Abstract
It has been reported that Korean Red Ginseng has been manufactured for 1,123 y as described in the GoRyeoDoGyeong record. The Korean Red Ginseng manufactured by the traditional preparation method has its own chemical component characteristics. The ginsenoside content of the red ginseng is shown as Rg1: 3.3 mg/g, Re: 2.0 mg/g, Rb1: 5.8 mg/g, Rc:1.7 mg/g, Rb2: 2.3 mg/g, and Rd: 0.4 mg/g, respectively. It is known that Korean ginseng generally consists of the main root and the lateral or fine roots at a ratio of about 75:25. Therefore, the red ginseng extract is prepared by using this same ratio of the main root and lateral or fine roots and processed by the historical traditional medicine prescription. The red ginseng extract is prepared through a water extraction (90°C for 14–16 h) and concentration process (until its final concentration is 70–73 Brix at 50–60°C). The ginsenoside contents of the red ginseng extract are shown as Rg1: 1.3 mg/g, Re: 1.3 mg/g, Rb1: 6.4 mg/g, Rc:2.5 mg/g, Rb2: 2.3 mg/g, and Rd: 0.9 mg/g, respectively. Arginine-fructose-glucose (AFG) is a specific amino-sugar that can be produced by chemical reaction of the process when the fresh ginseng is converted to red ginseng. The content of AFG is 1.0–1.5% in red ginseng. Acidic polysaccharide, which has been known as an immune activator, is at levels of 4.5–7.5% in red ginseng. Therefore, we recommended that the chemical profiles of Korean Red Ginseng made through the defined traditional method should be well preserved and it has had its own chemical characteristics since its traditional development.
Keywords: chemical components, historical document, preparation methods, Red ginseng (Panax ginseng Meyer)
1. Introduction
Korean ginseng (Panax ginseng Meyer, Araliaceae) is traditionally used as an important herbal medicine in Far East Asia. The root of ginseng is traditionally used as an adaptogen as it is stated to have the capacity to normalize body functions and strengthen systems that are compromised by stress. Adaptogens are reported to have a protective effect on health against a wide variety of environmental assaults and emotional conditions [1], [2], [3], [4]. In addition, the main biological activities of Korean Red Ginseng are known to include immune enhancement effects, the recovery of vital energy as well as the alleviation of fatigue, blood flow improvement, antioxidant effects, and the positive effects on memory enhancement and menopausal disorder [5], [6], [7], [8], [9]. Fresh ginseng is easily degraded at room temperature. Therefore, fresh ginseng is processed into red ginseng through the process of steaming and drying or processed into white ginseng by a simple drying process. According to general knowledge, red ginseng has significantly higher biological effects and fewer side effects compared with fresh and white ginseng.
Red ginseng (Ginseng Radix Rubra) and white ginseng (Ginseng Radix Alba) are individually regulated in Korean, Japanese, and Chinese Pharmacopoeias. These regulations imply that there is a difference in characteristics between red and white ginseng because these have the same origins of the plant but have different processing. The studies of the differences between red and white ginseng have been carried out since the early 1980s [10]. These reports described the changes of the ginseng's chemical profile due to the different processing methods. Thereafter classified according to the different processing methods of red ginseng, the components and pharmacological activities of red ginseng products have subsequently been reported in scientific research papers. Herein we will define the traditional preparation method of red ginseng and describe the characteristic chemical profiles of red ginseng and other preparations from it which were prepared by the traditional methods.
2. History of Korean Red Ginseng
Historically, the name “Red Ginseng” (Hongsam in Korean) has been reported in the Annals of King Jeongjo (1776–1800), which is a part of the Annals of the Joseon Dynasty. According to the records older than that literature, the process of steaming ginseng was introduced in GoRyeoDoGyeong (a record of personal experience in Korea, written in 1123) by Seo-Gung (1091–1153, Song Dynasty). According to GoRyeoDoGyeong, red ginseng (originally recorded as steamed ginseng) was prepared by steaming and drying fresh ginseng root. However, because the fragmentary records lack certain details, researchers could not identify the exact preparation method for red ginseng, i.e., undefined steaming time, numbers of repetition, and consecutive drying process. About 100 y later (late 1200s), more detailed records about red ginseng were written in the SohoDang miscellany by Taekyoung Kim (1850–1927). According to the record, ginseng roots were grown for > 6 y, their dirt shaken off, washed, and then steamed in a big steamer. The steamed ginseng roots were laid on bamboo racks in a drying warehouse and dried by heat from a fire or by sunlight and the wind.
Red ginseng is a product made by the primary processing of fresh ginseng. The preparation method of red ginseng applied in the modern age was written in detail in Samjung-Yolam (A Bulletin of Ginseng Policy, 1908, Ministry of Strategy and Finance, The Greater Korean Empire). According to this record, the steaming of ginseng was carried out as follows; “The fresh ginseng roots harvested were carefully washed with clear water and thereafter, classified based on their respective sizes. The washed fresh ginseng roots were then put in a large bamboo basket and moved directly to the steaming place. The baskets were put into a clayware steamer which was sealed. The time of steaming was about 50–90 min depending on the size of the ginseng”. The drying of steamed ginseng was carried out as follows: “The steamed ginseng roots were sufficiently dried in the drying room, and then thereafter, placed under the sunlight for 4–5 d. The ginseng produced by these processes is a transparent material that represents the color of the cherry blossoms. This was called red ginseng.” The growing years of fresh ginseng was ≥ 6 y as speculated by the SohoDang miscellany (Fig. 1A), which was written in the same period with Samjung-Yolam (Fig. 1B). Harvesting season for fresh ginseng was estimated to be between October to November by considering the daylight conditions and the harvesting times of common medicinal plants. According to the record, fresh ginseng was screened by size and then steamed. These facts are similar to the current screening by size and shape as has been done in the past, the fresh ginseng with a large size and good shape was considered to be a good product presumably. Ginseng was produced under the leadership of the government very strictly, and could not be manufactured and sold privately. The so-called red ginseng monopoly system (1908–1996) was enforced by the Japanese colonial government and the overall red ginseng industry was managed under the supervision of government. Therefore, the traditional preparation method of red ginseng was standardized and applied to the manufacturing system at that time. From that, the ginseng production technology that is currently followed has been developed from the methods applied in the past.
Fig. 1.
SohoDang miscellany. (A) Detailed records about red ginseng were written by Taekyoung Kim (1850–1927) and Samjung-Yolam. (B) A bulletin of ginseng policy, 1908, Ministry of Strategy and Finance, The Greater Korean Empire.
3. Red ginseng production method
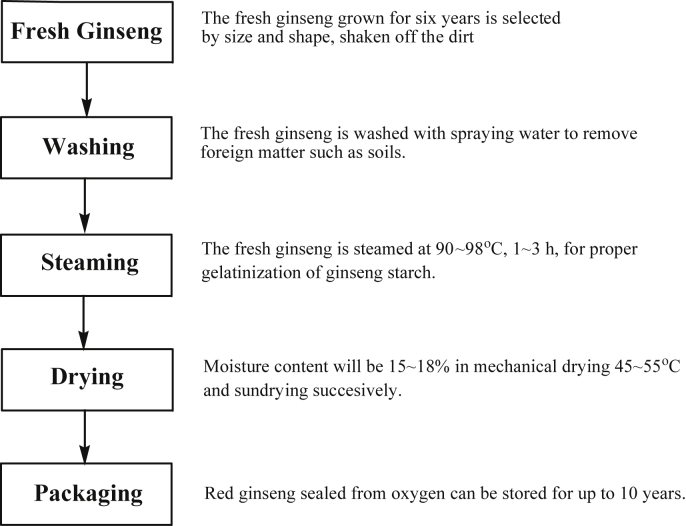
Currently in Korea, the dietary supplement products market has increased with the concern for health according to consumers increase of income. At the same time, consumer demand for red ginseng has rapidly increased. Reportedly, the ratio of primary processed to red ginseng from fresh ginseng was 67.1% in 2009. At that time, fresh ginseng production was 27,480 tons in Korea. Therefore, 18,439 tons of fresh ginseng were processed to red ginseng; however, red ginseng production facilities could not deal with these massive production demands [11]. Since 1908, the basic framework of traditional production methods for red ginseng was well structured and improved gradually. However, the ginseng manufacturing facilities scale was raised by the monopoly system and disappeared in 1996. The basic process of red ginseng production from fresh ginseng simply consists of three steps of washing, steaming, and drying. Fig. 2 is a ginseng process diagram from the major companies of red ginseng in Republic of Korea. Most manufacturers of red ginseng in the Republic of Korea are producing red ginseng by the utilization of the traditional production processes which can be summarized as follows. The fresh ginseng grown for 6 y is selected by size and shape, the dirt is shaken off, and then the root is washed with clean water. Subsequently, washed fresh ginseng is steamed for 1–3 h at 90–98°C (Fig. 2). Then the steamed ginseng is dried by hot air and laid in the sun until its moisture contents drops to 15% and 18%.
Fig. 2.
Manufacturing process of red ginseng from fresh ginseng.
4. Red ginseng extracts production method
The morphological ratio of ginseng is 75% of main root and 25% of the sum of lateral and fine roots, respectively. However, these morphological shapes from red ginseng manufacturing process are destroyed. Therefore, in general, the red ginseng extraction prepared in consideration of the part corresponding to the whole ginseng root is used as a raw material. Red ginseng extracts are one of the most important products that use the red ginseng root as a starting substance. In addition, it can be used as secondary raw material for liquid preparations formulated with various herbal extracts or commercialized by being sealed itself. But the traditional preparation methods or the records of red ginseng extracts have not yet been found. Because the related technology is developed and sophisticated very quickly, commercial manufacturers are applying the methods for optimizing their product yields and minimizing the production costs for securing their markets. Unfortunately, for them, to obtain the lucrative profits, excessive extraction times and temperatures are taken with the use of alcohol and low-cost starting substances (i.e., fine root, scraps, or small-sized main roots) as raw materials. For these reasons, there are problems related to the quality assurance of the red ginseng extraction as the nontraditional processes change the marked substances for physical, chemical, and biological properties severely. Therefore, strict quality control on the manufacturing process of the red ginseng extraction should be introduced. Optimized manufacturing process should be established based on the scientific references related to the extraction temperature, the extraction time, and the volume of extraction solvent for effective yield, reasonable amount of marked substances, and good sensory characteristics.
Fig. 3 is a schematic flow chart of the red ginseng extraction process that takes into consideration the various production process environments around the manufacturers in Republic of Korea. Most of red ginseng extracts are extracted with water which is in accordance with the extraction method originating from the traditional medicine prescription. However, there are some differences, such as the extraction time, solvents (i.e., alcohol) and temperature depending on the size, the drying state of red ginseng roots and the part of red ginseng in the case of producing the red ginseng extract.
Fig. 3.
Manufacturing process of red ginseng extracts from red ginseng.
5. Conversion of ginsenosides in the process of red ginseng
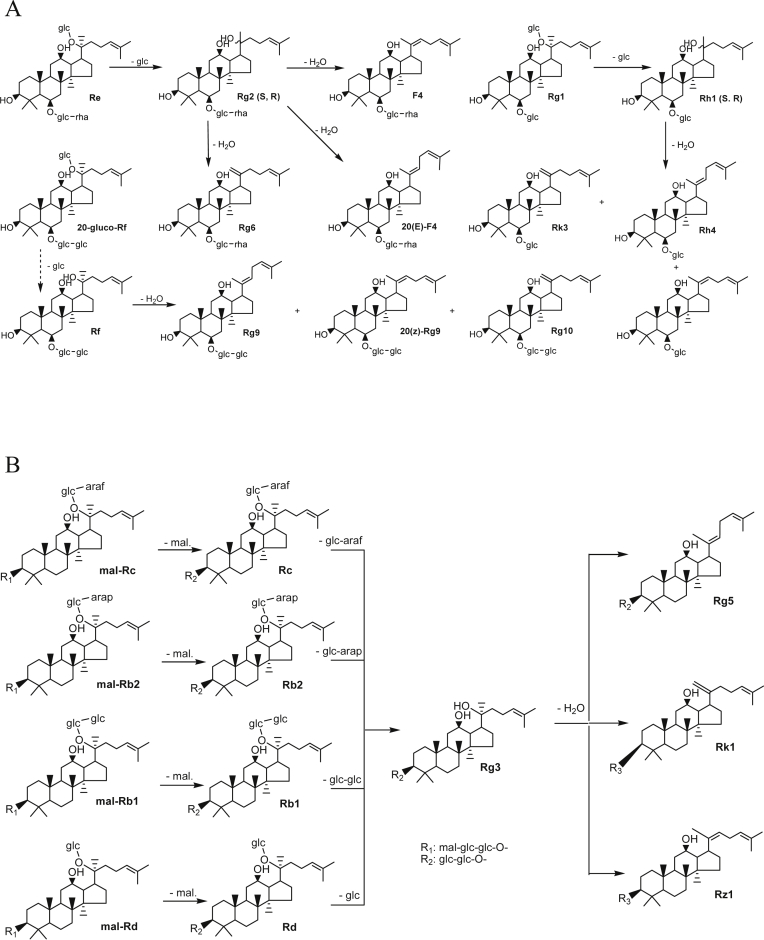
Dammarane ginsenosides are characteristic components of ginseng and are considered to be the primary pharmacologically effective components in ginseng. So far, about 50 kinds of ginsenosides have been identified from the ginseng root, which are defined as protopanaxadiol, and protopanaxatriol according to the dammarane skeleton [12]. Large numbers of ginsenosides in ginseng are converted into red ginseng by the heat treatment during the red ginseng production process. In particular, red ginseng is prepared by heat treatment, so it has relatively high concentration of the converted ginsenosides transformed from naturally occurred ginsenosides in the fresh ginseng. Ginsenosides Rg2, Rg6, F4, 20(E)-F4, Rh1, Rh4, Rk3, Rg3, Rg5, Rz1, Rk1, Rg9, and Rg10 were found in red ginseng, and these are converted from the major ginsenosides Rb1, Rb2, Rc, Rd, Rg1, and Re [13].
Generally, ginsenosides are denatured by the heat and acidic conditions. Red ginseng is treated with the heat process and the inner acidity get weak by citric acid and other organic acids. Thus, in red ginseng production processing, ginsenosides must be denatured to the several converted ginsenosides. Reportedly, the conversions from each ginsenoside were estimated as follows: [Rg1→Rh1→Rh4, Rk3], [Re→Rg2→F4, Rg6], [Rf→Rg9, 20Z-Rg9, Rg10], and [Rb1, Rc, Rb2, Rd→Rg3→Rg5, Rk1, Rz1]. These results explain that the contents of the converted ginsenosides such as Rg2, Rh1, and Rg3 progressively increase, and the contents of the natural ginsenosides such as Rg1, Re, Rb1, Rc, and Rd progressively decrease in the heat-processed red ginseng production (Fig. 4) [14].
Fig. 4.
The conversion mechanism of ginsenosides during red ginseng process. (A) Protopanaxatriol ginsenosides. (B) Protopanaxdiol ginsenosides.
6. The content variations of ginsenosides in red ginseng
Currently, red ginseng roots and extracts products available on the market have different chemical indexes depending on which part of the red ginseng and what kind of extraction processes are used. Commercially available red ginseng root consists of the main body and lateral roots including rhizome except for the mid-tail and fine hair roots of ginseng. Perhaps it is known that to do so is to have a good shape, although no clear reason has been presented yet theoretically. Practically, the contents of each part of the red ginseng ginsenosides show a lot of variances. The amounts of ginsenoside Rg1 and Rb1 among the ginsenosides in fine roots and the main root are very different. According to the report [15], the contents of each ginsenoside reveal significant differences between the epidermal part and inner part of the ginseng root. Some experimental results reveal (Table 1) that the concentration of panaxprotodiol ginsenosides is shown to be high in the epithermal parts but is low inside the body part. Therefore, the fine roots that have a high epithermal ratio have high concentrations of protopanaxdiol; ginsenoside Rb1, Rc, Rb2, and Rd. By contrast, the lateral roots and main roots have a low epithermal ratio, so they possess a relatively low concentration of propanaxdiol ginsenosides. The features of protopanaxtriol ginsenosides are evenly distributed in all parts of red ginseng relatively. The total ginsenoside amount of red ginseng roots such as in what is available on the market due to use of only lateral roots and main roots cannot be known comprehensively. Therefore, to obtain the ginsenoside profiles of red ginseng root, strictly selected samples as a standard red ginseng are needed. The standard ginsenoside profiles of the red ginseng root are shown in Table 2. Samples of the whole part of red ginseng produced by the red ginseng production process are presented in Fig. 2. In Table 2, the amount range of each major ginsenoside is shown as follows: Rg1, 3.3 mg/g; Re, 2.0 mg/g; Rb1, 5.8 mg/g; Rc, 1.7 mg/g; Rb2, 2.3 mg/g; and Rd: 0.4 mg/g. The amounts of some ginsenosides in red ginseng such as ginsenosides Rb1, Rc, Rb2, and Rd as the major protopanaxdiol ginsenosides are higher than the amounts of those fresh ginseng ginsenosides. The reason is that protopanaxdiol ginsenosides are contained as malonyl ginsenosides in fresh ginseng and the malonyl ginsenosides are demalonylated by heat and inner acidity in processing of red ginseng [16]. Unlike the existence of protopanaxdiol ginsenosides, malonyl type protopanaxtriol ginsenosides do not exist in red ginseng. The increased contents of protopanaxtriol in the red ginseng root are assumed to the sample deviation or variance analysis.
Table 1.
The contents of ginsenosides from each part of the red ginseng root1)
| Ginsenoside |
PPTs (mg/g, RSD%) |
PPDs (mg/g, RSD%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Rg1 | Re | Rf | Rh1 | Rg2(S) | Rg2(R) | Rb1 | Rc | Rb2 | Rd | Rg3(S) | Rg3(R) |
| Rhizome (地下莖) | 4.67 ± 1.90 (440.7) | 4.55 ± 1.08 (23.67) | 1.54 ± 0.57 (37.3) | 0.24 ± 0.12 (49.28) | 0.73 ± 0.23 (31.9) | 0.27 ± 0.14 (53.6) | 9.12 ± 2.83 (31.1) | 2.99 ± 0.77 (25.8) | 2.49 ± 0.67 (27.0) | 1.12 ± 0.51 (45.8) | 0.13 ± 0.03 (23.5) | 0.08 ± 0.02 (21.7) |
| Main root (主根) | 3.44 ± 1.64 (47.6) | 1.22 ± 0.26 (21.7) | 0.85 ± 0.36 (42.3) | 0.10 ± 0.08 (84.7) | 0.1 ± 0.08 (78.3) | 0.11 ± 0.12 (102.4) | 3.43 ± 1.31 (38.2) | 0.99 ± 0.22 (22.2) | 0.81 ± 0.21 (25.3) | 0.16 ± 0.11 (68.6) | 0.06 ± 0.02 (41.4) | 0.02 ± 0.02 (71.1) |
| Lateral root (枝根) | 4.29 ± 1.60 (37.3) | 6.53 ± 1.46 (22.35) | 1.84 ± 0.54 (29.7) | 0.14 ± 0.08 (50.0) | 1.17 ± 0.45 (38.1) | 0.42 ± 0.31 (73.8) | 13.54 ± 3.24 (23.9) | 7.2 ± 1.87 (25.9) | 6.24 ± 2.08 (33.4) | 1.52 ± 0.85 (56.1) | 0.17 ± 0.05 (27.5) | 0.09 ± 0.03 (33.9) |
| Fine root (細尾) | 2.86 ± 1.34 (46.9) | 10.2 ± 0.95 (9.32) | 1.78 ± 0.53 (35.4) | 0.17 ± 0.09 (52.7) | 1.99 ± 0.49 (24.6) | 0.70 ± 0.66 (94.9) | 21.00 ± 3.26 (15.5) | 11.4 ± 2.57 (22.6) | 9.35 ± 2.8 (29.9) | 2.97 ± 0.84 (28.4) | 0.24 ± 0.05 (18.8) | 0.13 ± 0.03 (21.2) |
Data are presented as mean ± SD (%)
PPT, Protopanaxtriol; PPD, Protopanaxdiol; RSD, Relative standard deviation; KFDA, Korea Food and Drug Administration.
Analyses were performed on HPLC/UV (203 nm) from the general analytical method of ginsenosides from KFDA [14]. Each different parts of the ginseng root from 40 objects made by traditional manufacturing methods. RSDs are relative standard deviation (n = 40)
Table 2.
The contents of ginsenosides from ginseng products
| Ginsenoside |
PPTs (mg/g, RSD%) |
PPDs (mg/g, RSD%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Rg1 | Re | Rf | Rh1 | Rg2(S) | Rg2(R) | Rb1 | Rc | Rb2 | Rd | Rg3(S) | Rg3(R) |
| Fresh ginseng1) | 2.01 ± 0.65 (32.5) | 1.75 ± 0.75 (42.9) | 0.54 ± 0.26 (47.8) | ND | 0.13 ± 0.07 (50.5) | ND | 2.02 ± 0.72 (35.5) | 0.66 ± 0.20 (30.6) | 0.63 ± 0.21 (33.3) | 0.15 ± 0.08 (53.8) | ND | ND |
| Red ginseng2) | 3.34 ± 0.98 (29.3) | 2.02 ± 0.63 (31.3) | 0.78 ± 0.25 (32.3) | 0.24 ± 0.12 (50.9) | 0.24 ± 0.07 (29.5) | ND | 5.80 ± 1.85 (31.9) | 1.78 ± 0.56 (31.5) | 2.31 ± 1.04 (45.1) | 0.44 ± 0.19 (48.8) | 0.16 ± 0.04 (25.5) | 0.06 ± 0.02 (27.8) |
| Red ginseng ex.3) | 1.31 ± 1.01 (75.9) | 1.35 ± 0.45 (33.5) | 0.97 ± 0.14 (14.5) | 0.99 ± 0.21 (21.3) | 1.09 ± 0.33 (29.8) | 0.52 ± 0.19 (36.8) | 6.43 ± 1.36 (21.1) | 2.58 ± 0.64 (24.9) | 2.37 ± 0.57 (24.1) | 0.94 ± 0.34 (35.8) | 1.87 ± 0.79 (42.2) | 0.74 ± 0.30 (40.3) |
The whole roots of fresh ginsengs are 6-y-old cultivated in Korea
The whole roots of red ginseng are made by traditional manufacturing methods from 6-y-old fresh ginseng cultivated in Korea
Red ginseng extracts are collected from several ginseng markets as the products of major ginseng producer in Korea. Analyses were performed on HPLC/UV (203 nm) from general analytical method of ginsenosides from KFDA [14]. RSDs are relative standard deviation (n = 40)
7. The content variations of ginsenosides in red ginseng extracts
Red ginseng extracts are prepared by extraction with water and they are concentrated to be the red ginseng extract concentration at suitable conditions. Generally, the whole chemical composition of red ginseng extracts all comes from the red ginseng roots. A noteworthy point is that the contents of ginsenosides from each part of red ginseng are significantly different (Table 1). Because protopanaxdiol (PPD) ginsenosides are heavily present in the epidermal part of red ginseng roots, lateral or fine roots relative to the high proportion of surface area which contains a lot of the protopanaxdiol ginsenosides compared to the main root. Data in Table 1 shows the chemical property of each ginsenoside. For this reason, red ginseng extracts mainly using lateral and fine roots are shown to have higher amounts of protopanaxdiol ginsenosides than extracts using the whole part of the red ginseng root. The red extract concentrates, which have exceptionally high contents of ginsenosides, are frequently found in the red ginseng extracts market. In such cases, those extracts are supposed to be prepared from lateral or fine roots having the large surface area. Numerous red ginseng extract manufacturers have tried to display high contents of ginsenosides to indicate the superiority of their products; however, when considering it logically, one red ginseng extract cannot have more than the total balance of the contents of ginsenosides in one object of red ginseng. Therefore, in order to eliminate the adulteration of ginsenosides content from red ginseng extracts, standard profiles of ginsenosides in red ginseng extracts is required. The standard profile of ginsenosides from red ginseng extracts is shown in Table 2. The samples used for quantitative analysis of ginsenosides are 40 types of red ginseng water extracts that are commercially available. Ginsenoside Rg1 remained at 1.31 mg/g in each extract evenly. Most of Rg1 are converted to ginsenoside Rh1, Rh4, and Rk3 [14]. Because the variation coefficiency of total Rg1 content is very large, we assume that the extraction conditions (i.e., temperature and time of extraction, concentration, and sterilization) are different for each extracted sample. In Table 2, the range of each major ginsenoside content is shown as follows: Rg1, 1.3 mg/g; Re, 1.3 mg/g; Rb1, 6.4 mg/g; Rc, 2.5 mg/g; Rb2, 2.3 mg/g; and Rd, 0.9 mg/g.
8. Arginine-fructose-glucose of red ginseng
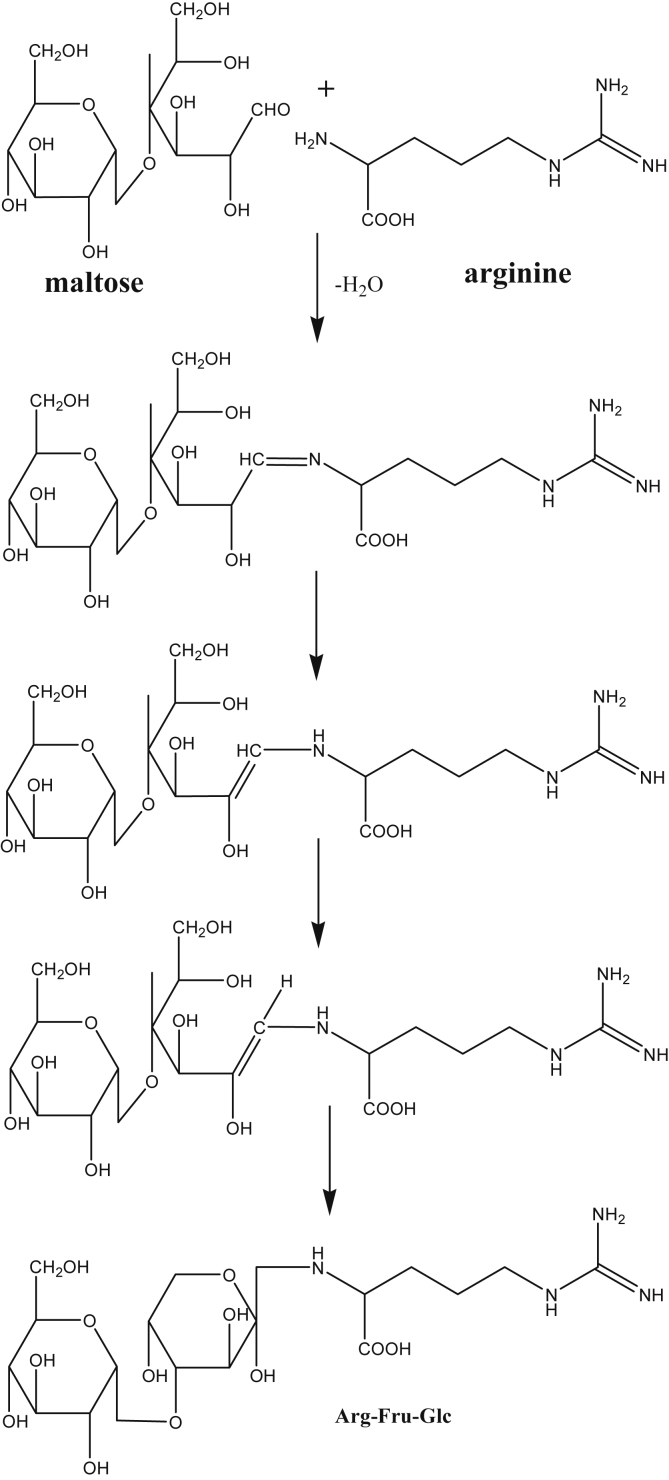
The roots of ginseng may also contain important nutritional components. The nutritional components include glucose, and fructose, sucrose, and maltose which are in bulk, and in addition, various amino acids are contained. Typically, arginine with a wide range of biological activities is contained in red ginseng together with some 21 types of amino acids [17]. These amino acids and sugars in fresh ginseng are reduced in amounts by the steaming process for red ginseng. The cause of this reduction is the Maillard reaction between amino acids and sugars. The first step of the reaction is the forming of amino sugars by Amadori rearrangement. Reportedly, 12 kinds of amino sugars were found in red ginseng [18]. Of their components, the major amino-sugars are arginine-fructose-glucose (AFG) and arginine-fructose (AF), which are produced by the thermal reaction of arginine and maltose or glucose (Fig. 5) [19]. During the steaming process, a marked decrease of starch and a considerable formation of maltose occur in the main roots of raw ginseng. After the drying process, the AFG and AF amounts are increased in steamed ginseng roots. According to the results of Okuda [20], the concentration of AFG containing the red ginseng and red ginseng extract is about 1.0–1.5% and 2.5%, respectively (Table 3). The amino sugars have an inhibitory effect on protein glycosylation in blood, attenuating various complications related to diabetes mellitus [21], [22] and hyperglycemia [23]. By the successive reactions, the amino sugars transform to several flavor substances, i.e., pyridines, pyrazines, pyrroles, and furanones. As a pyran, maltol, which is the representative flavor of red ginseng and the final product by Maillard reaction from amino sugars, is an important sensory characteristic compound. Changes in the amounts of amino acids and free sugars from fresh ginseng to red ginseng are shown with the total amino acid reduction from 17.9 mg/g to 12.2 mg/g. The facts illustrate that reduced amino acids transfer to variety amino sugars through combination with sugars. However, the amount of total amino acids severely decreased to 2.70 mg/g when the fresh ginseng was subjected to high temperatures [17]. In this case, the amino sugars underwent pyrolysis and transferred to melanoidins as a pigment rapidly.
Fig. 5.
Formation of Arg-Fru-Glc (AFG) from maltose and arginine by Maillard reaction.
Table 3.
The contents of acidic polysaccharide and arginine-fructose-glucose (AFG) of Korean red ginseng root from Okuda's [20] results
| Red ginseng parts | Acidic polysaccharide (%) | AFG (%) |
|---|---|---|
| Main root (主根) | 7.5 | 1.5 |
| Lateral (枝根) and fine root (細尾) | 4.7 | 1.0 |
| Extracts | 5.6 | 2.5 |
9. Acidic polysaccharides of red ginseng
It has been reported that P. ginseng polysaccharides have immunomodulation, anti-fatigue, antitumor, antiadhesive, antioxidant, antiulcer, antiradiation, hepatoprotective, hypoglycemic activities, and antihyperlipidemic activities [24], [25], [26], [27], [28], [29], [30]. Therefore, the acidic polysaccharides have most of the biological effect with ginsenosides. Red ginseng acidic polysaccharide which induces nitric oxide production with the enhancement of messenger RNA levels of inducible isoform nitric oxide synthase increased nuclear factor kappa B, AP-1 (activator protein 1), STAT-1 (signal transducer and activator of transcription 1), ATE-2 (arginine-tRNA protein transferase 2), and cAMP response element binding protein. At the same time ERK and JNK were found to be the most important signaling enzymes. So red ginseng acidic polysaccharide can be active in the macrophage function to stimulate immune responses through TLR2 mediated functional activation boosted by wortmannin targeted enzyme [31]. The effects of ginseng acidic polysaccharide on the physiological biomarkers of oxidative stress and the morphology of the mitochondria in striated skeletal muscle may indicate that ginseng acidic polysaccharide could strengthen the physical activity and enhance malondialdehyde and lactate dehydrogenase levels in serum [32]. The ginseng polysaccharides are categorized into neutral and acidic types. It is generally known that most biological activities are shown by acid polysaccharides as pectin combined with glucouronic and galacturonic acids [33]. The amount of the acidic polysaccharides in red ginseng is three times higher than the amount in white ginseng. The contents of acidic polysaccharides from ginseng (white ginseng) and red ginseng are 0.86% (white ginseng lateral root), 0.63% (white ginseng main root) compared to 4.7% (red ginseng lateral root), and 7.5% (red ginseng main root) (Table 3). The reason for increase of these components in red ginseng is the degradation of sugar components in the processes of steaming and drying fresh ginseng. However, the chemical characteristics and contents according to the production method are not sufficiently defined through the acidic polysaccharides in red ginseng; nevertheless, it is clear that the traditional red ginseng is more effective than white ginseng.
10. Polyacetylenes from red ginseng
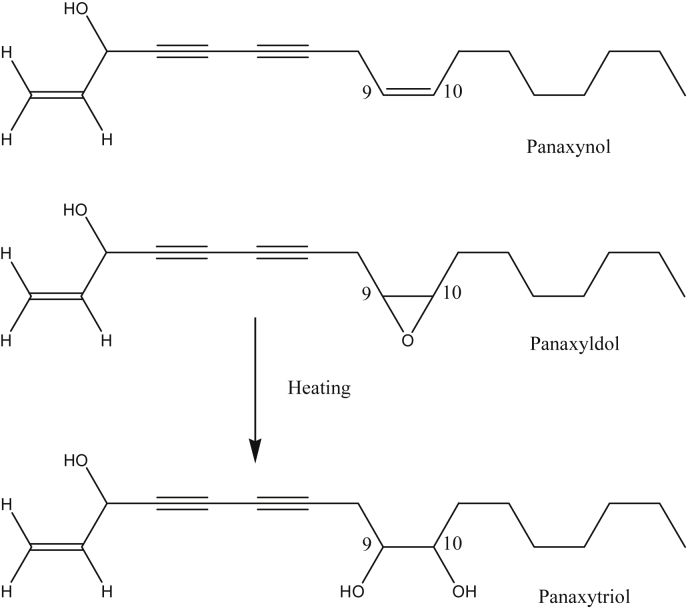
The roots of P. ginseng contain several polyactylene compounds that are representative non-water–soluble substances from ginseng. Panaxynol (heptadeca-1,9-diene-4,6-diyne-3-ol) as the first identified polyacetylenic compound from P. ginseng was isolated by Takahashi et al [34]. After that, panaxydol (heptadeca-1-ene-9,10-epoxy-4,6-diyne-3-ol) was isolated and identified from the petroleum ether soluble fraction of Panax ginseng by Poplawski et al [35]. These polyacetylenic substances have been found in 20 kinds to date [20]. As a characteristic polyacetylene compound from red ginseng, panaxytriol is a hydrated compound of epoxy ring from panaxydol by heat and acid (Fig. 6). Unfortunately, quantitative investigations about how much of the content of panaxytriol from red ginseng has not yet been made. These polyacetylenes of ginseng have some known toxicity and anticancer activities in vitro. However, when considered in pharmacological aspects, these polyacetylenes are not clearly shown to represent the in vivo efficacy because these compounds are chemically unstable. Therefore, a wide range of biological experiments are required in this regard.
Fig. 6.
Chemical structures of representative polyacetylene from Panax ginseng.
11. Conclusion
In this review, we discussed the traditional processing method of red ginseng production, the characteristic chemical profiles of red ginseng, and relevance of ingredient profiles and physiological activities. The various kinds of red ginseng products having the different chemical profiles have been manufactured using the producer-oriented red ginseng production techniques and distributed. However, the red ginseng that is not produced by the traditional manufacturing process has emphasized the amounts of ginsenoside contents with no valid physiological activity and safety based on solid scientific evidence. Some red ginseng manufacturers and salespeople stimulate consumer purchasing by emphasizing the higher concentration of a red ginseng specific compound, e.g., ginsenoside Rg1, and Rb1 and the other ginsenosides. However, the overall content of ginsenosides in red ginseng is not to be changed at all due to the consistency of chemical materials' balance. Therefore, when using the normal fresh ginseng, the ginsenosides amount of red ginseng is not changed. Ginsenosides Rg1 and Rb1 have been used as the markers for quality control of ginseng products, and the amounts of the ginsenosides in red ginseng products are not to be the direct indicator of physiological characteristics of its bioactivities. Under the current ginseng product-related regulations, the contents of ginsenosides in ginseng products is used to cite legally only if there is a minimal required amount that prove which is an authorized ginseng product.
When the market available red ginseng extracts products have been studied, some of them having unusually high content of specific ginsenosides are manufactured by using nontraditional red ginseng raw materials, i.e., fine root, scraps, and small size ginseng with nontraditional red ginseng process technology. Unfortunately, even if a high concentration of ginsenoside in red ginseng products sounds like it is good for health, there has been very limited research on the perspective of Chinese medicine of ginseng in terms of the physiologically bioactivity or efficacy of red ginseng. Ginseng product prescriptions have been used to improve human health for a long time in Asia. But it is rarely possible for ginseng products to find some concrete associations with a good effect to human health. We are reviewing the chemical profiles of the red ginseng products prepared by the traditional red ginseng production method that have been used for a long time to uncover some efficacies to human health. Therefore, red ginseng should have constant chemical composition profiles to carry its health benefits to humans. To get such efficacy from red ginseng, the growing time for raw ginseng (usually 6 y) should be used for the manufacturing red ginseng by the traditional process. Also, the red ginseng extracts should be prepared from strictly defined red ginseng with the proper ratio of main roots, lateral roots, and fine roots. There is no question that strictly and traditionally defined red ginseng and its extracts are to improve human health because they have been used for hundreds of years and have clinical efficacy proven by many oriental medical scientists. Therefore, the production process of red ginseng and the extracts should be based on their traditional prescription.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Sang Myung Lee, Email: smlee@mokwon.ac.kr.
Yi-Seong Kwak, Email: twostar@kgc.or.kr.
References
- 1.Brekhman I.I., Dardymov I.V. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419–430. doi: 10.1146/annurev.pa.09.040169.002223. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y.J., Lee O.R., Lee S.Y., Kim K.T., Yang D.C. Isolation and characterization of the glutathione s-transferase gen from Panax ginseng C.A. Meyer. J Ginseng Res. 2012;36:449–460. doi: 10.5142/jgr.2012.36.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C.X., Xio P.G. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;35:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Li S.S., Fan Y.Y., Chen Y., Liu D., Cheng H.R., Gao X.G., Zhou Y.F. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C.A. Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Babiker L.B., Gadkariem E.A., Alashban R.M., Aljohar H.I. Investigation of stability of Korean ginseng in herbal drug product. Am J Appl Sci. 2014;11:160–170. [Google Scholar]
- 6.Zhang D.T., Yasuda Y.Y., Zheng P., Kawabata T. ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996;20:145–150. doi: 10.1016/0891-5849(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 7.Yun T.K., Choi S.Y., Yun H.Y. Epidemiological study on cancer prevention by ginseng: Are all kinds of cancers preventable by ginseng. J Korean Med Sci. 2001:S19–S27. doi: 10.3346/jkms.2001.16.S.S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joo S.S., Won T.J., Lee I. Reciprocal activity of ginsenosides in the production of proinflammatory repertoire and their potential roles in neuroprotection in vivo. Planta Med. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- 9.Jung C.H., Seog H.M., Choi I.W., Choi H.D., Cho H.Y. Effects of wild ginseng (panax ginseng C.A. Meyer) leaves on lipid peroxidation levels and antioxidant enzyme activities in streptozotocin diabetic rats. J Ethnopharmacol. 2005;98:245–250. doi: 10.1016/j.jep.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 10.Nam K.Y. The comparative understanding between red ginseng and white ginsengs processed ginsengs (Panax ginseng C.A. Meyer) J Ginseng Res. 2005;29:1–18. [Google Scholar]
- 11.Baeg I.H., So S.H. The world ginseng mark and ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen L.P. Ginsenosides: chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–73. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee S.M., Kim S.C., Oh J.S., Kim J.H., Na M.K. 20(R)-Ginsenoside Rf: A new ginsenoside from red ginseng extract. Phytochem Lett. 2013;6:620–624. [Google Scholar]
- 14.Lee S.M. Thermal conversion pathways of ginsenoside in red ginseng processing. Nat Prod Sci. 2014;20:119–125. [Google Scholar]
- 15.Ahn I.O., Lee S.S., Lee J.H., Lee M.J., Cho B.G. Comparison of ginsenoside contents and pattern similarity between root parts of new cultivars in Panax ginseng C.A. Meyer. J Ginseng Res. 2008;32:15–18. [Google Scholar]
- 16.Kitagawa I., Taniyama T., Hayashi T., Yoshikawa M. Malonylginsenoside Rb1, Rb2, Rc, and Rd, four new malonylated dammarane-type triterpen oligosaccharides from Ginseng Radix. Chem Pharm Bull. 1983;31:3353–3356. [Google Scholar]
- 17.Cho E.J., Piao X.L., Jang M.H., Baek S.H., Kim H.Y., Kang S.K., Kwon S.W., Park J.H. The effect of steaming on the free amino acid contents and antioxidant activity of Panax ginseng. Food Chem. 2008;107:876–882. [Google Scholar]
- 18.Du Q.Q., Liu S.Y., Xu R.F., Li M., Song F.R., Liu Z.Q. Studies on structures and activities of initial Maillard reaction products by electrospray ionization mass spectrometry combined with liquid chromatography in processing of red ginseng. Food Chem. 2012;135:832–838. doi: 10.1016/j.foodchem.2012.04.126. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y., Choi K.J., Uchida K., Ko S.R., Shon H.J., Park J.D. Arginyl-fructosyl-glucose and arginyl-fructose, compounds related to browning reaction in the model system of steaming and heat-drying process for the preparation of red ginseng. J Ginseng Res. 2004;28:143–148. [Google Scholar]
- 20.Okuda H. Inhibitory substances in Korean red ginseng toward toxohormone-L: A toxic substance secreted from tumor cells. The Ginsneg Rev. 1992;15:34–37. [Google Scholar]
- 21.Nadeem A.A., Moinuddin, Alam K., Ali A. Preferential recognition of amadori-rich lycine residues by serum antibodies in diabetes mellitus: Role of protein glycation in the disease process. Hum Immunol. 2009;70:417–424. doi: 10.1016/j.humimm.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Arif B., Ashraf J.M., Moinuddin, Ahmed J., Arif Z., Alam K. Structural and immunological characterization of amadori-rich human serum albumin: Role in diabetes mellitus. Arch Biochem Biophys. 2012;522:17–25. doi: 10.1016/j.abb.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Ha K.S., Jo S.H., Kang B.H., Apostolidis E., Lee M.S., Jang H.D., Kwon Y.I. In vitro and in vivo antihyperglycemic effect of 2 amadori rearrangement compounds, arginyl-fructose and arginyl-fructosyl-glucose. J Food Sci. 2011;76:H188–H193. doi: 10.1111/j.1750-3841.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 24.Fu P., Gao Q., Wan W., Jiang R., Zhong C., Jiao L. Chemical properties and anti-tumor activity of polysaccharides from roots of Panax ginseng. J Norman Bethune University of Medical Science. 1994;20:439–441. [Google Scholar]
- 25.Lee J.H., Shim J.S., Kim M.K., Chung M.S., Kim K.H. Pectin-like acid polysaccharide from Panax ginseng which selective anti-adhesive activity against pathogenic bacteria. Carbohydr Res. 2006;342:1154–1163. doi: 10.1016/j.carres.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Luo D., Fang B. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohydr Polym. 2008;72:376–381. [Google Scholar]
- 27.Shin M.J., Kim Y.S., Kwak Y.S., Song Y.B., Kim Y.S., Park J.D. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP) Planta Med. 2004;70:1033–1038. doi: 10.1055/s-2004-832643. [DOI] [PubMed] [Google Scholar]
- 28.Song J.Y., Han S.K., Son E.H., Pyo S.N., Yun Y.S., Yi S.Y. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002;2:857–865. doi: 10.1016/s1567-5769(01)00211-9. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki Y., Hikino H. Mechanism of hypoglycemic activity of Panaxans A and B. glycans of Panax ginseng roots: Effects on the key enzymes of glucose metabolism in the liver of mice. Phytother Res. 1989;3:15–19. [Google Scholar]
- 30.Kwak Y.S., Kyung J.S., Kim J.S., Cho J.Y., Rhee M.H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 31.Byeon S.E., Lee J.H., Kim J.H. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators of Inflammation. 2012;732860:7. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Sun C., Zheng Y., Pan H., Zhou Y., Fan Y. The effective mechanism of the polysaccharides from Panax ginseng on chronic fatigue syndrome. Arch Pharm Res. 2014;37:530–538. doi: 10.1007/s12272-013-0235-y. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X., Yu L., Bi H., Li X., Ni W., Han H., Li N., Wang B., Zhou Y., Tai G. Total fractionation and characterization of the water soluble polysaccharides isolated from Panax ginseng C.A. Meyer. Carbohydr Polym. 2009;77:544–552. [Google Scholar]
- 34.Takahashi M., Yoshikura M. Studies on the components of Panax ginseng C. A. Meyer. V. On the structure of a new acetylene derivative ‘Panaxynol’ (3). Synthesis of 1,9-(cis)-heptadecadiene- 4,6-diyn-3-O. Yakugaku Zasshi. 1966;86:1053–1055. doi: 10.1248/yakushi1947.86.11_1053. [DOI] [PubMed] [Google Scholar]
- 35.Poplawski I., Wrobel J.J., Glinka M. Panaxydol, a new polyacetylenic epoxide from Panax ginseng roots. Phytochemistry. 1980;19:1539–1541. [Google Scholar]