Abstract
Background
UV-irradiated keratinocytes secrete various proinflammatory cytokines. UV-induced skin damage is mediated by growth factors and proinflammatory cytokines such as granulocyte macrophage colony stimulating factor (GM-CSF). In a previous study, we found that the saponin of Korean Red Ginseng (SKRG) decreased the expression of GM-CSF in UVB-irradiated SP-1 keratinocytes. In this study, we attempted to find the inhibitory mechanism of SKRG on UVB-induced GM-CSF expression in SP-1 keratinocytes.
Methods
We investigated the inhibitory mechanism of SKRG and ginsenosides from Panax ginseng on UVB-induced GM-CSF expression in SP-1 keratinocytes.
Results
Treatment with SKRG decreased the expression of GM-CSF mRNA and protein induced by irradiation of UVB in SP-1 keratinocytes. The phosphorylation of ERK was induced by UVB at 10 min, and decreased with SKRG treatment in SP-1 keratinocytes. In addition, treatment with SKRG inhibited the UVB-induced phosphorylation of epidermal growth factor receptor (EGFR), which is known to be an upstream signal of ERK. From these results, we found that the inhibition of GM-CSF expression by SKRG was derived from the decreased phosphorylation of EGFR. To identify the specific compound composing SKRG, we tested fifteen kinds of ginsenosides. Among these compounds, ginsenoside-Rh3 decreased the expression of GM-CSF protein and mRNA in SP-1 keratinocytes.
Conclusion
Taken together, we found that treatment with SKRG decreased the phosphorylation of EGFR and ERK in UVB-irradiated SP-1 keratinocytes and subsequently inhibited the expression of GM-CSF. Furthermore, we identified ginsenoside-Rh3 as the active saponin in Korean Red Ginseng.
Keywords: ginsenoside-Rh3, GM-CSF, Korean Red Ginseng, Panax ginseng, UVB
1. Introduction
Keratinocytes (90%) and melanocytes (5–10%) are located in the epidermis, which is the outermost layer of skin. UV radiation is comprised of UVC (100–280 nm), UVB (280–320 nm), and UVA (320–400 nm) [1]. When skin is exposed to solar UV radiation [2], it results in skin damage. UV-induced skin damage is mediated by growth factors and proinflammatory cytokines [3] such as granulocyte macrophage colony stimulating factor (GM-CSF). GM-CSF is a major immune regulator for the survival of a variety of cell types such as granulocytes and macrophages [4]. GM-CSF stimulates the proliferation, differentiation, transmigration, and survival of various types of cutaneous cells including dendritic cells, melanocytes, and keratinocytes [5], [6].
The expression of GM-CSF is regulated by mitogen-activated protein kinase (MAPK), extra cellular signal-related kinase (ERK) 1/2, p38, and c-Jun terminal kinase (JNK) pathways [7], [8]. The activation of the ERK signaling pathway is regulated by various stimuli such as growth factors, cytokines, and UV irradiation [9].
The phosphorylation of EGFR and a number of other cell surface receptors are also activated by UVB irradiation [10]. EGFR activation is initiated through EGF-EGFR complex dimerization and autophosphorylation by UV irradiation [11], [12]. After UV exposure, EGFR phosphorylation is detected within minutes in UV irradiated keratinocytes [13], [14]. It is known that UVB induces the phosphorylation of EGFR in keratinocytes [15]. In addition, EGFR is known to be an important upstream modulator of GM-CSF [16].
Saponin of Korean Red Ginseng (SKRG) is an important component in medicine and various health supplements because it has antiaging, antioxidant, and immune modulating effects, and a low rate of side effects [17], [18].
We have previously demonstrated that expression of GM-CSF increased in UV-irradiated SP-1 keratinocytes along with activated proliferation of melan-a cells (immortalized mouse melanocytes). Also, treatment with SKRG decreased the GM-CSF secreted from UV-irradiated SP-1 keratinocytes [17].
In this study, we attempted to elucidate the inhibitory mechanism of SKRG on UV-induced GM-CSF expression in SP-1 keratinocytes.
2. Materials and methods
2.1. Materials
SKRG was provided by Korea Ginseng Corporation (Daejeon, Korea). Newborn calf serum (NBCS) was purchased from Gibco Invitrogen (Carlsbad, CA, USA).
2.2. Cell culture
Murine SP-1 keratinocytes were derived from SENCAR mice and were generously provided by Dr. Stuart H. Yuspa (Laboratory of Cellular Carcinogenesis and Tumor Promotion, NCI, Bethesda, Maryland 20892-4255, USA). SP-1 keratinocytes were generally cultured in Eagle's minimum essential medium (EMEM) containing 0.05mM Ca2+, 8% Chelex treated heat-inactivated NBCS, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere of 10% CO2 and 95% air.
2.3. Cell viability assay
SP-1 keratinocytes cell were seeded in 96 well plates (2 × 104 cells/well). After 24 h, the media was replaced with media containing various concentrations of (A) SKRG, or (B) ginsenoside Rh3. Control cells were treated with dimethyl sulfoxide (DMSO) at a final concentration of 0.1%. After 24 h, the media containing the compounds or DMSO was replaced with media containing 10% EZ-Cytox (Daeil Lab Service, Seoul, Korea). The cells were then incubated at 37°C for 1 h, and the absorbance was measured using a microplate reader (Tecan, Mannedorf, Switzerland) at a wavelength of 450 nm. All assays were performed in triplicate.
2.4. UVB irradiation of SP-1 keratinocytes
Keratinocytes were seeded in a 60 mm cell culture dish or 24 well plates. After 24 h, the cells were washed with Dulbecco's phosphate-buffered saline (DPBS) and replaced with serum-free EMEM containing the SKRG for serum starvation. At 24 h after starvation, monolayer cultures were washed with DPBS and exposed to UVB by a UV irradiation system (VILBER Loumat, Marne la Vallée, France). The cells were irradiated with the UVB after being washed three times with DPBS. Immediately after irradiation, the cells were cultured in a medium containing 2% serum with or without SKRG for the indicated times.
2.5. Reverse transcription-polymerase chain reaction
Total RNA was isolated from SP-1 keratinocytes with TRIzol reagent (Takara, Shiga, Japan). The quality and quantity of the RNA were determined using NanoDrop2000 (Thermo Scientific, Loughborough, UK). To synthesize cDNA, 1 μg quantities of total RNA was mixed with 100 pmol quantities of oligo(dT) (ELPIS, Daejeon, Korea), followed by denaturation at 70°C for 5 min and chilling on ice for 5 min. The annealed samples were then incubated with reverse transcriptase (RT) and 2mM dNTPs (Fermentas, Hanover, MD, USA) for 1 h at 42°C. RT was terminated by heating for 10 min at 70°C. For amplification, the cDNA was mixed with HiPi polymerase chain reaction (PCR) Mix (ELPIS) and each of the following primer sets: GM-CSF forward: 5′ - GCC ATC AAA GAA GCC CTG AA - 3′, reverse: 5′ - GCG GGT CTG CAC ACA TGT TA - 3′.
The resulting PCR products were visualized by electrophoretic separation on 2% agarose gels with staining RedSafe Nucleic Acid Staining Solution (cat. no. 21141, Intron Biotechnology, Sangdaewon-Dong, Gyeonggi-do, Korea). Specific primers for β-actin were added as a control.
2.6. Quantitative real-time RT-PCR
SP-1 keratinocytes were harvested and total RNA was isolated using the TRIzol reagent (Takara, Shiga, Japan). Real-time RT-PCR was performed using a FastStart Essential DNA Probe Master Kit (Roche, Mannheim, Germany). The reaction was carried out according to the manufacturer's protocol. The probes for GM-CSF were designed with the Probe Library Assay Design Center (Universal ProbeLibrary, Probe #79. ref 04 689 020 001, Roche, Mannheim, Germany). The cycling condition was 600 s at 95°C, 40 cycles at 95°C for 20 s, and 60°C for 40 s on Lightcycler Nano (Roche, Mannheim, Germany). The mRNA expression level of the target gene was normalized to β-actin (Universal ProbeLibrary Mouse ACTB Gene Assay, ref 05 046 190 001, Roche, Mannheim, Germany). The obtained cDNA was amplified with the following primer (Bioneer, Daejeon, Korea): GM-CSF forward: 5′- GGC CTT GGA AGC ATG TAG AA -3′; reverse: 5′- TCT GCA CAC ATG TTA GCT TCT TG -3′.
2.7. Western blot
SP-1 keratinocytes were harvested and lysed with RIPA solution (Noble Bio, Suwon, Korea), protease inhibitor cocktail (Sigma-Aldrich, St. Loius, MO, USA), and 1mM phenylmethanesulfonyl fluoride (PMSF, Sigma) for 30 min. The lysates were sonicated with ice and centrifuged at 13,000 × g and 4°C for 20 min. Then, the supernatants were harvested and the protein concentration of each sample was determined using a Pierce Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL, USA) with bovine serum albumin (BSA) as the standard. The protein samples were stored at −20°C. The samples were mixed with LDS sample buffer and sample reducing buffer (Invitrogen, Carlsbad, CA, USA) and boiled at 95°C for 5 min. The reduced samples were loaded on NuPAGE 10% bis-tris gel and run with MOPS SDS running buffer (Invitrogen). The proteins were then transferred to a polyvinylidene fluoride transfer membrane (PVDF membrane, PALL Corporation, Port Washington, NY, USA). The membrane was blocked with 5% skim milk in TBS-T buffer (0.1% Tween 20, 100mM NaCl, and 10mM Tris-HCl, pH 7.5) or 3% BSA and incubated with primary antibodies using mouse monoclonal anti-β-actin antibody (1/10,000, Sigma), rabbit polyclonal anti-GM-CSF antibody (0.02 μg/mL, Abcam, ab9741, Cambridge, UK), rabbit monoclonal anti-p42/44 MAPK (ERK1/2) antibody (1/1,000, Cell Signaling, Beverly, MA, USA), rabbit monoclonal anti-Phospho-p42/44 MAPK (ERK1/2) antibody (1/2,000, Cell Signaling), rabbit monoclonal anti-EGF receptor antibody (1/1,000, Cell Signaling), rabbit monoclonal anti-phospho-EGF receptor antibody (1/1,000, Cell Signaling), donkey anti-rabbit IgG antibody (1/5,000, Bethyl Laboratories, Montgomery, TX, USA), and goat anti-mouse IgG antibody (1/10,000, Bio-Rad, Hercules, CA, USA). Bands were detected with WEST-ZOL plus a Western blot detection system (INtRON Biotechnology, Sungnam, Korea) and visualized with ChemiDoc XRS (Bio-Rad).
2.8. Enzyme-linked immunosorbent assay
The production of cytokines in SP-1 keratinocytes cells was measured by an enzyme-linked immunosorbent assay ELISA kit (eBioscience, San Diego, CA, USA). SP-1 keratinocytes were seeded in 24 well plates. After 24 h, the SP-1 keratinocytes in 24 well plates were washed with DPBS and replenished with serum-free EMEM containing ginsenosides. After starvation for 24 h, SP-1 keratinocytes in the 24 well plates were washed with DPBS and exposed to a radiation dose of 30 mJ/cm2 of UVB by a UV irradiation system. After irradiation, the cells were replaced with 2% NBCS EMEM containing ginsenosides. After 24 h, conditioned media of the UV-irradiated SP-1 keratinocytes were collected and stored at −80°C until ELISA was used for the measurement of cytokine production. 96-well immune plates were coated with the cytokine capture antibody overnight at 4°C. The cytokine capture antibody was removed, and the wells were washed five times with washing buffer (0.05% Tween 20 in PBS) and blocked with 1X assay diluent for 1 h at room temperature. The wells were washed five times, 100 μL aliquots of standard (recombinant mouse cytokine) and samples were added to each well, and the plates were incubated for 2 h at room temperature. The plate was washed five times with wash buffer, the cytokine detection antibody was added, and then incubation was continued for 1 h at room temperature. The solutions in the wells were removed and the wells were washed five times with wash buffer. Next, 100 μL of avidin-HRP was added to each well and the plate was incubated for 30 min at room temperature. After the wells were washed seven times, 100 μL aliquots of 1X TMB substrate solution were added to each well, and the plate was incubated for 15 min at room temperature. The color reaction was quenched with stop solution (50 μL, 1N H2SO4). The absorbance was measured using a microplate reader at a wavelength of 450 nm.
3. Results
3.1. Effect of SKRG or ginsenoside-Rh3 on SP-1 keratinocyte viability
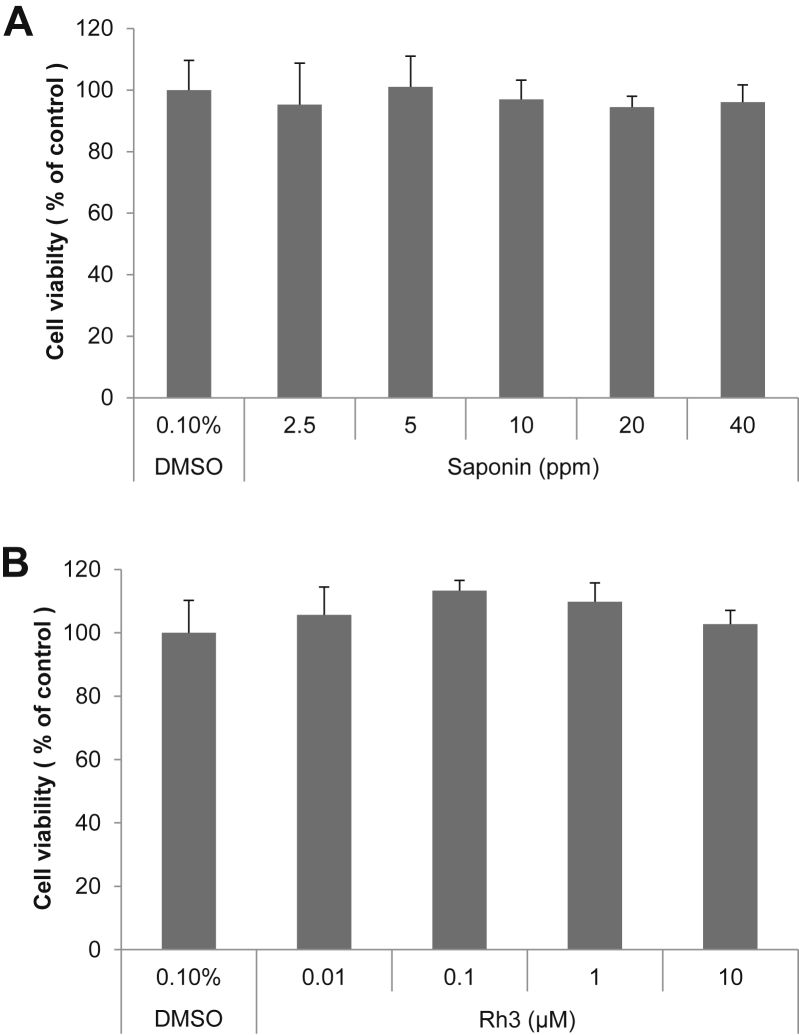
EZ-Cytox assay (Daeil Lab) was used to assess the effect of SKRG and ginsenoside-Rh3 on SP 1-keratinocytes viability. SKRG and ginsenoside-Rh3 showed no cytotoxic effect at all concentrations (Fig. 1).
Fig. 1.
Effect of SKRG and ginsenoside-Rh3 on SP-1 keratinocyte viability. Cell viability was measured in SP-1 keratinocytes. After incubation with various concentrations of (A) SKRG or (B) ginsenoside-Rh3 for 24 h, cell viability was measured using an EZ-Cytox assay kit (Daeil Lab Service, Seoul, Korea) based on the cleavage of the tetrazolium salt to water-soluble formazan by succinate-tetrazolium reductase. The experiment was repeated three times. SKRG, saponin of Korean Red Ginseng.
3.2. Effect of SKRG on UVB-induced expression of GM-CSF in SP-1 keratinocytes
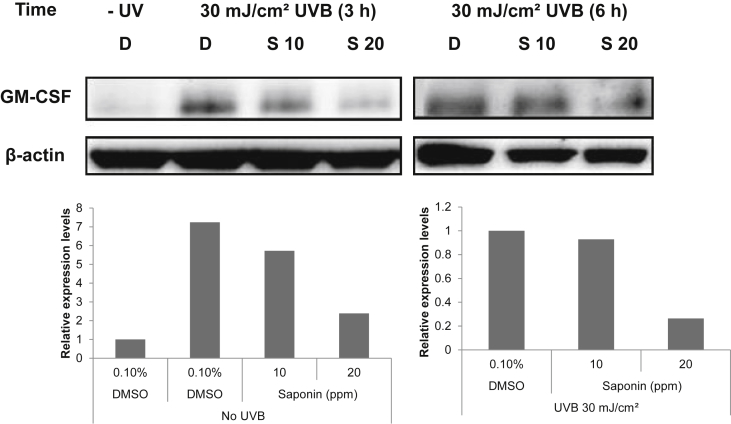
To elucidate the effect of SKRG on UVB-induced GM-CSF expression in SP-1 keratinocytes, SP-1 cells were exposed to 30 mJ/cm2 UVB for 3 h and 6 h. At various times after UV irradiation, the cells were collected for analysis of GM-CSF expression in SP-1 keratinocytes. The expression of GM-CSF was increased by UVB irradiation at 3 h until 6 h in SP-1 keratinocytes (Fig. 2). Treatment with SKRG dose-dependently decreased the expression of GM-CSF compared with the untreated irradiated control.
Fig. 2.
Effect of SKRG on UVB-induced expression of GM-CSF in SP-1 keratinocytes. Before UVB irradiation, SP-1 keratinocytes were treated with serum-free EMEM containing SKRG (10 ppm and 20 ppm). After 24 h, SP-1 keratinocytes were irradiated with UVB at a dose of 30 mJ/cm2. Immediately, the cells were treated with 2% newborn calf serum EMEM containing SKRG Cells were collected at 3 h and 6 h for Western blot analysis of GM-CSF. D, dimethyl sulfoxide (0.1%); EMEM, Eagle's minimum essential medium; GM-CSF, granulocyte macrophage colony stimulating factor; S 10, SKRG (10 ppm); S 20, SKRG (20 ppm); SKRG, saponin of Korean Red Ginseng.
3.3. Effect of SKRG on UVB-induced expression of GM-CSF mRNA in SP-1 keratinocytes
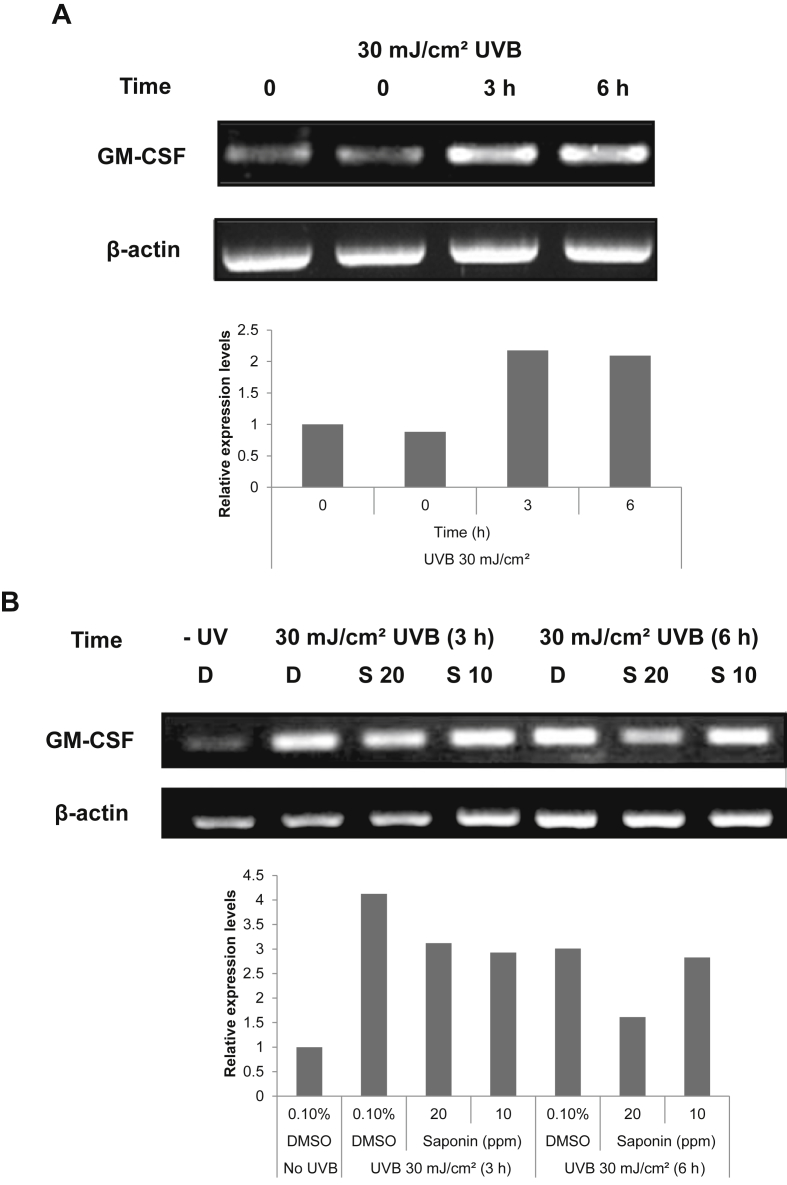
In a previous study, we found that SKRG decreased the expression of GM-CSF in UVB-irradiated SP-1 keratinocytes. We analyzed GM-CSF mRNA expression induced by UVB in SP-1 keratinocytes. Cells were exposed to 30 mJ/cm2 UVB for 0 h, 3 h, and 6 h. Total RNA was isolated from UVB-irradiated SP-1 keratinocytes and GM-CSF mRNA expression was analyzed by RT-PCR. GM-CSF mRNA expression was significantly increased at 3 h and 6 h after UVB irradiation (Fig. 3A). Treatment with SKRG (20 ppm) decreased GM-CSF mRNA expression induced by UVB in SP-1 keratinocytes at 3 h and 6 h (Fig. 3B).
Fig. 3.
Effect of SKRG on the UVB-induced expression of GM-CSF mRNA in SP-1 keratinocytes. Before UVB irradiation, SP-1 keratinocytes were treated with serum-free EMEM containing SKRG (10 ppm and 20 ppm). After 24 h, SP-1 keratinocytes were irradiated with UVB at a dose of 30 mJ/cm2. Immediately, the cells were treated with 2% newborn calf serum EMEM containing SKRG. Cells were harvested at the times indicated and RNA was isolated for RT-PCR of GM-CSF and β-actin. (A) Cells were treated with DMSO as the solvent control. (B) Cells were treated with SKRG (10 ppm and 20 ppm). D, dimethyl sulfoxide (0.1%); DMSO, dimethyl sulfoxide; EMEM, Eagle's minimum essential medium; RT-PCR, reverse transcriptase polymerase chain reaction; GM-CSF, granulocyte macrophage colony stimulating factor; S 10, SKRG (10 ppm); S 20, SKRG (2 ppm); SKRG, saponin of Korean Red Ginseng.
3.4. Effect of SKRG on UVB-induced phosphorylation of ERK in SP-1 keratinocytes
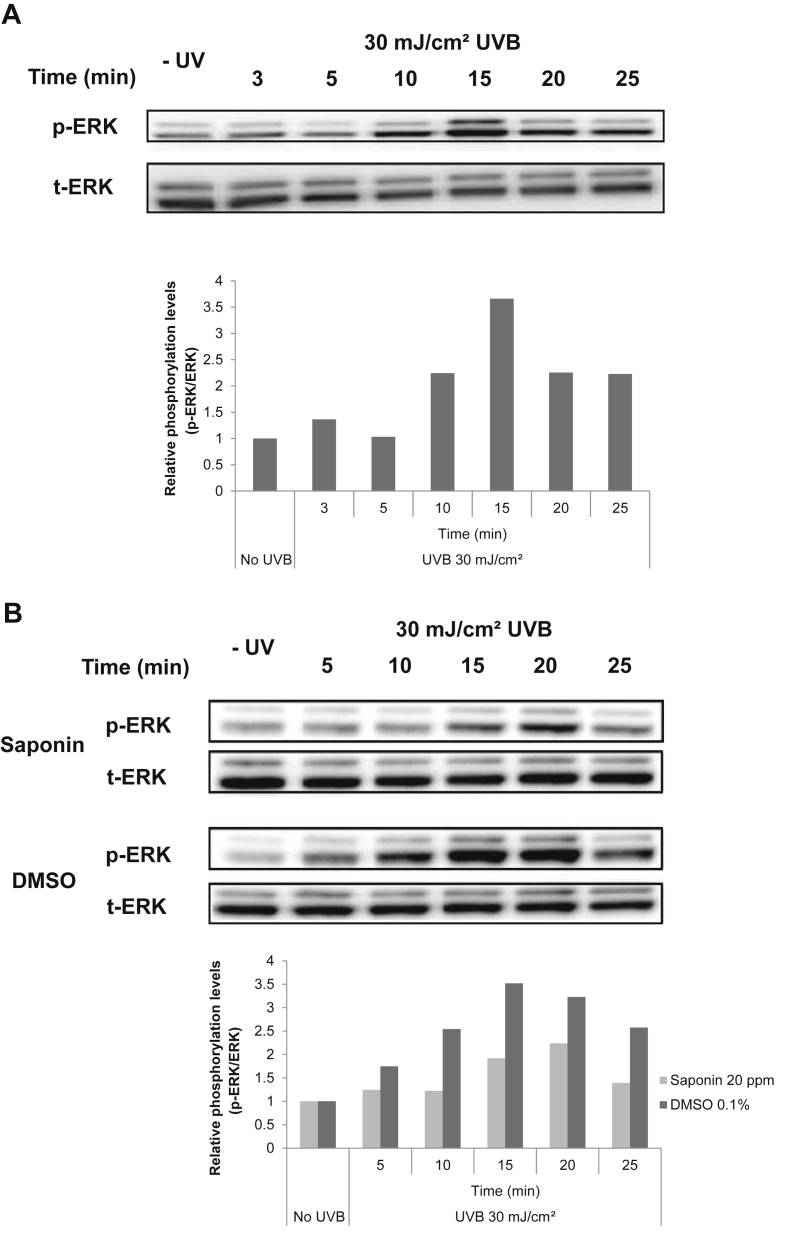
It has been reported that the expression of GM-CSF is regulated by ERK. We examined the effect of SKRG on UVB-induced phosphorylation of ERK in SP-1 keratinocytes. SP-1 keratinocytes were exposed to 30 mJ/cm2 UVB. The rapid activation of ERK was observed at 10 min after UVB irradiation and peaked at 15 min (Fig. 4A). Treatment with SKRG decreased ERK phosphorylation induced by UVB irradiation (Fig. 4B).
Fig. 4.
Effect of SKRG on the UVB-induced phosphorylation of ERK in SP-1 keratinocytes. Before UVB irradiation, SP-1 keratinocytes were treated with serum-free EMEM containing the SKRG (20 ppm). After 24 h, SP-1 keratinocytes were irradiated with UVB at a dose of 30 mJ/cm2. Immediately, the cells were treated with 2% newborn calf serum EMEM containing the SKRG. Cells were harvested at the times indicated for Western blot analysis of phospho-ERK (p-ERK) and total-ERK (t-ERK). (A) Cells were treated with DMSO as the solvent control. (B) Cells were treated with the SKRG (20 ppm). DMSO, dimethyl sulfoxide; EMEM, Eagle's minimum essential medium; SKRG, saponin of Korean Red Ginseng.
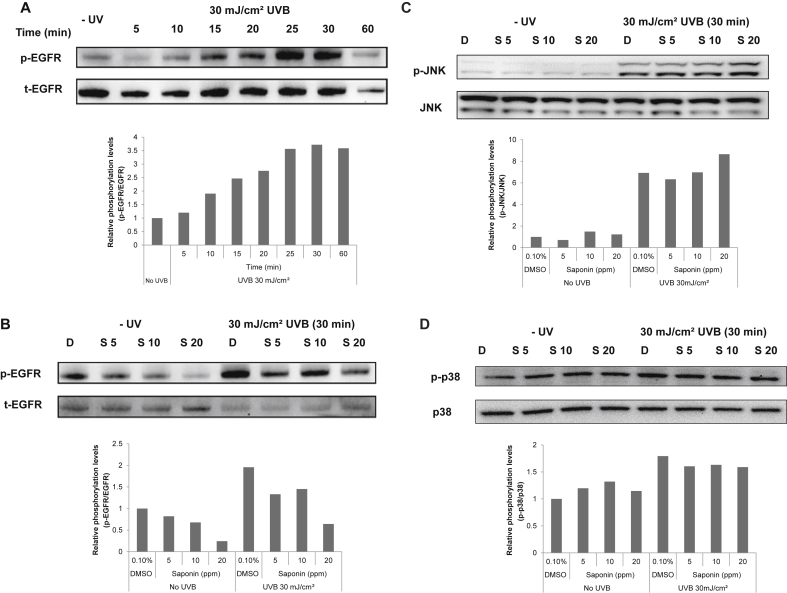
3.5. Effect of SKRG on the UVB-induced phosphorylation of EGFR in SP-1 keratinocytes
To examine whether SKRG affects the phosphorylation of EGFR which is a well-known main upstream signal of GM-CSF expression, SP-1 keratinocytes were irradiated with 30 mJ/cm2 UVB. At various times after UVB irradiation, the SP-1 cells were collected for analysis of the phosphorylation of EGFR. The phosphorylation of EGFR rapidly increased after 10 min of UVB exposure, and then dropped to the basal level within 60 min (Fig. 5A). SKRG treatment decreased UVB-induced phosphorylation of EGFR in a concentration-dependent manner (Fig. 5B). But, SKRG did not affect the other MAPKs such as JNK and p38 at the same conditions. The phosphorylations of JNK and p38 were increased by UVB exposure in SP-1 keratinocyte, but SKRG had no detectable effect (Figs. 5C and 5D).
Fig. 5.
Effect of SKRG on the UVB-induced phosphorylation of EGFR in SP-1 keratinocytes. Before UVB irradiation, SP-1 keratinocytes were treated with serum-free EMEM containing the SKRG (20 ppm). After 24 h, SP-1 keratinocytes were irradiated with UVB at a dose of 30 mJ/cm2. Immediately, the cells were treated with 2% newborn calf serum EMEM containing the SKRG. Cells were harvested at the times indicated for western blot analysis of phospho-EGFR (p-EGFR) and total-EGFR (t-EGFR) (A, B), phospho-JNK (p-JNK) and total-JNK (JNK) (C), phospho-p38 (p-p38) and total-p38 (p38) (D). D, dimethyl sulfoxide (0.1%); EMEM, Eagle's minimum essential medium; S 5, SKRG (5 ppm); S 10, SKRG (10 ppm); S 20, SKRG (20 ppm); SKRG, saponin of Korean Red Ginseng.
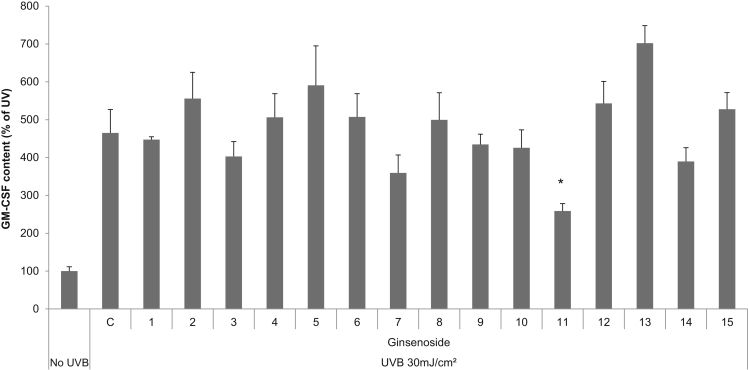
3.6. Effect of ginsenosides on the expression of GM-CSF in UVB-irradiated SP-1 keratinocytes
To determine what kinds of ginsenosides affect UVB-induced GM-CSF expression in SP-1 keratinocytes, we measured the production of GM-CSF using an ELISA kit from 24 h culture supernatants. UVB irradiation increased GM-CSF expression in SP-1 keratinocytes compared with the non-irradiated control. Ginsenoside-Rh3 decreased the expression of GM-CSF by 40% compared with control (Fig. 6).
Fig. 6.
Effect of ginsenosides on the expression of GM-CSF in UVB-irradiated SP-1 keratinocytes. Before UVB irradiation, SP-1 keratinocytes were treated with serum-free EMEM containing ginsenosides (1: ginsenoside-K, 2: -F1, 3: -F2, 4: -Rb1, 5: -Rb2, 6: -Rb3, 7: -Rc, 8: -Rd, 9: -Re, 10: -Rh1, 11: -Rh3, 12: -Rg1, 13: -Rg2, 14: -Rf, 15: -Ro 10 ppm). After 24 h, SP-1 keratinocytes were irradiated with UVB at a dose of 30 mJ/cm2. Immediately, the cells were treated with 2% newborn calf serum EMEM containing ginsenosides. After 24 h, the conditioned media was collected and granulocyte macrophage colony stimulating factor (GM-CSF) was measured by enzyme linked immunosorbent assay (ELISA), as described in the materials and methods section. Data were analyzed using the Student unpaired t test (*p < 0.05). D, dimethyl sulfoxide (0.1%); EMEM, Eagle's minimum essential medium; GM-CSF, granulocyte macrophage colony stimulating factor.
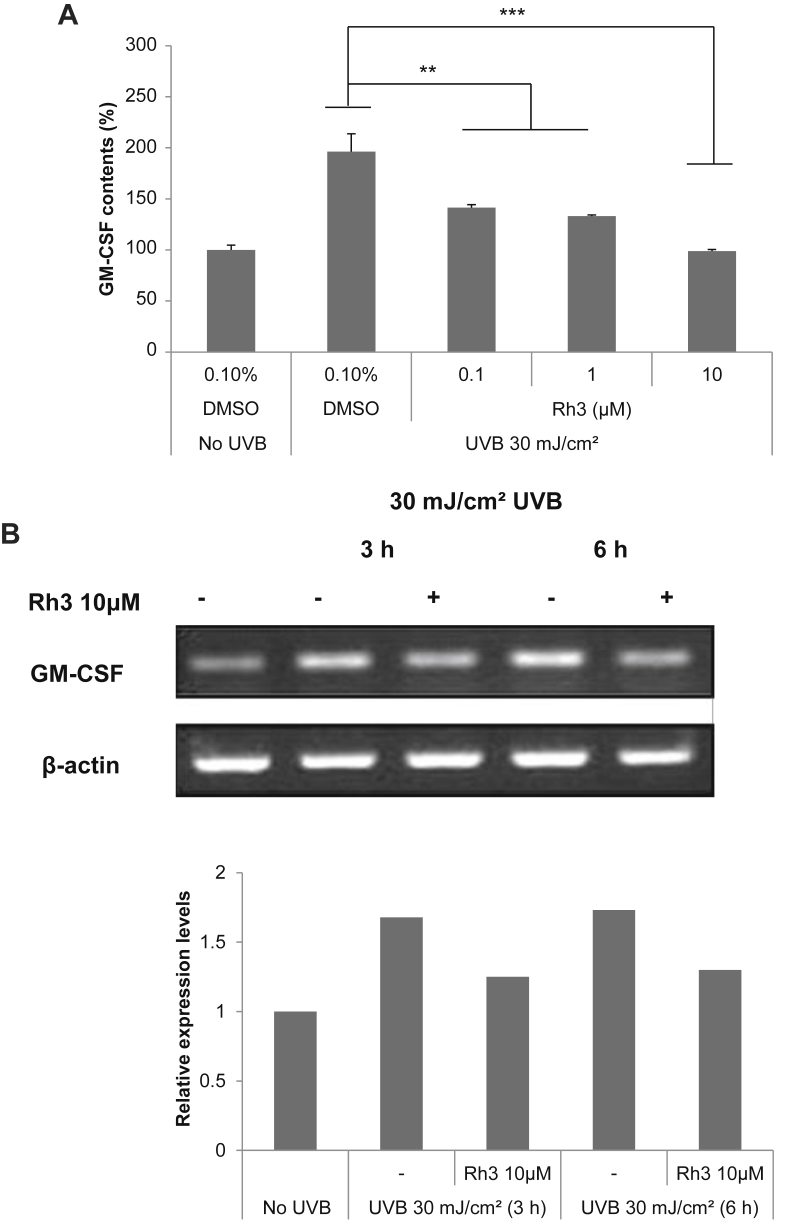
3.7. Effect of ginsenoside-Rh3 on the UVB-induced expression of GM-CSF mRNA and protein level in SP-1 keratinocytes
We examined the effect of ginsenoside-Rh3 on GM-CSF expression in UVB-irradiated SP-1 keratinocytes. Ginsenoside-Rh3 decreased expression of GM-CSF in a dose dependent manner (Fig. 7A). We also examined the effect of ginsenoside-Rh3 on GM-CSF mRNA expression in UVB-irradiated SP-1 keratinocytes. Using RT-PCR and quantitative real-time RT-PCR, we measured GM-CSF mRNA expression induced by UVB irradiation in SP-1 keratinocytes. After 3 h and 6 h, the GM-CSF mRNA expression induced by UVB irradiation was decreased with ginsenoside-Rh3 (Fig. 7B).
Fig. 7.
Effect of ginsenoside-Rh3 on the UVB-induced expression of GM-CSF protein and mRNA in SP-1 keratinocytes. Before UVB irradiation, SP-1 keratinocytes were treated with serum-free EMEM containing ginsenoside-Rh3 (0.1μM, 1μM, 10μM). After 24 h, SP-1 keratinocytes were irradiated with UVB at a dose of 30 mJ/cm2. Immediately, the cells were treated with 2% newborn calf serum EMEM containing ginsenoside-Rh3. (A) After 24 h, the conditioned media was collected and GM-CSF was measured by enzyme linked immunosorbent assay (ELISA), as described in the materials and methods section. Data were analyzed using the Student unpaired t test (**p < 0.01, ***p < 0.001). (B) The cells were harvested at the times indicated and RNA was isolated for RT-PCR. EMEM, Eagle's minimum essential medium; GM-CSF, granulocyte macrophage colony stimulating factor; RT-PCR, reverse transcriptase polymerase chain reaction.
4. Discussion
UVB irradiation can induce skin damage such as erythema, wrinkles, cancer, and skin pigmentation through changes in numerous signaling pathways [19], [20], [21], [22]. Among the various cytokines induced by UVB, GM-CSF is one of the leading promoters of skin pigmentation by inducing the proliferation of melanocytes [6]. GM-CSF can be produced by various cells and regulates cell proliferation, differentiation, and migration of cells [23].
In our previous study, GM-CSF increased significantly when irradiated with UVB in SP-1 keratinocytes and it had activated the proliferation of melan-a cells. The content of GM-CSF in cultured media from UV-irradiated SP-1 keratinocytes was higher than that in non-irradiated keratinocytes [17]. We showed that the GM-CSF protein levels decreased with treatment of SKRG in a concentration-dependent manner at 3 h and 6 h (Fig. 2). GM-CSF mRNA expression was increased by UVB in a time-dependent manner (Fig. 3A). In addition, treatment with SKRG decreased GM-CSF mRNA expression in UV-irradiated SP-1 keratinocytes (Fig. 3B). UVB activates four major families of growth factor receptors: EGFR, platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR), and insulin receptor (IR) [24]. EGFR belongs to a receptor tyrosine kinase (RTK) and regulates survival, proliferation, migration, differentiation, and the transformation of the cell [25], [26]. EGFR and its ligands participate in the response to skin damage [16]. EGFR family ligands include transforming growth factor (TGF)-α, amphiregulin, and epiregulin [13]. The complex of EGF family ligands and EGFR triggers diverse signal transduction pathways [25]. The EGFR-ERK pathway mediates survival and the proliferation of keratinocytes. It has been reported that EGFR activates the phosphorylation of ERK [13], and phosphorylated ERK has been shown to contribute to the generation of GM-CSF [27], [28]. We found out UVB irradiation induced the phosphorylation of ERK (Fig. 4A) and treatment with SKRG reduced the UVB-induced phosphorylation of ERK in SP-1 keratinocytes (Fig. 4B).
Moreover, it has been reported that EGFR activated by UV irradiation enhanced the expression of GM-CSF [16]. We found that EGFR phosphorylation increased when irradiating 10 min post-UVB, with activity peaks at 30 min that lasted until approximately 1 h (Fig. 5A). Treatment with SKRG decreased EGFR phosphorylation induced by UVB irradiation (Fig. 5B). Although the phosphorylation of JNK and p38 were also induced by UVB irradiation in SP-1 keratinocytes, treatment of SKRG showed no effect at all (Figs. 5C, 5D). From these results, we can conclude that the effect of SKRG on GM-CSF expression was regulated more specifically by EGFR pathway.
Ginsenosides isolated from ginseng have anti-tumor, anti-metastatic and anti-angiogenic activities that are related to the inhibition of cell proliferation [18], [29]. We showed that the secretion of GM-CSF protein (induced by UVB) is suppressed by ginsenoside-Rh3 (Fig. 6, Fig. 7A). Furthermore, ginsenoside-Rh3 decreased GM-CSF mRNA expression levels (Fig. 7B).
In conclusion, these results showed that SKRG inhibited UVB-induced GM-CSF expression by blocking the EGFR-ERK signal pathway. We concluded that a major source of this SKRG could be ginsenoside-Rh3 derived from Korean Red Ginseng.
Conflicts of interest
Authors have no conflict of interest.
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. HN11C0045) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2007330).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Han W., He Y.Y. Requirement for metalloproteinase-dependent ERK and AKT activation in UVB-induced G1-S cell cycle progression of human keratinocytes. Photochem Photobiol. 2009;85:997–1003. doi: 10.1111/j.1751-1097.2008.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh B., Schneider M., Knyazev P., Ullrich A. UV-induced EGFR signal transactivation is dependent on proligand shedding by activated metalloproteases in skin cancer cell lines. Int J Cancer. 2009;124:531–539. doi: 10.1002/ijc.23974. [DOI] [PubMed] [Google Scholar]
- 3.Lee E.J., Jeon M.-S., Kim B.-D., Kim J.-H., Kwon Y.-G., Lee H., Lee Y.S., Yang J.-H., Kim T.-Y. Capsiate inhibits ultraviolet B-induced skin inflammation by inhibiting Src family kinases and epidermal growth factor receptor signaling. Free Radical Bio Med. 2010;48:1133–1143. doi: 10.1016/j.freeradbiomed.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 5.Kanda N., Watanabe S. Histamine enhances the production of granulocyte-macrophage colony-stimulating factor via protein kinase C alpha and extracellular signal-regulated kinase in human keratinocytes. J Invest Dermatol. 2004;122:863–872. doi: 10.1111/j.0022-202X.2004.22432.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirobe T., Furuya R., Akiu S., Ifuku O., Fukuda M. Keratinocytes control the proliferation and differentiation of cultured epidermal melanocytes from ultraviolet radiation B-induced pigmented spots in the dorsal skin of hairless mice. Pigm Cell Res. 2002;15:391–399. doi: 10.1034/j.1600-0749.2002.02052.x. [DOI] [PubMed] [Google Scholar]
- 7.Peus D., Vasa R.A., Beyerle A., Meves A., Krautmacher C., Pittelkow M.R. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J Invest Dermatol. 1999;112:751–756. doi: 10.1046/j.1523-1747.1999.00584.x. [DOI] [PubMed] [Google Scholar]
- 8.Larbi A., Douziech N., Fortin C., Linteau A., Dupuis G., Fulop T., Jr. The role of the MAPK pathway alterations in GM-CSF modulated human neutrophil apoptosis with aging. Immun Ageing. 2005;2:6. doi: 10.1186/1742-4933-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Carpio P.A., Trelles M.A. Cutaneous epidermal growth factor receptor system following ultraviolet irradiation: exploring the role of molecular mechanisms. Photodermatol Photo. 2010;26:250–256. doi: 10.1111/j.1600-0781.2010.00534.x. [DOI] [PubMed] [Google Scholar]
- 10.Huang R.-P., Wu J.-X., Fan Y., Adamson E.D. UV activates growth factor receptors via reactive oxygen intermediates. J Cell Biol. 1996;133:211–220. doi: 10.1083/jcb.133.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J., Ramachandiran S., Tikoo K., Jia Z., Lau S.S., Monks T.J. EGFR-independent activation of p38 MAPK and EGFR-dependent activation of ERK1/2 are required for ROS-induced renal cell death. Am J Physiol-Renal. 2004;287:F1049–F1058. doi: 10.1152/ajprenal.00132.2004. [DOI] [PubMed] [Google Scholar]
- 12.Emlet D.R., Moscatello D.K., Ludlow L.B., Wong A.J. Subsets of epidermal growth factor receptors during activation and endocytosis. J Biol Chem. 1997;272:4079–4086. doi: 10.1074/jbc.272.7.4079. [DOI] [PubMed] [Google Scholar]
- 13.Pastore S., Mascia F., Mariani V., Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 14.Alexandrescu D.T., Kauffman C.L., Dasanu C.A. The cutaneous epidermal growth factor network: can it be translated clinically to stimulate hair growth? Dermatol Online J. 2009;15:1. [PubMed] [Google Scholar]
- 15.Yao Y., Wolverton J.E., Zhang Q., Marathe G.K., Al-Hassani M., Konger R.L., Travers J.B. Ultraviolet B radiation generated platelet-activating factor receptor agonist formation involves EGF-R-mediated reactive oxygen species. J Immunol. 2009;182:2842–2848. doi: 10.4049/jimmunol.0802689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascia F., Cataisson C., Lee T.-C., Threadgill D., Mariani V., Amerio P., Chandrasekhara C., Adeva G.S., Girolomoni G., Yuspa S.H. EGFR regulates the expression of keratinocyte-derived granulocyte/macrophage colony-stimulating factor in vitro and in vivo. J Invest Dermatol. 2010;130:682–693. doi: 10.1038/jid.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh C.T., Park J.I., Jung Y.R., Joo Y.A., Shin D.H., Cho H.J., Ahn S.M., Lim Y.-H., Park C.K., Hwang J.S. Inhibitory effect of Korean Red Ginseng on melanocyte proliferation and its possible implication in GM-CSF mediated signaling. J Ginseng Res. 2013;37:389. doi: 10.5142/jgr.2013.37.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung S.H., Woo M.S., Kim S.Y., Kim W.K., Hyun J.W., Kim E.J., Kim D.H., Kim H.S. Ginseng saponin metabolite suppresses phorbol ester–induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. Int J Cancer. 2006;118:490–497. doi: 10.1002/ijc.21356. [DOI] [PubMed] [Google Scholar]
- 19.Clydesdale G.J., Dandie G.W., Muller H.K. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. 2001;79:547–568. doi: 10.1046/j.1440-1711.2001.01047.x. [DOI] [PubMed] [Google Scholar]
- 20.Akazaki S., Imokawa G. Mechanical methods for evaluating skin surface architecture in relation to wrinkling. J Dermatol Sci. 2001;27(Suppl 1):S5–S10. doi: 10.1016/s0923-1811(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 21.Herrlich P., Sachsenmaier C., Radler-Pohl A., Gebel S., Blattner C., Rahmsdorf H.J. The mammalian UV response: mechanism of DNA damage induced gene expression. Adv Enzyme Regul. 1994;34:381–395. doi: 10.1016/0065-2571(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 22.Tyrrell R.M. Activation of mammalian gene expression by the UV component of sunlight–from models to reality. BioEssays. 1996;18:139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- 23.Van Nieuwenhuijze A., Koenders M., Roeleveld D., Sleeman M.A., Van den Berg W., Wicks I.P. GM-CSF as a therapeutic target in inflammatory diseases. Mol Immunol. 2013;56:675–682. doi: 10.1016/j.molimm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y., Voorhees J.J., Fisher G.J. Epidermal growth factor receptor is a critical mediator of ultraviolet B irradiation-induced signal transduction in immortalized human keratinocyte HaCaT cells. The Am J Pathol. 2006;169:823–830. doi: 10.2353/ajpath.2006.050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall C.J. MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 26.Jost M., Kari C., Rodeck U. The EGF receptor–an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505–510. [PubMed] [Google Scholar]
- 27.Meja K.K., Seldon P.M., Nasuhara Y., Ito K., Barnes P.J., Lindsay M.A., Giembycz M.A. p38 MAP kinase and MKK-1 co-operate in the generation of GM-CSF from LPS-stimulated human monocytes by an NF-κB-independent mechanism. Brit J Pharmacol. 2000;131:1143–1153. doi: 10.1038/sj.bjp.0703684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knobloch J., Peters H., Jungck D., Muller K., Strauch J., Koch A. TNFalpha-induced GM-CSF release from human airway smooth muscle cells depends on activation of an ET-1 autoregulatory positive feedback mechanism. Thorax. 2009;64:1044–1052. doi: 10.1136/thx.2008.111047. [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa H., Sung J.H., Huh J.H. Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch Pharm Res. 1997;20:539–544. doi: 10.1007/BF02975208. [DOI] [PubMed] [Google Scholar]