Abstract
Background
Panax ginseng has a wide range of biological activities including anti-inflammatory, antioxidant, and immunomodulatory functions. Wild ginseng cambial meristematic cells (CMCs) were obtained from P. ginseng cambium. This study examined the protective mechanism of wild ginseng CMCs against d-galactosamine (GalN)-induced liver injury. GalN, a well-known hepatotoxicant, causes severe hepatocellular inflammatory damage and clinical features similar to those of human viral hepatitis in experimental animals.
Methods
Hepatotoxicity was induced in rats using GalN (700 mg/kg, i.p.). Wild ginseng CMCs was administered orally once a day for 2 wks, and then 2 h prior to and 6 h after GalN injection.
Results
Wild ginseng CMCs attenuated the increase in serum aminotransferase activity that occurs 24 h after GalN injection. Wild ginseng CMCs also attenuated the GalN-induced increase in serum tumor necrosis factor-α, interleukin-6 level, and hepatic cyclooxygenase-2 protein and mRNA expression. Wild ginseng CMCs augmented the increase in serum interleukin -10 and hepatic heme oxygenase-1 protein and mRNA expression that was induced by GalN, inhibited the increase in the nuclear level of nuclear factor-kappa B, and enhanced the increase in NF-E2-related factor 2.
Conclusion
Our findings suggest that wild ginseng CMCs protects liver against GalN-induced inflammation by suppressing proinflammatory mediators and enhancing production of anti-inflammatory mediators.
Keywords: d-galactosamine, heme oxygenase-1, hepatitis, Panax ginseng, wild ginseng cambial meristematic cells
1. Introduction
Hepatitis is a serious clinical problem caused by exposure to different agents such as viruses, alcohol, and chemicals, and by autoimmune diseases. Hepatitis may occur with limited or no symptoms and often leads to anorexia, jaundice, and hepatic carcinoma [1]. Although extensive studies into the treatment of liver disease with several oral hepatoprotective agents have been carried out, few beneficial liver drugs are currently available in the clinic. d-Galactosamine (GalN) is a specific hepatotoxicant that depletes the uridine triphosphate pool and thereby inhibits macromolecule synthesis, inducing hepatotoxicity that resembles that of human viral and drug-induced hepatitis [2]. In GalN-induced acute liver injury, overproduction of reactive oxygen species from hepatocytes, infiltrated leukocytes, and activated Kupffer cells, accompanied by enhanced activity of the proinflammatory cytokine signaling pathway, contributes to liver damage [3]. Heme oxygenase (HO)-1 is an endogenous cytoprotective enzyme induced in response to cellular and environmental stresses. The critical role of HO-1 in the protection against in vivo and in vitro inflammatory disease models has been reported, and upregulation of HO-1 exerts an anti-inflammatory effect in experimental settings of hepatic ischemia/reperfusion and acute hepatitis [4].
Panax ginseng Meyer (Araliaceae) is a medicinal herb that has been used worldwide for > 5,000 yrs. P. ginseng has been widely used for treatment of inflammation, cardiovascular diseases, trauma, and bleeding caused by injury. Several investigators have reported the biologic role of P. ginseng in experimental models of liver diseases, including fatty liver disease, liver fibrosis, hepatic carcinoma, and chemical-induced liver injury [5]. Its biologic activities are primarily attributable to ginsenosides, which produce an array of pharmacologic responses [6]. Ginsenosides are located in the root, leaf, seed, and flower of P. ginseng, and have antitumor and immunomodulatory activity in various organs [7]. However, the yield of active components from natural sources can be highly variable depending on the source plant, location, season of harvest, and the prevailing environmental conditions [8]. In addition, a dramatic depletion of the plant population has occurred as a result of its consumption for medicinal purposes. The isolation of cambial meristematic cells (CMCs) from selected P. ginseng species may ensure a stable ginsenoside content and provide a sustainable supply for therapeutic applications [9]. However, there are no reports that demonstrate the biologic activity of natural compounds isolated from wild ginseng CMCs.
The objective of this study is to investigate the cytoprotective effects of wild ginseng CMCs against GalN-induced acute liver injury, particularly the modulation of inflammatory responses.
2. Materials and methods
2.1. Materials/chemicals
The followings are the materials and chemicals we used in this study: a 3-L bioreactor and a 20-L bioreactor (Samsung Science, Seoul, Korea), a 250-L bioreactor (Fermentec Co. Ltd, Cheongwon, Korea), 0.05% trifluoroacetic acid (Daejung Chemicals & Metals, Siheung, Korea), ginsenoside 20(S)-Rg3 and ginsenoside 20(S)-Rh2 (Chromadex, Irvine, CA, USA), mixture of ginsenoside Rk1 and Rg5 (Ambo Institute, Seoul, Korea), acetonitrile (Merck, Darmstadt, Germany), methanol (J.T. Baker, Center Valley, PA, USA), GalN and silymarin (Sigma-Aldrich, St. Louis, MO, USA), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) assay kits (IVDLab Co., Uiwang, Korea), tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10 enzyme-linked immunosorbent assay kits (BD Science, San Diego, CA, USA), PRO-PREP and enhanced chemiluminescence detection system (iNtRON Biotechnology, Seongnam, Korea), NE-PER and BCA Protein Assay Kits (Thermo Fisher Scientific Inc., Rockford, IL, USA), polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA), Semi-Dry Trans-Blot Cell (Bio-Rad Laboratories, Hercules, CA, USA), RNAiso Plus and EcoDry cDNA Synthesis Premix (Takara Bio Inc., Shiga, Japan), Thermocycler and SYBR Green detection System (Roche Applied Science, Mannheim, Germany). The following antibodies were used in this study: cyclooxygenase-2 (COX-2; Cayman Chemicals, Ann Arbor, MI, USA), HO-1 (Enzo Life Sciences, Farmingdale, NY, USA), kelch-like ECH-associated protein 1 (Keap1) and lamin b1 (Abcam, Cambridge, MA, USA), nuclear factor-kappa B (NF-κB)/p65, p-cJun and NF-E2-related factor 2 (Nrf2; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (Sigma-Aldrich).
2.2. Plant cell culture
Wild ginseng CMCs were obtained from the cambium of P. ginseng Meyer (wild ginseng; native to Kangwon province, Korea, for > 50 yrs; appraised by the Korea Association of Wild Ginseng Appraiser) [10]. Wild ginseng CMCs were gradually scaled up from 250-mL flasks to a 3-L bioreactor, a 20-L bioreactor, and a 250-L bioreactor. The bioreactors were all the airlift type. The working volume of the 250-L bioreactor was 210 L, 84% of the total volume. Wild ginseng CMCs were cultured in two stages: (1) a proliferation stage to obtain biomass in the 250-L bioreactor and (2) a production stage to obtain secondary metabolites such as ginsenosides. In general, plant growth regulators, such as 2,4-dichlorophenoxyacetic acid or naphthaleneacetic acid, are used to induce cell division. However, to obtain biomass under the proliferation culture condition without using growth regulators, wild ginseng CMCs underwent a cell habituation process for 1 yr without growth regulators [9]. Wild ginseng CMCs obtained through this process were maintained for 37 mo. Culture conditions for the proliferation stage to obtain biomass of wild ginseng CMCs were Murashige and Skoog medium (MS medium) containing 3% sucrose, pH 5.8, cell inoculum of 2.09 g/L [dry cell weight (DCW)], and aeration rate of 0.038 vvm (air volume/media volume/min), 0.052 vvm, and 0.066 vvm for Days 1–5, Days 5–10, and Days 11–13 (Dwyer Instruments, Michigan City, IN, USA), respectively. When proliferation was complete, wild ginseng CMCs were subcultured every 13 days. Culture conditions for the production stage to obtain secondary metabolites from wild ginseng CMCs were 1/2 MS media containing 3% brown sugar, cell inoculum of 3.94 g/L (DCW), and 100μM methyl jasmonate. The air flow rate was 0.06 vvm, and the cells were cultured for 7 d. Proliferation and production culture were performed in the same room in the dark at a temperature of 21 ± 1°C. After proliferation and production culture, biomass and major ginsenosides (Rb1, Rb2, Rc, Rd) of wild ginseng CMCs were obtained. The cells were then heat-treated in an extractor at 95°C for 48 h to obtain rare ginsenosides. During the heat treatment, major ginsenosides were converted to Rg3, Rh2, Rg5, and Rk1. The biomass was harvested and freeze-dried.
2.3. HPLC analysis (chromatographic analysis of wild ginseng CMCs)
An Agilent HPLC 1260 DAD system (Agilent Technologies, Santa Clara, CA, USA) and Agilent Zorbax Eclipse plus C18 column (4.6 × 100 mm, 3.5 μm, Agilent Technologies) were used for the analysis of rare ginsenosides in wild ginseng CMCs. The detection wavelength was 203 nm, the temperature of the column was 30°C, and the mobile phase was 0.05% trifluoroacetic acid in water and 0.05% trifluoroacetic acid in acetonitrile with a flow rate of 1 mL/min. Standards used for the analysis were ginsenoside 20(S)-Rg3, ginsenoside 20(S)-Rh2, and a mixture of ginsenoside Rk1 and Rg5. Solvents used for the analysis were acetonitrile, methanol, and trifluoroacetic acid. Each standard was weighed and the concentration was adjusted to 0.5 mg/mL using methanol. Freeze-dried wild ginseng CMCs were ground to a fine powder, and 80% of methanol solution was added to 0.5 g of ground wild ginseng CMCs to a volume of 10 mL. This solution was extracted for 2 h under ultrasonic waves, centrifuged, and filtered through a 0.2-μm syringe filter (Toyo Roshi Kaisha, Ltd., Tokyo, Japan) for HPLC analysis.
2.4. Treatment of animals
Male Sprague–Dawley rats weighing 250–300 g (Orient Bio, Seongnam, Korea) were fasted overnight but given access to water ad libitum. All animal experiments were approved by the Animal Care Committee of Sungkyunkwan University School of Pharmacy, Suwon, Korea (SUSP14-04) and performed in accordance with the guidelines of National Institutes of Health (NIH publication No. 86-23, revised 1985). To generate GalN-induced hepatitis model, rats were injected intraperitoneally with 700 mg/kg of GalN dissolved in phosphate-buffered saline. The dose of GalN was selected according to previous reports [11], [12]. There was no mortality in each experimental group, and all animals were shown to induce hepatotoxicity after GalN administration. Wild ginseng CMCs were suspended in distilled water and administered orally once a day for 2 wks and then 2 h prior to and 6 h after GalN injection. Animals were randomly assigned among the following seven groups (n = 10 per group): (1) vehicle-treated control (control), (2) wild ginseng CMCs (300 mg/kg)-treated control (wild ginseng CMCs + control), (3) vehicle-treated and GalN-treated (vehicle + GalN), (4–6) wild ginseng CMCs (75 mg/kg, 150 mg/kg, and 300 mg/kg)-treated and GalN-treated (wild ginseng CMCs 75 mg/kg, 150 mg/kg, and 300 mg/kg + GalN), and (7) silymarin (positive control, 50 mg/kg)-treated and GalN-treated (silymarin 50 mg/kg + GalN). Silymarin was suspended in 10% Tween 80 saline and administered orally once a day for 2 wks. The animals were euthanized under overdose of ether 24 h after GalN injection, and blood samples were taken from the inferior vena cava. The liver was isolated and used immediately for the preparation of mRNA, which was stored at −75°C for later analysis. A portion of the left lobe of the liver was used for histologic analysis.
2.5. Serum aminotransferase activities
Serum alanine ALT and AST activities were determined with standard spectrophotometric procedures using the ChemiLab ALT and AST assay kits, respectively.
2.6. Histologic analysis
Liver tissues were removed from a portion of the left lobe, fixed immediately in 10% neutral-buffered formalin, embedded in paraffin, and sectioned at 5-μm thickness. Serial sections were stained with hematoxylin and eosin for evaluation of portal inflammation, hepatocellular necrosis, and inflammatory cell infiltration. Sections were examined in a blinded manner under an Olympus CKX 41 microscope (Olympus Optical Co. Ltd., Tokyo, Japan).
2.7. Serum TNF-α, IL-6, and IL-10 levels
Serum levels of TNF-α, IL-6, and IL-10 were quantified at 24 h after GalN injection using commercial mouse ELISA kits according to the method described by Kang et al [13].
2.8. Preparation of protein extracts and Western blot immunoassay
Fresh liver tissue was isolated and homogenized in PRO- for whole protein samples and in NE-PER for extraction of nuclear and cytosolic protein samples, according to the manufacturer's instructions. Protein concentrations were determined using the BCA Protein Assay kit. Whole protein samples (20 μg) were used to determine the content of COX-2 and HO-1, cytosolic protein samples (20 μg) were used to determine the content of Keap1, and nuclear protein samples (10 μg) were used to determine the content of nuclear NF-κB/p65, phospho-cJun (p-cJun), and Nrf2. Protein samples were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes using the Semi-Dry Trans-Blot Cell. After the transfer, the membranes were washed with 0.1% Tween 20 in Tris-buffered saline and blocked for 1 h at room temperature with 5% (w/v) skim milk powder in Tris-buffered saline. Blots were incubated overnight at 4°C with primary antibodies against COX-2 (1:4,000), HO-1 (1:4,000), Keap1 (1:4,000), NF-κB/p65 (1:4,000), p-cJun (1:4,000), and Nrf2 (1:2,000). On the following day, the blots were incubated with the appropriate secondary antibodies and detected using an enhanced chemiluminescence detection system according to the manufacturer's instructions. Immunoreactive bands were evaluated densitometrically with ImageQuant TL software (GE Healthcare, Piscataway, NJ, USA). Signals were normalized to that achieved with antibodies to β-actin (1:4,000) for whole and cytosolic proteins, or lamin B1 (1:10,000) for nuclear proteins.
2.9. Total RNA extraction and real time-polymerase chain reaction
For mRNA analysis, total RNA was extracted from liver tissue of rats in the different treatment groups using RNAiso Plus, and cDNA was synthesized using a reverse transcription reaction. The cDNA was amplified using real-time polymerase chain reaction with a thermocycler and the SYBR Green detection system. The amplification cycling conditions were as follows: 45 cycles of 30 s at 94°C, 30 s at 56°C, and 30 s at 72°C for TNF-α and HO-1; 32 cycles of 30 s at 94°C, 45 s at 58°C, 30 s at 72°C for IL-6; 45 cycles of 30 s at 94°C, 30 s at 64°C, and 30 s at 72°C for IL-10; 45 cycles of 30 s at 94°C, 30 s at 65°C, and 30 s at 72°C for COX-2; and 45 cycles of 30 s at 94°C, 30 s at 62°C, and 30 s at 72°C for β-actin. The gene-specific primers used are listed in Table 1. mRNA expression levels were normalized to the expression level of β-actin and analyzed with respect to the average of all delta Ct values in each sample using the cycle threshold (Ct) method. All samples were run in duplicate to ensure amplification integrity.
Table 1.
Real-time RT-PCR primers used in study and the amplified product length
| Gene | Accession number | Primer sequences (5′–3′) | Product length |
|---|---|---|---|
| TNF-α | NM_012675 | Sense: AGA ACA GCA ACT CCA GAA CAC CCT | 148 |
| Antisense: ATC TCG GAT CAT GCT TTC CGT GCT | |||
| IL-6 | NM_012589 | Sense: ATA TAC CAC TTC ACA AGT CGG | 151 |
| Anti-sense: GGC AAA TTT CCT GGT TAT ATC C | |||
| IL-10 | NM_012854 | Sense: GAC CAG CTG GAC AAC ATA CT | 143 |
| Antisense: GAG GGT CTT CAG CTT CTC TC | |||
| COX-2 | NM_017232 | Sense: TCC AGT ATC AGA ACC GCA TTG CCT | 149 |
| Antisense: AGC AAG TCC GTG TTC AAG GAG GAT | |||
| HO-1 | NM_012580 | Sense: AAC AAG CAG AAC CCA GTC TAT GC | 212 |
| Antisense: GGC CCA CGC ATA TAC CCG CTA CCT | |||
| β-Actin | NM_031144 | Sense: CTA CAA TGA GCT GCG TGT GGC | 229 |
| Antisense: CAG GTC CAG ACG CAG GAT GGC |
COX, cyclooxygenase; HO, heme oxygenase; IL, interleukin; RT-PCR, reverse transcription-polymerase chain reaction; TNF-α, tumor necrosis factor alpha
2.10. Statistical analysis
All results are presented as mean ± standard deviation. The overall significance of the data was analyzed with one-way analysis of the variance. Differences between the groups were considered significant at p < 0.05 with the appropriate Bonferroni correction made for multiple comparisons.
3. Results
3.1. Qualitative and quantitative analysis of wild ginseng CMCs
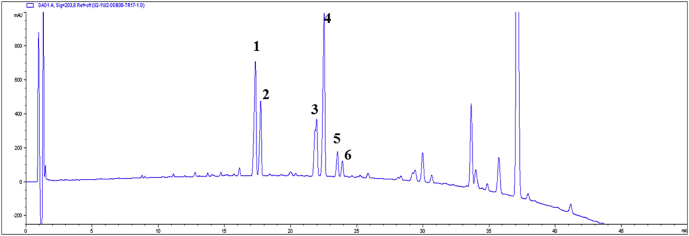
Wild ginseng CMCs were ultrasonically extracted and analyzed through HPLC. The analyzed chromatogram is as shown in Fig. 1. Major ginsenosides Rb1, Rb2, Rc, and Rd that were detected at the end of production culture stage were not detected after heat treatment, confirming that all these major ginsenosides have transferred to rare ginsenosides. Rare ginsenosides contained in wild ginseng CMCs were isolated and identified, and each compound was confirmed through electrospray ionization-mass spectrometry and nuclear magnetic resonance. In the analysis condition, compounds that were detected at 17.1 min, 17.7 min, 21.8 min, 22.3 min, 23.4 min, and 24.0 min were 20(S)-Rg3, 20(R)-Rg3, Rk1, Rg5, 20(S)-Rh2, and 20(R)-Rh2, respectively. The contents of Rg3, Rk1, Rg5, and Rh2 were confirmed through their standard compounds as shown in Table 2. To calculate the contents of Rk1 and Rg5, areas of the individual peaks of Rk1 and Rg5 were added. Rg3 and Rh2 were detected as isomers of the S form and R form, and their contents were calculated by summing the area of the individual peaks. Contents of rare ginsenosides, such as Rg3, Rh2, and Rk1 + Rg3 in dry cell of wild ginseng CMCs were 25.5 mg/g, 13.0 mg/g, and 29.0 mg/g, respectively. Rare ginsenosides are reported to be contained in red ginseng in a small amount, but wild ginseng CMCs produced high contents of rare ginsenosides. In particular, 20(S)-Rh2, 20(R)-Rh2, Rk2, Rh3, and 20(S)-PPD, which are rarely detected or are not contained in red ginseng, were highly produced in wild ginseng CMCs.
Fig. 1.
HPLC chromatogram of ginsenosides detected from the wild ginseng CMCs. Numbers on the HPLC chromatogram indicate: (1) ginsenoside 20(S)-Rg3, (2) ginsenoside 20(R)-Rg3, (3) ginsenoside Rk1, (4) ginsenoside Rg5, (5) ginsenoside 20(S)-Rh2, and (6) ginsenoside 20(R)-Rh2. CMC, cambial meristematic cells. CMCs, cambial meristematic cells.
Table 2.
Analysis of ginsenosides content (mg/g, DCW) of wild ginseng CMCs
| Rare ginsenosides | Rg3 | Rk1 + Rg5 | Rh2 | Total content |
|---|---|---|---|---|
| Content (mg/g, DCW) | 25.5 | 29.0 | 13.0 | 67.5 |
CMCs, cambial meristematic cells; DCW, dry cell weight
3.2. Serum aminotransferase activities
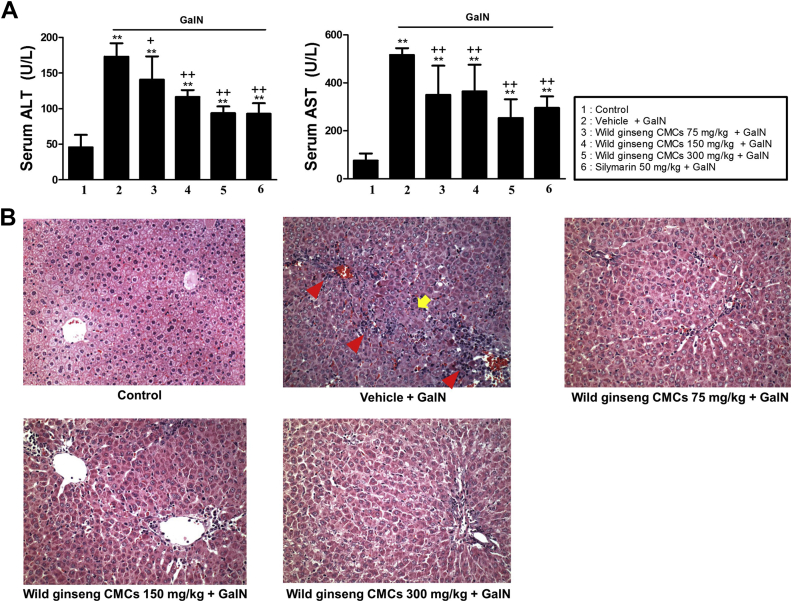
Serum ALT and AST activities in the control were 37.1 ± 17.6 U/L and 75.6 ± 30.3 U/L, respectively. At 24 h after GalN injection, serum ALT and AST activities significantly increased 4.8- and 6.8-fold, respectively, compared with the control. These increases were attenuated by treatment with wild ginseng CMCs at 75 mg/kg, 150 mg/kg, and 300 mg/kg and silymarin at 50 mg/kg. Wild ginseng CMCs alone (300 mg/kg) did not affect serum aminotransferase activities (Fig. 2A). Wild ginseng CMCs at 300 mg/kg was selected as the optimally effective dose for evaluating the molecular mechanisms by which wild ginseng CMCs attenuate GalN-induced hepatic injury.
Fig. 2.
Effects of wild ginseng CMCs on serum ALT, AST levels (A) and histological changes (B) in the rat liver after GalN (700 mg/kg) injection (original magnification ×400). All values are means ± SD of 10 rats per group. Rats received oral injection of vehicle or wild ginseng CMCs (75, 150, and 300 mg/kg) once daily for 2 wks and 2 h prior to and 6 h after GalN (700 mg/kg) treatment. **p < 0.05, significantly different from control group. †p < 0.05, ††p < 0.01, significantly different from GalN group. Histological features of liver sections stained with H&E at 24 h after GalN injection. Typical images were chosen from each experimental group (original magnification, ×400). Arrow and arrowhead indicate hemorrhagic necrosis and inflammatory cell infiltration, respectively. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CMCs, cambial meristematic cells; H&E, hematoxylin and eosin; GalN, d-galactosamine; SD, standard deviation.
3.3. Histologic analysis
The histologic features of livers from control animals indicated normal liver lobular architecture and cell structure (Fig. 2B). However, livers exposed to GalN showed obvious broad hemorrhagic necrosis, extensive areas of portal inflammation, and a moderate increase in inflammatory cell infiltration. These pathologic alterations were ameliorated in animals that were treated with wild ginseng CMCs.
3.4. Cytokine levels
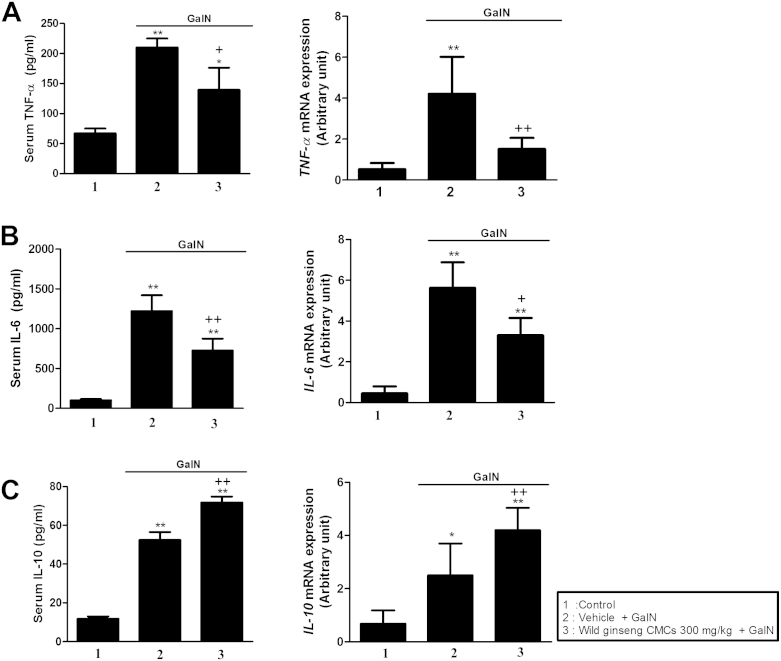
The serum level and hepatic mRNA expression of the proinflammatory cytokine TNF-α in the GalN-treated group were approximately 3.1- and 8.0-fold higher than that in the control group, respectively (Fig. 3A). Similarly, the serum level and hepatic mRNA expression of IL-6 in the GalN-treated group were approximately 13.5- and 12.2-fold higher than that in the control group, respectively. These changes were attenuated by wild ginseng CMCs (Fig. 3B). As shown in Fig. 3C, the serum level and hepatic mRNA expression of the anti-inflammatory cytokine IL-10 increased 4.5- and 3.1-fold at 24 h after GalN injection, respectively. These changes in IL-10 expression were augmented by wild ginseng CMCs.
Fig. 3.
Effects of wild ginseng CMCs (300 mg/kg) on levels of serum TNF-α and hepatic mRNA expression (A), and serum IL-10 and hepatic mRNA expression (B) after GalN (700 mg/kg) treatment. All values are means ± SD of 10 rats per group. *p < 0.05, **p < 0.01, significantly different from control group. †p < 0.05, ††p < 0.01, significantly different from GalN group. CMCs, cambial meristematic cells; GalN, d-galactosamine; IL, interleukin; TNF-α, tumor necrosis factor alpha; SD, standard deviation
3.5. Hepatic COX-2 and HO-1 protein and mRNA expression
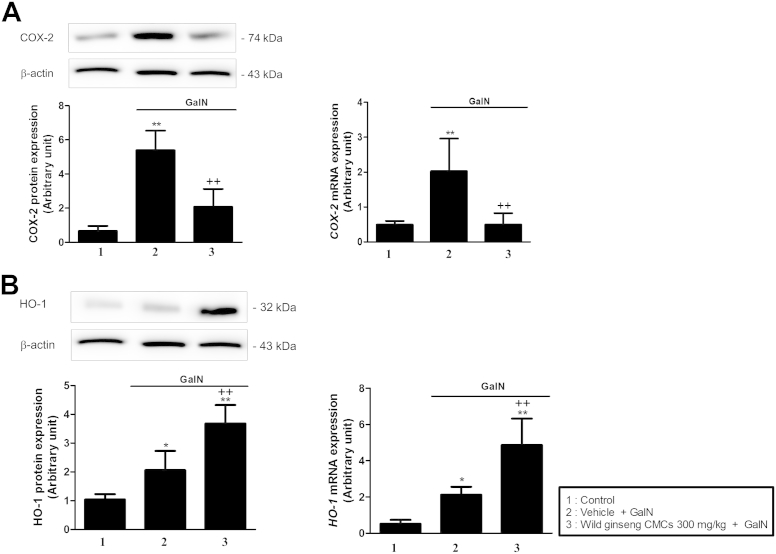
The levels of hepatic COX-2 protein and mRNA expression in rats in the GalN group were approximately 8.2- and 4.1-fold higher than that in the control group, respectively. The increased expression of COX-2 mRNA and protein expression was attenuated by wild ginseng CMCs (Fig. 4A). Fig. 4B shows that levels of hepatic HO-1 protein and mRNA expression increased 1.8- and 2.0-fold after GalN injection compared with those of the control. These increases were augmented by wild ginseng CMCs.
Fig. 4.
Effects of wild ginseng CMCs (300 mg/kg) on levels of hepatic COX-2 protein and mRNA expression (A), and hepatic HO-1 protein and mRNA expression (B) after GalN (700 mg/kg) treatment. All values are means ± SD of 10 rats per group. *p < 0.05, **p < 0.01, significantly different from control group. †p < 0.05, ††p < 0.01, significantly different from GalNgroup. CMCs, cambial meristematic cells; COX-2, cyclooxygenase-2; GalN, d-galactosamine; HO-1, heme oxygenase-1; SD, standard deviation.
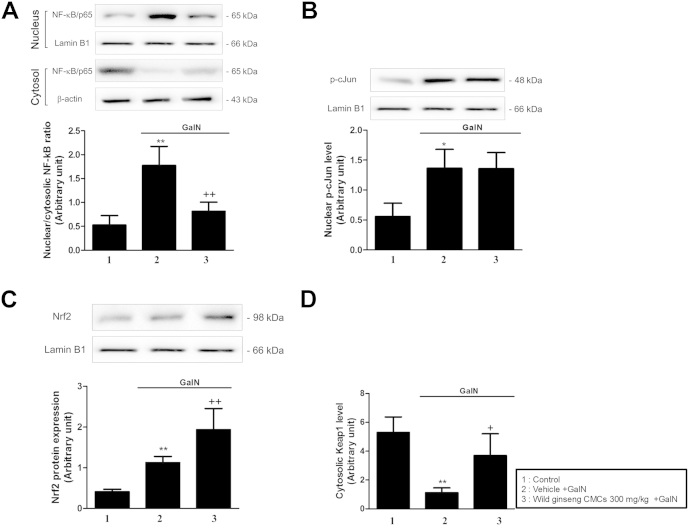
3.6. Nuclear translocation of NF-κB, p-cJun, and Keap1/Nrf2
Nuclear levels of NF-κB/p65, p-cJun, and Nrf2, and the cytosolic level of Keap1 were measured using immunoblot analysis (Fig. 5). At 24 h after GalN injection, the nuclear level of NF-κB/p65 increased 4.5-fold compared with that of the control, whereas the cytosolic level of NF-κB/p65 decreased compared with that of the control. In the GalN-treated group, the nuclear protein level of p-cJun increased 2.3-fold compared with the control. Wild ginseng CMCs attenuated these changes in the levels of nuclear and cytosolic NF-κB/p65 but did not affect the level of nuclear p-cJun (Fig. 5A, B). At 24 hours after GalN injection, the nuclear level of Nrf2 increased 2.7-fold over the control, whereas the cytosolic level of Keap1 decreased. Wild ginseng CMCs augmented the increase in the nuclear level of Nrf2 and attenuated the decrease in the cytosolic level of Keap1 (Fig. 5C, D).
Fig. 5.
Effects of wild ginseng CMCs (300 mg/kg) on hepatic levels of nuclear NF-κB (A), p-cJun (B), and Nrf2 (C), and cytosolic Keap1 (D) protein expression. All values are means ± SD of 10 rats per group. *p < 0.05, **p < 0.01, significantly different from control group. †p < 0.05, ††p < 0.01, significantly different from GalN group. CMCs, cambial meristematic cells; GalN, d-galactosamine; Keap1, kelch-like ECH-associated protein 1; NF-κB, nuclear factor-kappa B; Nrf2, NF-E2-related factor 2; TNF, tumor necrosis factor.
4. Discussion
P. ginseng is one of the most abundantly used herbal medicines in Korea, China, and Japan. Many studies have reported that P. ginseng possesses antidiabetic, antihepatotoxic, antifatigue, antistress, and blood pressure regulatory effects [14]. Wild ginseng, however, is rarely available, and the price is very high owing to its rarity. In addition, extensive efforts on quality control are required because the contents of ginsenosides vary depending on the ginseng species and cultivation environments [15]. To overcome these problems, plant cell culture emerged as an attractive alternative. Various tissue culture techniques are being used to produce large amounts of wild ginseng with increased yield of ginsenosides in a relatively shorter time [16]. However, it is often not commercially viable because of the difficulties associated with culturing dedifferentiated plant cells on an industrial scale and stable material production. To bypass the dedifferentiation step, some of us established a technique of isolation and culture of innately undifferentiated CMCs. The isolation of CMCs from selected P. ginseng species may ensure a stable method to provide high contents of ginsenosides and sustainable supply for therapeutic applications [10].
GalN is a highly specific hepatotoxic agent that induces liver injury closely resembling viral hepatitis when given to rats. Administration of GalN disrupts the permeability of the plasma membrane, causing leakage of enzymes from the cell. In our study, a significant elevation of serum ALT and AST was detected after GalN injection, and this increase was attenuated by treatment with wild ginseng CMCs. Normal lobular architecture and cell structure were observed in the control group, whereas increased hepatocellular necrosis, portal inflammation, and inflammatory cell infiltration were observed in the liver section obtained from GalN-treated animals. These pathologic changes were attenuated by treatment with wild ginseng CMCs, suggesting that wild ginseng CMCs may have a potential clinical application in the treatment of liver disease.
The liver is an inflammatory organ, and inflammatory processes contribute to a number of pathologic events. Kupffer cells, the resident macrophages of the liver, preside in a strategic position within the liver sinusoids. They interact with hepatocytes, leukocytes, and various mediators from the gut and therefore represent the first line of defense against microorganisms crossing the gut mucosal barrier and entering the portal circulation. Moreover, activated Kupffer cells produce a number of signaling molecules that promote inflammatory reactions and cause cell dysfunction and enhanced expression of adhesion molecules in endothelial cells. Stachlewitz et al [17] demonstrated the integral role of Kupffer cells in inflammatory cell interactions after GalN injection. Furthermore, the interaction of neutrophils with endothelial cells and subsequent neutrophil infiltration are observed in GalN-induced hepatic injury [18]. TNF-α is one of the key proinflammatory cytokines involved in the responses that ultimately result in liver failure [19]. TNF-α participates in necrotic, apoptotic, and inflammatory pathways in GalN-induced hepatotoxicity. IL-6, promptly and transiently produced in response to infections and tissue injuries, contributes to host defense through the stimulation of acute phase responses, hematopoiesis, and immune reactions. IL-6 produced by Kupffer cells in response to liver damage also plays a critical role in the acute phase response in the liver [20]. In our study, we found that the serum level and hepatic gene expression of TNF-α and IL-6 increased after GalN injection, and that these increases were attenuated by wild ginseng CMCs.
COX-2 plays a key role in the pathogenesis of inflammation and its expression is markedly upregulated by inflammatory stimuli leading to increased synthesis of prostanoids, which are potent lipid inflammatory mediators, in inflamed tissues. A previous report demonstrated that genetic overexpression of COX-2 accelerates endotoxin-induced inflammation and subsequent liver damage [21]. Modulation of COX-2 expression is an effective strategy for regulating inflammation in an animal model of GalN-induced hepatic failure. We demonstrated increased mRNA and protein expression of hepatic COX-2 in GalN-treated animals, which was ameliorated by wild ginseng CMCs. These results indicate that wild ginseng CMCs suppress GalN-induced production of proinflammatory mediators at the transcriptional level.
Toll-like receptors (TLRs) represent a family of pattern recognition receptors that are essential for infectious and inflammatory disease states. In particular, hepatic TLR2 and TLR4 mRNA and protein expression were increased in patients with hepatitis and cirrhosis [22], [23]. Recent investigations demonstrated that GalN-induced hepatotoxicity in rat involved the increase of expression of TLR2 and TLR4 mRNA [24]. Accumulating data have shown the involvement of transcription factors such as NF-κB and AP-1 in the amplification of inflammatory response in animal models of GalN-induced hepatotoxicity. NF-κB is a ubiquitous transcription factor, and the functional importance of NF-κB in inflammation is based on its ability to regulate the transcription of multiple inflammatory genes, including TNF-α and COX-2 [25]. A significant decrease in hepatic NF-κB activation was noted in TLR4 knockout mice compared to wild type mice following the induction of fulminant hepatic failure by GalN/lipopolysaccharide (LPS). AP-1 is a group of dimeric transcription factors consisting of cJun, cFos, activating transcription factor, and musculoaponeurotic fibrosarcoma, and the combination of proteins in the AP-1 complex affects its DNA binding activity. The TNF-α gene contains a cJun binding site in its promoter. Mitogen-activated protein kinases phosphorylation by stimulation of macrophage TLR4 activates the cJun, which subsequently promotes synthesis of proinflammatory mediators [26]. In our study, elevated nuclear NF-κB/p65 and p-cJun protein levels were observed in GalN-treated animals. Wild ginseng CMCs reduced the increased nuclear level of NF-κB/p65 protein, but did not affect the level of nuclear p-cJun protein. These results suggest that wild ginseng CMCs inhibit the transactivation of NF-κB. Unfortunately, we did not find a way to induce TLR2 and TLR4 mRNA and protein expression in GalN-treated rats; however, this could be a result of the differences in the injury models used by our group (700 mg/kg GalN) and other groups (800 mg/kg GalN or 800 mg/kg GalN + 40 μg/kg LPS) [27]. Further investigations are needed to elucidate the role of TLRs in GalN-induced hepatotoxicity.
A proper balance between pro- and anti-inflammatory responses is essential for maintaining an adequate immune response in GalN-induced liver injury, and the upregulation of anti-inflammatory mediators by therapeutic agent(s) may be helpful in hepatoprotection. IL-10 is an anti-inflammatory cytokine that was initially identified as a product of T helper cells (Th2) and has since been reported to be produced by Kupffer cells, implying a role in the regulation of local immune responses in the liver sinusoids [28]. IL-10 can suppress serum TNF-α, interferon-γ, and IL-1 levels and reduce the mortality induced by GalN/LPS [29]. In the present study, wild ginseng CMCs augmented the GalN-induced increase in serum IL-10 protein level and its mRNA expression.
HO-1 has been implicated in the cellular adaptive response against stress conditions. The primary function of HO-1 is to catabolize the oxidative degradation of heme into carbon monoxide (CO), free iron, and biliverdin, and the CO produced by its activity exerts powerful anti-inflammatory and anti-apoptotic effects. There is accumulating evidence for a critical role of HO-1 signaling in the protection against several inflammatory diseases [30]. Previous reports demonstrated that pharmacologic induction of HO-1 has an anti-inflammatory effect in sterile inflammatory settings of hepatic ischemia/reperfusion, and that genetic overexpression by adenovirus gene transfer of HO-1 (Ad-HO-1) confers cytoprotection [31]. Nrf2 is a transcription factor that promotes the transcription of a series of cytoprotective genes in response to cellular stress. Under basal conditions, Nrf2 is sequestered by Keap1 in the cytosol. In response to stress, Nrf2 is released from Keap1 and translocates into the nucleus, where it induces an array of cytoprotective genes as an adaptive response. Alam and Cook [32] demonstrated that, upon activation, Nrf2 enters the nucleus where it binds to the ARE in the HO-1 promoter region to trigger gene expression. During acute liver injury, and independent of the toxicants that caused it, Nrf2 plays a protective role by inducing antioxidant enzymes such as NAD(P)H quinone oxidoreductase 1 (Nqo1), glutathione, and many proteins involved in the repair of oxidized proteins, and by attenuating the expression of inflammatory genes including IL-1β, IL-6, and TNF-α [33]. Increased translocation of HO-1 and Nrf2 to protect the liver has been documented in GalN-induced acute liver injury [34]. In our study, rats treated with GalN showed a decrease in cytosolic levels of Keap1, and an increase in the nuclear level of Nrf2 and hepatic mRNA expression of HO-1. Treatment with wild ginseng CMCs augmented the increase in nuclear Nrf2 level and hepatic HO-1 mRNA expression, and attenuated the decrease in cytosolic Keap1 levels. These results suggest that wild ginseng CMCs may reduce the inflammatory response that occurs after GalN injection via the enhancement of Nrf2 nuclear translocation and subsequent induction of HO-1.
In summary, our findings suggest that wild ginseng CMCs protect against GalN-induced acute liver injury. The mechanism of action of wild ginseng CMCs appear to involve its ability to inhibit overexpression of proinflammatory mediators and nuclear translocation of NF-κB, and to induce the Nrf2/HO-1 anti-inflammatory pathway. Thus, we propose that wild ginseng CMCs are a potential therapeutic medication for the prevention of acute liver injury in clinical settings. Moreover, our findings may provide important information for novel therapeutic approaches using natural plant products from cultured multipotent cells.
Conflicts of interest
The authors declare they have no conflict of interests.
Acknowledgments
This research was financially supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT), and Honam Institute for Regional Program Evaluation through the Leading Industry Development for Economic Region.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Bernal W., Auzinger G., Dhawan A., Wendon J. Acute liver failure. Lancet. 2010;376:190–201. doi: 10.1016/S0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 2.Decker K., Keppler D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev Physiol Biochem Pharmacol. 1974;71:77–106. doi: 10.1007/BFb0027661. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh M., Das J., Sil P.C. D(+) galactosamine induced oxidative and nitrosative stress-mediated renal damage in rats via NF-kappaB and inducible nitric oxide synthase (iNOS) pathways is ameliorated by a polyphenol xanthone, mangiferin. Free Radic Res. 2012;46:116–132. doi: 10.3109/10715762.2011.644240. [DOI] [PubMed] [Google Scholar]
- 4.Immenschuh S., Baumgart-Vogt E., Mueller S. Heme oxygenase-1 and iron in liver inflammation: a complex alliance. Curr Drug Targets. 2010;11:1541–1550. doi: 10.2174/1389450111009011541. [DOI] [PubMed] [Google Scholar]
- 5.Shukla R., Kumar M. Role of Panax ginseng as an antioxidant after cadmium-induced hepatic injuries. Food Chem Toxicol. 2009;47:769–773. doi: 10.1016/j.fct.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 7.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 8.Yun U.W., Yan Z., Amir R., Hong S., Jin Y.W., Lee E.K., Loake G.J. Plant natural products: history, limitations and the potential of cambial meristematic cells. Biotechnol Genet Eng Rev. 2012;28:47–59. doi: 10.5661/bger-28-47. [DOI] [PubMed] [Google Scholar]
- 9.Asano S., Otobe K. Production of phytochemicals by using habituated and long-term cultured cells. Plant Biotechnol J. 2011;28:51–62. [Google Scholar]
- 10.Lee E.K., Jin Y.W., Park J.H., Yoo Y.M., Hong S.M., Amir R., Yan Z., Kwon E., Elfick A., Tomlinson S. Cultured cambial meristematic cells as a source of plant natural products. Nat Biotechnol. 2010;28:1213–1217. doi: 10.1038/nbt.1693. [DOI] [PubMed] [Google Scholar]
- 11.Choi J.H., Kang J.W., Kim D.W., Sung Y.K., Lee S.M. Protective effects of mg-CUD against d-galactosamine-induced hepatotoxicity in rats. Eur J Pharmacol. 2011;657:138–143. doi: 10.1016/j.ejphar.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Kang J.W., Kim S.J., Kim H.Y., Cho S.H., Kim K.N., Lee S.G., Lee S.M. Protective effects of HV-P411 complex against d-galactosamine-induced hepatotoxicity in rats. Am J Chin Med. 2012;40:467–480. doi: 10.1142/S0192415X1250036X. [DOI] [PubMed] [Google Scholar]
- 13.Kang T.J., Moon J.S., Lee S.Y., Yim D.S. Polyacetylene compound from Cirsium japonicum var ussuriense inhibits the LPS-induced inflammatory reaction via suppression of NF-κB activity in RAW 264.7 Cells. Biomol Ther. 2011;19:97–101. [Google Scholar]
- 14.Yun T.K. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:3–5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn I.O., Lee S.S., Lee J.H., Lee M.J., Jo B.G. Comparison of ginsenoside contents and pattern similarity between root parts of new cultivars in Panax ginseng C.A. Meyer. J Ginseng Res. 2008;32:15–18. [Google Scholar]
- 16.Kim Y.S., Hahn E.J., Murthy H.N., Paek K.Y. Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett. 2004;26:1619–1622. doi: 10.1007/s10529-004-3183-2. [DOI] [PubMed] [Google Scholar]
- 17.Stachlewitz R.F., Seabra V., Bradford B., Bradham C.A., Rusyn I., Germolec D., Thurman R.G. Glycine and uridine prevent d-galactosamine hepatotoxicity in the rat: role of Kupffer cells. Hepatology. 1999;29:737–745. doi: 10.1002/hep.510290335. [DOI] [PubMed] [Google Scholar]
- 18.Komatsu Y., Shiratori Y., Kawase T., Hashimoto N., Han K., Shiina S., Matsumura M., Niwa Y., Kato N., Tada M. Role of polymorphonuclear leukocytes in galactosamine hepatitis: mechanism of adherence to hepatic endothelial cells. Hepatology. 1994;20:1548–1556. doi: 10.1002/hep.1840200626. [DOI] [PubMed] [Google Scholar]
- 19.Shito M., Balis U.J., Tompkins R.G., Yarmush M.L., Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci. 2001;46:1700–1708. doi: 10.1023/a:1010653504568. [DOI] [PubMed] [Google Scholar]
- 20.Wanner G.A., Ertel W., Muller P., Hofer Y., Leiderer R., Menger M.D., Messmer K. Liver ischemia and reperfusion induces a systemic inflammatory response through Kupffer cell activation. Shock. 1996;5:34–40. doi: 10.1097/00024382-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Han C., Li G., Lim K., DeFrances M.C., Gandhi C.R., Wu T. Transgenic expression of cyclooxygenase-2 in hepatocytes accelerates endotoxin-induced acute liver failure. J Immunol. 2008;181:8027–8035. doi: 10.4049/jimmunol.181.11.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berzsenyi M.D., Roberts S.K., Preiss S., Woollard D.J., Beard M.R., Skinner N.A., Bowden D.S., Visvanathan K. Hepatic TLR2 & TLR4 expression correlates with hepatic inflammation and TNF-α in HCV & HCV/HIV infection. J Viral Hepat. 2011;18:852–860. doi: 10.1111/j.1365-2893.2010.01390.x. [DOI] [PubMed] [Google Scholar]
- 23.Soares J.B., Pimentel-Nunes P., Afonso L., Rolanda C., Lopes P., Roncon-Albuquerque R., Jr., Goncalves N., Boal-Carvalho I., Pardal F., Lopes S. Increased hepatic expression of TLR2 and TLR4 in the hepatic inflammation–fibrosis-carcinoma sequence. Innate Immun. 2012;18:700–708. doi: 10.1177/1753425912436762. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud M.F., Hamdan D.I., Wink M., El-Shazly A.M. Hepatoprotective effect of limonin, a natural limonoid from the seed of Citrus aurantium var. bigaradia, on d-galactosamine-induced liver injury in rats. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:251–261. doi: 10.1007/s00210-013-0937-1. [DOI] [PubMed] [Google Scholar]
- 25.Poligone B., Baldwin A.S. Positive and negative regulation of NF-kappaB by COX-2: roles of different prostaglandins. J Biol Chem. 2001;276:38658–38664. doi: 10.1074/jbc.M106599200. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T., Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 27.Kang J.W., Kim D.W., Choi J.S., Kim Y.S., Lee S.M. Scoparone attenuates d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure through inhibition of toll-like receptor 4 signaling in mice. Food Chem Toxicol. 2013;57:132–139. doi: 10.1016/j.fct.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Knolle P., Schlaak J., Uhrig A., Kempf P. Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22:226–229. doi: 10.1016/0168-8278(95)80433-1. [DOI] [PubMed] [Google Scholar]
- 29.Nagaki M., Tanaka M., Sugiyama A., Ohnishi H., Moriwaki H. Interleukin-10 inhibits hepatic injury and tumor necrosis factor-alpha and interferon-gamma mRNA expression induced by staphylococcal enterotoxin B or lipopolysaccharide in galactosamine-sensitized mice. J Hepatol. 1999;31:815–824. doi: 10.1016/s0168-8278(99)80282-7. [DOI] [PubMed] [Google Scholar]
- 30.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Sun L., Shi T., Qiao H., Jiang X., Jiang H., Krissansen G.W., Sun X. Hepatic overexpression of heme oxygenase-1 improves liver allograft survival by expanding T regulatory cells. J Surg Res. 2011;166:187–194. doi: 10.1016/j.jss.2010.11.917. [DOI] [PubMed] [Google Scholar]
- 32.Alam J., Cook J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Wu K.C., Lu Y.F., Ekuase E., Klaassen C.D. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev. 2013;2013:305861. doi: 10.1155/2013/305861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das J., Ghosh J., Roy A., Sil P.C. Mangiferin exerts hepatoprotective activity against d-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFkappaB pathways. Toxicol Appl Pharmacol. 2012;260:35–47. doi: 10.1016/j.taap.2012.01.015. [DOI] [PubMed] [Google Scholar]