Abstract
Background
Ginseng (the roots of Panax ginseng Meyer) is a well-known traditional Oriental medicine and is now widely used as a health food. It contains several types of ginsenosides, which are considered the major active medicinal components of ginseng. It has recently been reported that the qualitative and quantitative properties of ginsenosides found in ginseng may differ, depending on cultivation regions, ages, species, and so on. Therefore, it is necessary to study these variations with respect to cultivation ages and regions.
Methods
In this study, 3–6-yr-old roots of P. ginseng were collected from three different cultivation regions. The contents of five ginsenosides (Rb1, Rd, Rc, Re, and Rgl) were measured by rapid resolution liquid chromatography coupled with quadruple–time-of-flight mass spectrometry. The Kruskal–Wallis Rank sum test and multiple t test were used for comparative analysis of the data to evaluate the dynamic changes in the accumulation of these ginsenosides affected by cultivation regions and ages.
Results
The content and composition of ginsenosides varied significantly among specimens collected from different cultivation regions and having different cultivation ages. For all samples, the content of Rg1 and Re ginsenosides increases with age and this rate of increase is different for each sample. The contents of Rb1, Rc, and Rd varied with cultivation ages in samples from different cultivation regions; especially, Rb1 from a 6-yr-old root showed approximately twofold variation among the samples from three cultivation regions. Furthermore, the content of Rb1 highly correlated with that of Rd (r = 0.89 across all locations and ages).
Conclusion
In our study, only the contents of ginsenosides Rg1 and Re were affected by the root age. Ginsenosides Rb1, Rc, and Rd varied widely with ages in samples from different cultivation regions.
Keywords: cultivation, ginsenosides, harvest age, Panax ginseng, regions
1. Introduction
Ginseng (the roots of Panax ginseng Meyer) is a valuable agricultural commodity used for thousands of years as a traditional Oriental medicine in many Oriental countries [1]. P. ginseng has wide pharmacological properties, such as antifatigue, antidiabetes, vasodilation, and antidepressant effects, and is effective in stimulating the memory as well as in the prevention of cancer and the aging process [2]. Ginsenosides are the main active constituents in P. ginseng. To date, more than 80 kinds of ginsenosides have been isolated from P. ginseng. Based on the differences in their chemical constitutions, most ginsenosides are generally classified into the following three types: protopanaxadiol (PPD), protopanaxatriol, and oleanolic acid [3]. The main ginsenosides isolated from P. ginseng (Rb1, Rc, Rd, Re, and Rg1) typically account for more than 70% of the total ginsenoside content [4], [5], [6], [7], [8]. These ginsenosides are often used as markers for quality assessment of ginseng products [9]. However, the bioactive properties of ginsenosides differ with respect to individual ginsenoside and biological system [10]. American and Asian ginsengs have contradictory effects on the vascular system [11] and acute glycemia [12]. Recent laboratory studies have verified that the different bioactivities are due to the variation in the ratio of major ginsenosides. Mochizuki et al reported that Asian ginseng had a high Rg1:Rb1 ginsenoside ratio and Rg1 was shown to promote wound healing. By contrast, American ginseng had a low Rg1:Rb1 ratio and Rb1 was shown to inhibit tumor growth [13]. The heterogeneity of ginsenosides is a remarkable and significant property because this has different or even totally opposite pharmacological activities. The changes in the content of ginsenosides with age are related to the cultivation region of ginseng; for example, Osinski et al [14] determined and compared the major ginsenosides in American ginseng root, which showed changes based on cultivation region and age. Zhang Kun et al [15] demonstrated that the content of ginsenosides Rg1, Re, Rbl, Rc, Rb2, Rb3, and Rd in ginseng increased with the age of the plant.
Variations in the content of total and individual ginsenosides have been reported between populations and even among individual roots within a single ginseng population [16]. Because the efficacy and bioactive components of ginseng roots may differ depending on the cultivation region and age, it is important to know the components of ginsenosides in ginseng roots from different sources. The study on the variation of the main ginsenosides in ginseng could lead to a better understanding of the natural effects on ginsenosides contributed by cultivation regions and age. Moreover, the results of this research have potential contribution to quality assessment of ginseng products.
In this study, we used rapid resolution liquid chromatography coupled with quadruple–time-of-flight mass spectrometry method for the acquisition and analysis of the accumulation characteristics of monomer ginsenosides (Rb1, Rc, Rd, Re, and Rg1) in ginseng roots aged between 3 yr and 6 yr and from different cultivation regions. The objective of this research was to determine the relative contribution of age or location to the ginsenoside levels in P. ginseng collected from a geographically limited region.
2. Experimental analysis
2.1. Standard preparation
All ginsenoside standards were obtained from the Chinese Medical and Biological Products Institute (Changchun, China). The standards of ginsenosides Rg1, Re, Rb1, Rc, and Rd (1.01 mg, 1.02 mg, 0.98 mg, 0.99 mg, and 1.04 mg, respectively) were accurately weighed and dissolved in 10 mL of methanol to prepare a mixed stock solution. All solvents and samples were filtered through 0.45-μm membrane filters before analysis.
2.2. Plant material and extraction procedure
All cultivated samples were grown from seeds that purportedly had been collected during the past few decades from wild Jilin populations and harvested at ages of 3 yr, 4 yr, 5 yr, and 6 yr from three ginseng cooperative associations (Changchun, Jilin Agricultural University, Jingyu, The Second plantation of ginseng. Ji'an, Yisheng Pharmaceutical Co.) of the Jilin Province in China. The collected ginseng samples were dried at 40°C to a constant weight, and then pulverized. According to Chinese pharmacopoeia, the ginseng root sample is prepared as follows: 1.0 g of ginseng powder (40 meshes) was accurately weighed and refluxed with moderate ether solution for 4 h in a Soxhlet extractor. After removing the ether, a moderate solution of methanol was added. The sample solution was refluxed for 4 h, after which the methanol was removed. Then, chromatographic-grade methanol was added to dissolve the mixture and make the volume of the mixture solution to 10 mL. The sample solution was obtained by filtering the supernatant through a nylon filter membrane (0.45 μm) prior to the reverse-phase liquid chromatography analysis. For reliable results, five replicates of each sample group (aged from 3 yr to 6 yr) were prepared. Results are reported as percent ginsenoside on a dry-weight basis.
2.3. Liquid chromatography–mass spectrometry
Analyses were performed on an Agilent 1200 RRLC system equipped with a binary pump, micro degasser, an autoplate, and a thermostatically controlled sampler column apartment. Sample extracts were separated via reversed phase on a Thermo Scientific Hypersil GOLD C18 column (3.0 × 100 mm × 1.8 μm). The mobile phase consisted of water/0.1% formic acid (A) and acetonitrile/0.1% formic acid (B) and the flow rate was set to 0.3 mL/min. A linear gradient was used, starting with 19% B. This proportion was held constant for 5 min, and then increased linearly as follows: to 25% from 5 min to 15 min, to 30% from 15 min to 20 min, to 33% from 20 min to 25 min, to 50% from 25 min to 35 min, to 60% from 35 min to 40 min, to 75% from 40 min to 45 min, and then to 80%. The gradient was held constant at 80% until 50 min, and then returned to the initial composition (i.e., 19% B) at 50.5 min, and again held constant for 4.5 min to re-equilibrate the column. The column and sample managers were maintained at 30°C, respectively. The mass spectrometer was operated in the negative-ion mode and set to the total ion chromatogram mode. The optimized mass spectrometry conditions were as follows: capillary voltage of 2,800 V, cone voltage of 35 V, source temperature of 100°C, desolvation temperature of 250°C, and desolvation gas flow rate of 600 L/h. To ensure that the mass was measured accurately, leucine–enkephalin was used as the reference lock-mass compound at a concentration of 500 pg/μL at a flow rate of 2 μL/min, and the [M–CH3COO]– ion at 545.2615 Da was detected over 15 min of analysis. Xcalibur version 2.1 SP1 (Thermo Scientific) was used for data acquisition.
2.4. Calibration curves of ginsenoside standards
The stock solution of standards containing five ginsenosides (Rg1, Re, Rb1, Rc, and Rd) in methanol was diluted to the concentrations of 10 μg/mL, 20 μg/mL, 40 μg/mL, 80 μg/mL, and 100 μg/mL. Approximately 10 μL of the mixed-standard solutions were injected in triplicate, and then the calibration curves were constructed by plotting extracted ion peak area versus the amounts (μg) of each analyte. The contents (mg/g of herbs, μg/mL) of the five ginsenosides in these samples were measured according to the standard curves of five ginsenosides.
2.5. Experimental design and statistical analysis
Because of the large unexplained variation and small samples sizes, the experimental analysis used the average value to describe the central tendency of data. The number of replicate root samples (n) for each cultivation age is indicated in parentheses for the Changchun, Jingyu and Ji'an locations, respectively, as follows: CC-(3, 4, 5, 6), JY-(3, 4, 5, 6), and JA-(3, 4, 5, 6). Randomly selected five plant samples for each cultivation age from each of the three locations are analyzed five times, and the mean value for the ginsenoside content is calculated. The ginsenoside content was expressed as the weight of ginsenoside relative to the root dry weight (mg/g). Spearman nonparametric rank correlations among ginsenoside variables (mg/g; Rg1, Re, Rb1, Rc, and Rd) were analyzed using PROC CORR in SAS (SAS Institute Inc, Cary, NC, USA). First, we used the Kruskal–Wallis rank sum test, and then subsequently multiple t test to determine whether there were any significant differences in the ginsenoside contents or compositions among the samples from different location and of different harvest ages. The statistical package R3.0.2 was used for all analysis. In general, p values less than 0.05 were considered statistically significant.
3. Results and discussion
3.1. Chromatographic analysis and quantitative methods
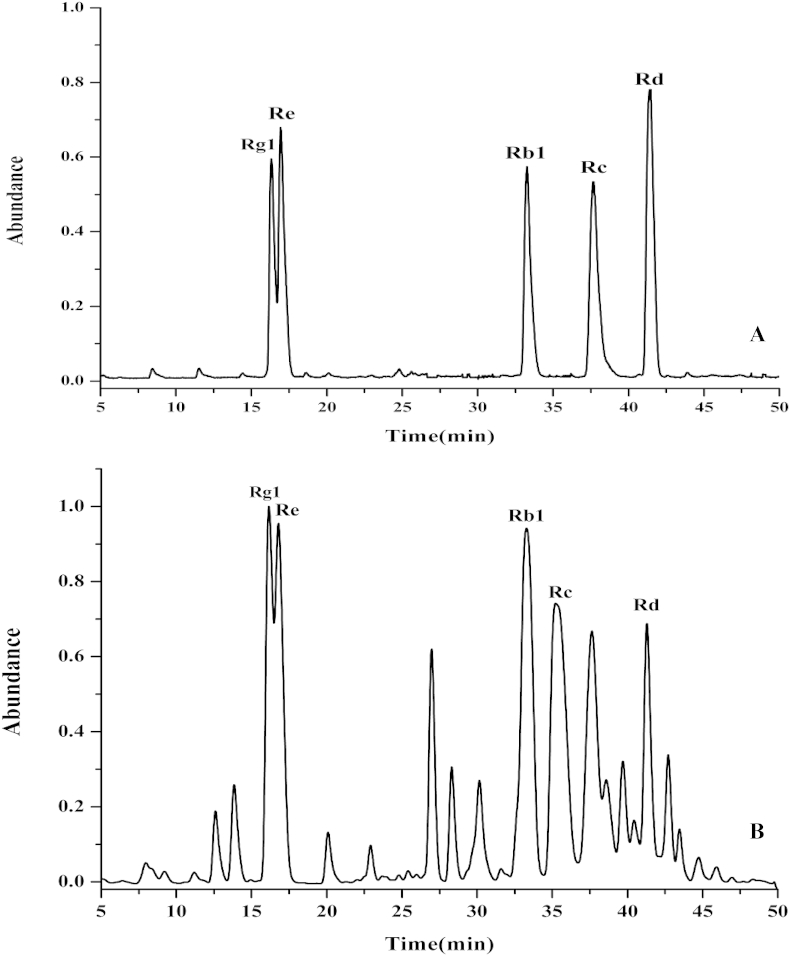
Based on the chromatograms (Fig. 1A), all of the five ginsenosides were well separated except for the ginsenosides Rg1 and Re. The linearity, regression, and precision ranges of the five tested ginsenosides were calculated according to a previous study [17]. In addition, the corresponding peak areas calculated by integrating extracted ion chromatogram [M+CH3COO]– from each standard concentration were used to make the standard curves, which were used to quantify individual ginsenosides in root samples. The higher correlation coefficient values (R2 > 0.99) indicated good correlations between investigated ginsenosides amounts and their area of extraction peaks within the test ranges (Table 1). The injection precision was obtained by analyzing the peak area variations of six injections of a mixture of the five standard ginsenosides. The intraday and interday (6 d) precisions are 3.1–6.1% (n = 6) and 2.51–6.0% (n = 6), respectively. The recoveries of the ginsenosides were determined with spiked samples. The stock solution of standards containing the five ginsenosides was added to 1 g of P. ginseng root and extracted using the microwave-assisted extraction method. The recoveries of all five ginsenosides were within the range of 97.29–99.50% (n = 6). In this study, each value is the average of three replicated samples.
Fig. 1.
Rapid resolution liquid chromatography coupled with electrospray ionization quadruple–time-of-flight mass spectrometry total ion chromatograms of (A) ginsenoside standard mixture and (B) alcohol extracts from the ginseng roots cultivated in Changchun. The five ginsenosides were identified by the retention time and qualitative fragments.
Table 1.
Calibration curve and concentration range of five ginsenosides
| Ginsenosides | Calibration curve | r2 | Linearity range (μg) | LOQ (ng) |
|---|---|---|---|---|
| Rg1 | Y = 16,675,697X + 296,894 | 0.9996 | 0.518–22.0 | 92–167 |
| Re | Y = 5,026,234X + 180,910 | 0.9984 | 0.464–17.7 | 91–174 |
| Rc | Y = 18,707,368X + 327,473 | 0.9988 | 0.565–20.1 | 94–184 |
| Rd | Y = 20,072,946X + 827,473 | 0.9987 | 0.374–15.7 | 97–197 |
| Rb1 | Y = 1,495,613X + 297,081 | 0.9982 | 0.427–16.7 | 109–217 |
LOQ, limit of quantification.
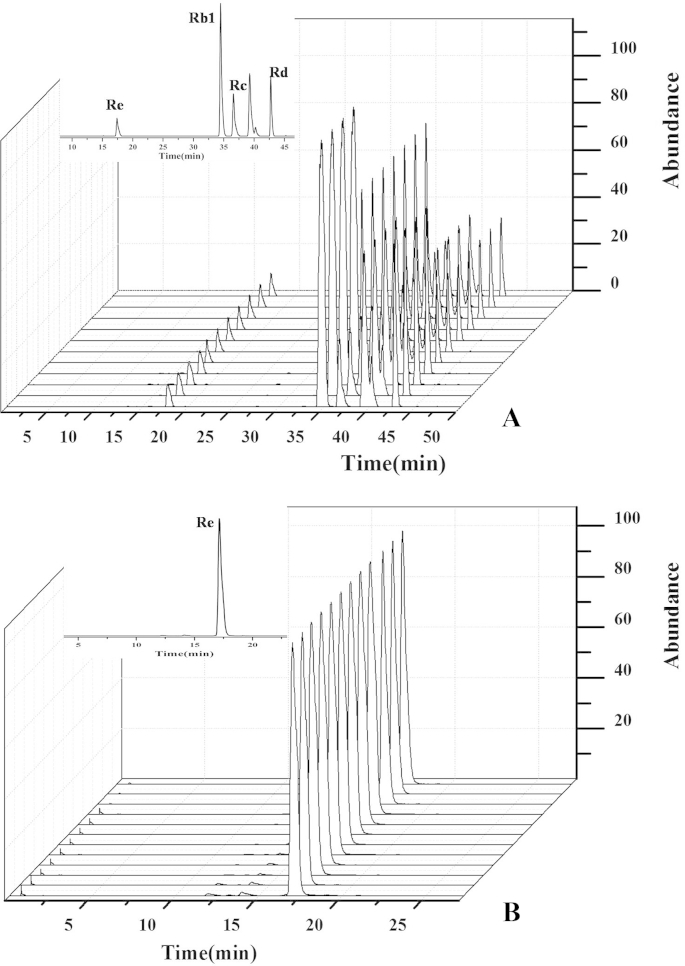
The total ion chromatogram of ginsenosides in ginseng is shown in Fig. 1B. The other chromatograms are not shown because of their similarly with the one presented in this figure. The confirmatory analysis for five ginsenosides in the ginseng root was performed with the retention time and qualitative fragments obtained by the data-dependent scan mode [18]. The quantitative analysis was carried out by acquiring the accurate mass of quasi-molecular ion and the peak area in the extracted chromatogram with accurate mass [19], [20]. The quasimolecular ion peak of Rg1, Re, Rb1, Rc, and Rd, respectively, were obtained at m/z 845.49, 991.55, 1,153.60, 1,123.58, and 991.53. The extraction ion chromatograms of the five ginsenosides in ginseng obtained from three cultivation regions are shown in Fig. 2.
Fig. 2.
Rapid resolution liquid chromatography coupled with quadruple–time-of-flight mass spectrometry chromatograms for extracts obtained from different cultivation regions. (A) Extracted chromatogram of Re, Rb1, Rc, and Rd. The quasimolecular ion peak of Re, Rb1, Rc, and Rd, respectively, at m/z 991.55, 1,153.60, 1,123.58, and 991.53. (B) Extracted chromatogram of Rg1. The quasimolecular ion peak of Rg1 at m/z 845.49.
3.2. Analysis of the content of ginsenosides Rg1 and Re
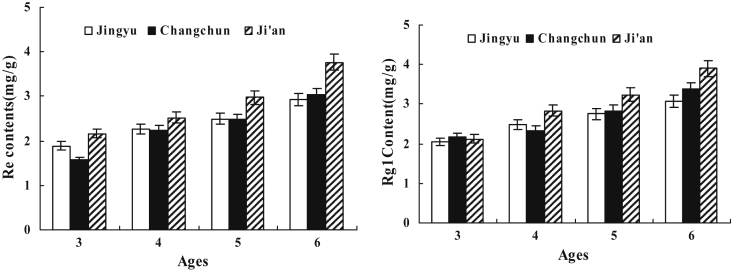
The average content of Rg1 and Re ginsenosides for samples from three locations having different cultivation ages is shown in Fig. 3. In all the samples from three different cultivation regions, the content of both Rg1 and Re increased with cultivation age, but the rate of increase was different (Fig. 4). In particular, the contents of ginsenosides Rg1 and Re in roots of different harvest ages at the same location were significantly different (p < 0.001). Shi et al [21] studied the content of ginsenosides in P. ginseng samples of different ages from Jingyu, and reported that the content of Re increases with age. The content of Rg1 in P. ginseng increases from 1 yr to 4 yr followed by a decrease [21]. The results obtained in our study are different from those of the previous study, which may be due to the difference in the variety, cultivation conditions, and geographic location of P. ginseng.
Fig. 3.
The average content of Rg1 and Re ginsenosides for samples from three locations having different cultivation ages. The changes of ginsenoside contents (mg/g dry plant material) for the samples between ages 3 yr and 6 yr are shown. Five plant samples for each cultivation age from each of the three locations are analyzed five times (n = 25).
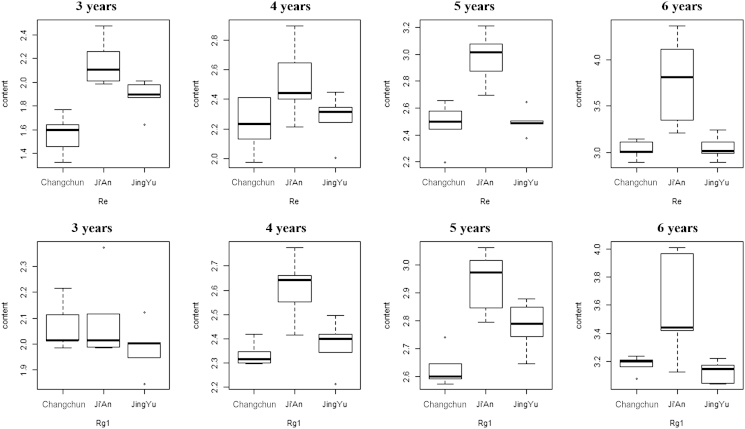
Fig. 4.
Box plot of the Rg1 and Re ginsenoside content (mg/g) in samples from different locations by age group. Box plots displaying the extremes, the upper and lower quartiles, and the median of the maximum difference between locations. The contents were assessed using the analysis of variance and repeated for five times (n = 25). The statistical significance was set at p ≤ 0.05.
Comparing the differences in ginsenosides content among all the samples from three cultivation regions, it can be seen that the contents of Re and Rg1 varied apparently. For samples with the same cultivation age, the content of Re for samples from Ji'an was significantly higher than that of samples from the other two cultivation regions (p < 0.01). Between the remaining two regions, Changchun and Jingyu, there was a less significant difference in the content of Re (p < 0.05). The content of Rg1 in the ginseng samples from all the three regions exhibited an identical proportional distribution to that of Re. The content of Rg1 in the Ji'an ginseng sample had a significant difference compared with the samples from the other two regions (p < 0.01) for the same cultivation age. Between the samples from Changchun and Jingyu, the p value was less than 0.05.
Lim et al [18] studied the relative abundance of ginsenosides in American ginseng (roots), and observed an inverse relationship between the levels of ginsenosides Rg1 and Re. Another study showed that “low Rg1/high Re chemotype” is common in American ginseng, whereas roots with high Rg1 and low Re content, which are described as “high Rg1/low Re chemotype,” are rarely observed [22]. The results obtained in our study showed that the content of Rg1 was slightly higher than that of Re, and the ratio of Rg1:Re is approximately equal to 1.0. In addition, the inverse relationship between the contents of ginsenosides Rg1 and Re was not observed. Moreover, the contents of Rg1 and Re in American ginseng were found to vary among populations (and in some cases, among roots within a population) [23]. Another study suggested that the Re and Rg1 content was affected by genotype, and the Rg1 content was also influenced by location [18]. In our study, the content of Re and Rg1 was found to change with the cultivation age. The results showed that there were differences between ginsenosides contents in P. ginseng and American ginseng, which may explain the variation in their bioactivity.
3.3. Analysis of the content of ginsenosides Rd, Rb1, and Rc
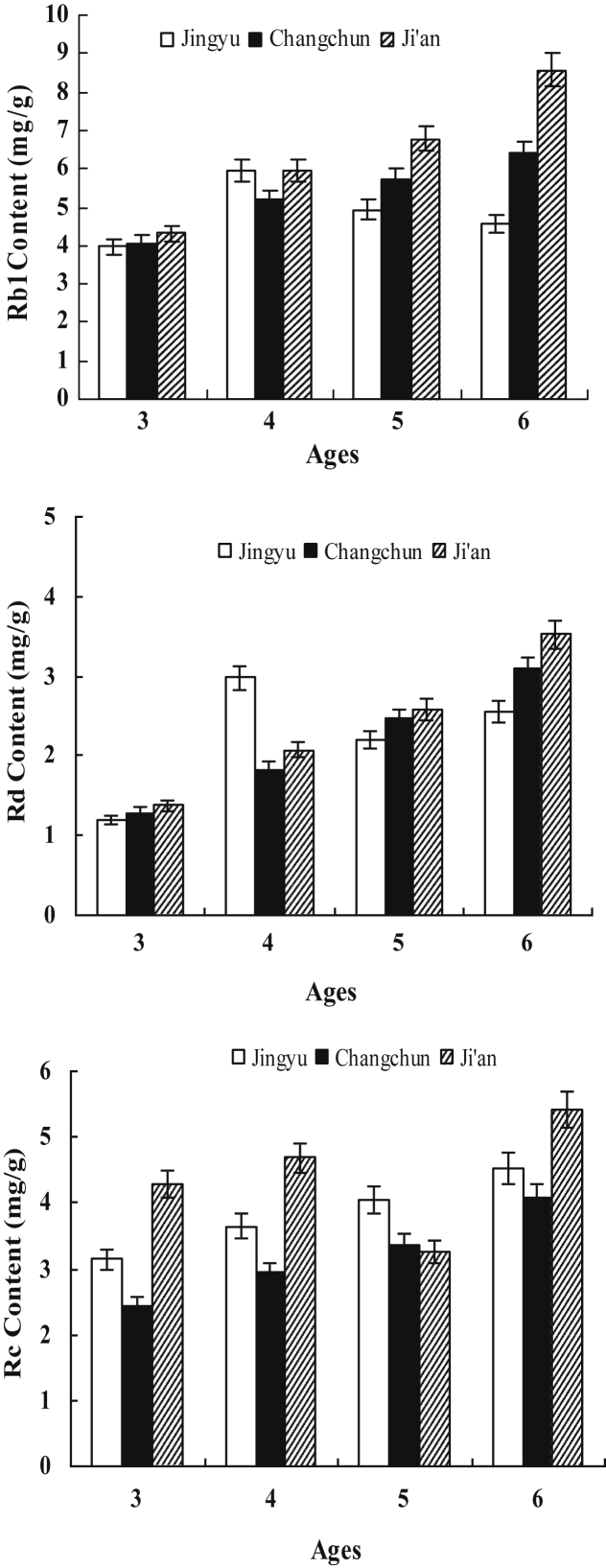
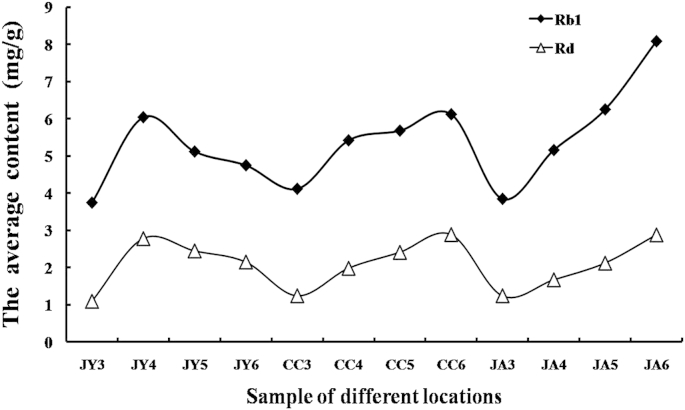
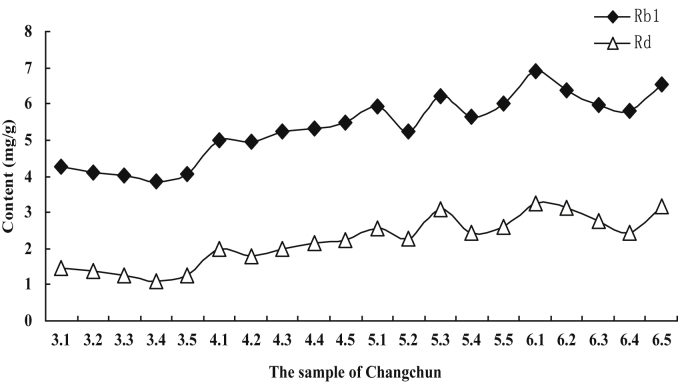
The other three PPD-type ginsenosides, Rb1, Rc, and Rd, were identified and analyzed. The nature of the interaction among ginsenosides was worthy of a further investigation. In this study, the monomer ginsenoside contents in all the samples varied with cultivation ages (Fig. 5). The content of the ginsenoside Rc in the Jingyu ginseng samples increased with cultivation age. The average contents of ginsenosides Rb1 and Rd increased to the highest amount in the 4th cultivation year followed by a decrease, as shown in Fig. 5. In the Changchun ginseng samples, the content of ginsenosides Rb1, Rc, and Rd increased with cultivation age from the 3rd yr to the 6th yr. The variation tendency of ginsenosides Rb1 and Rd in the Ji'an ginseng samples was the same as that of the Changchun samples, with both exhibiting a sustained growth in the ginsenosides content with cultivation ages, except for the ginsenoside Rc, whose content was low in the 5th cultivation year. For the three different region samples with the same cultivation age, the significant difference in the content of Rc and Rd was on the 0.01 level (p < 0.01) and on the 0.001 level for Rb1 (p < 0.001). Schlag and McIntosh [22] used PROC CORR in SAS to analyze nonparametric rank correlations among ginsenoside variables (% w/w of total ginsenosides, Rg1, Re, Rb1, Rc, and Rd). They found that Rb1 was highly correlated with the total ginsenoside content in American ginseng (r = 0.96, p < 0.001). In this study, we used the same method to analyze nonparametric rank correlations among ginsenosides (Rb1, Rc, Rd, Rg1, and Re). The result showed that the content of ginsenoside Rb1 was highly correlated with that of Rd (r = 0.89, p < 0.001). The correlated relationship between the Rb1 and Rd levels was apparent not only among individual roots within one region (Fig. 6), but also among locations based on the level of each of these ginsenosides averaged across all roots in each of the three locations. Fig. 7 shows the root-to-root variation in the ginsenosides Rb1 and Rd contents of 15 ginseng root samples in Changchun for representation. The sum of Rb1 and Rd content in ginseng root is more than 20% of the total ginsenosides, and the content of Rd in ginseng root is four to five times less than that of Rb1 [24]. Recently, more reports showed the biotransformation of ginsenoside Rb1 into Rd, in particular using microorganisms.
Fig. 5.
The average content of Rb1, Rc, and Rd ginsenosides in the samples from three locations with different cultivation ages. The changes of ginsenoside contents (mg/g dry plant material) for the samples between ages 3 yr and 6 yr are shown. Five plant samples for each cultivation age from each of the three locations are analyzed five times (n = 25).
Fig. 6.
Dynamic analysis diagram of the contents of Rb1 and Rd ginsenosides (across all locations and cultivation ages). Every value is the average calculated using five plant samples for each cultivation age from each of the three locations (n = 25). CC3-6 refers to the sample from Changchun aged 3–6 yr; JA3-6 refers to the sample from Ji'an aged 3–6 yr; JY3-6 refers to the sample from Jingyu aged 3–6 yr.
Fig. 7.
Dynamic analysis diagram of the contents of Rb1 and Rd ginsenosides (Changchun location). Every value is the average calculated using five plant samples for each cultivation age from each of the three locations (n = 25). 3.1–3.5 represents five Panax ginseng plants aged 3 yr. 4.1–4.5 represents five P. ginseng plants aged 4 yr. 5.1–5.5 represents five P. ginseng plants aged 5 yr; 6.1–6.5 represents five P. ginseng plants aged 6 yr.
A report by Sengupta et al [25] showed that a low Rg1:Rb1 ratio (1–0.15) produced uniform ginsenoside profiles, which is considered the characteristic in Asian ginseng, and pointed out that the ratio of Rg1:Rb1 ginsenoside is high in American ginseng [25]. In our study, the Rg1:Rb1 ratio was 0.4, less than 1.0, and the highest ratio was in the 6th cultivation year. Therefore, the Rg1:Rb1 ratio between 1 and 0.15 was considered as the characteristic of Asian ginseng, which is identical to the literature. Overall, the different biological properties between Asian and American ginseng may be not only due to a variety of ginsenoside structure but also due to the composition of total ginsenosides.
4. Conclusions
Based on the aforementioned results, the following conclusions are made:
-
•
Although there were apparent discrepancies among the contents of ginsenoside for samples of same age from the three cultivation areas, most of the five kinds of ginsenosides showed an identical variation trend in content, that is, constant increase in content with cultivation age between 3 yr and 6 yr, with the exception of Rb1 and Rd in Jingyu, as well as Rc in Ji'an, which showed a decrease in the 5th cultivation year.
-
•
Among samples with the same cultivation age, the ginseng sample from Ji'an exhibited much higher ginsenoside content than that of ginseng samples from the other two cultivation areas (Jingyu and Changchun), which may imply a potentially better quality of ginseng in Ji'an.
-
•
In all the four cultivation-age levels, Rb1 had the highest content among all the five kinds of ginsenosides, regardless of the cultivation regions.
-
•
Considering the positive correlation between the content of Rb1 and Rd and the steady ginseng cultivation environment, it could be inferred that Rb1 and Rd may experience an identical synthetic and metabolic pathway in P. ginseng.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research work was supported by the National Ministry of Science and Technology of China (Grant No. 2011BAI03B00) and the National Science Foundation of China (Grant No. 21175127 and 21475012).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Pu Z., Zhang P., Yugang G., Jianxun H., Yaxing W., Ran L., He Y., Ping L., Zhou D., Zhang L. The evaluation of contents of nine ginsenoside monomers in ginseng hairy roots by high performance liquid chromatography (HPLC) J Med Plants Res. 2011;5:5513–5516. [Google Scholar]
- 2.Shu-ping C., Li-xing N., Gang-li W., Rui-chao L. HPLC simultaneous determination of nine ginsenosides in Shenmai injection. Chinese J Pharm Anal. 2011;31(3):476–478. [Google Scholar]
- 3.Fuzzati N., Gabetta B., Jayakar K., Pace R., Peterlongo F. Liquid chromatography–electrospray mass spectrometric identification of ginsenosides in Panax ginseng roots. J Chromatogr A. 1999;854:69–79. doi: 10.1016/s0021-9673(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim N., Kim K., Choi B.Y., Lee D., Shin Y.S., Bang K.H., Cha S.W., Lee J.W., Choi H.K., Jang D.S. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Tof MS. J Agric Food Chem. 2011;59:10435–10441. doi: 10.1021/jf201718r. [DOI] [PubMed] [Google Scholar]
- 5.Ruan C.C., Liu Z., Li X., Liu X., Wang L.J., Pan H.Y., Zheng Y.N., Sun G.Z., Zhang Y.S., Zhang L.X. Isolation and characterization of a new ginsenoside from the fresh root of Panax ginseng. Molecules. 2010;15:2319–2325. doi: 10.3390/molecules15042319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assinewe V.A., Baum B.R., Gagnon D., Arnason J.T. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) J Agric Food Chem. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- 8.Lee J.S., Kwon K.A., Jung H.S., Kim J.H., Hahm K.B. Korea red ginseng on Helicobacter pylori-induced halitosis: newer therapeutic strategy and a plausible mechanism. Digestion. 2009;80:192–199. doi: 10.1159/000229997. [DOI] [PubMed] [Google Scholar]
- 9.Do J.H., Lee H.O., Lee S.K., Jang J.K., Lee S.D., Sung H.S. Colorimetric determination of acidic polysaccharide from Panax ginseng, its extraction condition and stability. Korean J Ginseng Sci. 1993;7:139–144. [Google Scholar]
- 10.McGraw J.B. Evidence for decline in stature of American ginseng plants from herbarium specimens. Biol Conserv. 2001;98:25–32. [Google Scholar]
- 11.Ki Y.N., Ko S.R., Choi K.J. Relationship of saponin and non-saponin for the quality of ginseng. J Ginseng Res. 1998;22:272–283. [Google Scholar]
- 12.Schluter C., Punja Z.K. Genetic diversity among natural and cultivated field populations and seed lots of American ginseng (Panax quinquefolius L.) in Canada. Int J Plant Sci. 2002;163:427–439. [Google Scholar]
- 13.Mochizuki M., Yoo Y.C., Matsuzawa K., Sato K., Saiki I., Tono-oka S., Samukawa K., Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 14.Anbao W., Chong Z-W., Ji A-W., Osinsk J., Chun S-Y. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem Anal. 2005;16(4):272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 15.Kun Z., Xiao W., Lan D., Juan L., Chen-ling Q., Li-gang C., Hai-yan J. Determination of seven major ginsenosides in different parts of Panax quinquefolius L. (American Ginseng) with different ages. Chem Res. 2008;24(6):707–711. [Google Scholar]
- 16.Attele A.S., Zhou Y.P., Xie J.T., Wu J.A., Zhang L., Dey L., Pugh W., Rue P.A., Polonsky K.S., Yuan C.S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 17.Sheng-Ji P. Ethnobotanical approaches of traditional medicine studies: some experiences from Asia. Pharm Biol. 2001;39:74–79. doi: 10.1076/phbi.39.s1.74.0005. [DOI] [PubMed] [Google Scholar]
- 18.Lim W., Mudge K.W., Vermeylen F. Effects of population, age, and cultivation methods on ginsenoside content of wild American ginseng (Panax quinquefolium) J Agric Food Chem. 2005;53:8498–8505. doi: 10.1021/jf051070y. [DOI] [PubMed] [Google Scholar]
- 19.Ligor T., Ludwiczuk A., Wolski T., Buszewski B. Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal Bioanal Chem. 2005;383:1098–1105. doi: 10.1007/s00216-005-0120-8. [DOI] [PubMed] [Google Scholar]
- 20.van Breemen R.B., Huang C.-R., Lu Z.-Z., Rimando A., Fong A., Fong H.H.S., Fitzloff J.F. Electrospray liquid chromatography/mass spectrometry of ginsenosides. Anal Chem. 1995;67:3985–3989. [Google Scholar]
- 21.Shi W., Wang Y., Li J., Zhang H., Ding L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007;102:664–668. [Google Scholar]
- 22.Schlag E.M., McIntosh M.S. Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry. 2006;67:1510–1519. doi: 10.1016/j.phytochem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Li T.S.C., Mazza G., Cottrell A.C., Gao L. Ginsenosides in roots and leaves of American ginseng. J Agric Food Chem. 1996;44:717–720. [Google Scholar]
- 24.Wang A., Wang C.Z., Wu J.A., Osinski J., Yuan C.S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem Anal. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 25.Sengupta S., Toh S.A., Sellers L.A., Skepper J.N., Koolwijk P., Leung H.W., Yeung H.W., Wong R.N., Sasisekharan R., Fan T.P. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]