Abstract
Ginseng, a perennial plant belonging to the genus Panax of the Araliaceae family, is well known for its medicinal properties that help alleviate pathological symptoms, promote health, and prevent potential diseases. Among the active ingredients of ginseng are saponins, most of which are glycosides of triterpenoid aglycones. So far, numerous saponins have been reported as components of Panax ginseng, also known as Korean ginseng. Herein, we summarize available information about 112 saponins related to P. ginseng; >80 of them are isolated from raw or processed ginseng, and the others are acid/base hydrolysates, semisynthetic saponins, or metabolites.
Keywords: Araliaceae, dammarane, ginsenoside, Panax ginseng, triterpene
1. Introduction
Ginseng has been one of the most important components in a number of East Asian herbal remedies. In fact, the term ginseng, without any modifier, refers particularly to the species Panax ginseng Meyer or sometimes even more specifically to the root of the plant species. As the name ginseng carries authority and veneration in East Asian medicine, other plants that have some properties in common with P. ginseng have been allegedly called “ginseng”. Eventually, ginseng has become a blanket term that encompasses >10 species of perennial plants belonging to the genus Panax of the family Araliaceae [1], [2]. Currently, 14 plants, including 12 species and two infraspecific taxa, have been recognized as members of the genus Panax, as shown in Table 1 [3]. Some of the Panax plants have common names, which stem from their countries of origin: P. ginseng, Panax japonicus, Panax notoginseng, Panax quinquefolius, and Panax vietnamensis are also called Korean ginseng, Japanese ginseng, Chinese ginseng, American ginseng, and Vietnamese ginseng, respectively. Of the Panax plants, Korean ginseng, Chinese ginseng, and American ginseng have been commercially cultivated; Vietnamese ginseng has recently been introduced for agriculture. Most ginseng species are native to Asia, especially East Asia. Thus, the use of equivocal names, such as Asian ginseng that often refers to P. ginseng, is discouraged.
Table 1.
Scientific and common names of panax plants
| Scientific name | Rank | Common name |
|---|---|---|
| Panax bipinnatifidus Seem | Species | |
| Panax bipinnatifidus var. angustifolius | Infraspecific taxon | |
| Panax bipinnatifidus var. bipinnatifidus | Infraspecific taxon | |
| Panax ginseng C. A. Mey. | Species | Korean ginseng, Ginseng |
| Panax japonicus (T. Nees) C. A. Mey. | Species | Japanese ginseng |
| Panax notoginseng (Burkill) F. H. Chen | Species | Chinese ginseng, sanchi |
| Panax pseudoginseng Wall. | Species | |
| Panax quinquefolius L. | Species | American ginseng |
| Panax sokpayensis Shiva K. Sharma & Pandit | Species | |
| Panax stipuleanatus H. T. Tsai & K. M. Feng | Species | |
| Panax trifolius L. | Species | |
| Panax vietnamensis Ha & Grushv. | Species | Vietnamese ginseng |
| Panax wangianus S. C. Sun | Species | |
| Panax zingiberensis C. Y. Wu & Feng | Species |
While the variety of species renders some pharmacological effects specific to certain species, ginseng, in general, displays restorative, tonic, and revitalizing properties [4]. Thus far, >6,000 articles regarding the traditional uses, chemical constituents, and biological and pharmacological effects of ginseng have been published since Petkov [5] reported the pharmacological properties of P. ginseng extracts in the 1950s. Such pharmacological activities of ginseng have been found to be mainly attributed to ginseng saponins, also known as ginsenosides [6], [7], [8], [9], [10], [11].
Since the first isolation of six ginsenosides from P. ginseng in the 1960s [12], plenty of ginsenosides have been isolated and identified from the species. In this review, we recapitulate the chemical structures, molecular masses, and monoisotopic masses of saponins from various parts of P. ginseng, including roots, flower buds, fruits, and leaves. In addition, we furnish available information about artifactual saponins formed during physicochemical and/or biological treatment and compounds synthesized from saponins isolated from P. ginseng.
2. Classification of ginseng saponins according to their genin structures
Most ginseng saponins are believed to be biosynthesized from 2,3-oxidosqualene, which is also the precursor of β-sitosterol, a steroid commonly found in plants [13]. It has been suggested that the action of three different enzymes on 2,3-oxidosqualene leads to the formation of cycloartenol, dammarenediol-II, and β-amyrin, the latter two of which are eventually biotransformed into ginseng saponins. Fig. 1 shows the proposed biosynthetic pathway of ginseng saponins and β-sitosterol. Dammarenediol-II is the precursor of dammarane-type saponins, including ginsenosides Rb1, Rb2, Re, and Rg1, which account for a significant portion of saponins found in ginseng species. Dammarane-type saponins are further classified into various groups. By contrast, oleanane-type saponins are biosynthesized from β-amyrin. In P. ginseng, however, oleanane-type saponins other than ginsenoside Ro are rare and often practically undetectable.
Fig. 1.
Biosynthetic pathways of ginseng saponins. 2,3-Oxidosqualene may be cyclized into three different compounds, two of which are dammarenediol-II and β-amyrin, the precursor of dammarane-type saponins and oleanane-type saponins, respectively.
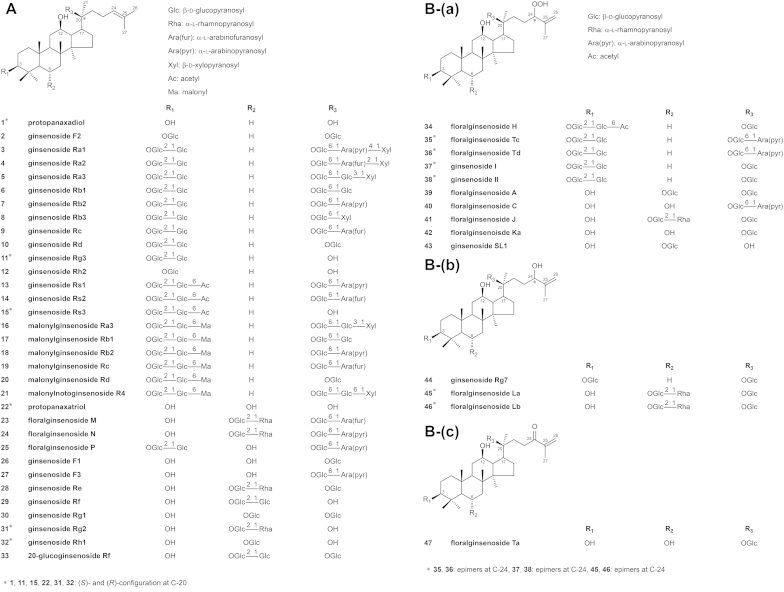
Table 2 [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67] displays the molecular formulas, molecular masses, monoisotopic masses, and parts of ginseng saponins, isolated from or related to P. ginseng, that are used. We categorize the ginseng saponins based upon the position of hydroxyl group(s) and/or double bond(s) of their genins.
Table 2.
Useful information about saponins isolated from p. ginseng, synthetic saponins, and saponin metabolites
| No. | Saponin | Formula | Backbone (Fig. 2) | Molecular mass (u) 1) | Monoisotopic mass (u) 1) | Plant part (process) | Refs |
|---|---|---|---|---|---|---|---|
| Saponins from Panax ginseng | |||||||
| 1 | Protopanaxadiol | C30H52O3 | A | 460.73 | 460.3916 | (Hydrolysis) | [14], [15] |
| 2 | Ginsenoside F2 | C42H72O13 | A | 785.01 | 784.4973 | Leaves | [16] |
| 3 | Ginsenoside Ra1 | C58H98O26 | A | 1,211.38 | 1,210.6346 | Roots | [17] |
| 4 | Ginsenoside Ra2 | C58H98O26 | A | 1,211.38 | 1,210.6346 | Roots | [17] |
| 5 | Ginsenoside Ra3 | C59H100O27 | A | 1,241.41 | 1,240.6452 | Roots | [18] |
| 6 | Ginsenoside Rb1 | C54H92O23 | A | 1,109.29 | 1,108.6029 | Roots | [19], [20], [21], [22] |
| 7 | Ginsenoside Rb2 | C53H90O22 | A | 1,079.27 | 1,078.5924 | Roots | [19], [20], [21] |
| 8 | Ginsenoside Rb3 | C53H90O22 | A | 1,079.27 | 1,078.5924 | Roots | [23] |
| 9 | Ginsenoside Rc | C53H90O22 | A | 1,079.27 | 1,078.5924 | Roots | [19], [20], [21] |
| 10 | Ginsenoside Rd | C48H82O18 | A | 947.15 | 946.5501 | Roots | [19], [20], [21], [24] |
| 11 | Ginsenoside Rg3 | C42H72O13 | A | 785.01 | 784.4973 | Steamed roots | [20], [25] |
| 12 | Ginsenoside Rh2 | C36H62O8 | A | 622.87 | 622.4445 | Steamed roots | [25] |
| 13 | Ginsenoside Rs1 | C55H92O23 | A | 1,121.31 | 1,120.6029 | Roots | [20] |
| 14 | Ginsenoside Rs2 | C55H92O23 | A | 1,121.31 | 1,120.6029 | Roots | [20] |
| 15 | Ginsenoside Rs3 | C44H74O14 | A | 827.05 | 826.5079 | Steamed roots | [26] |
| 16 | Malonylginsenoside Ra3 | C62H102O30 | A | 1,327.46 | 1,326.6456 | Roots | [27] |
| 17 | Malonylginsenoside Rb1 | C57H94O26 | A | 1,195.34 | 1,194.6033 | Roots | [28] |
| 18 | Malonylginsenoside Rb2 | C56H92O25 | A | 1,165.31 | 1,164.5928 | Roots | [28] |
| 19 | Malonylginsenoside Rc | C56H92O25 | A | 1,165.31 | 1,164.5928 | Roots | [28] |
| 20 | Malonylginsenoside Rd | C51H84O21 | A | 1,033.20 | 1,032.5505 | Roots | [28] |
| 21 | Malonylnotoginsenoside R4 | C62H102O30 | A | 1,327.46 | 1,326.6456 | Roots | [29] |
| 22 | Protopanaxatriol | C30H52O4 | A | 476.73 | 476.3866 | (Hydrolysis) | [30] |
| 23 | Floralginsenoside M | C53H90O22 | A | 1,079.27 | 1,078.5924 | Flower buds | [31] |
| 24 | Floralginsenoside N | C53H90O22 | A | 1,079.27 | 1,078.5924 | Flower buds | [31] |
| 25 | Floralginsenoside P | C53H90O23 | A | 1,095.27 | 1,094.5873 | Flower buds | [31] |
| 26 | Ginsenoside F1 | C36H62O9 | A | 638.87 | 638.4394 | Leaves | [16] |
| 27 | Ginsenoside F3 | C41H70O13 | A | 770.99 | 770.4816 | Leaves | [16] |
| 28 | Ginsenoside Re | C48H82O18 | A | 947.15 | 946.5501 | Roots | [20], [21], [24], [32] |
| 29 | Ginsenoside Rf | C42H72O14 | A | 801.01 | 800.4922 | Roots | [20], [32] |
| 30 | Ginsenoside Rg1 | C42H72O14 | A | 801.01 | 800.4922 | Roots | [20], [21], [33] |
| 31 | Ginsenoside Rg2 | C42H72O13 | A | 785.01 | 784.4973 | Roots | [25], [32], [34], [35] |
| 32 | Ginsenoside Rh1 | C36H62O9 | A | 638.87 | 638.4394 | Steamed roots | [21], [25], [34] |
| 33 | 20-Glucoginsenoside Rf | C48H82O19 | A | 963.15 | 962.5450 | Roots | [23] |
| 34 | Floralginsenoside H | C50H84O21 | B-(a) | 1,021.19 | 1,020.5505 | Flower buds | [36] |
| 35 | Floralginsenoside Tc | C53H90O24 | B-(a) | 1,111.27 | 1,110.5822 | Flower buds | [37] |
| 36 | Floralginsenoside Td | C53H90O24 | B-(a) | 1,111.27 | 1,110.5822 | Flower buds | [37] |
| 37 | Ginsenoside I | C48H82O20 | B-(a) | 979.15 | 978.5400 | Flower buds | [38] |
| 38 | Ginsenoside II | C48H82O20 | B-(a) | 979.15 | 978.5400 | Flower buds | [38] |
| 39 | Floralginsenoside A | C42H72O16 | B-(a) | 833.01 | 832.4820 | Flower buds | [39] |
| 40 | Floralginsenoside C | C41H70O15 | B-(a) | 802.99 | 802.4715 | Flower buds | [39] |
| 41 | Floralginsenoside J | C48H82O20 | B-(a) | 979.15 | 978.5400 | Flower buds | [36] |
| 42 | Floralginsenoside Ka | C36H62O11 | B-(a) | 670.87 | 670.4292 | Flower buds | [40] |
| 43 | Ginsenoside SL1 | C36H62O11 | B-(a) | 670.87 | 670.4292 | Steamed leaves | [41] |
| 44 | Ginsenoside Rg7 | C36H60O9 | B-(b) | 636.86 | 636.4237 | Leaves | [42] |
| 45 | Floralginsenoside La | C48H82O19 | B-(b) | 963.15 | 962.5450 | Flower buds | [36] |
| 46 | Floralginsenoside Lb | C48H82O19 | B-(b) | 963.15 | 962.5450 | Flower buds | [36] |
| 47 | Floralginsenoside Ta | C36H60O10 | B-(c) | 652.86 | 652.4187 | Flower buds | [37] |
| 48 | Floralginsenoside E | C42H72O15 | C-(a) | 817.01 | 816.4871 | Flower buds | [39] |
| 49 | Floralginsenoside F | C42H72O15 | C-(a) | 817.01 | 816.4871 | Flower buds | [39] |
| 50 | Floralginsenoside G | C50H84O21 | C-(a) | 1,021.19 | 1,020.5505 | Flower buds | [36] |
| 51 | Floralginsenoside K | C48H82O21 | C-(a) | 995.15 | 994.5349 | Flower buds | [36] |
| 52 | Floralginsenoside O | C53H90O22 | C-(a) | 1,079.27 | 1,078.5924 | Flower buds | [31] |
| 53 | Floralginsenoside B | C42H72O16 | C-(a) | 833.01 | 832.4820 | Flower buds | [39] |
| 54 | Floralginsenoside D | C41H70O15 | C-(a) | 802.99 | 802.4715 | Flower buds | [39] |
| 55 | Floralginsenoside I | C48H82O20 | C-(a) | 979.15 | 978.5400 | Flower buds | [36] |
| 56 | Ginsenoside Rh6 | C36H62O11 | C-(a) | 670.87 | 670.4292 | Leaves | [42] |
| 57 | Ginsenoside ST2 | C36H62O10 | C-(b) | 654.87 | 654.4343 | Steamed leaves | [43] |
| 58 | Ginsenoside Ki | C36H62O10 | C-(c) | 654.87 | 654.4343 | Leaves | [44] |
| 59 | Ginsenoside Km | C36H62O10 | C-(c) | 654.87 | 654.4343 | Leaves | [44] |
| 60 | Floralginsenoside Kb | C45H76O19 | D-(a) | 921.07 | 920.4981 | Flower buds | [40] |
| 61 | Floralginsenoside Kc | C45H76O20 | D-(a) | 937.07 | 936.4930 | Flower buds | [40] |
| 62 | Floralginsenoside Tb | C35H62O11 | D-(b) | 658.86 | 658.4292 | Flower buds | [37] |
| 63 | 25-Hydroxyprotopanaxadiol | C30H54O4 | E | 478.75 | 478.4022 | Fruits | [45] |
| 64 | 25-Hydroxyprotopanaxatriol | C30H54O5 | E | 494.75 | 494.3971 | Fruits | [45] |
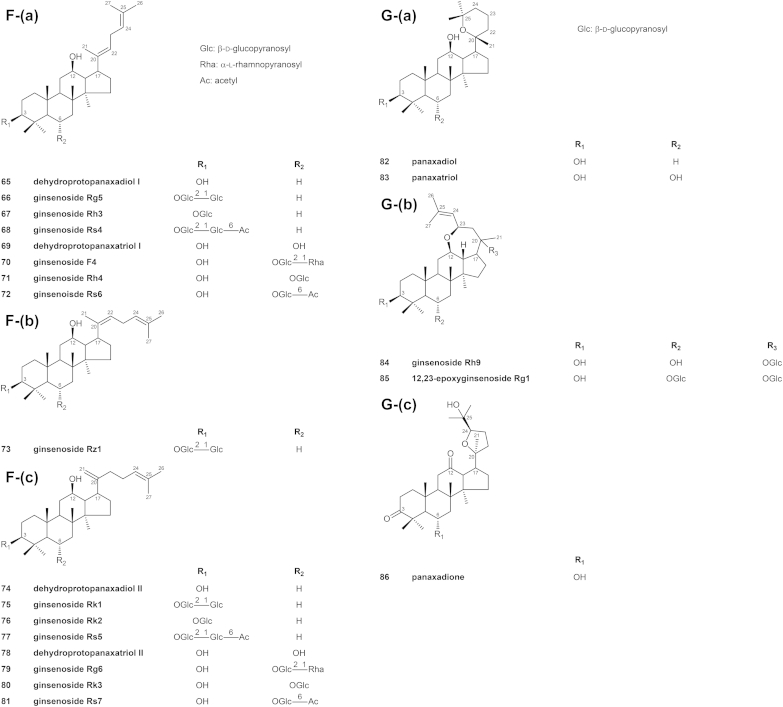
| 65 | Dehydroprotopanaxadiol I | C30H50O2 | F-(a) | 442.72 | 442.3811 | Steamed roots | [46] |
| 66 | Ginsenoside Rg5 | C42H70O12 | F-(a) | 767.00 | 766.4867 | Steamed roots | [47], [48] |
| 67 | Ginsenoside Rh3 | C36H60O7 | F-(a) | 604.86 | 604.4339 | Steamed roots | [47], [49] |
| 68 | Ginsenoside Rs4 | C44H72O13 | F-(a) | 809.03 | 808.4973 | Steamed roots | [46] |
| 69 | Dehydroprotopanaxatriol I | C30H50O3 | F-(a) | 458.72 | 458.3760 | Steamed roots | [46] |
| 70 | Ginsenoside F4 | C42H70O12 | F-(a) | 767.00 | 766.4867 | Leaves | [50] |
| 71 | Ginsenoside Rh4 | C36H60O8 | F-(a) | 620.86 | 620.4288 | Steamed roots | [47], [51] |
| 72 | Ginsenoside Rs6 | C38H62O9 | F-(a) | 662.89 | 662.4394 | Steamed roots | [46] |
| 73 | Ginsenoside Rz1 | C42H70O12 | F-(b) | 767.00 | 766.4867 | Steamed roots | [52] |
| 74 | Dehydroprotopanaxadiol II | C30H50O2 | F-(c) | 442.72 | 442.3811 | Steamed roots | [46] |
| 75 | Ginsenoside Rk1 | C42H70O12 | F-(c) | 767.00 | 766.4867 | Steamed roots | [47] |
| 76 | Ginsenoside Rk2 | C36H60O7 | F-(c) | 604.86 | 604.4339 | Steamed roots | [47] |
| 77 | Ginsenoside Rs5 | C44H72O13 | F-(c) | 809.03 | 808.4973 | Steamed roots | [46] |
| 78 | Dehydroprotopanaxatriol II | C30H50O3 | F-(c) | 458.72 | 458.3760 | Steamed roots | [46] |
| 79 | Ginsenoside Rg6 | C42H70O12 | F-(c) | 767.00 | 766.4867 | Steamed roots | [53] |
| 80 | Ginsenoside Rk3 | C36H60O8 | F-(c) | 620.86 | 620.4288 | Steamed roots | [47] |
| 81 | Ginsenoside Rs7 | C38H62O9 | F-(c) | 662.89 | 662.4394 | Steamed roots | [46] |
| 82 | Panaxadiol | C30H52O3 | G-(a) | 460.73 | 460.3916 | (Hydrolysis) | [54], [55] |
| 83 | Panaxatriol | C30H52O4 | G-(a) | 476.73 | 476.3866 | (Hydrolysis) | [30] |
| 84 | Ginsenoside Rh9 | C36H60O9 | G-(b) | 636.86 | 636.4237 | Leaves | [42] |
| 85 | 12,23-Epoxyginsenoside Rg1 | C42H70O14 | G-(b) | 799.00 | 798.4766 | Leaves | [56] |
| 86 | Panaxadione | C30H48O5 | G-(c) | 488.70 | 488.3502 | Seeds | [57] |
| 87 | Ginsenoside Rh5 | C36H60O9 | H-(a) | 636.86 | 636.4237 | Steamed roots | [42] |
| 88 | Ginsenoside Rh7 | C36H60O9 | H-(b) | 636.86 | 636.4237 | Leaves | [42] |
| 89 | Ginsenoside Rh8 | C36H60O9 | H-(c) | 636.86 | 636.4237 | Leaves | [42] |
| 90 | Ginsenoside Ro | C48H76O19 | H-(d) | 957.11 | 656.4981 | Roots | [19], [22], [58] |
| 91 | Ginsenoside SL2 | C42H70O14 | I-(a) | 799.00 | 798.4766 | Steamed leaves | [41] |
| 92 | Ginsenoside ST1 | C36H60O10 | I-(a) | 652.86 | 652.4187 | Steamed leaves | [43] |
| 93 | Ginsenoside SL3 | C42H70O14 | I-(b) | 799.00 | 798.4766 | Steamed leaves | [41] |
| 94 | Hexanordammaran | C24H40O4 | I-(c) | 392.57 | 392.2927 | Leaves | [59] |
| 95 | Isoprotopanaxadiol | C30H52O3 | I-(d) | 460.73 | 460.3916 | (Hydrolysis) | [60] |
| Synthetic saponins | |||||||
| 96 | Ginsenoside DM1 | C48H84O9 | J-(a) | 805.18 | 804.6115 | (Synthesis) | [61] |
| 97 | Ginsenoside PM1 | C52H92O9 | J-(a) | 861.28 | 860.6741 | (Synthesis) | [61] |
| 98 | Ginsenoside SM1 | C54H96O9 | J-(a) | 889.33 | 888.7054 | (Synthesis) | [61] |
| 99 | C-X1 | C53H90O23 | J-(a) | 1,095.27 | 1,094.5873 | (Synthesis) | [62] |
| 100 | C-Y1 | C53H90O23 | J-(a) | 1,095.27 | 1,094.5873 | (Synthesis) | [62] |
| 101 | C-Y2 | C42H72O14 | J-(a) | 801.01 | 800.4922 | (Synthesis) | [62] |
| 102 | Ginsenoside ORh1 | C44H76O10 | J-(a) | 765.07 | 764.5439 | (Synthesis) | [63] |
| 103 | Ocotillol derivative 3a | C36H62O10 | J-(b) | 654.87 | 654.4343 | (Synthesis) | [64] |
| 104 | Ocotillol derivative 3b | C36H62O10 | J-(b) | 654.87 | 654.4343 | (Synthesis) | [64] |
| 105 | Ginsenoside Rp1 | C42H74O12 | J-(c) | 771.03 | 770.5180 | (Synthesis) | [65] |
| Saponin metabolites | |||||||
| 106 | M1 (Compound K) | C36H62O8 | K | 622.87 | 622.4445 | (Metabolization) | [66] |
| 107 | M2 (Compound Y) | C41H70O12 | K | 754.99 | 754.4867 | (Metabolization) | [66] |
| 108 | M3 (Ginsenoside Mc) | C41H70O12 | K | 754.99 | 754.4867 | (Metabolization) | [66] |
| 109 | M6 | C47H80O17 | K | 917.13 | 916.5396 | (Metabolization) | [66] |
| 110 | M7 (Ginsenoside Mb) | C47H80O17 | K | 917.13 | 916.5396 | (Metabolization) | [66] |
| 111 | M9 (Gp-LXXV) | C48H82O18 | K | 947.15 | 946.5501 | (Metabolization) | [66] |
| 112 | M13 (Gp-XVII) | C42H72O13 | K | 785.01 | 784.4973 | (Metabolization) | [66] |
The calculations are based upon the latest atomic mass data from the International Union of Pure and Applied Chemistry (IUPAC) [67].
2.1. Protopanaxadiol, protopanaxatriol, and their glycosides
As shown in Fig. 1, dammarenediol-II is hydroxylated to protopanaxadiol (PPD), 3β,12β,20-trihydroxydammar-24-ene. Ultimately, a number of saponins are biosynthesized by O-glycosylation of PPD that involves the attachment of saccharide(s) to C-3 and/or C-20. Typical PPD-type saponins include ginsenosides Rb1, Rb2, Rc, and Rd, which are found in the roots [19], [20], flower buds [21], and leaves [21] of P. ginseng.
PPD may further be hydroxylated to protopanaxatriol (PPT), 3β,6α,12β,20-tetrahydroxydammar-24-ene. A variety of saponins are biosynthesized by O-glycosylation of PPT that involves the attachment of saccharide(s) to C-6 and/or C-20. Typically, the hydroxyl group at C-3 remains free in PPT-type ginsenosides. The two most abundant PPT-type saponins in P. ginseng are ginsenosides Re and Rg1.
Fig. 2A illustrates the structures of PPD- and PPT-type saponins. While most naturally occurring ginsenosides are of the (S)-configuration at C-20, some artifactual ginsenosides exist in two epimeric forms at the carbon.
Fig. 2.
Structures of ginseng saponins. A. Structures of ginseng saponins whose genin is 3β,12β,20-trihydroxydammar-24-ene (protopanaxadiol)/3β,6α,12β,20-tetrahydroxydammar-24-ene (protopanaxatriol); B. Structures of ginseng saponins whose genin is (a) 3β,12β,20-trihydroxy-24-hydroperoxydammar-25-ene/3β,6α,12β,20-tetrahydroxy-24-hydroperoxydammar-25-ene; (b) 3β,12β,20,24-tetrahydroxydammar-25-ene/3β,6α,12β,20,24-pentahydroxydammar-25-ene; (c) 3β,6α,12β,20-tetrahydroxydammar-24-one-25-ene; C. Structures of ginseng saponins whose genin is (a) (E)-3β,12β,20-trihydroxy-25-hydroperoxydammar-23-ene/(E)-3β,6α,12β,20-tetrahydroxy-25-hydroperoxydammar-23-ene; (b) (E)-3β,6α,12β,20,25-pentahydroxydammar-23-ene; (c) 3β,6α,12β,26-tetrahydroxydammar-24-ene/3β,6α,12β,27-tetrahydroxydammar-24-ene; D. Structures of ginseng saponins whose genin is (a) 3β,12β,20-trihydroxy-25,26,27-trinordammar-24-al/3β,12β,20,23-tetrahydroxy-25,26,27-trinordammar-24-al; (b) 3β,6α,12β,20-tetrahydroxy-24,24-dimethoxy-25,26,27-trinordammarane; E. Structures of ginseng saponins whose genin is 3β,12β,20,25-tetrahydroxydammarane/3β,6α,12β,20,25-pentahydroxydammarane; F. Structures of ginseng saponins whose genin is (a) (E)-3β,12β-dihydroxydammar-20(22),24-diene/(E)-3β,6α,12β-trihydroxydammar-20(22),24-diene; (b) (Z)-3β,12β-dihydroxydammar-20(22),24-diene; (c) 3β,12β-dihydroxydammar-20(21),24-diene/3β,6α,12β-trihydroxydammar-20(21),24-diene; G. Structures of ginseng saponins whose genin is (a) 3β,12β-dihydroxy-20,25-epoxydammarane (panaxadiol)/3β,6α,12β-trihydroxy-20,25-epoxydammarane (panaxatriol); (b) 3β,6α,20-trihydroxy-12,23-epoxydammar-24-ene; (3) 6α,25-dihydroxy-20,24-epoxydammar-3,12-dione; H. Structure of a ginseng saponin whose genin is (a) 3β,6α,12β,24-tetrahydroxydammar-20(22),25-diene; (b) 3β,7β,12β,20-tetrahydroxydammar-5,24-diene; (c) 3β,6α,20-trihydroxydammar-12-one-24-ene; (d) oleanolic acid; I. Structures of ginseng saponins whose genin is (a) 3β,6α,12β-trihydroxy-24-hydroperoxydammar-20(22),25-diene; (b) 3β,6α,12β-trihydroxy-23-hydroperoxydammar-20(21),24-diene; (c) 3β,6α,12β-trihydroxy-22,23,24,25,26,27-hexanordammar-20-one; (d) (E)-3β,12β,25-trihydroxydammar-20(22)-ene; J. Structures of synthesized saponins whose genin is (a) 3β,12β,20-trihydroxydammar-24-ene (protopanaxadiol)/3β,6α,12β,20-tetrahydroxydammar-24-ene (protopanaxatriol); (b) 3β,12β,25-trihydroxy-20,24-epoxydammarane/3β,6α,12β,25-tetrahydroxy-20,24-epoxydammarane; (c) 3β,12β-dihydroxydammarane; K. Structures of ginseng saponin metabolites whose genin is 3β,12β,20-trihydroxydammar-24-ene (protopanaxadiol)/3β,6α,12β,20-tetrahydroxydammar-24-ene (protopanaxatriol).
2.2. Peroxidation products of PPD- and PPT-type saponins
Some saponins isolated from the flower buds of P. ginseng have an aglycone that is believed to be produced via the peroxidation of PPD or PPT [68]. In most cases, the peroxidation occurs at or around the double bond between C-24 and C-25, and eventually leads to various structures. Fig. 2B, C show the structures of ginsenosides whose genin appears to be produced via the peroxidation of PPD or PPT. Fig. 2B-(a) shows the structures of some saponins that have a hydroperoxyl group at C-24 and a double bond between C-25 and C-26. Fig. 2B-(b) contains the genin structure that has a hydroxyl group at C-24, which would be reduced from the hydroperoxyl group shown in Fig. 2B-(a). In addition, Fig. 2B-(c) shows the structure of floralginsenoside Ta, a glycoside of 3β,6α,12β,20-tetrahydroxydammar-24-one-25-ene, which may be considered to be formed by the dehydration of floralginsenoside Ka, whose structure is illustrated in Fig. 2B-(a).
In a similar fashion, Fig. 2C-(a,b) show the structures of ginseng saponins whose genin has a hydroperoxyl group and a hydroxyl group, respectively, at C-25 and a double bond between C-23 and C-24. While geometric isomerism is possible in compounds with a double bond between C-23 and C-24, most of those reported are the (E)-form isomers rather than the (Z)-form. In addition, Fig. 2C-(c) illustrates the structures of ginsenosides whose genin has a hydroxyl group either at C-26 or at C-27, which would be reduced from the hydroperoxyl group formed around the double bond between C-24 and C-25.
2.3. Cleavage products of PPD- and PPT-type saponins
The oxidative cleavage of the double bond of some saponins yields an aldehyde with three fewer carbon atoms, that is, 3β,12β,20-trihydroxy-25,26,27-trinordammar-24-al, and its derivatives, which are found mainly in the flower buds of ginseng.
Fig. 2D-(a) shows the structures of ginsenosides whose genin is considered to be formed by the oxidative cleavage of the double bond of PPD or 23-hydroxyprotopanaxadiol. Fig. 2D-(b) shows the structure of floralginsenoside Tb, whose genin is an acetal of 3β,6α,12β,20-tetrahydroxy-25,26,27-trinordammar-24-al, which appears to be formed from PPT.
2.4. Hydration and dehydration products of PPD- and PPT-type saponins
The hydration of the double bond of PPD or PPT yields a dammarane derivative with a hydroxyl group at C-25 and no double bond. Fig. 2E illustrates the structures of the saponins 25-hydroxyprotopanaxadiol and 25-hydroxyprotopanaxatriol.
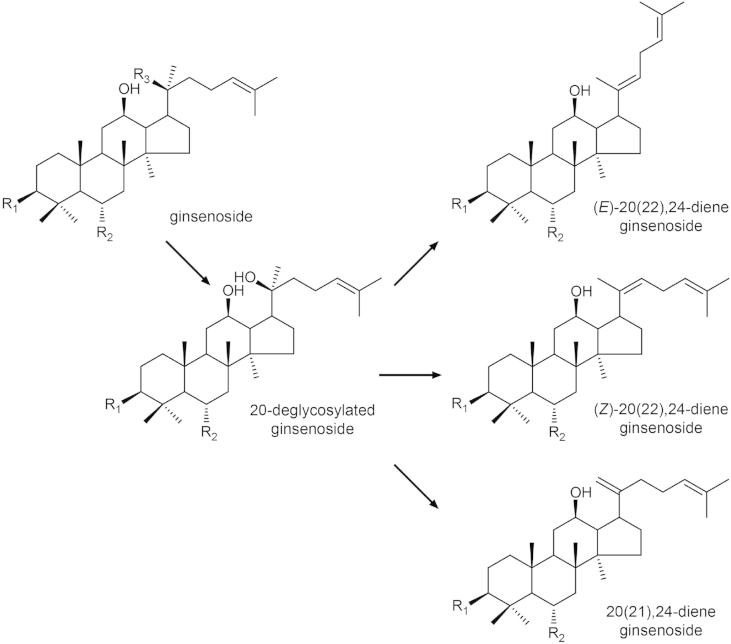
Most PPD- and PPT-type ginsenosides tend to be deglycosylated and dehydrated at C-20 when steamed or heat processed. The resultant double bond is formed either between C-20 and C-21 or between C-20 and C-22. In the latter case, the (E)/(Z) geometric isomerism exists. Fig. 3 illustrates the probable pathways of the formation of artifactual saponins owing to heating. Fig. 2F shows the structures of saponins that are considered to be the dehydration products of the PPD- and PPT-type saponins shown in Fig. 2A.
Fig. 3.
Probable pathways of the formation of artifactual saponins owing to heating. Deglycosylation and dehydration may occur at C-20 when a PPD- or PPT-type ginsenoside is steamed or heat-processed. The resultant double bond is formed either between C-20 and C-21 or between C-20 and C-22, leading to positional and geometric isomerism. PPD, protopanaxadiol; PPT, protopanaxatriol.
2.5. Saponins with an epoxy group
The acid hydrolysis of a PPD-type and a PPT-type saponin leads to the formation of a six-membered ring containing oxygen, yielding panaxadiol, 3β,12β-dihydroxy-20,25-epoxydammarane, and panaxatriol, 3β,6α,12β-trihydroxy-20,25-epoxydammarane, respectively. Fig. 2G-(a) shows the structures of panaxadiol and panaxatriol. Moreover, some saponins are derivatives of 3β,6α,20-dihydroxy-12,23-epoxydammar-24-ene or 6α,25-dihydroxy-20,24-epoxydammar-3,12-dione. Fig. 2G-(b,c) show the structures of saponins with an epoxy group between C-12 and C-23 and between C-20 and C-24, respectively.
2.6. Saponins isolated from P. ginseng with other aglycones
The genins of some saponins isolated from P. ginseng are different from those aforementioned. Fig. 2H, I illustrate the structures of ginsenosides with other backbones.
2.7. Synthetic saponins
Synthetic compounds whose structures are related to saponins isolated from P. ginseng have been reported. In most cases, derivatives of dammarane are synthesized from isolated ginsenosides to enhance biological activity. Indeed, several acylated saponins have been found to have antitumor activity [61], [63]. In addition, some derivatives of ocotillol have shown myocardial ischemia protective effect [64]. Fig. 2J illustrates the structures of some synthetic saponins.
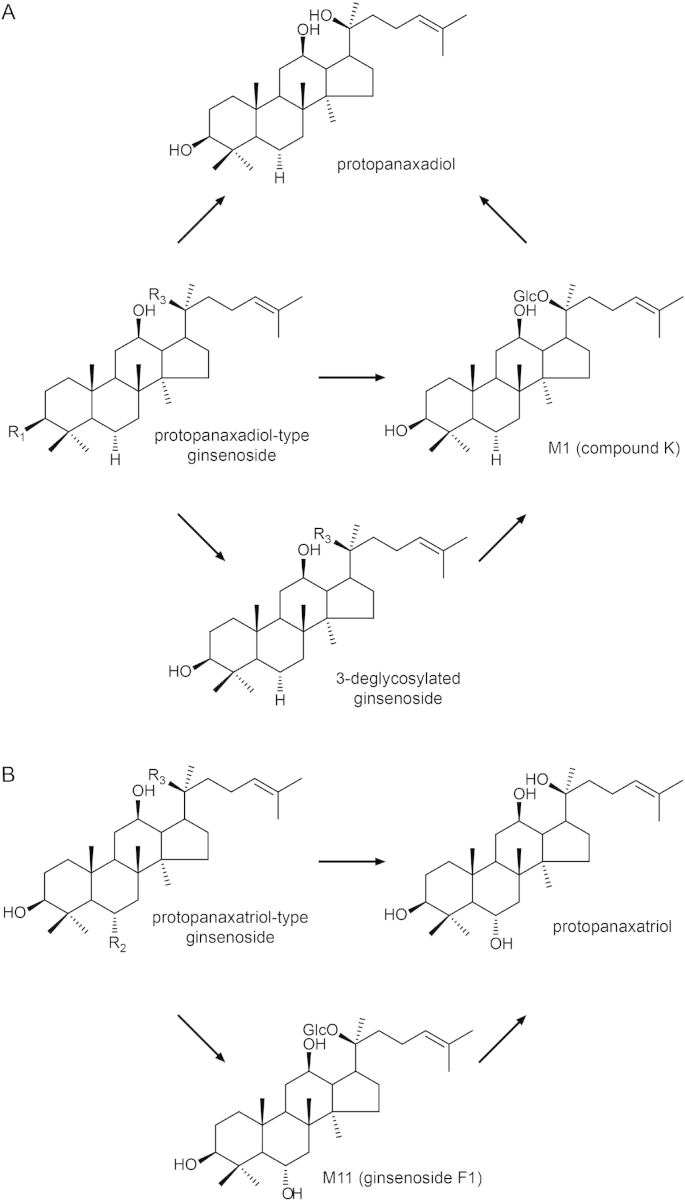
2.8. Saponin metabolites
Most ginsenosides are metabolized by intestinal bacteria. Fig. 4 shows the suggested metabolic pathways of PPD- and PPT-type ginsenosides. The former is deglycosylated at C-3 and transformed to either M1 (compound K) or PPD. By contrast, the latter is deglycosylated at C-6 and/or C-20, and eventually transformed to PPT. Fig. 2K shows the structures of the saponin metabolites that have not been reported as being present in raw or processed ginseng.
Fig. 4.
Suggested metabolic pathways of PPD- and PPT-type ginsenosides. PPD-type ginsenosides tend to be deglycosylated at C-3, and M1 (compound K) may result. PPT-type ginsenosides are deglycosylated at C-6 and/or C-20. PPD, protopanaxadiol; PPT, protopanaxatriol.
3. Concluding remarks
Ginseng is well known for its beneficial biological effects on the human body. While the plant contains various ingredients, ginsenosides play a more significant role in exerting pharmacological actions than any other constituents. Of the great number of ginsenosides present in P. ginseng, fewer than 10 account for most ginsenoside contents. In particular, ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1 are most abundant in the roots of raw ginseng. Intriguingly, chemical reactions during the processing of ginseng, such as oxidation, hydrolysis, and/or dehydration, lead to the formation of artifactual compounds, which often have enhanced biological activities. Besides, orally administered ginsenosides undergo biotransformations in the gastrointestinal tract, and some metabolites produced by the action of bacteria have structures different from those of naturally occurring ginsenosides. Here, >100 ginsenosides have been classified according to their structural features.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by the Next-Generation BioGreen 21 Program (PJ008202) from the Rural Development Administration of Korea and the Bio-Synergy Research Project (NRF-2012M3A9C4048796) from the National Research Foundation funded by the Ministry of Science, ICT, and Future Planning.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Yun T.K. Brief introduction of Panax ginseng C. A. Meyer. J Korean Med Sci. 2001;16:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu S.Y. The genus Panax (Ginseng) in Chinese medicine. Econ Bot. 1976;30:11–28. [Google Scholar]
- 3.Roskov Y., Kunze T., Orrell T., Abucay L., Paglinawan L., Culham A., Bailly N., Kirk P., Bourgoin T., Baillargeon G., editors. Species 2000 & ITIS catalogue of life. 2014. www.catalogueoflife.org/annual-checklist/2014 2014 Annual Checklist. Digital resource at. Species 2000: Naturalis, Leiden, the Netherlands. [Google Scholar]
- 4.Hu S.Y. A contribution to our knowledge of ginseng. Am J Chin Med. 1977;5:1–23. doi: 10.1142/s0192415x77000026. [DOI] [PubMed] [Google Scholar]
- 5.Petkov W. Pharmacological studies of the drug P. ginseng C. A. Meyer. Arzneim Forsch. 1959;9:305–311. [PubMed] [Google Scholar]
- 6.Kim S.K., Park J.H. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee D.H., Cho H.J., Kim H.H., Rhee M.H., Ryu J.H., Park H.J. Inhibitory effects of total saponin from Korean red ginseng via vasodilator-stimulated phosphoprotein-Ser157 phosphorylation on thrombin-induced platelet aggregation. J Ginseng Res. 2013;37:176–186. doi: 10.5142/jgr.2013.37.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siddiqi M.H., Siddiqi M.Z., Ahn S.G., Kang S., Kim Y.J., Sathishkumar N., Yang D.U., Yang D.C. Ginseng saponins and the treatment of osteoporosis: mini literature review. J Ginseng Res. 2013;37:261–268. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang K.S., Ham J.Y., Kim Y.J., Park J.H., Cho E.J., Yamabe N. Heat-processed Panax ginseng and diabetic renal damage: active components and action mechanism. J Ginseng Res. 2013;37:379–388. doi: 10.5142/jgr.2013.37.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S., Kim M.G., Ko S.K., Kim H.K., Leem K.H., Kim Y.J. Protective effect of ginsenoside Re on acute gastric mucosal lesion induced by compound 48/80. J Ginseng Res. 2014;38:89–96. doi: 10.1016/j.jgr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elyakov G.B., Strigina L.I., Uvarova N.I., Vaskovsky V.E., Dzizenko A.K., Kochetkov N.K. Glycosides from ginseng roots. Tetrahedron Lett. 1964;5:3591–3597. [Google Scholar]
- 13.Tansakul P., Shibuya M., Kushiro T., Ebizuka Y. Dammarenediol-II synthase, the first dedicated enzyme for ginsenoside biosynthesis, in Panax ginseng. FEBS Lett. 2006;580:5143–5149. doi: 10.1016/j.febslet.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Shibata S., Tanaka O., Sado M., Tsushima S. On genuine sapogenin of ginseng. Tetrahedron Lett. 1963;4:795–800. [Google Scholar]
- 15.Shibata S., Tanaka O., Ando T., Sado M., Tsushima S., Ohsawa T. Chemical studies on oriental plant drugs. XIV. Protopanaxadiol, a genuine sapogenin of ginseng saponins. Chem Pharm Bull. 1966;14:595–600. doi: 10.1248/cpb.14.595. [DOI] [PubMed] [Google Scholar]
- 16.Yahara S., Tanaka O., Komori T. Saponins of the leaves of Panax ginseng C. A. Meyer. Chem Pharm Bull. 1976;24:2204–2208. [Google Scholar]
- 17.Besso H., Kasai R., Saruwatari Y., Fuwa T., Tanaka O. Ginsenoside-Ra1 and ginsenoside-Ra2, new dammarane-saponins of ginseng roots. Chem Pharm Bull. 1982;30:2380–2385. [Google Scholar]
- 18.Matsuura H., Kasai R., Tanaka O., Saruwatari Y., Kunihiro K., Fuwa T. Further studies on dammarane-saponins of ginseng roots. Chem Pharm Bull. 1984;32:1188–1192. [Google Scholar]
- 19.Sanada S., Kondo N., Shoji J., Tanaka O., Shibata S. Studies on the saponins of ginseng. I. Structures of ginsenoside-Ro, -Rb1, -Rb2, -Rc and -Rd. Chem Pharm Bull. 1974;22:421–428. [Google Scholar]
- 20.Kasai R., Besso H., Tanaka O., Saruwatari Y., Fuwa T. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–2125. [Google Scholar]
- 21.Yahara S., Kaji K., Tanaka O. Further study on dammarane-type saponins of roots, leaves, flower-buds, and fruits of Panax ginseng C. A. Meyer. Chem Pharm Bull. 1979;27:88–92. [Google Scholar]
- 22.Kondo N., Shoji J., Tanaka O. Studies on the constituents of Himalayan ginseng, Panax pseudoginseng. I. The structures of the saponins. (1) Chem Pharm Bull. 1973;21:2705–2711. doi: 10.1248/cpb.23.3282. [DOI] [PubMed] [Google Scholar]
- 23.Sanada S., Shoji J. Studies on the saponins of ginseng. III. Structures of ginsenoside-Rb3 and 20-glucoginsenoside-Rf. Chem Pharm Bull. 1978;26:1694–1697. [Google Scholar]
- 24.Yahara S., Matsuura K., Kasai R., Tanaka O. Saponins of buds and flowers of Panax ginseng C. A. Meyer. (1). Isolation of ginsenosides-Rd, -Re, and -Rg1. Chem Pharm Bull. 1976;24:3212–3213. [Google Scholar]
- 25.Kitagawa I., Yoshikawa M., Yoshihara M., Hayashi T., Taniyama T. Chemical studies on crude drug precession. I. On the constituents of Ginseng Radix rubra (1) Yakugaku Zasshi. 1983;103:612–622. [PubMed] [Google Scholar]
- 26.Baek N.I., Kim J.M., Park J.H., Ryu J.H., Kim D.S., Lee Y.H., Park J.D., Kim S.I. Ginsenoside Rs3, a genuine dammarane-glycoside from Korean red ginseng. Arch Pharm Res. 1997;20:280–282. doi: 10.1007/BF02976158. [DOI] [PubMed] [Google Scholar]
- 27.Ruan C.C., Liu Z., Li X., Liu X., Wang L.J., Pan H.Y., Zheng Y.N., Sun G.Z., Zhang Y.S., Zhang L.X. Isolation and characterization of a new ginsenoside from the fresh root of Panax ginseng. Molecules. 2010;15:2319–2325. doi: 10.3390/molecules15042319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitagawa I., Taniyama T., Hayashi T., Yoshikawa M. Malonyl-ginesnosides Rb1, Rb2, Rc, and Rd, four new malonylated dammarane-type triterpene oligoglycosides from Ginseng Radix. Chem Pharm Bull. 1983;31:3353–3356. [Google Scholar]
- 29.Sun G.Z., Li X.G., Liu Z., Wang J.Y., Zheng Y.N., Yang X.W. Isolation and structure characterization of malonyl-notoginsenoside-R4 from the root of Panax ginseng. Chem J Chin Univ. 2007;28:1316–1318. [Google Scholar]
- 30.Shibata S., Tanaka O., Sôma K., Iida Y., Ando T., Nakamura H. Studies on saponins and sapogenins of ginseng: the structure of panaxatriol. Tetrahedron Lett. 1965;6:207–213. doi: 10.1016/s0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa M., Sugimoto S., Nakamura S., Sakumae H., Matsuda H. Medicinal flowers. XVI. New dammarane-type triterpene tetraglycosides and gastroprotective principles from flower buds of Panax ginseng. Chem Pharm Bull. 2007;55:1034–1038. doi: 10.1248/cpb.55.1034. [DOI] [PubMed] [Google Scholar]
- 32.Sanada S., Kondo N., Shoji J., Tanaka O., Shibata S. Studies on the saponins of ginseng. II. Structures of ginsenoside-Re, -Rf and -Rg2. Chem Pharm Bull. 1974;22:2407–2412. [Google Scholar]
- 33.Iida Y., Tanaka O., Shibata S. Studies on saponins of ginseng: the structure of ginsenoside-Rg1. Tetrahedron Lett. 1968;9:5449–5453. doi: 10.1016/s0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhou J.U.N., Wu M., Taniyasu S., Besso H., Tanaka O., Saruwatari Y., Fuwa T. Dammarane-saponins of sanchi-ginseng, roots of Panax notoginseng (BURK.) F. H. Chen (Araliaceae) : structures of new saponins, notoginsenosides-R1 and -R2, and identification of ginsenosides-Rg2 and -Rh1. Chem Pharm Bull. 1981;29:2844–2850. [Google Scholar]
- 35.Lin T., Kondo N., Shoji J. Studies on the constituents of panacis japonici rhizoma. V. The structures of chikusetsusaponin I, Ia, Ib, IVa and glycoside P1. Chem Pharm Bull. 1976;24:253–261. [Google Scholar]
- 36.Nakamura S., Sugimoto S., Matsuda H., Yoshikawa M. Structures of dammarane-type triterpene triglycosides from the flower buds of Panax ginseng. Heterocycles. 2007;71:577–588. [Google Scholar]
- 37.Nguyen H.T., Song G.Y., Kim J.A., Hyun J.H., Kang H.K., Kim Y.H. Dammarane-type saponins from the flower buds of Panax ginseng and their effects on human leukemia cells. Bioorg Med Chem Lett. 2010;20:309–314. doi: 10.1016/j.bmcl.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 38.Qiu F., Ma Z.Z., Xu S.X., Yao X.S., Che C.T., Chen Y.J. A pair of 24-hydroperoxyl epimeric dammarane saponins from flower-buds of Panax ginseng. J Asian Nat Prod Res. 2001;3:235–240. doi: 10.1080/10286020108041396. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa M., Sugimoto S., Nakamura S., Matsuda H. Medicinal flowers. XI. Structures of new dammarane-type triterpene diglycosides with hydroperoxide group from flower buds of Panax ginseng. Chem Pharm Bull. 2007;55:571–576. doi: 10.1248/cpb.55.571. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen H.T., Song G.Y., Nhiem N.X., Ding Y., Tai B.H., Jin L.G., Lim C.M., Hyun J.W., Park C.J., Kang H.K. Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity. J Agric Food Chem. 2010;58:868–874. doi: 10.1021/jf903334g. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen H.T., Song G.Y., Minh C.V., Kiem P.V., Jin L.G., Boo H.J., Kang H.K., Kim Y.H. Steamed ginseng-leaf components enhance cytotoxic effects on human leukemia HL-60 cells. Chem Pharm Bull. 2010;58:1111–1115. doi: 10.1248/cpb.58.1111. [DOI] [PubMed] [Google Scholar]
- 42.Dou D.Q., Chen Y.J., Liang L.H., Pang F.G., Shimizu N., Takeda T. Six new dammarane-type triterpene saponins from the leaves of Panax ginseng. Chem Pharm Bull. 2001;49:442–446. doi: 10.1248/cpb.49.442. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen H.T., Song G.Y., Kang H.K., Kim Y.H. New dammarane saponins from the steamed ginseng leaves. Bull Korean Chem Soc. 2010;31:2094–2096. [Google Scholar]
- 44.Nguyen H.T., Song G.Y., Park Y.J., Kim Y.H. Two new dammarane-type saponins from the leaves of Panax ginseng. Chem Pharm Bull. 2009;57:1412–1414. doi: 10.1248/cpb.57.1412. [DOI] [PubMed] [Google Scholar]
- 45.Wang W., Zhao Y., Rayburn E.R., Hill D.L., Wang H., Zhang R. In vitro anti-cancer activity and structure–activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- 46.Park I.H., Han S.B., Kim J.M., Piao L.Z., Kwon S.W., Kim N.Y., Kang T.L., Park M.K., Park J.H. Four new acetylated ginsenosides from processed ginseng (sun ginseng) Arch Pharm Res. 2002;25:837–841. doi: 10.1007/BF02977001. [DOI] [PubMed] [Google Scholar]
- 47.Park I.H., Kim N.Y., Han S.B., Kim J.M., Kwon S.W., Kim H.J., Park M.K., Park J.H. Three new dammarane glycosides from heat processed ginseng. Arch Pharm Res. 2002;25:428–432. doi: 10.1007/BF02976595. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.I., Park J.H., Ryu J.H., Park J.D., Lee Y.H., Park J.H., Kim T.H., Kim J.M., Baek N.I. Ginsenoside Rg5, a genuine dammarane glycoside from Korean red ginseng. Arch Pharm Res. 1996;19:551–553. doi: 10.1007/BF02976158. [DOI] [PubMed] [Google Scholar]
- 49.Kim D.S., Baek N.I., Lee Y.H., Park J.D., Kim S.I. Preparation and structure determination of a new glycoside, (20E)-ginsenoside Rh3, and its isomer from diol-type ginseng saponins. Yakhak Hoeji. 1996;39:86–93. [Google Scholar]
- 50.Ryu J.H., Park J.H., Kim T.H., Sohn D.H., Kim J.M., Park J.H. A genuine dammarane glycoside, (20E)-ginsenoside F4 from Korean red ginseng. Arch Pharm Res. 1996;19:335–336. [Google Scholar]
- 51.Baek N.I., Kim D.S., Lee Y.H., Park J.D., Lee C.B., Kim S.I. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 52.Lee S.M., Shon H.J., Choi C.S., Hung T.M., Min B.S., Bae K.H. Ginsenosides from heat processed ginseng. Chem Pharm Bull. 2009;57:92–94. doi: 10.1248/cpb.57.92. [DOI] [PubMed] [Google Scholar]
- 53.Ryu J.H., Park J.H., Eun J.H., Jung J.H., Sohn D.H. A dammarane glycoside from Korean red ginseng. Phytochemistry. 1997;44:931–933. [Google Scholar]
- 54.Shibata S., Fujita M., Itokawa H., Tanaka O., Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XI. Panaxadiol, a sapogenin of ginseng roots. (1) Chem Pharm Bull. 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 55.Shibata S., Tanaka O., Nagai M., Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XII. Panaxadiol, a sapogenin of ginseng roots. (2) Chem Pharm Bull. 1963;11:762–765. doi: 10.1248/cpb.11.762. [DOI] [PubMed] [Google Scholar]
- 56.Wang L.B., Wu Z.H., Gao H.Y., Huang J., Sun B.H., Wu L.J. A new compound with cytotoxic activities from the leaves of Panax ginseng C.A. Meyer. Chin Chem Lett. 2008;19:837–840. [Google Scholar]
- 57.Sugimoto S., Nakamura S., Matsuda H., Kitagawa N., Yoshikawa M. Chemical constituents from seeds of Panax ginseng: structure of new dammarane-type triterpene ketone, panaxadione, and HPLC comparisons of seeds and flesh. Chem Pharm Bull. 2009;57:283–287. doi: 10.1248/cpb.57.283. [DOI] [PubMed] [Google Scholar]
- 58.Kondo N., Marumoto Y., Shoji J. Studies on the constituents of Panacis Japonici Rhizoma. IV. The structure of chikusetsusaponin V. Chem Pharm Bull. 1971;19:1103–1107. [Google Scholar]
- 59.Wu L.J., Wang L.B., Gao H.Y., Wu B., Song X.M., Tang Z.S. A new compound from the leaves of Panax ginseng. Fitoterapia. 2007;78:556–560. doi: 10.1016/j.fitote.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Tao L.N., Meng Q., Yin J.Y., Xing R., Guo H.R. A new panaxadiol from the acid hydrolysate of Panax ginseng. Chin Chem Lett. 2009;20:687–689. [Google Scholar]
- 61.Lei J., Li X., Gong X.J., Zheng Y.N. Isolation, synthesis and structures of cytotoxic ginsenoside derivatives. Molecules. 2007;12:2140–2150. doi: 10.3390/12092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ko S.R., Suzuki Y., Kim Y.H., Choi K.J. Enzymatic synthesis of two ginsenoside Re-β-xylosides. Biosci Biotechnol Biochem. 2001;65:1223–1226. doi: 10.1271/bbb.65.1223. [DOI] [PubMed] [Google Scholar]
- 63.Han M., Hou J.G., Dong C.M., Li W., Yu H.L., Zheng Y.N., Chen L. Isolation, synthesis and structures of ginsenoside derivatives and their anti-tumor bioactivity. Molecules. 2010;15:399–406. doi: 10.3390/molecules15010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yi B., Tian W., Qingguo M., Jiangfen Z., Liang W., Qiang L., Fenglan Z., Haijun S. Synthesis and myocardial ischemia protective effect of ocotillol-type derivatives. Rec Nat Prod. 2012;6:242–254. [Google Scholar]
- 65.Kumar A., Kumar M., Panwar M., Samarth R.M., Park T.Y., Park M.H., Kimura H. Evaluation of chemopreventive action of ginsenoside Rp1. BioFactors. 2006;26:29–43. doi: 10.1002/biof.5520260104. [DOI] [PubMed] [Google Scholar]
- 66.Hasegawa H. Proof of the mysterious efficacy of ginseng: basic and clinical trials: metabolic activation of ginsenoside: deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- 67.Wieser M.E., Holden N., Coplen T.B., Böhlke J.K., Berglund M., Brand W.A., Bièvre P.D., Gröning M., Loss R.D., Meija J. Atomic weights of the elements 2011 (IUPAC Technical Report) Pure Appl Chem. 2013;85:1047–1078. [Google Scholar]
- 68.Niki E., Yoshida Y., Saito Y., Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005;338:668–676. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]