Abstract
Background
The beneficial effects of ginsenoside species have been well demonstrated in a number of studies. However, the function of ginsenoside Ro (GRo), an oleanane-type saponin, has not been sufficiently investigated. Thus, the aim of the present study was to investigate the anti-inflammatory effects of GRo in vitro using the Raw 264.7 mouse macrophage cell line treated with lipopolysaccharide (LPS), and to clarify the possible mechanism of GRo involving heme oxygenase-1 (HO-1), which itself plays a critical role in self-defense in the presence of inflammatory stress.
Methods
Raw 264.7 cells were pretreated with GRo (up to 200μM) for 1 h before treatment with 1 μg/mL LPS, and both cell viability and inflammatory markers involving HO-1 were evaluated.
Results
GRo significantly increased cell viability in a dose dependent manner following treatment with LPS, and decreased levels of reactive oxygen species and nitric oxide. GRo decreased inflammatory cytokines such as nitric oxide synthase and cyclooxygenase-2 induced by LPS. Moreover, GRo increased the expression of HO-1 in a dose dependent manner. Cotreatment of GRo with tin protoporphyrin IX, a selective inhibitor of HO-1, not only inhibited upregulation of HO-1 induced by GRo, but also reversed the anti-inflammatory effect of GRo in LPS treated Raw 264.7 cells.
Conclusion
GRo induces anti-inflammatory effects following treatment with LPS via upregulation of HO-1.
Keywords: ginsenoside Ro, heme oxygenase-1, inflammation, lipopolysaccharide, macrophage
1. Introduction
Inflammation is a complex biological response against harmful stimuli and plays a critical role in immune defense under various external and internal pathogens [1]. Harmful stimuli such as lipopolysaccharides (LPS), a toxic molecule derived from gram-negative bacteria cell walls, activate macrophages to release various proinflammatory molecules, including nitric oxide synthase (NOS), nitric oxide (NO), and cytokines including cyclooxygenase 2 (COX-2) [2]. NO has a critical role in various diseases involving the immune system such as atherosclerosis, auto-immune disease, and neurodegenerative disorders [3]. NO is produced by inducible NOS (iNOS), which is the major form induced in response to inflammatory stimuli including LPS [4]. Likewise, COX-2 is responsible for the production of numerous prostaglandins and reactive oxygen species (ROS) at sites of inflammation [5], [6]. Therefore, a number of studies concerning the development of anti-inflammatory agents have proposed targeting the production of harmful stimuli as a potential strategy for the treatment of inflammatory diseases.
Heme oxygenase (HO) has three isoforms, namely, HO-1, HO-2, and HO-3, that serve as cyto-protective enzymes that catalyze heme degradation, producing carbon monoxide (CO), iron, and biliverdin as the final products [7], [8]. Among the isoforms of HO, HO-1 is the major functional protein induced by various stimuli including UV radiation and oxidative stress [9]. Previous studies have examined the relationship between HO-1 and LPS as a potential strategy for mediating anti-inflammatory effects [10], [11]. As a result, it has been established that HO-1 plays an important role in regulating immune responses.
The ginseng plant has been traditionally used as a medicine in East Asia [12]. Numerous alternative and complementary medicine studies have examined the function of ginseng. Ginseng contains various bioactive components including ginsenoside, polyacetylenes, polyphenolic compounds, and acidic polysaccharides [13]. Previous studies have shown that the most functional molecules in ginseng are ginsenoside, which are a specific type of triterpene saponin [13], [14]. Ginsenoside Ro (GRo), which possesses an oleanane-type aglycone, is the major ginsenoside constituent in the Panax ginseng rhizome [15]. However, only one report of the anti-inflammatory effects of GRo has been published, and was limited to the effects of GRo on suppression of inflammatory symptoms in arthritic rats [16]. However, there have been no studies that have looked at the underlying mechanisms of GRo and its anti-inflammatory effects in Raw 264.7 cells, which are a model of immune macrophage cells. Thus, the aim of the present study was to explore the anti-inflammatory effects of GRo associated with the HO-1 pathway in Raw 264.7 cells treated with LPS.
2. Materials and methods
2.1. Chemicals
GRo was purchased from the Ambo Institute (Daejeon, Korea). LPS, Griess reagent, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 2′,7′-dichlordihydrofluorescin diacetate (DCF-DA) was purchased from Invitrogen (Carlsbad, CA, USA). Tin protoporphyrin IX (SnPP), an inhibitor of HO activity, was purchased from Porphyrin Products (Logan, UT, USA). Primary antibodies, including HO-1, COX-2, iNOS, and the appropriate secondary antibodies used for immunoblotting analysis, were purchased from Cell Signaling Technology (Danvers, MA, USA). Cell culture medium and other in vitro ingredients were purchased from Hyclone (Logan, UT, USA).
2.2. Cell culture and treatments
The Raw 264.7 mouse macrophage cell line was obtained from the Korean Cell Line Bank (KCLB; Seoul, Korea). Cells were cultured at 37°C under a humidified, 5% CO2 atmosphere in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% Fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL of streptomycin. In all experiments, cells were allowed to acclimate for 24 h before treatment. After reaching confluence, cells were incubated with GRo for 1 h at different concentrations up to 200μM followed by 1 μg/mL LPS for 24 h. In some experiments, Raw 264.7 cells were also pretreated with 10μM SnPP for 1 h prior to GRo and/or LPS treatment.
2.3. Cell viability assay
Cell viability was determined with an MTT assay kit. Briefly, Raw 264.7 cells were plated in 48-well plates at a density of 2.0 × 104 cells per well, incubated for 24 h, and treated with various concentrations of GRo for 24 h. We then investigated how 1 h of pretreatment with GRo (50μM, 100μM, and 200μM) affected the viability of Raw 264.7 cells treated with 1 μg/mL LPS for 24 h. After the incubation period, 10 μL of MTT reagent was added to each well and incubated for 3 h at 37°C in 5% CO2. The resulting formazan crystals were subsequently dissolved in MTT solubilization solution. The absorbance was determined at 540 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
2.4. Determination of ROS production
ROS production was quantified by fluorescence using DCF-DA. Cells were grown in 48-well plates and incubated with the indicated treatments for 3 h. After the incubation period, cells were washed with phosphate-buffered saline (PBS) and stained with DCF-DA in PBS for 30 min in the dark. The plates were then washed twice with PBS and extracted with 0.1% Tween-20 in PBS for 10 min at 37°C. Fluorescence was recorded using an excitation wavelength of 490 nm and an emission wavelength of 525 nm.
2.5. Determination of NO production
Production of NO was determined by measuring accumulated levels of nitrite, an indicator of NO in the supernatant, after treatment with LPS for 24 h under the indicated conditions. The nitrite concentration in the supernatant of cultured medium was measured using Griess reagent. Briefly, samples were mixed with equal volumes of Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride, and 2.5% phosphoric acid) and incubated for 20 min at room temperature. Absorbance was then measured at 540 nm on a microplate reader.
2.6. Immunoblotting analysis
Cells were washed three times with phosphate buffered saline (PBS), scraped off plates, and lysed with lysis buffer (1% Triton X-100, 1% deoxycholate). The protein concentration of lysates was determined using Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA), and equal amounts of protein were separated electrophoretically using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidenedifluoride membranes (Bio-Rad Laboratories). Membranes were blocked in 5% skim milk in PBS and incubated with primary antibodies against HO-1, COX-2, iNOS, and β-actin diluted 1:500 in 1% skim milk in PBS overnight at 4°C. The blots were then incubated with a peroxidase-conjugated goat antirabbit IgG diluted 1:5000 for 1 h. Protein bands were visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, San Jose, CA, USA) and analyzed using a chemiImager analyzer system (Alpha Innotech, San Leandro, CA, USA).
2.7. Cell morphology observations
Raw 264.7 cells in 48-well plates were treated with the indicated conditions for 24 h. After incubation, plates were observed and photographed using an Observer A1 microscope at 100× magnification (Carl Zeiss, München, Germany).
2.8. Statistical analyses
Results are presented as the mean ± standard error. Data were analyzed using Student t test (for 2 groups), one-way analysis of variance, and Tukey test (for > 2 groups). A p value < 0.05 was considered statistically significant. All analyses were performed using the SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. GRo increases cell viability attenuated by LPS
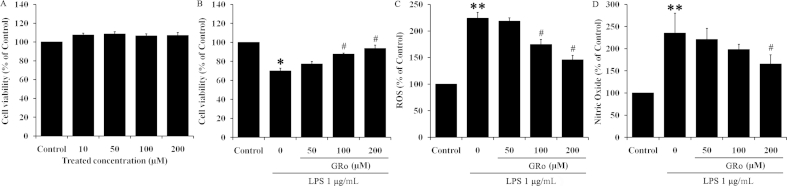
MTT assay was performed to determine the effect of GRo on cell viability in LPS-treated Raw 264.7 cells. To assess the toxicity of GRo in Raw 264.7 cells, they were first treated with various concentrations (10μM, 50μM, 100μM, and 200μM) of GRo for 24 h. GRo exhibited no significant dose dependent toxicity (Fig. 1A). We next determined the effect of GRo on both cell viability and ROS levels, a marker of oxidative stress, following treatment with 1 μg/mL LPS (Fig. 1B, 1C). LPS reduced cell viability by ∼70% compared with nontreated controls. As shown in Fig. 1B, pretreatment with 100μM and 200μM GRo for 1 h prior to 1 μg/mL LPS incubation for 24 h led to a significant increase in cell viability. The changes in ROS levels and NO production were consistent with the effects of GRo on viability (Fig. 1C, 1D). Together, these results suggested that GRo increases cell viability in a dose dependent manner while decreasing ROS and NO production induced by LPS.
Fig. 1.
Effects of GRo on cell viability and production of ROS and NO in LPS-induced Raw 264.7 cells. Cells were incubated with GRo up to 200μM for 24 h, after which (A) cell viability was determined. Next, cells were pretreated with GRo for 1 h and then incubated with 1 μg/mL LPS for 24 h, after which (B) cell viability was determined. In addition, cells were pretreated with GRo for 1 h followed by 1 μg/mL LPS for 3 h and (C) ROS production was examined. (D) NO assay using supernatant from the same samples used for the cell viability assay. Data are presented as mean ± SEM (n = 3). *p < 0.05 and **p < 0.01, as compared with the control. #p < 0.05, as compared with LPS treatment alone. GRo, ginsenoside Ro; LPS, lipopolysaccharide; NO, nitric oxide; ROS, reactive oxygen species.
3.2. GRo inhibits proinflammatory cytokines in LPS-induced Raw 264.7 cells
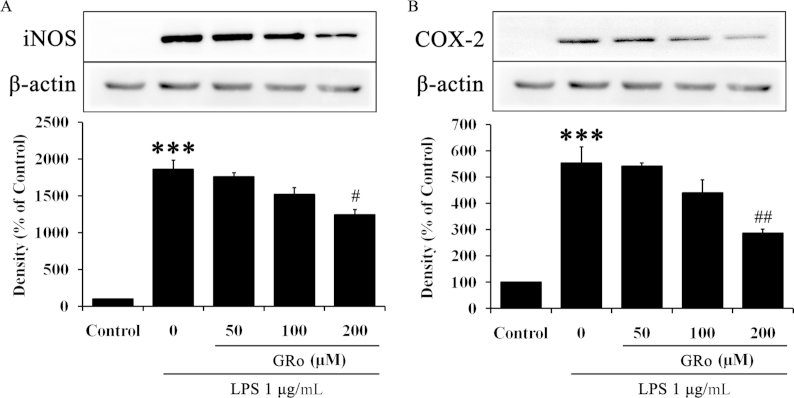
To examine the anti-inflammatory effects of GRo on LPS-induced inflammation, immunoblotting was performed with cells pretreated with various concentration of GRo for 1 h followed by LPS. As shown in Fig. 2A, GRo significantly reduced protein expression of iNOS induced by LPS. Moreover, protein expression of COX-2 was significantly reduced in GRo-treated cells induced by LPS (Fig. 2B). These results showed that the anti-inflammatory effects of GRo were associated with a marked reduction in levels of proinflammatory cytokines induced by LPS.
Fig. 2.
Effects of GRo on proinflammatory cytokines in LPS-induced Raw 264.7 cells. Cells were pretreated with GRo up to 200μM for 1 h and incubated with 1 μg/mL LPS for 24 h. (A and B) The protein expression levels of iNOS and COX-2 were determined by immunoblotting analysis. Data are presented as mean ± SEM (n = 3). ***p < 0.001, as compared with the control. #p < 0.05 and ##p < 0.01, as compared with LPS treatment alone. COX-2, cyclooxygenase-2; GRO, ginsenoside Ro; iNOS, inducible NOS; LPS, lipopolysaccharide.
3.3. GRo induces upregulation of HO-1 in Raw 264.7 cells
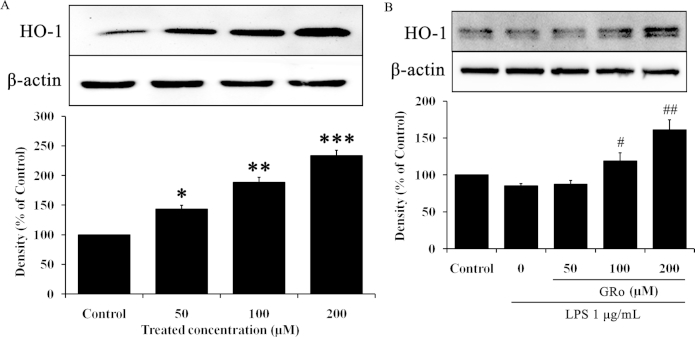
To identify the anti-inflammatory mechanism of GRo, we first confirmed the expression of HO-1, a candidate for the anti-inflammatory effects of GRo, in Raw 264.7 cells. Specifically, we examined whether induction of HO-1 is involved in the anti-inflammatory properties of GRo. As shown in Fig. 3A, GRo increased HO-1 expression in a dose dependent manner, and cells treated with 200μM GRo exhibited significantly increased expression of HO-1. In addition, we investigated whether GRo induces HO-1 expression in the presence of LPS. Cells were pretreated with various concentrations of GRo for 1 h prior to LPS treatment for 24 h. GRo significantly increased HO-1 expression despite the presence of LPS (Fig. 3B). Importantly, these results suggested the possibility that HO-1 mediates the anti-inflammatory effect of GRo.
Fig. 3.
Effects of GRo on HO-1 expression in LPS-induced Raw 264.7 cells. Cells were incubated with GRo up to 200μM for 24 h, and then (A) the protein expression of HO-1 was determined by immunoblotting analysis. Cells were pretreated with GRo up to 200μM for 1 h and incubated with 1 μg/mL LPS for 24 h. (B) The protein expression of HO-1 was determined by immunoblotting analysis. Data are presented as mean ± SEM (n = 3). *p < 0.05, **p < 0.01, and ***p < 0.001, as compared with the control. #p < 0.05 and ##p < 0.01, as compared with LPS treatment alone. GRo, ginsenoside Ro; HO-1, heme oxygenase-1; LPS, lipopolysaccharide.
3.4. GRo inhibits proinflammatory cytokines via HO-1 induction
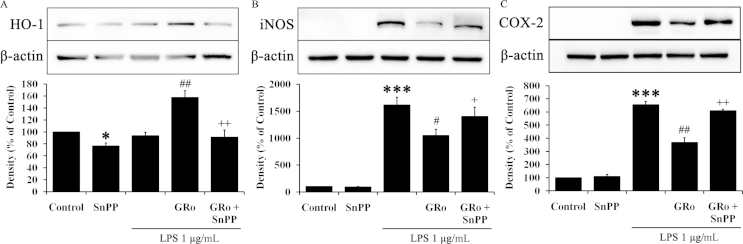
To investigate the anti-inflammatory effects of GRo via HO-1 induction, we used SnPP, a selective inhibitor of HO activity [17]. SnPP induces light oxidation processes followed by inhibit metabolism of porphyrin. As a result, essential prosthetic factor, such as heme was not synthesized and enzyme activity of HO-1 was also decreased. Specifically, cells were pretreated with 10μM SnPP for 1 h prior to sequential treatment with GRo and then LPS for 24 h. The expression of HO-1 was significantly diminished by SnPP. Moreover, SnPP significantly counteracted HO-1 expression increased by GRo in the presence of LPS (Fig. 4A). Consistent with these data, the patterns of iNOS and COX-2 were opposite to that of HO-1 (Fig. 4B, 4C). Specifically, cells treated with SnPP alone did not exhibit significant changes in iNOS (Fig. 4B) or COX-2 (Fig. 4C). SnPP treatment notably counteracted iNOS and COX-2 expression attenuated by GRo. Taken together, these results suggested that GRo attenuates proinflammatory cytokines by inducing expression of HO-1.
Fig. 4.
Effects of GRo on the relationship between HO-1 and proinflammatory cytokines in LPS-induced Raw 264.7 cells. Cells were pretreated with 10μM SnPP for 1 h prior to addition of 200μM GRo for 1 h, followed by incubation with 1 μg/mL LPS for 24 h. (A, B, and C) The protein expression of HO-1, iNOS, and COX-2 were determined by immunoblotting analysis. Data are presented as mean ± SEM (n = 3). *p < 0.05 and ***p < 0.001, as compared with the control. #p < 0.05 and ##p < 0.01, as compared with LPS treatment alone. +p < 0.05 and ++p < 0.01, as compared with cells treated with GRo followed by LPS. GRo, ginsenoside Ro; HO-1, heme oxygenase-1; LPS, lipopolysaccharide.
3.5. GRo exerts its anti-inflammatory effect via induction of HO-1
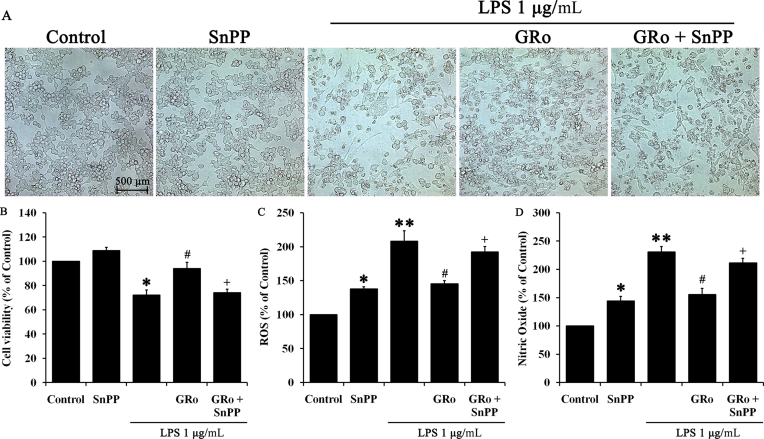
Finally, we investigated changes in cell morphology under different conditions, and examined these changes in the presence of SnPP and in the context of our previous findings on cell viability and ROS. Specifically, LPS treatment induced morphological changes in Raw 264.7 cells through generation of spindle shaped cells and massive clusters. Moreover, LPS significantly reduced the number of attached cells as determined by microscopy, whereas GRo treatment led to maintenance of normal cell shape and morphology and also increased the number of adherent cells; SnPP significantly counteracted the effects of GRo (Fig. 5A). Naturally, results of cell viability, ROS, and NO reflected our observations of cell morphology. As shown in Fig. 5B, SnPP significantly inhibited the increased cell viability facilitated by GRo in the presence of LPS, whereas ROS and NO assay results exhibited an opposite tendency consistent with the changes in cell viability (Fig. 5C, 5D). Interestingly, NO production was increased in cells treated with SnPP alone (Fig. 5D), suggesting the possibility that additional factors are induced by inhibition of HO-1. Taken together, these results suggested that GRo exerts its anti-inflammatory effects via induction of HO-1.
Fig. 5.
Effects of GRo on cell protection in LPS-induced Raw 264.7 cells via HO-1 induction. Cells were pretreated with 10μM SnPP for 1 h prior to 200μM GRo for 1 h, followed by incubation with 1 μg/mL LPS for 24 h. (A) Cell morphology was photographed using an Observer A1 microscope at 100× magnification, and (B) cell viability was determined. Cells were pretreated with 10μM SnPP for 1 h prior to the addition of 200μM GRo for 1 h, followed by incubation with 1 μg/mL LPS for 3 h, after which (C) ROS production was examined. (D) NO assay performed using supernatant from the same samples used for cell viability assays. Data are presented as mean ± SEM (n = 3). *p < 0.05 and **p < 0.01, as compared with the control. #p < 0.05 as compared with LPS treatment alone. +p < 0.05, as compared with cells treated with GRo followed by LPS. GRo, ginsenoside Ro; HO-1, heme oxygenase-1; LPS, lipopolysaccharide; ROS, reactive oxygen species.
4. Discussion
Ginsenosides are specific saponin constituents only found in ginseng. Numerous pharmacological effects of ginsenosides have been previously reported, including anti-inflammation, anticancer, antiaging, antioxidant, anticarcinogenic, and antihypertension effects as well as improved blood circulation [12], [18], [19], [20]. The present study demonstrated that GRo, one of the major physiologically-active ingredients in ginseng, protects macrophages against LPS-induced inflammation. Moreover, HO-1 induction was identified for the first time as the possible underlying mechanism responsible for the anti-inflammatory effects of GRo.
LPS is produced by bacteria as a toxicant and trigger of proinflammatory cytokine expression, iNOS, and NO production in various mammalian cells, including macrophages [21]. To evaluate the properties of GRo on macrophages, which are major immune cells associated with inflammation, we initially confirmed its effects on cell viability and production of ROS and NO in LPS-induced Raw 264.7 cells, which were used as a model of inflammatory macrophages. As shown in Fig. 1B–1D, GRo improved the condition of cells treated with LPS. Accordingly, we confirmed that GRo affected the expression of proinflammatory cytokines at the protein level. NO can produce other proinflammatory cytokines and is a harmful stimuli in the cell environment. Additionally, COX-2 triggers sustained ROS production and has been shown to be induced by proinflammatory cytokine signaling and iNOS production of NO, both of which are active in mammalian cells [10]. As shown in Fig. 2, GRo decreased levels of iNOS and COX-2 expression induced by LPS in a dose dependent manner. Having shown that GRo facilitates anti-inflammatory effect against LPS, we next attempted to determine the possible underlying mechanism.
HO-1 is an intrinsic cell-protective enzyme and is expressed in various cell types. We first confirmed the relationship between GRo and HO-1, the latter of which was previously shown to play a critical role in immune defense against inflammation-induced damage in the body [22]. Specifically, HO-1 is the inducible isoform of Heme oxygenase, and exerts significant anti-inflammatory, antioxidant, and antiapoptotic effects. With respect to the anti-inflammatory activities of HO-1, previous studies have reported that innate deficiency of HO-1 leads to severe inflammation, whereas HO-1 overexpression leads to enhanced anti-inflammatory effects [23], [24], [25]. Moreover, HO-1 expression plays a critical role in preventing inflammation in LPS-induced macrophages [26]. As expected, HO-1 expression was increased by GRo treatment in a dose dependent manner (Fig. 3A), as well as under LPS-induced inflammation conditions (Fig. 3B). Accordingly, we used SnPP, which is a selective inhibitor of HO activity, to clarify the relationship between GRo and its anti-inflammatory effects. As shown in Fig. 4A, SnPP treatment reduced HO-1 expression as expected. Moreover, cells treated with SnPP exhibited increased levels of proinflammatory cytokines due to inhibition of the downstream effects of GRo on HO-1 expression (Fig. 4B, 4C). Lastly, SnPP counteracted the effects of GRo on cell viability and production of ROS and NO in LPS-treated cells (Fig. 5).
In the present study, we examined the potential involvement of HO-1 in the anti-inflammatory effect triggered by GRo. We found that GRo notably induced HO-l expression in Raw 264.7 cells, and that this induction of HO-1 correlated with decreased proinflammatory molecules such as iNOS and COX-2, and impaired NO production induced by LPS. Inhibition of HO-1 activity by SnPP counteracted the effects of GRo, demonstrating that the anti-inflammatory properties of GRo require the activity of HO-1. Based on these results, our findings suggest that GRo may serve as a potential therapeutic strategy to prevent inflammation caused by various harmful immune system stimuli.
Conflicts of interest
We declare that no financial conflict of interest exists in relation to the publication of this work.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea, funded by the Korean Government (NRF-2012R1A1B3003531 and NRF-2013R1A1A2065158).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Ran S., Montgomery K.E. Macrophage-mediated lymphangiogenesis: the emerging role of macrophages as lymphatic endothelial progenitors. Cancers (Basel) 2012;4:618–657. doi: 10.3390/cancers4030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossol M., Heine H., Meusch U., Quandt D., Klein C., Sweet M.J., Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C., Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- 6.Tsatsanis C., Androulidaki A., Venihaki M., Margioris A.N. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Maines M.D., Trakshel G.M., Kutty R.K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411–419. [PubMed] [Google Scholar]
- 8.McCoubrey W.K., Jr., Huang T.J., Maines M.D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 9.Idriss N.K., Blann A.D., Lip G.Y. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008;52:971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Tsoyi K., Kim H.J., Shin J.S., Kim D.H., Cho H.J., Lee S.S., Ahn S.K., Yun-Choi H.S., Lee J.H., Seo H.G. HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal. 2008;20:1839–1847. doi: 10.1016/j.cellsig.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Maines M.D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988;2:2557–2568. [PubMed] [Google Scholar]
- 12.Jia L., Zhao Y., Liang X.J. Current evaluation of the millennium phytomedicine-ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang A., Wang C.Z., Wu J.A., Osinski J., Yuan C.S. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using high-performance liquid chromatography. Phytochem Anal. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- 14.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 15.Murata K., Takeshita F., Samukawa K., Tani T., Matsuda H. Effects of ginseng rhizome and ginsenoside Ro on testosterone 5alpha-reductase and hair regrowth in testosterone-treated mice. Phytother Res. 2012;26:48–53. doi: 10.1002/ptr.3511. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda H., Samukawa K., Kubo M. Anti-inflammatory activity of ginsenoside Ro. Planta Med. 1990;56:19–23. doi: 10.1055/s-2006-960875. [DOI] [PubMed] [Google Scholar]
- 17.Sato K., Balla J., Otterbein L., Smith R.N., Brouard S., Lin Y., Csizmadia E., Seviqny J., Robson S.C., Vercellotti G. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H., Samukawa K., Kubo M. Antihepatitic activity of ginesenoside ro1. Planta Med. 1991;57:523–526. doi: 10.1055/s-2006-960198. [DOI] [PubMed] [Google Scholar]
- 19.Yu J.L., Dou D.Q., Chen X.H., Yang H.Z., Hu X.Y., Cheng G.F. Ginsenoside-Ro enhances cell proliferation and modulates Th1/Th2 cytokines production in murine splenocytes. Yao Xue Xue Bao. 2005;40:332–336. [PubMed] [Google Scholar]
- 20.Murthy H.N., Georgiev M.I., Kim Y.S., Jeong C.S., Kim S.J., Park S.Y. Ginsenosides: prospective for sustainable biotechnological production. Appl Microbiol Biotechnol. 2014;98:6243–6254. doi: 10.1007/s00253-014-5801-9. [DOI] [PubMed] [Google Scholar]
- 21.Zuo Z., Johns R.A. Inhalational anesthetics upregulate constitutive and lipopolysaccharide-induced inducible nitric oxide synthase expression and activity. Mol Pharmacol. 1997;52:606–612. doi: 10.1124/mol.52.4.606. [DOI] [PubMed] [Google Scholar]
- 22.Fujii H., Takahashi T., Nakahira K., Uehara K., Shimizu H., Matsumi M., Morita K., Hirakawa M., Akaqi R., Sassa S. Protective role of heme oxygenase-1 in the intestinal tissue injury in an experimental model of sepsis. Crit Care Med. 2003;31:893–902. doi: 10.1097/01.CCM.0000050442.54044.06. [DOI] [PubMed] [Google Scholar]
- 23.Jun M.S., Ha Y.M., Kim H.S., Jang H.J., Kim Y.M., Lee Y.S., Kim H.J., Seo H.G., Lee J.H., Lee S.H. Anti-inflammatory action of methanol extract of Carthamus tinctorius involves in heme oxygenase-1 induction. J Ethnopharmacol. 2011;133:524–530. doi: 10.1016/j.jep.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 24.Motterlini R., Foresti R., Bassi R., Green C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 25.Otterbein L.E., Soares M.P., Yamashita K., Bach F.H. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 26.Li Q.F., Zhu Y.S., Jiang H., Xu H., Sun Y. Heme oxygenase-1 mediates the anti-inflammatory effect of isoflurane preconditioning in LPS-stimulated macrophages. Acta Pharmacol Sin. 2009;30:228–234. doi: 10.1038/aps.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]