. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 9.Kurl S., Laukkanen J.A., Niskanen L., Laaksonen D., Sivenius J., Nyyssönen K., Salonen J.T. Metabolic syndrome and the risk of stroke in middle-aged men. Stroke. 2006;37:806–811. doi: 10.1161/01.STR.0000204354.06965.44. [DOI] [PubMed] [Google Scholar]
- 10.Kjeldsen S.E.J., Julies S., Hedner T., Hansson L. Stroke is more common than myocardial infarction in hypertension: analysis based on 11 major randomized intervention trials. Blood Press. 2001;10:190–192. doi: 10.1080/08037050152669684. [DOI] [PubMed] [Google Scholar]
- 11.Feigin V.L., Lawes C.M., Bennett D.A., Anderson C.S. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 12.Ayala C., Croft J.B., Greenlund K.J., Keenan N.L., Donehoo R.S., Malarcher A.M., Mensah G.A. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke. 2002;33:1197–1201. doi: 10.1161/01.str.0000015028.52771.d1. [DOI] [PubMed] [Google Scholar]
- 13.Nabavi S., Dean O., Turner A., Sureda A., Daglia M., Nabavi S. Oxidative stress and post-stroke depression: possible therapeutic role of polyphenols? Curr Med Chem. 2015;22:343–351. [PubMed] [Google Scholar]
- 14.Nabavi S., Turner A., Dean O., Sureda A., Nabavi S. Post-stroke depression therapy: where are we now? Curr Neurovasc Res. 2014;11:279–289. doi: 10.2174/1567202611666140522123504. [DOI] [PubMed] [Google Scholar]
- 15.Slomski A. Insomnia increases stroke risk. JAMA. 2014;311:2056. [Google Scholar]
- 16.Psaltopoulou T., Sergentanis T.N., Panagiotakos D.B., Sergentanis I.N., Kosti R., Scarmeas N. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74:580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 17.Weimar C., Kraywinkel K., Rödl J., Hippe A., Harms L., Kloth A., Diener H.C. Etiology, duration, and prognosis of transient ischemic attacks: an analysis from the German Stroke Data Bank. Arch Neurol. 2002;59:1584–1588. doi: 10.1001/archneur.59.10.1584. [DOI] [PubMed] [Google Scholar]
- 18.Saver J.L., Johnston K.C., Homer D., Wityk R., Koroshetz W., Truskowski L.L., Haley E.C. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. Stroke. 1999;30:293–298. doi: 10.1161/01.str.30.2.293. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen B., Oda K., Knutsen S., Fraser G. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: the Adventist Health Study, 1976–88. Int J Epidemiol. 2009;38:245–252. doi: 10.1093/ije/dyn251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Ruotsalainen S., Moilanen L., Lepistö P., Laakso M., Kuusisto J. The metabolic syndrome predicts cardiovascular mortality: a 13-year follow-up study in elderly non-diabetic Finns. Eur Heart J. 2007;28:857–864. doi: 10.1093/eurheartj/ehl524. [DOI] [PubMed] [Google Scholar]
- 21.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukocyte Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H., Yoshioka H., Kim G.S., Jung J.E., Okami N., Sakata H., Maier C.M., Narasimhan P., Goeders C.E., Chan P.H. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–1517. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabavi S.M., Daglia M., Moghaddam A.H., Nabavi S.F., Curti V. Tea consumption and risk of ischemic stroke: a brief review of the literature. Curr Pharm Biotechnol. 2014;15:298–303. doi: 10.2174/1389201015666140617100945. [DOI] [PubMed] [Google Scholar]
- 24.Griep L.O., Verschuren W.M., Kromhout D., Ocké M.C., Geleijnse J.M. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur J Clin Nutr. 2011;65:791–799. doi: 10.1038/ejcn.2011.36. [DOI] [PubMed] [Google Scholar]
- 25.Hjartåker A., Knudsen M.D., Tretli S., Weiderpass E. Consumption of berries, fruits and vegetables and mortality among 10,000 Norwegian men followed for four decades. Eur J Nutr. 2014 doi: 10.1007/s00394-014-0741-9. [DOI] [PubMed] [Google Scholar]
- 26.Hu D., Huang J., Wang Y., Zhang D., Qu Y. Fruits and vegetables consumption and risk of stroke a meta-analysis of prospective cohort studies. Stroke. 2014;45:1613–1619. doi: 10.1161/STROKEAHA.114.004836. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y., Chen F., Zhang J., Wang T., Wei X., Wu J., Feng Y., Dai Z., Wu Q. Neuroprotective effect of resveratrol on ischemia/reperfusion injury in rats through TRPC6/CREB pathways. J Mol Neurosci. 2013;50:504–513. doi: 10.1007/s12031-013-9977-8. [DOI] [PubMed] [Google Scholar]
- 28.Sinha K., Chaudhary G., Kumar Gupta Y. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sci. 2002;71:655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 29.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Shutenko Z., Henry Y., Pinard E., Seylaz J., Potier P., Berthet F., Girard P., Sercombe R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem Pharmacol. 1999;57:199–208. doi: 10.1016/s0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 31.Nabavi S.F., Daglia M., Moghaddam A.H., Habtemariam S., Nabavi S.M. Curcumin and liver disease: from chemistry to medicine. Compr Rev Food Sci Food Saf. 2014;13:62–77. doi: 10.1111/1541-4337.12047. [DOI] [PubMed] [Google Scholar]
- 32.Nabavi S.M., Nabavi S.F., Eslami S., Moghaddam A.H. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012;132:931–935. [Google Scholar]
- 33.Nabavi S.F., Nabavi S.M., Mirzaei M., Moghaddam A.H. Protective effect of quercetin against sodium fluoride induced oxidative stress in rat's heart. Food Funct. 2012;3:437–441. doi: 10.1039/c2fo10264a. [DOI] [PubMed] [Google Scholar]
- 34.Curti V., Capelli E., Boschi F., Nabavi S.F., Bongiorno A.I., Habtemariam S., Nabavi S.M., Daglia M. Modulation of human miR-17–3p expression by methyl 3-O-methyl gallate as explanation of its in vivo protective activities. Mol Nutr Food Res. 2014;58:1776–1784. doi: 10.1002/mnfr.201400007. [DOI] [PubMed] [Google Scholar]
- 35.Slemmer J.E., Shacka J.J., Sweeney M., Weber J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 36.Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97–107. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabavi S.M., Marchese A., Izadi M., Curti V., Daglia M., Nabavi S.F. Plants belonging to the genus Thymus as antibacterial agents: from farm to pharmacy. Food Chem. 2015;173:339–347. doi: 10.1016/j.foodchem.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 38.Nabavi S.F., Nabavi S.M., Moghaddam A.H., Naqinezhad A., Bigdellou R., Mohammadzadeh S. Protective effects of Allium paradoxum against gentamicin-induced nephrotoxicity in mice. Food Funct. 2012;3:28–29. doi: 10.1039/c1fo10173k. [DOI] [PubMed] [Google Scholar]
- 39.Nabavi S.F., Nabavi S.M., Ebrahimzadeh M.A., Eslami B., Jafari N. In vitro antioxidant and antihemolytic activities of hydroalcoholic extracts of Allium scabriscapum Boiss. & Ky. aerial parts and bulbs. Int J Food Prop. 2013;16:713–722. [Google Scholar]
- 40.Alinezhad H., Azimi R., Zare M., Ebrahimzadeh M.A., Eslami S., Nabavi S.F., Nabavi S.M. Antioxidant and antihemolytic activities of ethanolic extract of flowers, leaves, and stems of Hyssopus officinalis L. Var. angustifolius. Int J Food Prop. 2013;16:1169–1178. [Google Scholar]
- 41.Nabavi S.F., Nabavi S.M., Setzer W.N., Nabavi S.A., Nabavi S.A., Ebrahimzadeh M.A. Antioxidant and antihemolytic activity of lipid-soluble bioactive substances in avocado fruits. Fruits. 2013;68:185–193. [Google Scholar]
- 42.Nabavi S.F., Russo G.L., Daglia M., Nabavi S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015;179:305–310. doi: 10.1016/j.foodchem.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Nabavi S.F., Nabavi S.M., Ebrahimzadeh M.A., Jafari N., Yazdanpanah S. Biological activities of freshwater algae, Spirogyra singularis Nordstedt. J Aquat Food Prod Technol. 2013;22:58–65. [Google Scholar]
- 44.Visioli F., Borsani L., Galli C. Diet and prevention of coronary heart disease: the potential role of phytochemicals. Cardiovasc Res. 2000;47:419–425. doi: 10.1016/s0008-6363(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 45.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 46.Park J.D., Rhee D.K., Lee Y.H. Biological activities and chemistry of saponins from Panax ginseng CA Meyer. Phytochem Rev. 2005;4:159–175. [Google Scholar]
- 47.Radad K., Gille G., Liu L., Rausch W.-D. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- 48.Choi K.-T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharm Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Li S., Fan Y., Chen Y., Liu D., Cheng H., Gao X., Zhou Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng CA Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 50.Coon J.T., Ernst E. Panax ginseng. Drug Saf. 2002;25:323–344. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 51.Yun T.K. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 52.Zhou W., Chai H., Lin P.H., Lumsden A.B., Yao Q., Chen C. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit. 2004;10:RA187–192. [PubMed] [Google Scholar]
- 53.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Kim J.H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shibata S., Fujita M., Itokawa H., Tanaka O., Ishii T. Studies on the constituents of Japanese and Chinese crude drugs: XI. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull. 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 56.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 57.Zhu J., Jiang Y., Wu L., Lu T., Xu G., Liu X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- 58.Park E.-K., Choo M.-K., Han M.J., Kim D.-H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 59.Chen X.C., Zhu Y.G., Zhu L.A., Huang C., Chen Y., Chen L.M., Fang F., Zhou Y.C., Zhao C.H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. EurJ Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- 60.Fu Y., Ji L.L. Chronic ginseng consumption attenuates age-associated oxidative stress in rats. J Nutr. 2003;133:3603–3609. doi: 10.1093/jn/133.11.3603. [DOI] [PubMed] [Google Scholar]
- 61.Chen X., Salwinski S., Lee T.F. Extracts of Ginkgo biloba and ginsenosides exert cerebral vasorelaxation via a nitric oxide pathway. Clin Exp Pharmacol Physiol. 1997;24:958–959. doi: 10.1111/j.1440-1681.1997.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 62.Lü J.-M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He F., Guo R., Wu S.-L., Sun M., Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–320. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 64.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 65.Jeon B.H., Kim C.S., Park K.S., Lee J.W., Park J.B., Kim K.J., Kim S.H., Chang S.J., Nam K.Y. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen Pharmacol Vasc Syst. 2000;35:135–141. doi: 10.1016/s0306-3623(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 66.Yamamoto M., Uemura T., Nakamura S., Uemiya M., Kumagai A. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. Am J Chin Med. 1983;11:96–101. doi: 10.1142/S0192415X83000161. [DOI] [PubMed] [Google Scholar]
- 67.Hong S.Y., Kim J.Y., Ahn H.Y., Shin J.-H., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J Agric Food Chem. 2012;60:3086–3091. doi: 10.1021/jf204447y. [DOI] [PubMed] [Google Scholar]
- 68.Ni H.-X., Yu N.-J., Yang X.-H. The study of ginsenoside on PPARγ expression of mononuclear macrophage in type 2 diabetes. Mol Biol Rep. 2010;37:2975–2979. doi: 10.1007/s11033-009-9864-0. [DOI] [PubMed] [Google Scholar]
- 69.Ye R., Yang Q., Kong X., Han J., Zhang X., Zhang Y., Li P., Liu J., Shi M., Xiong L. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Ye R., Li N., Han J., Kong X., Cao R., Rao Z., Zhao G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res. 2009;64:306–310. doi: 10.1016/j.neures.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Liu X., Wang L., Wen A., Yang J., Yan Y., Song Y., Liu X., Ren H., Wu Y., Li Z. Ginsenoside-Rd improves outcome of acute ischaemic stroke—a randomized, double-blind, placebo-controlled, multicenter trial. Eur J Neurol. 2012;19:855–863. doi: 10.1111/j.1468-1331.2011.03634.x. [DOI] [PubMed] [Google Scholar]
- 72.Attele A.S., Wu J.A., Yuan C.-S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 73.Lewis R., Wake G., Court G., Court J.A., Pickering A.T., Kim Y.C., Perry E.K. Non-ginsenoside nicotinic activity in ginseng species. Phytother Res. 1999;13:59–64. doi: 10.1002/(SICI)1099-1573(199902)13:1<59::AID-PTR423>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 74.Shi Y., Sun C., Zheng B., Li Y., Wang Y. Simultaneous determination of nine ginsenosides in functional foods by high performance liquid chromatography with diode array detector detection. Food Chem. 2010;123:1322–1327. [Google Scholar]
- 75.Mihalov J.J., Marderosian A.D., Pierce J.C. DNA identification of commercial ginseng samples. J Agric Food Chem. 2000;48:3744–3752. doi: 10.1021/jf000011b. [DOI] [PubMed] [Google Scholar]
- 76.Lim W., Mudge K.W., Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium) J Agric Food Chem. 2005;53:8498–8505. doi: 10.1021/jf051070y. [DOI] [PubMed] [Google Scholar]
- 77.Schlag E.M., McIntosh M.S. Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry. 2006;67:1510–1519. doi: 10.1016/j.phytochem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 78.Yun T.K., Lee Y.S., Lee Y.H., Kim S.I., Yun H.Y. Anticarcinogenic effect of Panax ginseng CA Meyer and identification of active compounds. J Korean Med Sci. 2001;16:S6–S18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan C.-S., Wu J.A., Osinski J. Ginsenoside variability in American ginseng samples. Am J Clin Nutr. 2002;75:600–601. doi: 10.1093/ajcn/75.3.600. [DOI] [PubMed] [Google Scholar]
- 80.Cheng L.-Q., Kim M.K., Lee J.-W., Lee Y.-J., Yang D.-C. Conversion of major ginsenoside Rb1 to ginsenoside F2 by Caulobacter leidyia. Biotechnol Lett. 2006;28:1121–1127. doi: 10.1007/s10529-006-9059-x. [DOI] [PubMed] [Google Scholar]
- 81.Chi H., Ji G.-E. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett. 2005;27:765–771. doi: 10.1007/s10529-005-5632-y. [DOI] [PubMed] [Google Scholar]
- 82.Hsu B.Y., Lu T.J., Chen C.H., Wang S.J., Hwang L.S. Biotransformation of ginsenoside Rd in the ginseng extraction residue by fermentation with lingzhi (Ganoderma lucidum) Food Chem. 2013;141:4186–4193. doi: 10.1016/j.foodchem.2013.06.134. [DOI] [PubMed] [Google Scholar]
- 83.Kim M.K., Lee J.W., Lee K.Y., Yang D. Microbial conversion of major ginsenoside rb ∼1 to pharmaceutically active minor Ginsenoside Rd. J Microbiol Seoul. 2005;43:456–462. [PubMed] [Google Scholar]
- 84.Son J.-W., Kim H.-J., Oh D.-K. Ginsenoside Rd production from the major ginsenoside Rb1 by β-glucosidase from Thermus caldophilus. Biotechnol Lett. 2008;30:713–716. doi: 10.1007/s10529-007-9590-4. [DOI] [PubMed] [Google Scholar]
- 85.Yu H., Liu H., Zhang C., Tan D., Lu M., Jin F. Purification and characterization of gypenoside-α-l-rhamnosidase hydrolyzing gypenoside-5 into ginsenoside Rd. Process Biochem. 2004;39:861–867. [Google Scholar]
- 86.Zhang C., Yu H., Bao Y., An L., Jin F. Purification and characterization of ginsenoside-α-arabinofuranase hydrolyzing ginsenoside Rc into Rd from the fresh root of Panax ginseng. Process Biochem. 2002;37:793–798. [Google Scholar]
- 87.Zhao X., Wang J., Li J., Fu L., Gao J., Du X., Bi H., Zhou Y., Tai G. Highly selective biotransformation of ginsenoside Rb1 to Rd by the phytopathogenic fungus Cladosporium fulvum (syn. Fulvia fulva) J Ind Microbiol Biotechnol. 2009;36:721–726. doi: 10.1007/s10295-009-0542-y. [DOI] [PubMed] [Google Scholar]
- 88.Zhao X., Gao L., Wang J., Bi H., Gao J., Du X., Zhou Y., Tai G. A novel ginsenoside Rb1-hydrolyzing β-D-glucosidase from Cladosporium fulvum. Process Biochem. 2009;44:612–618. [Google Scholar]
- 89.Kochkin D.V., Kachala V.V., Shashkov A.S., Chizhov A.O., Chirva V.Y., Nosov A.M. Malonyl-ginsenoside content of a cell-suspension culture of Panax japonicus var. repens. Phytochemistry. 2013;93:18–26. doi: 10.1016/j.phytochem.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Wang J., Man S., Gao W., Zhang L., Huang L. Cluster analysis of ginseng tissue cultures, dynamic change of growth, total saponins, specific oxygen uptake rate in bioreactor and immuno-regulative effect of ginseng adventitious root. Ind Crops Prod. 2013;41:57–63. [Google Scholar]
- 91.Ye R., Kong X., Yang Q., Zhang Y., Han J., Zhao G. Ginsenoside Rd attenuates redox imbalance and improves stroke outcome after focal cerebral ischemia in aged mice. Neuropharmacology. 2011;61:815–824. doi: 10.1016/j.neuropharm.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 92.Ye R., Zhang X., Kong X., Han J., Yang Q., Zhang Y., Chen Y., Li P., Liu J., Shi M. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y., Zhou L., Zhang X., Bai J., Shi M., Zhao G. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci. 2012;33:1125–1131. doi: 10.1007/s10072-011-0916-6. [DOI] [PubMed] [Google Scholar]
- 94.Li X.Y., Liang J., Tang Y.B., Zhou J.G., Guan Y.Y. Ginsenoside Rd prevents glutamate-induced apoptosis in rat cortical neurons. Clin Exp Pharmacol Physiol. 2010;37:199–204. doi: 10.1111/j.1440-1681.2009.05286.x. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X., Shi M., Bjørås M., Wang W., Zhang G., Han J., Liu Z., Zhang Y., Wang B., Chen J. Ginsenoside Rd promotes glutamate clearance by up-regulating glial glutamate transporter GLT-1 via PI3K/AKT and ERK1/2 pathways. Front Pharmacol. 2013;4:152–160. doi: 10.3389/fphar.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu G., Wu Z., Yang F., Zhao H., Liu X., Deng Y., Shi M., Zhao G. Ginsenoside Rd blocks AIF mitochondrio-nuclear translocation and NF-κB nuclear accumulation by inhibiting poly(ADP-ribose) polymerase-1 after focal cerebral ischemia in rats. Neurol Sci. 2013;34:2101–2106. doi: 10.1007/s10072-013-1344-6. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X., Shi M., Ye R., Wang W., Liu X., Zhang G., Han J., Zhang Y., Wang B., Zhao J. Ginsenoside Rd attenuates Tau protein phosphorylation via the PI3K/AKT/GSK-3β pathway after transient forebrain ischemia. Neurochem Res. 2014:1–11. doi: 10.1007/s11064-014-1321-3. [DOI] [PubMed] [Google Scholar]
- 98.Liu X., Xia J., Wang L., Song Y., Yang J., Yan Y., Ren H., Zhao G. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol. 2009;16:569–575. doi: 10.1111/j.1468-1331.2009.02534.x. [DOI] [PubMed] [Google Scholar]