Abstract
Background
Ginseng is a semishade perennial plant cultivated in sloping, sun-shaded areas in Korea. Recently, owing to air-environmental stress and various fungal diseases, greenhouse cultivation has been suggested as an alternative. However, the optimal light transmission rate (LTR) in the greenhouse has not been established.
Methods
The effect of LTR on photosynthesis rate, growth, and ginsenoside content of ginseng was examined by growing ginseng at the greenhouse under 6%, 9%, 13%, and 17% of LTR.
Results
The light-saturated net photosynthesis rate (Asat) and stomatal conductance (gs) of ginseng increased until the LTR reached 17% in the early stage of growth, whereas they dropped sharply owing to excessive leaf chlorosis at 17% LTR during the hottest summer period in August. Overall, 6–17% of LTR had no effect on the aerial part of plant length or diameter, whereas 17% and 13% of LRT induced the largest leaf area and the highest root weight, respectively. The total ginsenoside content of the ginseng leaves increased as the LTR increased, and the overall content of protopanaxatriol line ginsenosides was higher than that of protopanaxadiol line ginsenosides. The ginsenoside content of the ginseng roots also increased as the LTR increased, and the total ginsenoside content of ginseng grown at 17% LTR increased by 49.7% and 68.3% more than the ginseng grown at 6% LTR in August and final harvest, respectively.
Conclusion
These results indicate that 13–17% of LTR should be recommended for greenhouse cultivation of ginseng.
Keywords: ginsenoside, greenhouse cultivation, light stress, Panax ginseng, photosynthesis
1. Introduction
Ginseng (Panax ginseng Meyer) is a semishade perennial plant whose growth diminishes beyond the optimum light intensity. It should be shaded from direct sunlight by an artificial facility in consideration of the light transmission rate (LTR), in order to increase the yield. For this reason, ginseng has been cultivated under a two-layered black polyethylene net with shading in Korea, a major ginseng exporter, for several decades [1]. Nevertheless, ginseng leaves, which play a critical role in the root yield [2], are easily damaged by air-environmental stresses [3] and various fungal diseases [4]—such as Alternaria blight by Alternaria panax, anthracnose by Colletotrichum gloeosporioides, and Phytophthora blight by Phytophthora cactorum—beyond redemption because of the open environment [5], the nonuniform LTR [6], and the high temperature of still air beneath the polyethylene net [3], [7]. To prevent early defoliation by pathogenic fungi in particular, agricultural chemicals are widely used; unfortunately, however, excessive use has led to today's pesticide residue problem. These negative aspects have a direct influence on the image of ginseng, which is used as a medicine.
Growing ginseng in greenhouses on account of incidence inhibition by rainproof cultivation and improving cultivation efficiency have become an affordable alternative solution to this comprehensive problem [8]. The data for cultivating ginseng in the greenhouses, however, are scarce, and little is known about the optimum LTR in shaded greenhouses because the majority of previous studies on the growth of ginseng have been based on analyses undertaken in slanted shading where the LTR is subject to changes in solar altitude. Lee et al [6] reported that there were notable differences of more than three times the light intensity among the lines and the rearmost line irrespective of time and weather. As a result of the different natural light influx occasioned by the slanted shading, ginseng growth shows a wide variation according to the planting position, even in the same shading [9]. So the optimal LTR in a greenhouse for ginseng is practically unestablished, and it has customarily been suspended in a black polyethylene net with a polyethylene film above the greenhouse for light interruption, without checking the light influx, although it has been shown that LTRs ranging from 8% to 20% are suitable for increasing the ginseng root yield on the basis of photosynthetic capability [1], [10], [11].
The purpose of the present study was to examine the photosynthetic and growth characteristics of ginseng grown at different LTRs in greenhouses, including its ginsenoside content, which is recognized as an important factor in determining the quality of ginseng under different natural light levels, and also to determine the suitable LTR for greenhouse cultivation because plant growth can be increased by improving photosynthetic efficiency in an environment similar to ginseng's natural habitat. Furthermore, our data provide valuable resources for the development of a suitable shading material for greenhouses in which ginseng is cultivated.
2. Materials and methods
2.1. Growth conditions of ginseng in the greenhouse
The experiments were conducted in four greenhouses (with a surface area of 42 m2 and a height of 2.5 m) at the Department of Herbal Crop Research, Eumseong (127°45′13.14″ E, 36°56′36.63″ N), Korea, from March 31, 2014 to October 10, 2014. The light intensities in the greenhouse were measured with a LI-250A quantum sensor (LI-COR, Lincoln, NE, USA) for 5 min both above and below the crop canopy three times during the growing seasons prior to solar noon under clear sky conditions. Light transmission in the greenhouse was controlled by covering polyethylene film with black polyethylene netting above the greenhouse for establishing 6%, 9%, 13%, and 17% of LTR. The average temperature differences around ginseng leaves, continuously logged by an air thermometer (Thermo Recorder TR-72Ui; T&D Corp, Tokyo, Japan) in the greenhouse according to LTR, were about 1.0°C during plant growth (Fig. 1). No additional covering material was installed during the growth of the ginseng plants. To keep identical soil conditions, a sterilized potting mix (Premier Ginseng 50L; Shinsung, Seoul, Korea) of peat moss, perlite, and leaf mold at the ratio of 5:3:2 (volume) was used as the growing medium in each container (with a surface area of 3.0 m2 and a depth of 0.3 m). The 1-yr-old ginseng seedlings were purchased from a local Eumseong grower and planted in each container without interplant competition. As no registered cultivars or selections are available, these seedlings are genetically diverse. Three containers per treatment along with a completely randomized design were used. The plants were irrigated once a week with tap water without using fertilizers.
Fig. 1.
The average temperature in greenhouse established at 6%, 9%, 13%, and 17% of light transmission rates (LTRs) from April 11, 2014 to October 3, 2014.
2.2. Determination of photosynthetic parameters
The light-saturated net photosynthetic rates (Asat) and stomatal conductance (gs) of the 2-yr-old ginseng leaves grown at different LTRs were measured using a LI 6400 portable photosynthesis system (LI-COR) equipped with an infrared gas analyzer. The middle of the fully expanded leaves was clamped to a 6-cm2 leaf chamber provided by a LI 6400-02 LED light source sensor head (LI-COR). The conditions inside the leaf chamber were controlled at an air influx rate of 500 μmol/s, a CO2 concentration of 400 μmol (similar to natural atmosphere concentration), a relative humidity of 30–40%, a block temperature of 20°C, and an irradiance level of 500 μmol/m2 s photosynthetic photon fluxes [12]. Measurements were taken twice, on June 2, 2014 (when ginseng leaves are fully expanded) and August 11, 2014, under an elevated temperature environment during the hot summer season of 2014. A minimum and maximum wait time for each step were set at 3 min and 5 min, respectively.
2.3. Measurements of chlorophyll contents and growth
The chlorophyll content of 2-yr-old ginseng leaves was measured with a chlorophyll meter (SPAD-502; Minolta, Tokyo, Japan) three times at the center of the leaves throughout the experiments [13]. The 2-yr-old ginseng leaves were harvested according to a completely randomized design under sunny conditions and directly measured for their growth characteristics using a general ruler, a pair of Digimatic calipers (caliper 500-182; Mitutoyo, Tokyo, Japan), or an electronic scale (RE260; CAS, Seoul, Korea). The leaf surface area was measured with a WindDIAS image analysis system (Delta-T; ADC, London, UK) when the aerial parts were fully foliated. Measurements were taken on June 2, 2014 and August 11, 2014. In the case of underground growth, measurement was taken once more on the final harvest day, which was October 3, 2014.
2.4. Cross section of leaf tissues
Ginseng leaf sections of 2 mm × 3 mm × 3mm were taken and added to 2.5% glutaraldehyde, and then bubbles including tissue were immediately removed. All processes were carried out at 4°C. For the first holding, washing was done four to five times at intervals of 15 min with 0.1M phosphate buffer (pH 7.2) after 90 min of treatment. In the second holding, treatment was done for 90 min at 4°C with 1% osmium tetroxide and then washed four to five at intervals of 20 min with 0.1M phosphate buffer (pH 7.2) and then passed one night in the final phosphate buffer. Dehydration was done with 40% ethanol, 60% ethanol, 80% ethanol, 90% ethanol, and 95% ethanol for 5 min each and with 100% ethanol for 5 min, 15 min, and 30 min. After dehydration, samples spent 15 min in a 1:1 mixed solution of propylene oxide and ethanol for easier penetration into epon tissues, and then soaked in pure propylene oxide for 5 min, 15 min, and 30 min. Finally, each was treated for 3 h in 2:1 and 1:1 mixed solutions of propylene oxide and epon to embed in the epon and then spent one night in pure epon. The next day, the epon was changed and treatment was done for 15 min, after which epon + D.M.P. 30 (1.5% addition of epon) was put in a silicon mold with sample sections and heat cured at 60°C for 4 d to make an epon block. Next, 10–15 epon blocks were made per treatment, and three epon blocks were randomly selected from among these. An ultrafine cutter (Ultracut R; Leica Co., Wetzlar, Germany) was used to cut samples to a thickness of 1,500 nm. Distilled water was dropped on a slide glass, and samples were placed on it and dried at 60°C for ≥ 5 h and then stained. For the staining process, manufactured tissue sections were soaked in a 0.5% periodic acid (H5IO6) solution for 30 min and then washed two to three times for 10 min with distilled water, treated for 15 min in Schiff's reagent, treated for 5 min in 1% sodium bisulfite solution, and then washed for 30 min in running water. Samples having completed staining were dried on a 60°C hot plate for ≥ 5 h for permanent preservation, and then Hystomount was dropped and covered with a cover glass and dried again. Hystomount stuck to the cover glass area was then cleanly removed, and an optical microscope (Axioskop 2; Carl Zeiss Co., Germany) was used to examine microscopically at 200 times and record.
2.5. Analysis of ginsenosides
Ginsenoside components such as ginsenoside Rb1, Rb2, Rb3, Rc, Rd, Re, Rf, Rg1, Rg2, and Rh1 were purchased from a chemical company (Chroma Dex Inc., Santa Anna, CA, USA). MeOH (Merck & Co Inc., Darmstadt, Germany) and other GR-grade solvents were used for the quantitative analysis of ginsenosides by an Agilent 1100 series HPLC system (Agilent Technologies, Palo Alto, CA, USA), using Sep-Pak Plus C18 cartridges (Waters Corp., Milford, MA, USA) for the solid phase extraction.
For each treatment, 100 mg of freeze-dried powder sampled from 30 freeze-dried ginseng plants was used for the extraction of ginsenosides. Each sample was suspended in 1 mL of 70% MeOH in a 2-mL microcentrifuge tube, placed in an ultrasonic bath (Powersonic 410; Hwashin Tech., Seoul, Korea), and extracted by sonication for 60 min. For the solid phase extraction, a Sep-Pak Plus C18 cartridge was eluted slowly using 3 mL MeOH for the lower conditioning, and again using 3 mL dd-H2O for the upper conditioning. One milliliter of the extracted solution (70% MeOH extract) was loaded into the cartridge and eluted slowly using 1 mL dd-H2O to remove any sugar soluble materials, then eluted slowly using 2 mL MeOH to extract the ginsenoside components. The final eluent was adjusted to an exact volume of 1 mL and filtered through a membrane filter (with a pore size of 0.45 μm), and then analyzed by HPLC on a Halo RP-Amide (4.6 mm × 150 mm, 2.7 μm at 50°C with a flow rate of 0.5–0.8 mL/min). The optimized LC elution conditions were as follows: 0–1 min, 27% B; 1–6 min, 28% B; 6–10 min, 28% B; 10–30 min, 34% B; 30–33 min, 80% B and 33–35 min, 27% B. The extracts were analyzed with a UV detector at 203 nm.
2.6. Statistical analysis
All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC, USA). The statistical significance of the differences was determined using Duncan's multiple tests and one-way analysis of variance, evaluating significant differences at p < 0.05. All data were at the 5% significance level and were reported as mean ± standard deviation.
3. Results and discussion
3.1. Effect of LTR on photosynthesis and stomatal conductance
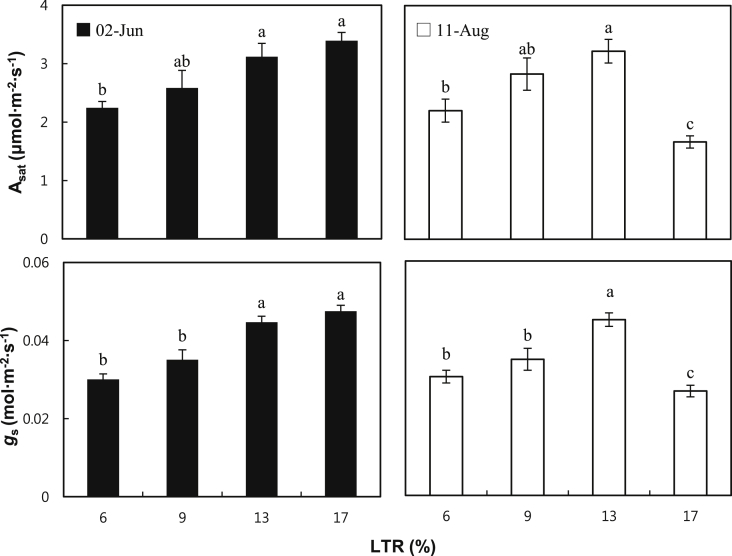
The degree of light transmission could affect the cultivation of ginseng in greenhouses because it directly affects the photosynthesis rate. As photosynthetic parameters, the Asat and gs were measured in P. ginseng grown at 6%, 9%, 13%, and 17% of LTR in the greenhouse on June 2, 2014 and August 11, 2014, as shown in Fig. 2. Both Asat and gs of ginseng grown at the higher LTR were significantly higher than those of the lower LTRs in June, i.e., 17% (3.39 μmol/m2 s) > 13% (3.12 μmol/m2 s) > 9% (2.58 μmol/m2 s) > 6% (2.24 μmol/m2 s) on Asat, and 17% (0.047 mol/m2 s) > 13% (0.045 mol/m2 s) > 9% (0.035 mol/m2 s) > 6% (0.030 mol/m2 s) on gs. However, in August, 2014, both parameters similarly increased until 13% of LRT, but they rapidly decreased at 17% of LTR, i.e., 13% (3.22 μmol/m2 s) > 9% (2.83 μmol/m2 s) > 6% (2.20 μmol/m2 s) > 17% (1.66 μmol/m2 s) on Asat, and 13% (0.045 μmol/m2 s) > 9% (0.035 μmol/m2 s) > 6% (0.030 μmol/m2 s) > 17% (0.027 μmol/m2 s) on gs. The results for the photosynthetic parameters in June indicated that ginseng leaves are more adaptable to sunlight by 17%, and that photosynthesis may occur more readily at high light intensity during the early season when there is no high-temperature stress. These results were found to be in agreement with those of previous studies, i.e., that the Asat and gs of ginseng were higher in the front rows of the beds under a relatively much higher light influx environment than in the rear rows of the beds [14]. When ginseng that has adapted to a low intensity of radiation is exposed to a high intensity of radiation, the photosynthetic parameter is lower. The reason for this is that as the plant grows under conditions of a low intensity of radiation, the size of photosystem II's antenna is bigger than that of the plant growing under a high intensity of radiation [15], [16]. The zeaxanthin in the xanthophyll cycle formation ability also becomes lower and causes the activity of the D1 protein to decrease [17]. Conversely, many researchers reported that the Asat of ginseng rapidly decreased as the LTR increased owing to a reduction of the chlorophyll content [18], [19]. This also seems to be similar to the result obtained in August, 2014 in this study, where ginseng grown at an LTR of 17% was observed to have lower Asat compared with that in other LTR conditions. It is assumed that the reduction in photochemical efficiency in photosystem II in excessively bleached leaves caused by photooxidation reduced the gs and, subsequently, the Asat under an elevated temperature environment during the hot summer season of July and early August [20], because the continuous inflow of too much light causes chronic damage to the photosynthesis system, which decreases quantum efficiency and Asat [21].
Fig. 2.
Light-saturated net photosynthesis rate, Asat, and stomatal conductance, gs, of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of the light transmission rates (LTRs) in a greenhouse on June 2, 2014 and August 11, 2014. The vertical error bars represent the standard errors (n = 5). The same letters at the top of each bar indicate statistically no difference at p < 0.05 using Duncan's multiple range test.
3.2. Effect of LTR on chlorophyll contents
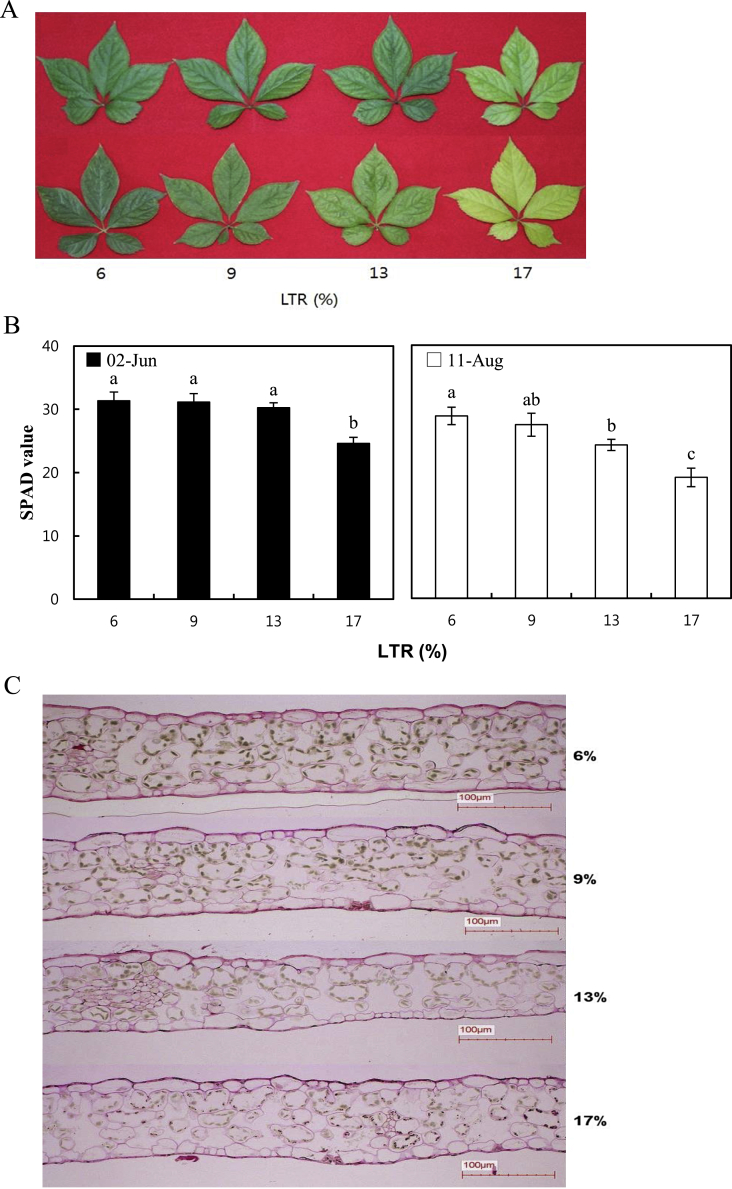
In addition to the decrease in the photosynthetic parameter Asat at 17% of LTR in August, 2014, we observed yellowish leaves, particularly in ginseng grown at higher LTRs, as shown in Fig. 3A. Thus, the contents of chlorophyll in leaves were examined using a chlorophyll meter. The respective SPAD value of ginseng grown at 6%, 9%, 13%, and 17% of LTRs were 31.4, 31.1, 30.3, and 24.6 in June, 2014, and 28.8, 27.4, 24.2, and 19.1 in August, 2014, respectively, indicating a steady decrease in chlorophyll over time during the hot summer season (p = 0.0338; Fig. 3B). The reduction rates between June and August of the SPAD value in ginseng grown at 6%, 9%, 13%, and 17% of LTR were 8.1% (2.54), 11.9% (3.72), 20.0% (6.04), and 22.4% (5.50), respectively, and tended to rise as the LTR increased. Consistently, when the status of chloroplasts in leaves of ginseng grown at 6%, 9%, 13%, and 17% of LTRs were observed using a light microscope, the intensity of the green color in chloroplasts decreased as LTR increased (Fig. 3C).
Fig. 3.
The chlorophyll contents in ginseng grown at the indicated light transmission rate (LTR) in the greenhouse. (A) Leaf color of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of LTRs in a greenhouse. Photos were taken on June 2, 2014 (top) and August 11, 2014 (bottom). (B) SPAD value of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of LTRs in a greenhouse on June 2, 2014 and August 11, 2014. The vertical error bars represent the standard errors (n = 10). The same letters at the top of each bar indicate statistically no difference at p < 0.05 using Duncan's multiple range test. (C) The status of chloroplasts observed by light microscope (× 200). Greenish spots indicate chloroplasts.
In the case of plants growing under a regular sunny condition, the light saturation point exceeds 1,000 μmol/m2 s [22]. By contrast, ginseng has a fairly low amount of intensity of radiation of about 250 μmol/m2 s [10], [11], and photoinhibition takes place, suppressing the electron transport system's (ETS) electron transfer [23]. In the case of a shade plant that has a higher photosystem II/I ratio than a plant growing under a regular sunny condition, a large amount of photon gets absorbed by photosystem II [24]. This causes the ETS to saturate faster, whereas the electrons and energy that did not get used react with O2 to create singlet oxygen (1O2) with a strong acidic toxicity [25]. If one can maintain an adequate amount and decrease the overabsorption of the photon in photosystem II for the ETS of the ginseng to use, one can expect to prevent damages such as pigment bleaching and lipid peroxidation, as well as achieve the maximum root yield. During the early growth stage in June 2014, the SPAD value did not have any significance besides the ginseng grown at 17% LTR; however, when compared to the 17% LTR, the 1O2 creation would be small up to 13% LTR, as the ETS did not saturate. However, the decrease in SPAD value was noticeable in August 2014, when the temperature was higher. The high temperature greatly decreased the ETS activity of the ginseng [14], and the ginseng grown at 13% LTR had a lower SPAD value compared with the one grown at below 9% LTR. In this study, the steady decrease of chlorophyll in ginseng leaves as the LTR increased reaffirmed the similar results obtained in previous studies. Cheon et al [1] reported that the chlorophyll contents of ginseng leaves decreased remarkably according to increases of 5%, 10%, 15%, and 20% under shade, and that ginseng leaves grown at 30% LTR withered because the rate is too high for the plant to endure the breakdown of chlorophylls inside the leaves.
3.3. Effect of LTR on aerial and subterranean growth of ginseng
Previous reports showed different results about ginseng growth depending on LTR, although the growth environment and species of ginseng used in their experiments were different. Cheon et al [1] reported that as the LTR increases up to 30% in the shading, growth of the aerial part continues to decrease, except for the diameter of 2- and 4-yr-old P. ginseng plants, and that underground growth increases up to 20%. By contrast, Proctor et al [26] reported that both the aerial and underground growth of 2-yr-old P. quinquefolius increases as the LTR increases up to 30%. Kim et al [27] reported that the leaves of P. ginseng that grew under a large amount of light in the front row had bigger leaves than the ones in the back row. Because of these discrepancies, growth of aerial and subterranean parts of ginseng was measured separately, depending on different LTRs.
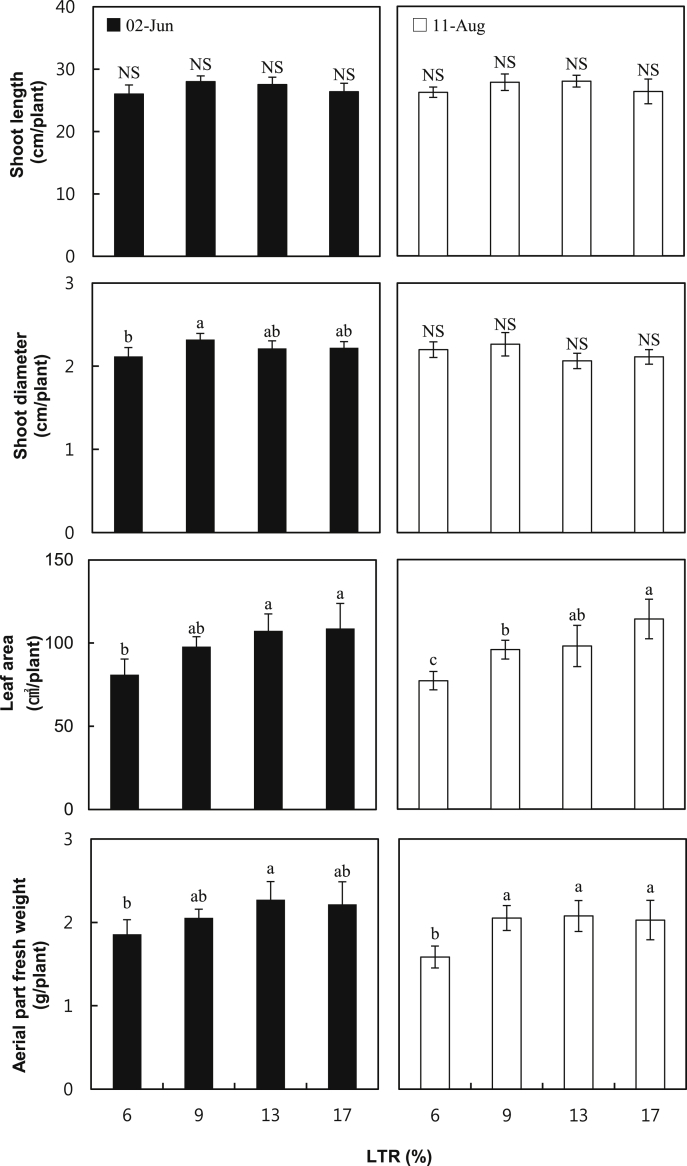
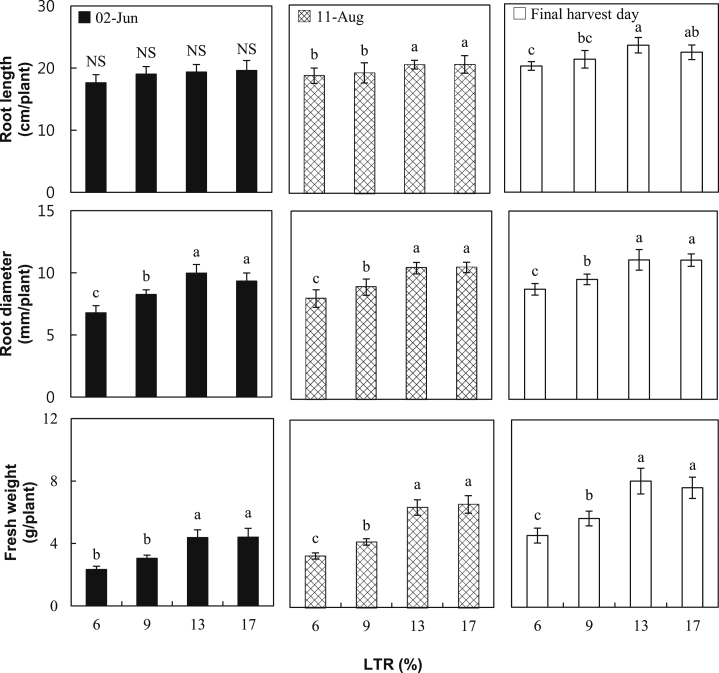
In this study, shoot length, shoot diameter, leaf area, and aerial part fresh weight were measured to examine the aerial part growth of ginseng grown at different LTRs. Shoot length and diameter were not different at all LTRs, whereas the leaf area increased as the LTR increased (Fig. 4). The fresh weight of the aerial part of the ginseng that grew under 6% LTR in our experiment was lower than at other LTRs, and this may be attributable to the difference in the decrease of the leaves' surface area because there were no significant difference between shoot length and stem diameter (Fig. 4). Such a result agrees with the results of other studies that reported that the plants have lower aerial part growth when grown under a low intensity of light [28]. Root length, root diameter, and root fresh weight were also measured to examine the underground growth of ginseng grown at different LTRs. The root length of ginseng grown under 6% LTR was shorter than that of other samples at the final harvest day (Fig. 5). In the case of root diameter and fresh weight, they were bigger or heavier at higher LTRs than at lower LTRs, and they gradually increased by 13% LTR. However, at 17% LTR both were not different from those at 13% LTR. This might indicate that approximately 13% LTR is the optimum condition for both aerial part and root growth of ginseng in a greenhouse. Based on previous studies performed in shading when the average solar radiation intensity was 4.29 kW h/m2/d between April and October during a 28-yr period (from 1982 to 2010) in Korea [1], [6], [10], [18], [29], roughly 10–20% of LTR was assumed to be optimal. Our results could help optimize the LTR level for ginseng growth in a greenhouse.
Fig. 4.
Growth of the aerial part of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of light transmission rates (LTRs) in a greenhouse on June 2, 2014 and August 11, 2014. The vertical error bars represent the standard errors (n = 30). The same letters at the top of each bar indicate statistically no difference at p < 0.05 using Duncan's multiple range test. NS, not significant.
Fig. 5.
Growth of the subterranean part of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of light transmission rates (LTRs) in a greenhouse on June 2, 2014, August 11, 2014, and the final harvest day. The vertical error bars represent the standard errors (n = 30). The same letters at the top of each bar indicate statistically no difference at p < 0.05 using Duncan's multiple range test.
Previous reports showed that early defoliation by leaf chlorosis in ginseng grown at 20% and 30% LTR in conventional shading led to a decrease of 14% and 20%, respectively, than at 10% LTR in the yield of ginseng root [1]. Our results also showed leaf chlorosis and lower photosynthetic rates at 17% LTR, compared to 13% LTR, particularly on August 11, 2014 (Fig. 2, Fig. 3). However, both aerial part and root fresh weights were not significantly decreased at 17% LTR, compared to 13% LTR (Fig. 4, Fig. 5). This could be explained by two ways. First, aerial growth of 2-yr-old ginseng in a greenhouse probably already reaches the maximum or near maximum before leaf chlorosis observed on August 11, 2014 occurs. In fact, the final values of shoot length and fresh weight measured on June 02, 2014 and August 11, 2014 were very similar (Fig. 4). Second, the amount of nutrients provided to roots may be kept stable during the entire growing period. In fact, the values of root diameter or fresh weight were slightly higher on August 11, 2014 compared with those recorded on June 2, 2014, and were highest on the final harvest day (Fig. 5).
3.4. Effect of LTR on ginsenoside contents in ginseng leaves and roots
Ginsenosides are known as one of the main medicinal properties of ginseng and as an indicator of quality in evaluations of ginseng's properties [30]. Moreover, ginsenosides are ginseng's innate defensive substances against stresses that increase steadily under various environmental conditions, including high light levels (30% of solar radiation) [31], [32]. Many factors affecting ginsenoside contents in ginseng have been previously reported [33]. In our study, the effect of LTR on ginsenoside contents in ginseng grown at 6%, 9%, 13%, and 17% of LTR in the greenhouse was examined. For this purpose, 10 major types of ginsenosides—including five panaxadiol line ginsenosides (PD), Rb1, Rb2, Rb3, Rc, and Rd and five panaxatriol line ginsenosides (PT), Re, Rf, Rg1, Rg2, and Rh1—were measured separately.
Changes in the content of ginsenosides followed by light stress were very clear in our experiment. In ginseng leaves, the trend whereby ginsenoside content increases as the LTR increases was more evident in the PT than in the PD line ginsenosides regardless of time (Table 1). Likewise, Lee et al [34] also reported that until LTR increases by 20%, the total ginsenoside content increased significantly, but the increase in the PT line was larger, with the PD/PT eventually decreasing. This may be because when ginseng is exposed to light stress, the protopanaxadiol contained in its leaves is more likely to be converted into protopanaxatriol than into Rd as a defense mechanism. In the early stage of growth, the total ginsenoside content decreased as the fresh weight of the root was heavier, owing to the dilution of the concentrations of storage root ginsenosides caused by the increase in size of the storage root (Table 2). That is, the absolute amounts of ginsenosides in storage roots from plants grown at different LTRs did not differ, and the concentrations of ginsenosides decreased only because the biomass increased. However, after August, when exposure to light stress was relatively higher, all the ginsenoside content in the roots evenly increased as the LTR increased, regardless of root size (Table 2). The reason for this may be that plant defense mechanisms such as jasmonate-response genes might be consistently stimulated by photooxidation stress, causing the ginsenoside content to increase [35], [36]. To prove such results, further studies are needed to confirm the expression level of jasmonate-response genes, PgSS or PgSE genes, in ginseng depending on the various degrees of light stress.
Table 1.
Ginsenoside contents in leaves of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of light transmission rate (LTR) in a greenhouse on June 2, 2014 and August 11, 2014
| Ginsenoside contents in leaves (mg/g leaf dry weight) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTR | Panaxadiol (PD) |
Panaxatriol (PT) |
PD/PT | Total | ||||||||||
| Rb1 | Rb2 | Rb3 | Rc | Rd | Total | Re | Rf | Rg1 | Rg2 | Rh1 | Total | |||
| 2 June | ||||||||||||||
| 6 | 1.13(c)1) | 2.02(c) | 0.26NS | 2.90(c) | 5.87(c) | 12.33NS | 5.44(c) | 0.16(a) | 11.53(b) | 0.38(c) | ND | 17.54(d) | 0.7029 | 29.83(c) |
| 9 | 1.23(b) | 2.23(b) | 0.29 | 3.20(b) | 7.25(a) | 14.35 | 7.54(b) | 0.17(a) | 11.96(b) | 0.42(b) | ND | 20.08(c) | 0.7146 | 34.43(b) |
| 13 | 1.27(b) | 2.42(a) | 0.34 | 3.67(a) | 6.76(b) | 14.63 | 7.29(b) | 0.12(b) | 13.52(a) | 0.52(a) | ND | 21.46(b) | 0.6817 | 36.09(a,b) |
| 17 | 1.41(a) | 2.20(b) | 0.28 | 3.15(b) | 5.89(c) | 14.91 | 9.05(a) | 0.17(a) | 14.07(a) | 0.52(a) | ND | 23.82(a) | 0.6259 | 36.89(a) |
| 11 August | ||||||||||||||
| 6 | 2.83(a) | 5.26(a) | 5.02(c) | 7.89(a) | 14.29(a) | 34.63(a) | 12.95(c) | 0.15(b) | 24.38(c) | 0.82(d) | ND | 37.32(d) | 0.9279 | 73.60(b) |
| 9 | 1.51(c) | 2.86(d) | 5.53(b) | 4.33(d) | 9.99(c) | 24.22(c) | 18.32(a) | 0.19(a) | 22.22(d) | 0.89(c) | ND | 41.61(c) | 0.5821 | 65.83(c) |
| 13 | 2.32(b) | 3.43(c) | 6.86(a) | 5.41(c) | 10.98(b) | 29.00(b) | 16.73(b) | 0.15(b) | 33.12(a) | 1.24(a) | ND | 51.24(a) | 0.5659 | 80.24(a) |
| 17 | 2.76(a) | 4.72(b) | 6.66(a) | 7.14(b) | 13.62(a) | 34.91(a) | 16.84(b) | 0.18(a) | 29.90(b) | 1.14(b) | ND | 48.06(b) | 0.7264 | 82.97(a) |
ND, not detected; NS, not significant
The same letters at the top of each bar indicate statistically no difference at p < 0.05 using Duncan's multiple range test (n = 30)
Table 2.
Ginsenoside contents in roots of 2-yr-old Panax ginseng grown at 6%, 9%, 13%, and 17% of light transmission rate (LTR) in a greenhouse on June 2, 2014, August 11, 2014, and on the final harvest day
| Ginsenoside contents in roots (mg/g root dry weight) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTR | Panaxadiol (PD) |
Panaxatriol (PT) |
PD/PT | Total | ||||||||||
| Rb1 | Rb2 | Rb3 | Rc | Rd | Total | Re | Rf | Rg1 | Rg2 | Rh1 | Total | |||
| 2 June | ||||||||||||||
| 6 | 2.98(a)1) | 0.92(a) | 0.20(a) | 1.84(a) | 1.02NS | 7.07(a) | 2.47(b) | 0.99(c) | 2.91(a) | 0.47(a) | 0.07(b) | 7.02(ab) | 1.0071 | 13.89(a) |
| 9 | 2.99(a) | 1.00(a) | 0.20(a) | 1.88(a) | 0.96 | 7.03(a) | 3.54(a) | 1.30(a) | 2.35(b) | 0.37(b) | 0.12(a) | 7.67(a) | 0.9166 | 14.70(a) |
| 13 | 2.17(b) | 0.75(b) | 0.15(b) | 1.47(b) | 0.75 | 5.30(b) | 2.67(b) | 1.14(b) | 2.17(b,c) | 0.35(bc) | 0.07(b) | 6.40(b) | 0.8281 | 11.70(c) |
| 17 | 2.36(b) | 0.74(b) | 0.15(b) | 1.44(b) | 1.09 | 5.78(b) | 3.48(a) | 1.36(a) | 1.96(c) | 0.31(c) | 0.11(a) | 7.22(a) | 0.8005 | 13.00(b) |
| 11 August | ||||||||||||||
| 6 | 2.88(c) | 0.76(b) | 0.18(c) | 1.79(b) | 0.77(b) | 6.44(c) | 1.84(b) | 0.86(c) | 2.37(c) | 0.37(c) | 0.05NS | 5.49(c) | 1.1730 | 11.88(c) |
| 9 | 3.22(b) | 0.82(b) | 0.19(b,c) | 1.96(b) | 0.74(b) | 6.93(bc) | 3.02(a) | 1.39(a) | 2.43(c) | 0.41(b) | 0.06 | 7.31(b) | 0.9480 | 14.25(b) |
| 13 | 3.35(b) | 0.81(b) | 0.20(b) | 1.95(b) | 0.73(b) | 7.05(b) | 2.74(a) | 1.14(b) | 2.83(b) | 0.44(b) | 0.05 | 7.20(b) | 0.9792 | 14.24(b) |
| 17 | 4.42(a) | 1.05(a) | 0.23(a) | 2.42(a) | 1.03(a) | 9.15(a) | 3.08(a) | 1.42(a) | 3.52(a) | 0.56(a) | 0.05 | 8.64(a) | 1.0590 | 17.79(a) |
| Final harvest | ||||||||||||||
| 6 | 2.61(b)1) | 0.56(c) | 0.15(c) | 1.02(c) | 0.38(c) | 4.73(b) | 2.07(b) | 1.01(c) | 2.86(c) | 0.33(b) | 0.03(b) | 6.31(c) | 0.7496 | 11.03(c) |
| 9 | 3.09(a) | 0.73(b) | 0.18(b) | 1.24(b) | 0.45(b) | 5.69(a) | 1.99(b) | 1.21(b) | 2.97(c) | 0.44(a) | 0.04(a) | 6.65(c) | 0.8556 | 12.34(b) |
| 13 | 2.94(a) | 0.68(b) | 0.19(b) | 1.44(a) | 0.46(b) | 5.71(a) | 2.03(b) | 1.12(b,c) | 3.58(b) | 0.45(a) | 0.03(b) | 7.23(b) | 0.7897 | 12.93(b) |
| 17 | 2.41(b) | 1.03(a) | 0.22(a) | 1.37(a) | 0.62(a) | 5.65(a) | 3.34(a) | 1.34(a) | 5.34(a) | 0.45(a) | 0.02(c) | 10.49(a) | 0.5386 | 16.14(a) |
NS, not significant
The same letters at the top of each bar indicate statistically no difference at p < 0.05 using Duncan's multiple range test (n = 30)
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was carried out with the support of “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01098501),” Rural Development Administration, Republic of Korea.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Cheon S.K., Mok S.K., Lee S.S., Shin D.Y. Effects of light intensity and quality on the growth and quality of Korean ginseng (Panax ginseng C.A. Meyer): I. Effects of light intensity on the growth and yield of ginseng plants. Kor J Ginseng Sci. 1991;15:21–30. [Google Scholar]
- 2.Proctor J.T.A. Source–sink relations in North American ginseng seedlings as influenced by leaflet removal. J Ginseng Res. 2008;32:337–340. [Google Scholar]
- 3.Jochum G.M., Mudge K.W., Thomas R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae) Am J Bot. 2007;94:819–826. doi: 10.3732/ajb.94.5.819. [DOI] [PubMed] [Google Scholar]
- 4.Park Y.H., Lee S.G., Ahn D.J., Kwon T.R., Park S.U., Lim H.S., Bae H. Diversity of fungal endophytes in various tissues of Panax ginseng Meyer cultivated in Korea. J Ginseng Res. 2012;36:211–217. doi: 10.5142/jgr.2012.36.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim Y.C., Lee J.H., Bae Y.S., Sohn B.K., Park S.K. Development of effective environmentally-friendly approaches to control Alternaria blight and anthracnose diseases of Korean ginseng. Eur J Plant Pathol. 2010;127:443–450. [Google Scholar]
- 6.Lee S.S., Kim J.M., Cheon S.K., Kim Y.T. Relationship between environmental conditions and the growth of ginseng plant in field: II. Light intensity under shading material and photosynthesis. Kor J Crop Sci. 1982;27:169–174. [Google Scholar]
- 7.Lee J.S., Lee J.H., Ahn I.O. Characteristics of resistant lines to high-temperature injury in ginseng (Panax ginseng C.A. Meyer) J Ginseng Res. 2010;34:274–281. [Google Scholar]
- 8.Kim D.W., Kim J.Y., You D.H., Kim C.S., Kim H.J., Park J.S., Kim J.M., Choi D.C., Oh N.K. Effect of cultivation using plastic-film house on yield and quality of ginseng in paddy field. Kor J Med Crop Sci. 2014;22:210–216. [Google Scholar]
- 9.Kwon S.G., Lee C.Y., Oh D.J., Li G.Y., Cha S.W., Lee S.W. Changes of growth characteristics and ginsenoside content by growth stages and different planting position in Panax ginseng C.A. Meyer. Kor J Med Crop Sci. 2010;18:51–55. [Google Scholar]
- 10.Hyun D.Y., Hwang J.K., Choi S.Y., Jo J.S. Photosynthetic characteristics of Panax ginseng C.A. Meyer: I. Photosynthetic response to changes of light intensity and leaf temperature. Kor J Ginseng Sci. 1993;17:240–245. [Google Scholar]
- 11.Lee S.S. Characteristics of photosynthesis among new cultivars of ginseng (Panax ginseng C.A. Meyer) J Ginseng Res. 2002;26:85–88. [Google Scholar]
- 12.Lee J.S., Lee D.Y., Lee J.H., Ahn I.O., In J.G. Photosynthetic characteristics of resistance and susceptible lines to high temperature injury in Panax ginseng Meyer. J Ginseng Res. 2012;36:461–468. doi: 10.5142/jgr.2012.36.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markwell J., Osterman J.C., Mitchell J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth Res. 1995;46:467–472. doi: 10.1007/BF00032301. [DOI] [PubMed] [Google Scholar]
- 14.Oh D.J., Lee C.Y., Kim S.M., Li G.Y., Lee S.J., Hwang D.Y., Son H.J., Won J.Y. Effects of chlorophyll fluorescence and photosynthesis characteristics by planting positions and growth stage in Panax ginseng C.A. Meyer. Kor J Med Crop Sci. 2010;18:65–69. [Google Scholar]
- 15.Anderson J.M. Photo-regulation of the composition, function, and structure of thylakoid membranes. Plant Physiol. 1986;37:93–136. [Google Scholar]
- 16.Terashima I. Anatomy of non-uniform leaf photosynthesis. Photosynth Res. 1992;31:195–212. doi: 10.1007/BF00035537. [DOI] [PubMed] [Google Scholar]
- 17.Tyystjarvi E., Koivuniemi A., Kettnen R., Aro E.M. Small light-harvesting antenna does not protect from photo-inhibition. Plant Physiol. 1991;97:477–483. doi: 10.1104/pp.97.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C.H. Effect of light intensity and temperature on the photosynthesis and respiration of Panax spp. Kor J Ginseng Sci. 1988;12:11–29. [Google Scholar]
- 19.Cho J.W., Park H.W., Kim M.J., Kim H.H., Choi J.E. Photosynthetic, morphological and growing characteristics by shading materials in Panax ginseng C.A. Meyer. Kor J Crop Sci. 2008;53:256–260. [Google Scholar]
- 20.Yang D.C., Yoo H.S., Yoon J.J. Investigation on the photo-oxidation of pigment in leaf-burning disease of Panax ginseng: II. Investigation and analysis of physiological reaction mechanism on the chlorophyll bleaching phenomenon. Kor J Ginseng Sci. 1987;11:101–110. [Google Scholar]
- 21.Osmond C.B. What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker N.R., Bowyer J.R., editors. Photoinhibition of photosynthesis: from molecular mechanisms to the field. BIOS Scientific; Oxford: 1994. [Google Scholar]
- 22.Ort D.R., Baker N.R. Consideration of photosynthetic efficiency at low light as a major determinant of crop photosynthetic performance. Plant Physiol Biochem. 1988;26:555–565. [Google Scholar]
- 23.Yang D.C., Chae Q., Lee S.J., Kim Y.H., Kang Y.H. Effects of light and photosynthetic electron transport system on the generation of singlet oxygen (1O2) in ginseng thylakoid membrane. Kor J Ginseng Sci. 1990;14:57–62. [Google Scholar]
- 24.Powles S.B. Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Biol. 1984;35:15–44. [Google Scholar]
- 25.Long S.P., Humphries S., Falkowski P.G. Photoinhibition of photo-synthesis in nature. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:633–662. [Google Scholar]
- 26.Proctor J.T.A., Palmer J.W., Follett J.M. Growth, dry matter partitioning and photosynthesis in North American ginseng seedlings. J Ginseng Res. 2010;34:175–182. [Google Scholar]
- 27.Kim J.M., Lee S.S., Cheon S.R., Cheon S.K. Relationship between environmental conditions and the growth of ginseng plant in field: I. Productive structures as affected by planting positions and ages. Kor J Crop Sci. 1982;27:94–98. [Google Scholar]
- 28.Lichtenthaler H.K., Buschmann C., Doll M., Fietz H.J., Bach T., Kozel U., Meier D., Rahmsdorf U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res. 1981;2:115–141. doi: 10.1007/BF00028752. [DOI] [PubMed] [Google Scholar]
- 29.Jo D.K., Yun C.Y., Kim K.D., Kang Y.H. Estimation of solar radiation distribution in Korea using a satellite. Kor J Sol Energy. 2011;31:99–106. [Google Scholar]
- 30.Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 31.Fournier A.R., Proctor J.T., Gauthier L., Khanizadeh S., Belanger A., Gosselin A., Dorais M. Understory light and root ginsenosides in forest-grown Panax quinquefolius. Phytochemistry. 2003;63:777–782. doi: 10.1016/s0031-9422(03)00346-7. [DOI] [PubMed] [Google Scholar]
- 32.Lee M.K., Park H., Lee C.H. Effect of growth conditions on saponins content and ginsenoside pattern of Panax ginseng. Kor J Ginseng Sci. 1987;11:233–251. [Google Scholar]
- 33.Li T.J., Lian M.L., Yu D., Shao C.H., Piao X.C. Effects of several factors on cell growth and ginsenoside accumulation of Panax ginseng suspension culture. Zhongguo Zhong Yao Za Zhi. 2013;38:4047–4051. [PubMed] [Google Scholar]
- 34.Lee J.C., Choi J.H., Cheon S.K., Lee C.H., Jo J.S. Studies on the optimum light intensity for growth of Panax ginseng: II. Effect of light intensity on the contents of saponins and free sugar in the ginseng leaf. Kor J Ginseng Sci. 1983;28:497–503. [Google Scholar]
- 35.Kim O.T., Bang K.H., Kim Y.C., Hyun D.Y., Kim M.Y., Cha S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tiss Organ Cult. 2009;98:25–33. [Google Scholar]
- 36.Choi D.W., Jung J.D., Ha Y.I., Park H.W., In D.S., Chung H.J., Liu J.R. Analysis of transcripts in methyl jasmonate-treated ginseng hairy roots to identify genes involved in the biosynthesis of ginsenosides and other secondary metabolites. Plant Cell Rep. 2005;23:557–566. doi: 10.1007/s00299-004-0845-4. [DOI] [PubMed] [Google Scholar]