Abstract
Background
Intracellular Ca2+([Ca2+]i) is a platelet aggregation-inducing molecule. Therefore, understanding the inhibitory mechanism of [Ca2+]i mobilization is very important to evaluate the antiplatelet effect of a substance. This study was carried out to understand the Ca2+-antagonistic effect of total saponin from Korean Red Ginseng (KRG-TS).
Methods
We investigated the Ca2+-antagonistic effect of KRG-TS on cyclic nucleotides-associated phosphorylation of inositol 1,4,5-trisphosphate receptor type I (IP3RI) and cyclic adenosine monophosphate (cAMP)-dependent protein kinase (PKA) in thrombin (0.05 U/mL)-stimulated human platelet aggregation.
Results
The inhibition of [Ca2+]i mobilization by KRG-TS was increased by a PKA inhibitor (Rp-8-Br-cAMPS), which was more stronger than the inhibition by a cyclic guanosine monophosphate (cGMP)-dependent protein kinase (PKG) inhibitor (Rp-8-Br-cGMPS). In addition, Rp-8-Br-cAMPS inhibited phosphorylation of PKA catalytic subunit (PKAc) (Thr197) by KRG-TS. The phosphorylation of IP3RI (Ser1756) by KRG-TS was very strongly inhibited by Rp-8-Br-cAMPS compared with that by Rp-8-Br-cGMPS. These results suggest that the inhibitory effect of [Ca2+]i mobilization by KRG-TS is more strongly dependent on a cAMP/PKA pathway than a cGMP/PKG pathway. KRG-TS also inhibited the release of adenosine triphosphate and serotonin. In addition, only G-Rg3 of protopanaxadiol in KRG-TS inhibited thrombin-induced platelet aggregation.
Conclusion
These results strongly indicate that KRG-TS is a potent beneficial compound that inhibits [Ca2+]i mobilization in thrombin–platelet interactions, which may result in the prevention of platelet aggregation-mediated thrombotic disease.
Keywords: Ca2+-mobilization; inositol 1,4,5-trisphosphate receptor type I (Ser1756) phosphorylation; Panax ginseng; protein kinase A catalytic subunit (Thr197) phosphorylation; total saponin from Korean Red Ginseng
1. Introduction
Platelet aggregation is absolutely essential for the formation of a hemostatic plug when normal blood vessels are injured. However, the interactions between platelets and thrombin can also cause circulatory disorders, such as thrombosis, atherosclerosis, and myocardial infarction [1]. Accordingly, inhibition of the platelet–thrombin interaction might be a promising approach for the prevention of thrombosis. It is well-known that thrombin, a platelet agonist, stimulates platelet aggregation by binding to the Gq-coupled proteinase-activated receptor, which is involved in activating phospholipase C-β (PLC-β). The activated PLC-β hydrolyzes phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DG) [2], [3], [4], [5]. Moreover, IP3 mobilizes cytosol-free Ca2+ ([Ca2+]i) from the dense tubular system by binding to the IP3 receptor type I (IP3RI). The increased [Ca2+]i activates both Ca2+/calmodulin-dependent phosphorylation of myosin light chain (20 kDa) and DG-dependent phosphorylation of pleckstrin (47 kDa) to induce granule secretion [adenosine triphosphate (ATP) secretion] and platelet aggregation [6], [7].The Ca2+-antagonistic effects of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are mediated through cAMP- and cGMP-dependent protein kinases (PKA and PKG, respectively), which phosphorylate the substrate protein IP3RI. The phosphorylation of IP3RI involves in inhibition of [Ca2+]i mobilization [8], [9], [10]. Therefore, understanding the mechanism of IP3RI phosphorylation is very useful for evaluating the Ca2+-antagonistic effect of various substances.
Ginseng, the root of Panax ginseng C.A. Meyer, has been used frequently in traditional Oriental medicine, and is known to possess various pharmacological effects such as anti-inflammatory, antioxidation, antitumor, antidiabetes, and antihepatotoxicity [11], [12]. Recently, it has been reported that Korean Red Ginseng (KRG) also has an effect on cardiovascular disease, which is characterized by reduction in blood pressure and arterial stiffness by inhibition of Rho kinase activity [13], anticoagulation by prolonging prothrombin and activated partial thromboplastin times [14], endothelium relaxation by the nitric oxide–cGMP pathway [15], and inhibition of hypercholesterolemia-induced platelet aggregation [16]. In a previous report, it has been demonstrated that total saponin from KRG (KRG-TS) inhibits the production of thromboxane A2 (TXA2) in platelet-mediated thrombotic disease by suppressing the activities of cyclooxygenase-1 (COX-1) and thromboxane A2 synthase (TXAS) [17]. In addition, KRG-TS was reported to be involved in increasing the cAMP level and subsequent reduction of [Ca2+]i mobilization in thrombin-induced rat platelet aggregation [18]. With regard to the effects of ginsenosides on platelet aggregation, it is well-known that ginsenoside Rg3 (G-Rg3) and its chemical derivatives (dihydroxyginsenoside Rg3 and ginsenoside Rp1) exhibit antiplatelet effects by regulating the aggregation-inhibiting molecule (cAMP) and aggregation-stimulating molecules (extracellular kinase 2, tyrosine kinase-dependent phosphoproteins, TXA2, intracellular Ca2+, integrin αIIb/β3, etc.) [19], [20], [21]. In this study, to understand the inhibition of [Ca2+]i mobilization by KRG-TS [18], we investigated whether KRG-TS is involved in phosphorylation of IP3RI. We also examined which cyclic nucleotide of cAMP and cGMP participates in IP3RI phosphorylation to attenuate [Ca2+]i mobilization in thrombin-induced human platelet aggregation. In addition, our results showed that only G-Rg3 of protopanaxadiol in KRG-TS inhibited thrombin-induced platelet aggregation.
2. Materials and methods
2.1. Materials
KRG-TS was obtained from Korea Ginseng Corporation R&D Headquarters (Daejeon, Korea). Thrombin was purchased from Chrono-log Corporation (Havertown, PA, USA). The ATP assay kit was purchased from Biomedical Research Service Center (Buffalo, NY, USA). Serotonin enzyme-linked immunosorbent assay (ELISA) kit was purchased from Labor Diagnostika Nord GmbH & Co. (Nordhorn, Germany). Fura 2-acetoxymethyl (Fura 2-AM) and other reagents were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Antiphospho-IP3RI (Ser1756), antiphospho-PKA catalytic subunit (PKAc; Thr197), antirabbit immunoglobulin G (IgG)–horseradish peroxidase conjugate (HRP), and lysis buffer were obtained from Cell Signaling (Beverly, MA, USA). Polyvinylidene difluoride (PVDF) membrane was obtained from GE Healthcare (Piscataway, NJ, USA). Enhanced chemiluminescence (ECL) solution was purchased from GE Healthcare (Chalfont St, Giles, Buckinghamshire, UK). Protopanaxadiol saponins [PPDSs; G-Ra1, G-Rb1, G-Rb2, G-Rb3, G-Rc, G-Rd, G-Rg3 (20R), G-Rg3 (20S), and G-Rh2 (20R)] and protopanaxatriol saponins [PPTSs; G-Re, G-Rf, G-Rg1, G-Rg2 (20R), and G-Rh1 (20S)] were purchased from Ambo Institute (Daejeon, Korea).
2.2. Preparation of washed human platelets
Human-platelet-rich plasma (PRP) anticoagulated with acid–citrate–dextrose solution (0.8% citric acid, 2.2% sodium citrate, and 2.45% glucose) was obtained from Korean Red Cross Blood Center (Changwon, Korea). The PRP was centrifuged for 10 min at 125g to remove little red blood cells, and was centrifuged for another 10 min at 1,300g to obtain the platelets. The platelets were washed two times with washing buffer (138mM NaCl, 2.7mM KCl, 12mM NaHCO3, 0.36mM NaH2PO4, 5.5mM glucose, and 1mM EDTA; pH 6.5). The washed platelets were then resuspended in suspension buffer (138mM NaCl, 2.7mM KCl, 12mM NaHCO3, 0.36mM NaH2PO4, 0.49mM MgCl2, 5.5mM glucose, and 0.25% gelatin; pH 6.9) to make up a final concentration of 5 × 108/mL. All of the aforementioned procedures were carried out at 25°C to avoid platelet aggregation at low temperatures. The Korea National Institute for Bioethics Policy Public Institutional Review Board (Seoul, Korea) approved these experiments (PIRB12-072).
2.3. Measurement of platelet aggregation
Washed platelet (108/mL) were preincubated for 3 min at 37°C in the presence of 2mM exogenous CaCl2 with or without (KRG-TS, A-kinase/G-kinase inhibitor), and then stimulated with thrombin (0.05 U/mL) for 5 min. Aggregation was monitored using an aggregometer (Chrono-log Corporation) at a constant stirring speed of 1,000 rpm. Each aggregation rate was evaluated as an increase in light transmission. The suspension buffer was used as the reference (transmission 0). KRG-TS was dissolved in the platelet suspension buffer (pH 6.9).
2.4. Determination of cytosolic-free Ca2+
PRP was incubated with 5μM Fura 2-AM at 37°C for 60 min. Because Fura 2-AM is light sensitive, the tube containing the PRP was covered with aluminum foil during loading. The Fura 2-loaded washed platelets were prepared using the aforementioned procedure and 108 platelets/mL were preincubated for 3 min at 37°C with or without KRG-TS in the presence of 2mM CaCl2, and then stimulated with thrombin (0.05 U/mL) for 5 min to evaluate [Ca2+]i. Fura 2 fluorescence was measured using a spectrofluorometer (SFM 25; BioTek Instruments, Milan, Italy) with an excitation wavelength that was changed every 0.5 s from 340 nm to 380 nm. The emission wavelength was set at 510 nm. The [Ca2+]i values were calculated using the method suggested by Schaeffer and Blaustein [22].
2.5. Determination of ATP and serotonin release
The washed platelets (108/mL) were preincubated for 3 min at 37°C with or without (KRG-TS, A-kinase/G-kinase inhibitor, A-kinase/G-kinase activator) in the presence of 2mM CaCl2, and then stimulated with thrombin (0.05 U/mL). The reaction was terminated and the mixture was centrifused with 200xg at 4°C for 10 min. The supernatants were used for the ATP and serotonin release assays. ATP release was measured in a luminometer (GloMax 20/20; Promega, Madison, WI, USA) using an ATP assay kit. Serotonin release was measured with a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, USA) using a serotonin ELISA kit.
2.6. Western blot analysis for evaluation of PKA catalytic subunit and IP3RI phosphorylation
Washed platelets (108/mL) were preincubated with or without KRG-TS in the presence of 2mM CaCl2 for 3 min and then stimulated with thrombin (0.05 U/mL) for 5 min at 37°C. The reactions were terminated by adding an equal volume (250 μL) of lysis buffer (20mM Tris–HCl, 150mM NaCl, 1mM Na2EDTA, 1mM ethylene glycol tetraacetic acid, 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM serine/threonine phosphatase inhibitor β-glycerophosphate, 1mM ATPase, alkaline and acid phosphatase, protein phosphotyrosine phosphatase inhibitor Na3VO4, 1 μg/mL serine and cysteine protease inhibitor leupeptin, and 1mM serine protease and acetylcholinesterase inhibitor phenylmethanesulfonyl fluoride; pH 7.5). Platelet lysates containing the same protein (15 μg) were used for analysis. Protein concentrations were measured using a bicinchoninic acid protein assay kit (Pierce Biotechnology, Waltham, MA, USA). The effects of various substances on PKA catalytic subunit (PKAc) and IP3RI phosphorylation were analyzed by Western blotting. A 6–8% sodium dodecyl sulfate polyacrylamide gel electrophoresis was used for electrophoresis and a PVDF membrane was used for protein transfer from the gel. The dilutions for antiphospho-IP3RI (Ser1756), antiphospho-PKAc (Thr197), and antirabbit IgG–HRP were 1:1,000, 1:1,000, and 1:10,000, respectively. The membranes were visualized using ECL. Blots were analyzed using the Quantity One version 4.5 software (BioRad, Hercules, CA, USA).
2.7. Statistical analyses
The experimental results were expressed as the mean ± standard deviation accompanied by the number of observations. Data were assessed by analysis of variance. If this analysis indicated significant differences among the group means, then each group was compared by the Newman–Keuls method. Statistical analysis was performed using SPSS 21.0.0 (SPSS Inc., Chicago, IL, USA). A p value < 0.05 was taken to be significant.
3. Results
3.1. Effects of KRG-TS on thrombin-induced human platelet aggregation
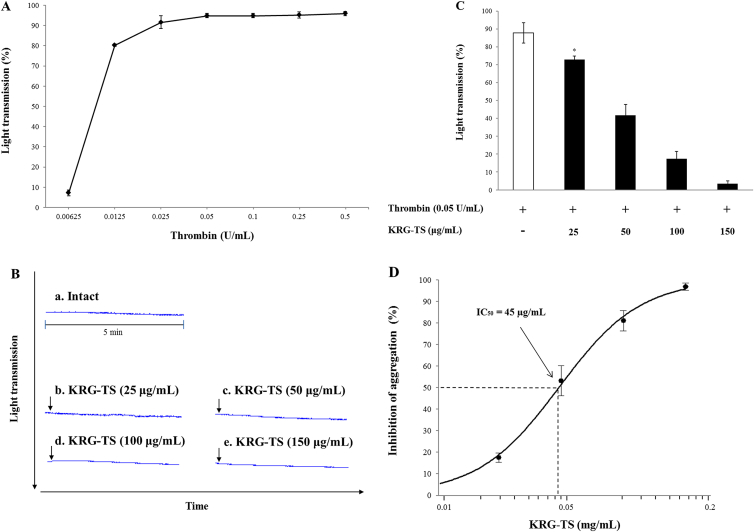
The concentration of thrombin-induced maximal human platelet aggregation was approximately 0.05 U/mL (Fig. 1A). Therefore, 0.05 U/mL thrombin was used as the human platelet agonist in this study. The light transmission in response to various concentrations of KRG-TS in intact platelets was 1.3 ± 0.6% (at 25 μg/mL of KRG-TS), 1.3 ± 0.6% (at 50 μg/mL of KRG-TS), 1.3 ± 0.6% (at 100 μg/mL of KRG-TS), and 1.3 ± 0.6% (at 150 μg/mL of KRG-TS), which was not significantly different from that observed in resting platelets [1.0 ± 0.0%; Fig. 1B(a–e)]. This result indicated that KRG-TS alone did not have an effect on platelet aggregation. When washed human platelets (108/mL) were activated with thrombin, the aggregation rate was increased up to 87.8 ± 5.7%. However, various concentrations of KRG-TS (25–150 μg/mL) significantly reduced thrombin-stimulated platelet aggregation in a dose-dependent manner (Fig. 1C), and its half-maximal inhibitory concentration (IC50) was approximately 45 μg/mL (Fig. 1D). This IC50 is low compared with that (81 μg/ml) by rat platelets [18]. In addition, 150 μg/mL of KRG-TS inhibited up to 95.9% of thrombin-induced human platelet aggregation (87.8 ± 5.7%).
Fig. 1.
Effects of total saponin from Korean Red Ginseng (KRG-TS) on thrombin-induced human platelet aggregation. (A) Concentration threshold of thrombin on human platelet aggregation. (B) Effects of KRG-TS on resting human platelets. (C) Effects of KRG-TS on thrombin-induced human platelet aggregation. (D) Half-maximal inhibitory concentration (IC50) value of KRG-TS in thrombin-stimulated human platelet aggregation. Measurement of platelet aggregation was carried out as described in the “Materials and methods” section. The rate of inhibition by KRG-TS was recorded as the percentage of thrombin-induced aggregation rate. The IC50 value of KRG-TS was calculated according to the four-parameter log-fit method. Data are expressed as the mean ± standard deviation (n = 4). *p < 0.05 versus the thrombin-induced human platelet aggregation.
3.2. Effects of KRG-TS on thrombin-induced human platelet aggregation in the presence of PKA or PKG inhibitor
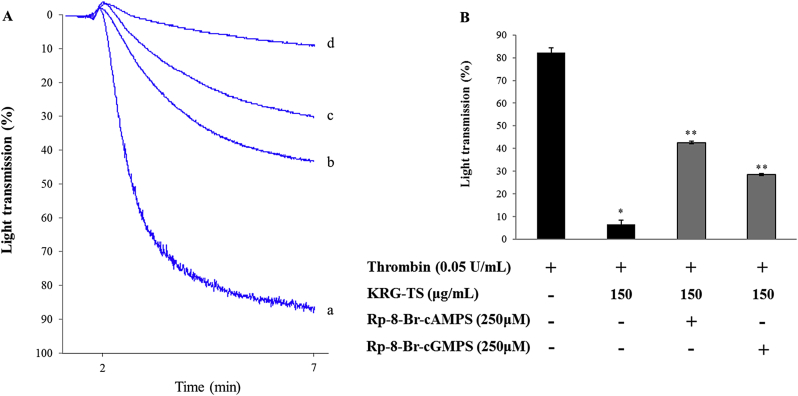
Because [Ca2+]i is essential for platelet aggregation, we investigated the effect of KRG-TS on thrombin-induced platelet aggregation in the presence of PKA or PKG inhibitor, both of which are intracellular Ca2+-antagonistic compounds. As shown in Fig. 2A, thrombin increased light transmission [Fig. 2A(a)], whereas KRG-TS decreased thrombin-induced light transmission [Fig. 2A(d)]. The PKA inhibitor Rp-8-Br-cAMPS [Figs. 2A(b) and 2B] and the PKG inhibitor Rp-8-Br-cGMPS [Figs. 2A(c) and 2B] increased KRG-TS-attenuated light transmission in thrombin-activated platelets. The inhibitory degree [Figs. 2A(b) and 2B] achieved by the PKA inhibitor was more stronger than that achieved by the PKG inhibitor [Figs. 2A(c) and 2B]. These results suggest that the inhibition of thrombin-induced human platelet aggregation by KRG-TS is greatly dependent on cAMP-dependent Ca2+-antagonistic condition than cGMP-dependent Ca2+-antagonistic condition.
Fig. 2.
Effects of total saponin from Korean Red Ginseng (KRG-TS) on thrombin-induced human platelet aggregation in the presence of cyclic adenosine monophosphate-dependent protein kinase (PKA) or cyclic guanosine monophosphate-dependent protein kinase (PKG) inhibitor. (A) Effects of KRG-TS, PKA inhibitor, or PKG inhibitor on thrombin-elevated light transmission: (a) thrombin (0.05 U/mL); (b) thrombin (0.05 U/mL) + KRG-TS (150 μg/mL) + Rp-8-Br-cAMPS (250μM); (c) thrombin (0.05 U/mL) + KRG-TS (150 μg/mL) + Rp-8-Br-cGMPS (250μM); and (d) thrombin (0.05 U/mL) + KRG-TS (150 μg/mL). (B) Effects of KRG-TS, PKA inhibitor, or PKG inhibitor on thrombin-induced human platelet aggregation. Washed human platelets (108/mL) were preincubated with KRG-TS (150 μg/mL) in the presence of PKA inhibitor (Rp-8-Br-cAMPS) or PKG inhibitor (Rp-8-Br-cGMPS) for 3 min at 37°C, and then thrombin (0.05 U/mL) was added. Data are expressed as the mean ± standard deviation (n = 4). *p < 0.05 versus the thrombin-stimulated human platelets. **p < 0.05 versus the thrombin-stimulated platelets in the presence of KRG-TS (150 μg/mL).
3.3. Effects of KRG-TS on [Ca2+]i mobilization
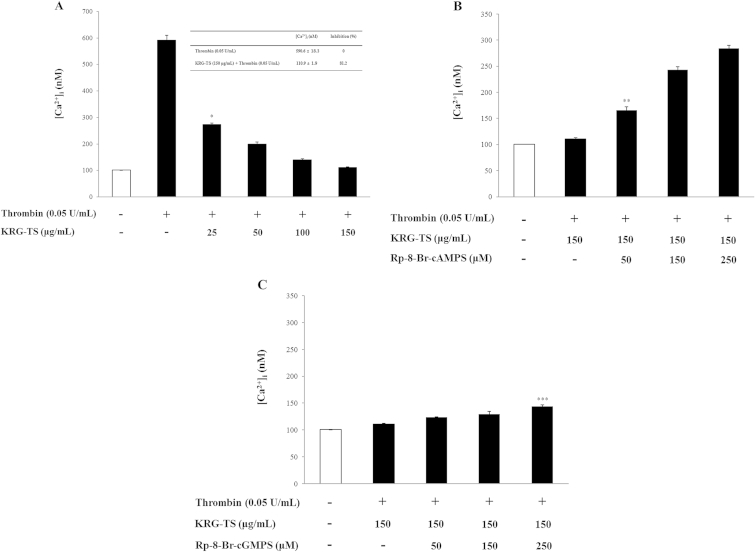
We investigated the effect of KRG-TS on Ca2+-antagonistic activity. As shown in Fig. 3A, thrombin increased the [Ca2+]i level from 101.2 ± 0.5nM (basal level) to 590.6 ± 18.3nM (Fig. 3A, inset table). However, this was significantly decreased by various concentrations (25–150 μg/mL) of KRG-TS in a dose-dependent manner (Fig. 3A). If the inhibition of [Ca2+]i mobilization by KRG-TS results from IP3R phosphorylation through the cAMP/PKA or cGMP/PKG pathway, then the KRG-TS-reduced [Ca2+]i level would be increased by the PKA or PKG inhibitor. Accordingly, we investigated the effects of PKA inhibitor (Rp-8-Br-cAMPS) and PKG inhibitor (Rp-8-Br-cGMPS) on KRG-TS-reduced [Ca2+]i mobilization. As shown in Fig. 3B, the PKA inhibitor Rp-8-Br-cAMPS (50–250μM) dose dependently increased the [Ca2+]i level compared with that (110.9 ± 1.9 nM) by KRG-TS (150 μg/mL) in the thrombin-induced platelet aggregation. By contrast, the PKG inhibitor Rp-8-Br-cGMPS (50–250μM) very weakly increased the [Ca2+]i level compared with that by KRG-TS (150 μg/mL; 110.9 ± 1.9nM) in thrombin-induced platelet aggregation (Fig. 3C). As shown in Table 1, Rp-8-Br-cAMPS (250μM) increased the [Ca2+]i level to 155.1% and Rp-8-Br-cGMPS (250μM) increased the [Ca2+]i level to 29.4%, indicating that the reduction of [Ca2+]i mobilization (Fig. 3A) by KRG-TS may be more dependent on the pathway of cAMP/PKA than cGMP/PKG. We, therefore, investigated the effect of KRG-TS on phosphorylation of PKAc as an indicator of cAMP/PKA activation.
Fig. 3.
Effects of total saponin from Korean Red Ginseng (KRG-TS) on thrombin-induced [Ca2+]i mobilization. (A) Inhibitory effects of KRG-TS on thrombin-induced [Ca2+]i mobilization. (B) Effects of KRG-TS on [Ca2+]i mobilization in the presence of cyclic adenosine monophosphate-dependent protein kinase inhibitor (Rp-8-Br-cAMPS). (C) Effects of KRG-TS on [Ca2+]i mobilization in the presence of cyclic guanosine monophosphate-dependent protein kinase inhibitor (Rp-8-Br-cGMPS). [Ca2+]i was determined as described in the “Materials and methods” section. Data are expressed as the mean ± standard deviation (n = 4). *p < 0.05 versus the thrombin-stimulated human platelets. **p < 0.05 versus the thrombin-stimulated human platelets in the presence of KRG-TS (150 μg/mL). ***p < 0.05 versus the thrombin-stimulated human platelets in the presence of KRG-TS (150 μg/mL).
Table 1.
Effects of Rp-8-Br-cAMPS and Rp-8-Br-cGMPS on [Ca2+]i mobilization
| [Ca2+]i (nM) | Increase (%) | |
|---|---|---|
| KRG-TS (150 μg/mL) + thrombin (0.05 U/mL) | 110.9 ± 1.9 | – |
| KRG-TS (150 μg/mL) + Rp-8-Br-cAMPS (250μM) + thrombin (0.05 U/mL) | 238.6 ± 5.9 | 155.11) |
| KRG-TS (150 μg/mL) + Rp-8-Br-cGMPS (250μM) + thrombin (0.05 U/mL) | 143.6 ± 2.8 | 29.42) |
Data are from Figs. 3B and 3C
KRG-TS, total saponin from Korean Red Ginseng
Increase (%) = [(KRG-TS + thrombin + Rp-8-Br-cAMPS) − (KRG-TS + thrombin)]/(KRG-TS + thrombin) × 100
Increase (%) = [(KRG-TS + thrombin + Rp-8-Br-cGMPS) − (KRG-TS + thrombin)]/(KRG-TS + thrombin) × 100
3.4. Effects of KRG-TS on PKAc (Thr197) phosphorylation
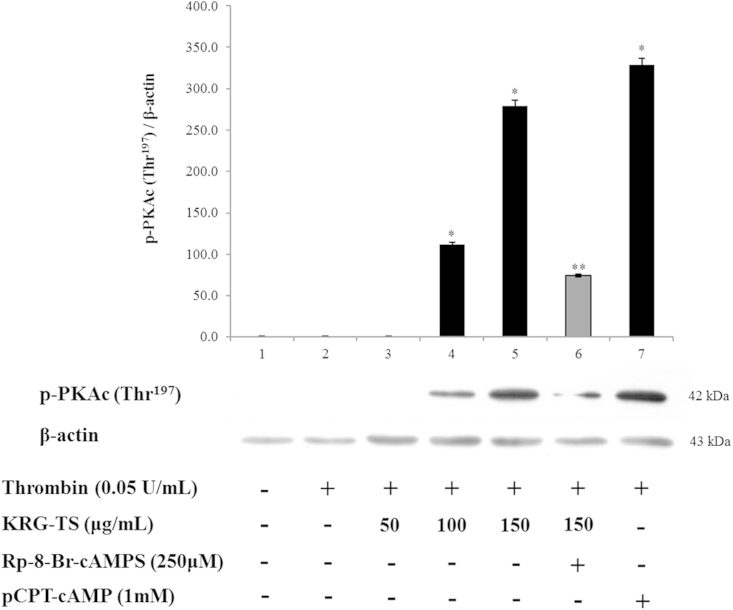
It is well-known that the phosphorylation of PKAc (Thr197) is necessary to produce biological functions through the cAMP/PKA pathway [23]. As shown in Fig. 4, KRG-TS dose dependently phosphorylated Thr197 in PKAc (42 kDa), and abruptly increased the ratio of phosphorylated PKAc (Thr197) to β-actin in thrombin-induced platelet aggregation (Fig. 4, lanes 4 and 5). The PKA inhibitor Rp-8-Br-cAMPS strongly decreased KRG-TS-elevated phosphorylation of PKAc (Thr197; Fig. 4, lane 6), meaning that the phosphorylation of PKAc (Thr197) by KRG-TS depends on the cAMP/PKA pathway. This is also supported by the fact that the PKA activator 8-(4-chlorophenylthio)-cAMP (pCPT-cAMP) increased the phosphorylation of PKAc (Thr197; Fig. 4, lane 7).
Fig. 4.
Effect of total saponin from Korean Red Ginseng (KRG-TS) on cyclic adenosine monophosphate-dependent protein kinase catalytic subunit (PKAc) phosphorylation. Lane 1, intact platelets (base); Lane 2, thrombin (0.05 U/mL); Lane 3, thrombin (0.05 U/mL) + KRG-TS (50 μg/mL); Lane 4, thrombin (0.05 U/mL) + KRG-TS (100 μg/mL); Lane 5, thrombin (0.05 U/mL) + KRG-TS (150 μg/mL); Lane 6, thrombin (0.05 U/mL) + KRG-TS (150 μg/mL) + Rp-8-Br-cAMPS (250μM); and Lane 7, thrombin (0.05 U/mL) + 8-(4-chlorophenylthio)-cyclic adenosine monophosphate (pCPT-cAMP; 1mM). Western blotting was performed as described in the “Materials and methods” section. Data are expressed as the mean ± standard deviation (n = 4). *p < 0.05 versus the thrombin-stimulated human platelets. **p < 0.05 versus the thrombin-stimulated human platelets in the presence of KRG-TS (150 μg/mL).
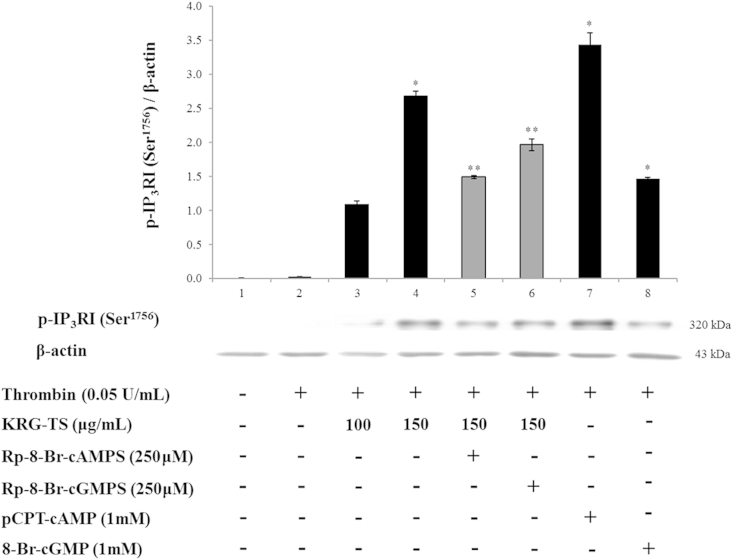
3.5. Effects of KRG-TS on IP3RI (Ser1756) phosphorylation in the presence of PKA and PKG inhibitors
We then investigated the IP3RI phosphorylation-associated PKAc (Thr197) phosphorylation. The phosphorylation of IP3RI (Ser1756) and the ratio of phosphorylated IP3RI (p-IP3RI; Ser1756) to β-actin were increased in the presence of the PKA activator pCPT-cAMP (Fig. 5, lane 7) in thrombin-induced human platelet aggregation, indicating that cAMP/PKA is involved in the phosphorylation of IP3RI (Ser1756). As shown in Fig. 5 (lanes 3 and 4), the phosphorylation of IP3RI (Ser1756) and the ratio of p-IP3RI (Ser1756) to β-actin were dose dependently increased in the presence of both thrombin and KRG-TS. To investigate whether cAMP/PKAc (Thr197) phosphorylation by KRG-TS (Fig. 4, lane 5) is involved in IP3RI (Ser1756) phosphorylation (Fig. 5, lane 4), we used the PKA inhibitor Rp-8-Br-cAMPS (Fig. 4, lane 6) that inhibited KRG-TS-induced PKAc (Thr197) phosphorylation. As shown in Fig. 5 (lane 5), the PKA inhibitor Rp-8-Br-cAMPS potently decreased p-IP3RI (Ser1756) and the ratio of p-IP3RI (Ser1756) to β-actin achieved by both thrombin and KRG-TS. These results indicate that the increase of p-IP3RI (Ser1756) by KRG-TS is dependent on cAMP/PKAc (Thr197). The PKG inhibitor Rp-8-Br-cGMPS also decreased KRG-TS-induced p-IP3RI (Ser1756; Fig. 5, lane 6). However, its inhibitory degree (22.6%) was lower compared with that of the PKA inhibitor Rp-8-Br-cAMPS (44.3%; Table 2), indicating that the increase of p-IP3RI (Ser1756) by KRG-TS is effected through the cAMP/PKA pathway.
Fig. 5.
Effect of total saponin from Korean Red Ginseng (KRG-TS) on inositol 1,4,5-trisphosphate receptor type I (IP3RI) (Ser1756) phosphorylation. Lane 1, intact platelets (base); Lane 2, thrombin (0.05 U/mL); Lane 3, thrombin (0.05 U/mL) + KRG-TS (100 μg/mL); Lane 4, thrombin (0.05 U/mL) + KRG-TS (150 μg/mL); Lane 5, thrombin (0.05 U/mL) + KRG-TS (150 μg/mL) + Rp-8-Br-cAMPS (250μM); Lane 6, thrombin (0.05 U/mL) + KRG-TS (150 μg/mL) + Rp-8-Br-cGMPS (250μM); Lane 7, thrombin (0.05 U/mL) + 8-(4-chlorophenylthio)-cyclic adenosine monophosphate (pCPT-cAMP; 1mM); and Lane 8, thrombin (0.05 U/mL) +8-Br-cGMP (1mM). Western blotting was performed as described in the “Materials and methods” section. Data are expressed as the mean ± standard deviation (n = 4). *p < 0.05 versus the thrombin-stimulated platelets, **p < 0.05 versus the thrombin-stimulated human platelets in the presence of KRG-TS (150 μg/mL).
Table 2.
Changes of p-IP3R to β-actin ratio
| Ratio of p-IP3R to β-actin | Δ (%) | |
|---|---|---|
| Thrombin (0.05 U/mL) | 2.7 ± 0.1 | |
| KRG-TS (150 μg/mL) + thrombin (0.05 U/mL) | 267.6 ± 7.3 | +9811.11) |
| KRG-TS (150 μg/mL) + thrombin (0.05 U/mL) + Rp-8-Br-cAMPS (250μM) | 148.9 ± 2.1 | −44.32) |
| KRG-TS (150 μg/mL) + thrombin (0.05 U/mL) + Rp-8-Br-cGMPS (250μM) | 196.2 ± 8.5 | −26.63) |
Data are from Fig. 5
IP3R, inositol 1,4,5-trisphosphate receptor; KRG-TS, total saponin from Korean Red Ginseng
Δ (%) = [(KRG-TS + thrombin) − thrombin]/thrombin × 100
Δ (%) = [(KRG-TS + thrombin + Rp-8-Br-cAMPS) − (KRG-TS + thrombin)]/(KRG-TS + thrombin) × 100
Δ (%) = [(KRG-TS + thrombin + Rp-8-Br-cGMPS) − (KRG-TS + thrombin)]/(KRG-TS + thrombin) × 100
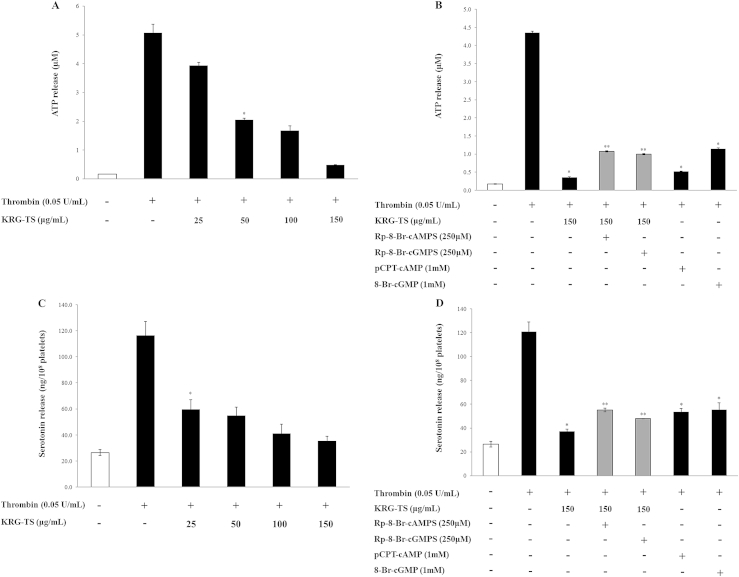
3.6. Effects of KRG-TS on ATP and serotonin release
Intracellular [Ca2+]i mobilization is known to be involved in the release of ATP and serotonin from a dense body of platelets [24]. Because KRG-TS inhibited thrombin-elevated [Ca2+]i mobilization (Fig. 3A), we investigated whether KRG-TS is involved in the inhibition of ATP and serotonin release. As shown in Fig. 6, KRG-TS dose dependently inhibited thrombin-induced ATP (Fig. 6A) and serotonin release (Fig. 6C). Because the inhibition of [Ca2+]i mobilization by KRG-TS was dependent on cAMP/PKA and cGMP/PKG pathways, we investigated whether the inhibition of ATP and serotonin release by KRG-TS resulted from cAMP/PKA and cGMP/PKG pathways. The PKA inhibitor Rp-8-Br-cAMPS (Fig. 6B) and the PKG inhibitor Rp-8-Br-cGMPS (Fig. 6D) increased KRG-TS-decreased ATP and serotonin release.
Fig. 6.
Effects of total saponin from Korean Red Ginseng (KRG-TS) on adenosine triphosphate (ATP) and serotonin release. (A) Effects of KRG-TS on ATP release in thrombin-activated platelets. (B) Effects of KRG-TS on ATP release in the presence of cyclic adenosine monophosphate-dependent protein kinase (PKA) or cyclic guanosine monophosphate-dependent protein kinase (PKG) inhibitor. (C) Effects of KRG-TS on serotonin release. (D) Effects of KRG-TS on serotonin release in the presence of PKA or PKG inhibitor. Data are expressed as mean ± standard deviation (n = 4). *p < 0.05 versus the thrombin-stimulated human platelets. **p < 0.05 versus the thrombin-stimulated human platelets in the presence of KRG-TS (150 μg/mL).
These results indicate that the inhibition of ATP and serotonin release by KRG-TS (Figs. 6A and 6C) is dependent on the cAMP/PKA and cGMP/PKG-pathways. The results are supported by the fact that the PKA activator pCPT-cAMP (Fig. 6B) and the PKG activator 8-Br-cGMP (Fig. 6D) inhibit thrombin-induced ATP and serotonin release.
3.7. Effects of ginsenosides in KRG-TS on thrombin-induced platelet aggregation
In a previous report [18], five ginsenosides (G-Rb1, G-Rb2, G-Rc, G-Rd, and G-Rg3) were reported as 20(S)-PPDS and three ginsenosides (G-Re, G-Rg1, and G-Rg2) as 20(S)-PPTS. Besides these ginsenosides, it is known that G-Ra1, G-Rb3, and G-Rh2 as PPDS and G-Rf and G-Rh1 as PPTS are also present in P. ginseng [25]. Therefore, to investigate which ginsenoside of KRG-TS was involved in the inhibition of thrombin-induced human platelet aggregation, we investigated the effect of ginsenosides in KRG-TS and other ginsenosides on thrombin-induced human platelet aggregation. In this study, G-Ra1, G-Rb1, G-Rb2, G-Rb3, G-Rc, G-Rd, and G-Rh2(20R) of PPDS did not inhibit thrombin-induced human platelet aggregation, but only G-Rg3 (20R, 20S) dose dependently inhibited thrombin-induced human platelet aggregation (Table 3). In addition, none of the PPTSs [G-Re, G-Rf, G-Rg1, G-Rg2 (20R), and G-Rh1 (20S)] inhibited thrombin-induced human platelet aggregation (Table 4).
Table 3.
Effects of protopanaxadiol on thrombin-induced human platelet aggregation
| Treatment (μM) | Aggregation (%) | Treatment (μM) | Aggregation (%) |
|---|---|---|---|
| Thrombin (0.05 U/mL) | 86.7 ± 1.5 | – | – |
| Ginsenoside Ra1 | Ginsenoside Rd | ||
| 50 | 83.3 ± 1.2 | 50 | 85.0 ± 3.0 |
| 10 | 83.0 ± 1.7 | 100 | 84.0 ± 1.7 |
| 200 | 83.0 ± 1.0 | 200 | 86.3 ± 1.5 |
| 300 | 86.7 ± 1.5 | 300 | 84.3 ± 4.0 |
| Ginsenoside Rb1 | Ginsenoside Rg3 (20R) | ||
| 50 | 83.0 ± 1.7 | 50 | 86.7 ± 1.5 |
| 100 | 84.3 ± 0.6 | 100 | 86.0 ± 2.0 |
| 200 | 85.3 ± 3.1 | 200 | 75.7 ± 3.2 |
| 300 | 83.0 ± 3.5 | 300 | 62.0 ± 2.0 |
| Ginsenoside Rb2 | Ginsenoside Rg3 (20S) | ||
| 50 | 86.7 ± 1.5 | 50 | 79.7 ± 2.1 * |
| 100 | 84.7 ± 0.6 | 100 | 39.7 ± 2.1 ** |
| 200 | 86.3 ± 2.1 | 200 | 9.7 ± 2.1 ** |
| 300 | 84.7 ± 0.6 | 300 | 3.7 ± 1.5 ** |
| Ginsenoside Rb3 | Ginsenoside Rh2 (20R) | ||
| 50 | 82.3 ± 3.5 | 50 | 83.3 ± 1.2 |
| 100 | 85.7 ± 1.2 | 100 | 84.7 ± 0.6 |
| 200 | 83.7 ± 1.5 | 200 | 85.3 ± 3.1 |
| 300 | 84.0 ± 3.6 | 300 | 81.0 ± 1.7 |
| Ginsenoside Rc | |||
| 50 | 84.7 ± 3.2 | – | – |
| 100 | 84.7 ± 0.6 | – | – |
| 200 | 86.3 ± 2.1 | – | – |
| 300 | 84.0 ± 2.6 | – | – |
Results are expressed as percentage of aggregation induced by thrombin. The data are expressed as the mean ± standard deviation (n = 4)
*p < 0.05, **p < 0.01 versus the thrombin-stimulated human platelets
Table 4.
Effects of protopanaxatriol on thrombin-induced human platelet aggregation
| Treatment (μM) | Aggregation (%) | Treatment (μM) | Aggregation (%) |
|---|---|---|---|
| Thrombin (0.05 U/mL) | 86.7 ± 1.5 | – | – |
| Ginsenoside Re | Ginsenoside Rg2 (20R) | ||
| 50 | 82.7 ± 1.2 | 50 | 84.0 ± 2.6 |
| 100 | 82.3 ± 1.5 | 100 | 87.0 ± 1.0 |
| 200 | 85.3 ± 2.5 | 200 | 85.3 ± 2.9 |
| 300 | 84.7 ± 0.6 | 300 | 86.0 ± 2.6 |
| Ginsenoside Rf | Ginsenoside Rh1 (20S) | ||
| 50 | 82.7 ± 2.1 | 50 | 84.3 ± 1.5 |
| 100 | 86.7 ± 1.5 | 100 | 83.3 ± 2.1 |
| 200 | 84.7 ± 0.6 | 200 | 82.7 ± 1.2 |
| 300 | 83.0 ± 1.0 | 300 | 85.3 ± 2.5 |
| Ginsenoside Rg1 | |||
| 50 | 84.3 ± 2.1 | – | – |
| 100 | 83.0 ± 1.7 | – | – |
| 200 | 84.7 ± 4.0 | – | – |
| 300 | 85.0 ± 1.0 | – | – |
Results are expressed as percentage of aggregation induced by thrombin. The data are expressed as the mean ± standard deviation (n = 4)
4. Discussion
Although KRG-TS elevated cAMP only in thrombin-induced human platelets [17], to confirm whether the cAMP/PKA or cGMP/PKG pathway contributed to the inhibition of platelet aggregation by KRG-TS, we investigated the effect of PKA inhibitor and PKG inhibitor on thrombin-induced human platelet aggregation in the presence of KRG-TS. The results showed that the PKA inhibitor Rp-8-Br-cAMPS and the PKG inhibitor Rp-8-Br-cGMPS increased KRG-TS-decreased light transmission in thrombin-induced human platelet aggregation. However, the elevation of light transmission by the PKA inhibitor was stronger than that by the PKG inhibitor in KRG-TS-inhibited thrombin-induced human platelet aggregation. These results show that the cAMP/PKA pathway mainly contributed to the inhibition of thrombin-induced platelet aggregation and [Ca2+]i mobilization by KRG-TS. Several aggregation-inducing molecules (Ca2+, TXA2, etc.) are commonly generated by agonists such as thrombin, collagen, and adenosine diphosphate (ADP). The IP3 mobilizes [Ca2+]i, and subsequently activates Ca2+-dependent PLC or phospholipase-A2 to separate the TXA2 precursor arachidonic acid (20:4) from glycerophospholipids, and TXA2 is produced by activation of COX-1/TXAS. TXA2 produces IP3 to mobilize [Ca2+]i through the G-protein-coupled receptor/PLC-β pathway, and constricts the blood vessel tract [5], [26], [27], [28], which enforces thrombus formation. Therefore, the inhibition of [Ca2+]i mobilization by IP3 and TXA2 production by COX-1/TXAS are very important to evaluate the antiplatelet effect of a substance. A previous report [17] confirmed that KRG-TS inhibits TXA2 production by attenuating COX-1 and TXAS activities. Although KRG-TS inhibited thrombin-induced [Ca2+]i mobilization, its inhibitory mechanism is unknown. The Ca2+-antagonistic reaction by cAMP and cGMP is mediated by both PKA/IP3RI and PKG/IP3RI phosphorylation pathways. Because KRG-TS elevated the level of cAMP [18], if KRG-TS stimulates IP3RI (Ser1756) phosphorylation in thrombin-activated human platelets, it is clear evidence that KRG-TS inhibits [Ca2+]i mobilization through the cAMP/PKA/IP3RI (Ser1756) phosphorylation pathway. In this report, we confirmed that KRG-TS inhibited [Ca2+]i mobilization through IP3RI (Ser1756) phosphorylation by cAMP/PKAc, which is supported by the fact that the cAMP inhibitor Rp-8-Br-cAMPS inhibited KRG-TS-elevated phosphorylation of both IP3RI (Ser1756) and PKAc (Thr197) in thrombin-induced human platelet aggregation, otherwise the cAMP inhibitor Rp-8-Br-cAMPS would not increase KRG-TS-decreased [Ca2+]i mobilization in thrombin-induced human platelet aggregation. It is known that IP3 induces serotonin release from platelet-dense bodies, meaning that IP3 is involved in serotonin release by elevating [Ca2+]i through IP3RI [29]. This reflects the fact that KRG-TS may be involved in the inhibition of serotonin release by phosphorylating IP3RI (Ser1756).
A lot of agonists such as collagen, thrombin, and ADP mobilize [Ca2+]i to phosphorylate Ca2+/calmodulin-dependent myosin light chain (20 kDa), which plays a role in secretion of granules such as serotonin and ATP [6], [7], and platelet aggregation. It is thought that the inhibition of ATP and serotonin secretion by KRG-TS results from the elevation of Ca2+-antagonistic molecule cAMP and subsequent inhibition of [Ca2+]i mobilization, which is also supported by the fact that KRG-TS stimulated the phosphorylation of both PKAc (Thr197) and IP3RI (Ser1756).
Platelet aggregation is generated at the site of vascular wall injury, and is involved in the formation of thrombus. During the formation of thrombus, platelets release cell growth proteins [e.g., platelet-derived growth factor (PDGF)] and vascular endothelial growth factor (VEGF) in α-granules [30], [31]. It is well-established that PDGF and VEGF induce the proliferation of fibroblast, vascular smooth cells, and epithelial cells, and subsequently enhance the rate of atherosclerosis lesion progression [32], [33], [34], [35], [36]. The progression of atherosclerosis is strongly induced by inflammatory cells such as monocytes/macrophages and neutrophils [37]. Although KRG-TS shows antiplatelet effects, if KRG-TS does not inhibit inflammation by leukocytes, progression of atherosclerosis lesion occurs at the site of vascular wall injury, which raises questions about the antiplatelet effects of KRG-TS. Byeon et al [38] reported that saponin fraction inhibits lipopolysaccharide (LPS)-induced inflammation, and it is well-known that ginsenosides exhibit anti-inflammatory effects by inhibiting the production of various proinflammatory mediators such as prostaglandin E2 and NO [39]. Recently, it was reported that saponin fractions of KRG downregulate LPS-induced proinflammatory mediators (i.e., NO and interleukin-1β) [40]. Considering these three previous reports [38], [39], [40], it is thought that KRG-TS may exhibit antithrombotic and antiatherosclerotic effects without causing inflammation and progression of atherosclerotic lesion at the site of vascular wall injury. Therefore, KRG-TS is highlighted as a “nontoxic antiplatelet compound,” and could be clinically applied to the prevention of platelet-mediated thrombosis. This result is supported by a previous study suggesting the protective effects of KRG on carotid artery thrombosis in vivo in rats [41]. In addition, both ginseng and ginsenosides are very useful for prevention of cardiovascular disease [42].
With regard to the antiplatelet effects of ginsenosides in KRG-TS, only G-Rg3 (20R, 20S) inhibited thrombin-induced platelet aggregation. This is in accordance with the reports that G-Rg3 (20R, 20S) inhibited arachidonic acid- or U46619-induced platelet aggregation [43], and its analog (G-Rp1, dihydroxy G-Rg3) inhibited collagen- or thrombin-induced platelet aggregation [19], [20]. Other reports also suggested that G-Rg1 [molecular weight (MW) = 800.94] and G-Rg2 (MW = 781.01) inhibit various agonists (i.e., thrombin, collagen, ADP)-induced platelet aggregation [44], [45], [46], but the inhibitory concentrations were 1 mg/mL (1.2mM) to 4 mg/mL (5mM) for G-Rg1 [44], [45] and 1 mg/mL (1.3mM) for G-Rg2 [45], [46]. These inhibitory concentrations (1.2–5mM) of G-Rg1 or G-Rg2 (1.3mM) [44], [45], [46] are very high compared with those of G-Rg3 and its analogues (G-Rp1, dihydroxy G-Rg3), which exhibited antiplatelet effect at concentrations in the range of micromoles [19], [20], [43]. Therefore, it is thought that the antiplatelet effects exhibited by G-Rg1 and G-Rg2 should be re-evaluated. Because only G-Rg3 in KRG-TS inhibited thrombin-induced human platelet aggregation, it is thought that G-Rg3 in KRG-TS might have contributed to the inhibition of platelet aggregation and [Ca2+]i mobilization, which also supports the report that G-Rg3 elevated Ca2+-antagonistic cAMP levels [20].
Thrombosis mainly results from the irreversible aggregation, which is closely related to the serotonin released from platelets activated by agonists (i.e., collagen and thrombin) [47], [48], [49]. In addition, the released serotonin is involved in causing migraine [50], [51], [52].
In conclusion, the most important result of this study is that KRG-TS significantly phosphorylates IP3RI (Ser1756) to inhibit thrombin-induced [Ca2+]i mobilization, which contributed to attenuating the release of ATP and serotonin. Therefore, our results suggest that KRG-TS may be a physiologically effective negative regulator in platelet aggregation, a cause of thrombosis, atherosclerosis, and myocardial infarction. Because KRG-TS also inhibits serotonin release, it should also be evaluated as an antimigraine substance.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This study was supported by a grant (Grant No. NRF-2011-0012143 to H.-J.P.) from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, Korea.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Schwartz S.M., Heimark R.L., Majesky M.W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J., Irvine R.F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 3.Cattaneo M. The P2 receptors and congenital platelet function defects. Semin Thromb Hemost. 2005;31:168–173. doi: 10.1055/s-2005-869522. [DOI] [PubMed] [Google Scholar]
- 4.Guidetti G.F., Lova P., Bernardi B., Campus F., Baldanzi G., Graziani A., Balduini C., Torti M. The Gi-coupled P2Y12 receptor regulates diacylglycerol-mediated signaling in human platelets. J Biol Chem. 2008;283:28795–28805. doi: 10.1074/jbc.M801588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings L.K. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103:4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaibuchi K., Sano K., Hoshijima M., Takai Y., Nishizuka Y. Phosphatidylinositol turnover in platelet activation; calcium mobilization and protein phosphorylation. Cell Calcium. 1982;3:323–335. doi: 10.1016/0143-4160(82)90020-3. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz U.R., Walter U., Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem Pharmacol. 2001;62:1153–1161. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 9.Cavallini L., Coassin M., Borean A., Alexandre A. Prostacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promote its phosphorylation. J Biol Chem. 1996;271:5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 10.Quinton T.M., Dean W.L. Cyclic AMP-dependent phosphorylation of the inositol-1,4,5-trisphosphate receptor inhibits Ca2+ release from platelet membranes. Biochem Biophys Res Commun. 1992;184:893–899. doi: 10.1016/0006-291x(92)90675-b. [DOI] [PubMed] [Google Scholar]
- 11.Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res. 2010;34:259–263. [Google Scholar]
- 12.Kim S.K., Park J.H. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung I.M., Lim J.W., Pyun W.B., Kim H. Korean Red Ginseng improves vascular stiffness in patients with coronary artery disease. J Ginseng Res. 2010;34:212–218. [Google Scholar]
- 14.Wee J.J., Kim Y.S., Kyung J.S., Song Y.B., Do J.H., Kim D.C., Lee S.D. Identification of anticoagulant components in Korean Red Ginseng. J Ginseng Res. 2010;34:355–362. [Google Scholar]
- 15.Jung Y.H., Park K.Y., Jeon J.H., Kwak Y.S., Song Y.B., Wee J.J., Rhee M.H., Kim T.W. Red Ginseng saponin fraction A isolated from Korean Red Ginseng by ultrafiltration on the porcine coronary artery. J Ginseng Res. 2011;35:325–330. doi: 10.5142/jgr.2011.35.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang S.Y., Son D.J., Kim I.W., Kim D.M., Sohn S.H., Lee J.J., Kim S.K. Korean Red Ginseng attenuates hypercholesterolemia-enhanced platelet aggregation through suppression of diacylglycerol liberation in high-cholesterol-diet-fed rabbits. Phytother Res. 2008;6:778–783. doi: 10.1002/ptr.2363. [DOI] [PubMed] [Google Scholar]
- 17.Lee D.H., Cho H.J., Kang H.Y., Rhee M.H., Park H.J. Total saponin from Korean Red Ginseng inhibits thromboxane A2 production associated microsomal enzyme activity in platelets. J Ginseng Res. 2012;36:40–46. doi: 10.5142/jgr.2012.36.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D.H., Cho H.J., Kim H.H., Rhee M.H., Ryu J.H., Park H.J. Inhibitory effects of total saponin from Korean Red Ginseng via vasodilator-stimulated phosphoprotein-Ser(157) phosphorylation on thrombin-induced platelet aggregation. J Ginseng Res. 2013;37:176–186. doi: 10.5142/jgr.2013.37.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endale M., Lee W.M., Kamruzzaman S.M., Kim S.D., Park J.Y., Park M.H., Park T.Y., Park H.J., Cho J.Y., Rhee M.H. Ginsenoside-Rp1 inhibits platelet activation and thrombus formation via impaired glycoprotein VI signalling pathway, tyrosine phosphorylation and MAPK activation. Br J Pharmacol. 2012;167:109–127. doi: 10.1111/j.1476-5381.2012.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee W.M., Kim S.D., Park M.H., Cho J.Y., Park H.J., Seo G.S., Rhee M.H. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp/60.11.0015. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.R., Park J.H., Kim N.D., Choi K.J. Inhibitory effects of ginsenoside Rg3 on platelet aggregation and its mechanism of action. J Ginseng Res. 1997;21:132–140. [Google Scholar]
- 22.Schaeffer J., Blaustein M.P. Platelet free calcium concentrations measured with fura-2 are influenced by the transmembrane sodium gradient. Cell Calcium. 1989;10:101–113. doi: 10.1016/0143-4160(89)90050-x. [DOI] [PubMed] [Google Scholar]
- 23.Jin H.X., Wu T.X., Jiang Y.J., Zou J.W., Zhuang S.L., Mao X., Yu Q.S. Role of phosphorylated Thr-197 in the catalytic subunit of cAMP-dependent protein kinase. J Mol Struct. 2007;805:9–15. [Google Scholar]
- 24.Charo I.F., Feinman R.D., Detwiler T.C. Inhibition of platelet secretion by an antagonist of intracellular calcium. Biochem Biophys Res Commun. 1976;72:1462–1467. doi: 10.1016/s0006-291x(76)80178-7. [DOI] [PubMed] [Google Scholar]
- 25.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 26.Moriyama T., Takamura H., Narita H., Tanaka K., Matsuura T., Kito M. Elevation of cytosolic free Ca2+ is directly evoked by thromboxane A2 in human platelets during activation with collagen. J Biochem. 1988;103:901–902. doi: 10.1093/oxfordjournals.jbchem.a122383. [DOI] [PubMed] [Google Scholar]
- 27.Charo I.F., Feinman R.D., Detwiler T.C. Interrelations of platelet aggregation and secretion. J Clin Invest. 1977;60:866–873. doi: 10.1172/JCI108841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moncada S., Vane J.R. Pharmacology and endogenous roles of prostaglandin endoperoxides, thromboxane A2, and prostacyclin. Pharmacol Rev. 1978;30:293–331. [PubMed] [Google Scholar]
- 29.Authi K.S., Evenden B.J., Crawford N. Metabolic and functional consequences of introducing inositol 1,4,5-trisphosphate into saponin-permeabilized human platelets. Biochem J. 1986;233:707–718. doi: 10.1042/bj2330707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castro-Malaspina H., Rabellino E.M., Yen A., Nachman R.L., Moore M.A. Human megakaryocyte stimulation of proliferation of bone marrow fibroblasts. Blood. 1981;57:781–787. [PubMed] [Google Scholar]
- 31.Holash J., Maisonpierre P.C., Compton D., Boland P., Alexander C.R., Zagzag D., Yancopoulos G.D., Wiegand S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 32.Nagai R., Suzuki T., Aizawa K., Shindo T., Manabe I. Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost. 2005;3:1569–1576. doi: 10.1111/j.1538-7836.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz S.M., Reidy M.A. Common mechanisms of proliferation of smooth muscle in atherosclerosis and hypertension. Hum Pathol. 1987;18:240–247. doi: 10.1016/s0046-8177(87)80006-0. [DOI] [PubMed] [Google Scholar]
- 34.Packham M.A., Mustard J.F. The role of platelets in the development and complications of atherosclerosis. Semin Hematol. 1986;23:8–26. [PubMed] [Google Scholar]
- 35.Schwartz S.M., Ross R. Cellular proliferation in atherosclerosis and hypertension. Prog Cardiovasc Dis. 1984;26:355–372. doi: 10.1016/0033-0620(84)90010-0. [DOI] [PubMed] [Google Scholar]
- 36.Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G.R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92:584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips D.R., Conley P.B., Sinha U., Andre P. Therapeutic approaches in arterial thrombosis. J Thromb Haemost. 2005;3:1577–1589. doi: 10.1111/j.1538-7836.2005.01418.x. [DOI] [PubMed] [Google Scholar]
- 38.Byeon S.E., Choi W.S., Hong E.K., Lee J., Rhee M.H., Park H.J., Cho J.Y. Inhibitory effect of saponin fraction from Codonopsis lanceolata on immune cell-mediated inflammatory responses. Arch Pharm Res. 2009;32:813–822. doi: 10.1007/s12272-009-1601-7. [DOI] [PubMed] [Google Scholar]
- 39.Park J.S., Cho J.Y. Anti-inflammatory effects of ginsenosides from Panax ginseng and their structural analogs. Afr J Biotechnol. 2009;8:3682–3690. [Google Scholar]
- 40.Lee Y.J., Han J.Y., Lee C.G., Heo K., Park S.I., Park Y.S., Kim J.S., Yang K.M., Lee K.J., Kim T.H. Korean Red Ginseng saponin fraction modulates radiation effects on lipopolysaccharide-stimulated nitric oxide production in RAW264.7 macrophage cells. J Ginseng Res. 2014;38:208–214. doi: 10.1016/j.jgr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean Red Ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee J.G., Lee Y.Y., Kim S.Y., Pyo J.S., Yun-Choi H.S., Park J.H. Platelet antiaggregating activity of ginsenosides isolated from processed ginseng. Pharmazie. 2009;64:602–604. [PubMed] [Google Scholar]
- 44.Zhou Q., Jiang L., Xu C., Luo D., Zeng C., Liu P., Yue M., Liu Y., Hu X., Hu H. Ginsenoside Rg1 inhibits platelet activation and arterial thrombosis. Thromb Res. 2014;133:57–65. doi: 10.1016/j.thromres.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 45.Kuo S.C., Teng C.M., Lee J.C., Ko F.N., Chen S.C., Wu T.S. Antiplatelet components in Panax ginseng. Planta Med. 1990;56:164–167. doi: 10.1055/s-2006-960916. [DOI] [PubMed] [Google Scholar]
- 46.Teng C.M., Kuo S.C., Ko F.N., Lee J.C., Lee L.G., Chen S.C., Huang T.F. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim Biophys Acta. 1989;990:315–320. doi: 10.1016/s0304-4165(89)80051-0. [DOI] [PubMed] [Google Scholar]
- 47.Mustard J.F., Packham M.A. Factors influencing platelet function: adhesion, release, and aggregation. Pharmacol Rev. 1970;22:97–187. [PubMed] [Google Scholar]
- 48.Holmsen H. Prostaglandin endoperoxide—thromboxane synthesis and dense granule secretion as positive feedback loops in the propagation of platelet responses during “the basic platelet reaction”. Thromb Haemost. 1977;38:1030–1041. [PubMed] [Google Scholar]
- 49.Borgdorff P., Tangelder G.J. Migraine: possible role of shear-induced platelet aggregation with serotonin release. Headache. 2012;52:1298–1318. doi: 10.1111/j.1526-4610.2012.02162.x. [DOI] [PubMed] [Google Scholar]
- 50.Kromer W. Drug treatment of pain. 4: Headache and migraine, drug interactions, contra-indications, use of analgesics in pregnancy and lactation. Fortschr Med. 1986;104:771–774. [Article in German] [PubMed] [Google Scholar]
- 51.Jernej B., Vladić A., Cicin-Sain L., Hranilović D., Banović M., Balija M., Bilić E., Sucić Z., Vukadin S., Grgicević D. Platelet serotonin measures in migraine. Headache. 2002;42:588–595. doi: 10.1046/j.1526-4610.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- 52.Danese E., Montagnana M., Lippi G. Platelets and migraine. Thromb Res. 2014;134:17–22. doi: 10.1016/j.thromres.2014.03.055. [DOI] [PubMed] [Google Scholar]