Abstract
Background
Ginseng belongs to the genus Panax. Its main active ingredients are the ginsenosides. Interstitial cells of Cajal (ICCs) are the pacemaker cells of the gastrointestinal (GI) tract. To understand the effects of ginsenoside Re (GRe) on GI motility, the authors investigated its effects on the pacemaker activity of ICCs of the murine small intestine.
Methods
Interstitial cells of Cajal were dissociated from mouse small intestines by enzymatic digestion. The whole-cell patch clamp configuration was used to record pacemaker potentials in cultured ICCs. Changes in cyclic guanosine monophosphate (cGMP) content induced by GRe were investigated.
Results
Ginsenoside Re (20–40μM) decreased the amplitude and frequency of ICC pacemaker activity in a concentration-dependent manner. This action was blocked by guanosine 5′-[β-thio]diphosphate [a guanosine-5'-triphosphate (GTP)-binding protein inhibitor] and by glibenclamide [an adenosine triphosphate (ATP)-sensitive K+ channel blocker]. To study the GRe-induced signaling pathway in ICCs, the effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (a guanylate cyclase inhibitor) and RP-8-CPT-cGMPS (a protein kinase G inhibitor) were examined. Both inhibitors blocked the inhibitory effect of GRe on ICC pacemaker activity. L-NG-nitroarginine methyl ester (100μM), which is a nonselective nitric oxide synthase (NOS) inhibitor, blocked the effects of GRe on ICC pacemaker activity and GRe-stimulated cGMP production in ICCs.
Conclusion
In cultured murine ICCs, GRe inhibits the pacemaker activity of ICCs via the ATP-sensitive potassium (K+) channel and the cGMP/NO-dependent pathway. Ginsenoside Re may be a basis for developing novel spasmolytic agents to prevent or alleviate GI motility dysfunction.
Keywords: gastrointestinal tract, ginsenoside Re, interstitial cells of Cajal, patch clamp configuration
1. Introduction
Ginseng belongs to the genus Panax, and its main active ingredients, the ginsenosides, are derivatives of the triterpenoid dammarane [1]. Ginsenosides have a four-ring, steroid-like structure with pendant sugar moieties. Approximately 30 ginsenosides have been isolated from Panax ginseng roots [2], [3]. Many reports have shown that the ginsenosides have wide-ranging biological effects, and they influence the central and peripheral nervous systems and the cardiovascular and immune systems [4], [5], [6]. Furthermore, ginsenosides affect the gastrointestinal (GI) tract. Ginseng increases mouse intestinal movement and promotes the relaxation of circular muscles in the gastric body [7]. In isolated guinea pig GI tract tissues, ginseng increases longitudinal muscle contraction in the ileum and distal colon [8]. In the rabbit intestine, ginseng stimulates intestinal motility [9]. Ginsenoside Re (GRe) is a major ginsenoside that has diverse effects. For example, it enhances small-conductance calcium (Ca2+)-activated potassium (K+) currents in human coronary artery endothelial cells [10], protects against the formation of acute gastric mucosal lesions induced by compound 48/80 [11], enhances the immune response to inactivated rabies virus vaccine in mice [12], and exhibits anticarcinogenic effects in human gastric cancer cells [13].
Interstitial cells of Cajal (ICCs) are the GI pacemaker cells that generate rhythmic oscillations in membrane potentials that are known as “slow waves.” [14], [15]. The loss of ICCs has been implicated in several motility disorders, which suggests they have an important role in regulating GI motility [16]. The pacemaker mechanism involves rhythmic oscillations in intracellular calcium concentrations and Ca2+ release from d-myo-inositol 1,4,5-trisphosphate (IP3) receptor-operated stores [17]. In the murine small intestine, increases in the pacemaker activity of ICCs primarily result from periodic activation of nonselective cation channels (NSCCs) [17], [18] or chloride (Cl−) channels [19], [20], [21]. Kim et al [16] suggest that transient receptor potential melastatin 7 (TRPM 7) is required for ICC pacemaker activity in the murine small intestine, and that a Ca2+-activated Cl− channel (CaCC) is involved in the slow waves generated by ICCs; this Cl− channel was later identified as transmembrane protein 16A, which is also called anoctamin-1 (ANO1) [20]. Kim et al [2] suggest that ginseng total saponins (GTS) modulate ICC pacemaker activity in the GI tract. We recently reported that in cultured murine ICCs, gintinin, a ginseng-derived G protein-coupled lysophosphatidic acid (LPA) receptor [22], [23], increased the membrane depolarization associated with pacemaker activity, and that ANO1 activation was coupled to the stimulation of GI contractility via LPA1/3 receptor pathways [24]. However, it remains unclear how GRe exerts its pharmacologic and physiologic effects on GI motility. In the present study, we examined whether GRe regulates the electrical properties of cultured ICC clusters derived from murine small intestine, and we characterized GRe-mediated signaling pathways.
2. Materials and methods
2.1. Preparation of cells and cell cultures
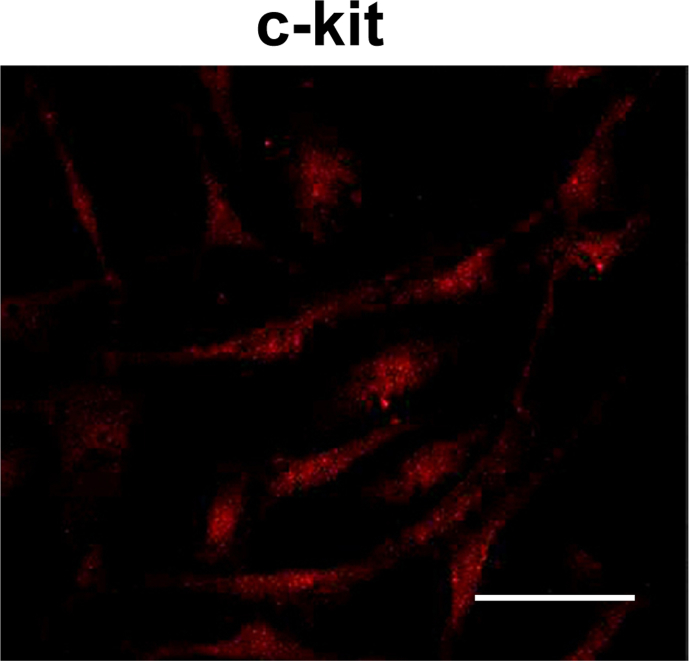
Animal care and experiments were conducted in accordance with the guidelines issued by the Ethics Committee of the Pusan National University (Yangsan, Republic of Korea). BALB/c mice were used throughout the study. Small intestines were excised (from 1 cm below the pyloric ring to the cecum) and opened along the mesenteric border. Luminal contents were removed using Krebs–Ringer bicarbonate solution, and the tissues were pinned to the bases of Sylgard dishes. The mucosae were removed by sharp dissection. Small tissue strips of intestine muscle (which consisted of circular and longitudinal muscles) were equilibrated for 30 min in Ca2+-free Hank's solution, which contained the following: potassium chloride (KCl), 5.36mM; sodium chloride (NaCl), 125mM; sodium hydroxide (NaOH), 0.34mM; sodium bicarbonate (Na2HCO3), 0.44mM; glucose, 10mM; sucrose, 2.9mM; and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 11mM; pH 7.4). Cells were then dispersed in an enzyme solution containing collagenase (Worthington Biochemical, Lakewood, NJ, USA; 1.3 mg/mL), bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA; 2 mg/mL), trypsin inhibitor (Sigma-Aldrich; 2 mg/mL), and ATP (0.27 mg/mL). The cells were plated onto sterile glass coverslips coated with murine collagen (2.5 μg/mL; Falcon/BD, Franklin Lakes, NJ, USA) in 35 mm culture dishes. The cells were then cultured at 37°C in a 95% oxygen–5% carbon dioxide incubator in a smooth muscle growth medium (Clonetics, San Diego, CA, USA) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, USA) and murine stem cell factor (5 ng/mL; Sigma-Aldrich). All experiments on ICC clusters were performed after they were cultured for 12 h. The ICCs were identified immunologically using an anti-c-Kit antibody, phycoerythrin-conjugated rat anti-mouse c-Kit monoclonal antibody (eBioscience, San Diego, CA, USA), at a dilution of 1:50 for 20 min (Fig. 1). Because the morphology of the ICCs differs from other cell types in the culture, it was possible to identify them under a phase contrast microscope after incubation with the anti-c-Kit antibody.
Fig. 1.
Cultured ICCs from the murine small intestine. The tunica muscularis of the small bowel was digested with collagenase, and the dispersed cells were cultured for 12 h. The confocal microscope image shows the Kit-immunopositive ICC network in the culture. The scale bar represents 50 μm. GRe, ginsenoside Re; ICC, interstitial cells of Cajal.
2.2. Patch clamp experiments
Physiological salt solution was used to bathe cultured ICC clusters (Na+-Tyrode) and contained the following: KCl, 5mM; NaCl, 135mM; calcium chloride (CaCl2), 2mM; glucose, 10mM; magnesium chloride (MgCl2), 1.2mM; and HEPES, 10mM (adjusted to pH 7.4 with NaOH). The pipette solution used to examine pacemaker activity contained the following: KCl, 140mM; MgCl2, 5mM; dipotassium ATP (K2ATP), 2.7mM; sodium GTP (NaGTP), 0.1mM; creatine phosphate disodium, 2.5mM; HEPES, 5mM; and ethylene glycol tetra-acetic acid, 0.1mM (adjusted to pH 7.2 with potassium hydroxide). Patch clamp techniques were conducted in whole-cell configuration to record the membrane currents (i.e., voltage clamp mode) and the potentials (i.e., current-clamp mode) from cultured ICCs using Axopatch I-D and Axopatch 200B amplifiers (Axon Instruments, Foster, CA, USA). Command pulses were applied using an IBM-compatible personal computer (Compaq; Houston, TX, USA) and pClamp software (versions 6.1 and 10.0; Axon Instruments). Data were filtered at 5 kHz and displayed on an oscilloscope, a computer monitor, and/or a pen recorder (Gould 2200; Gould, Valley View, OH, USA). Results were analyzed using pClamp and Origin software (version 6.0, Microcal, Northampton, MA, USA). All experiments were performed at 30–33°C.
2.3. The cGMP assay
Interstitial cells of Cajal were preincubated with 100μM IBMX (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C to inhibit cGMP degradation, and then incubated with GRe (100μM) for 10 min. After homogenization in a buffer containing 4mM EDTA to prevent the degradation of enzymatic cGMP, the homogenates were heated for 5 min in a boiling water bath to coagulate the proteins, and then centrifuged at 3950 × g for 5 min. The supernatants thus obtained were transferred to new tubes and stored at 4°C. Samples were assayed for cGMP using cGMP enzyme-linked immunosorbent assay kits (Enzo Life Science, Farmingdale, NY, USA).
2.4. Drugs
Ginsenoside Re was purchased from LKT Laboratories (St. Paul, MN, USA). All other drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA). Stock solutions were prepared and stored in accordance with the manufacturers' instructions. Chemicals were dissolved in Na+-Tyrode solution to their final concentrations immediately before use.
2.5. Statistical analysis
The results are expressed as the mean ± the standard deviation. Statistical analysis was performed using the Student t test or by analysis of variance (ANOVA), followed by the Tukey multiple comparison test, as appropriate. The analysis was performed using GraphPad Prism, version 6 (GraphPad Software, Inc., La Jolla, CA, USA); values of p < 0.05 were considered statistically significant. The n values reported in the text refer to the number of cells used in the patch clamp experiments.
3. Results
3.1. Effects of GRe on the pacemaker activity of cultured ICCs
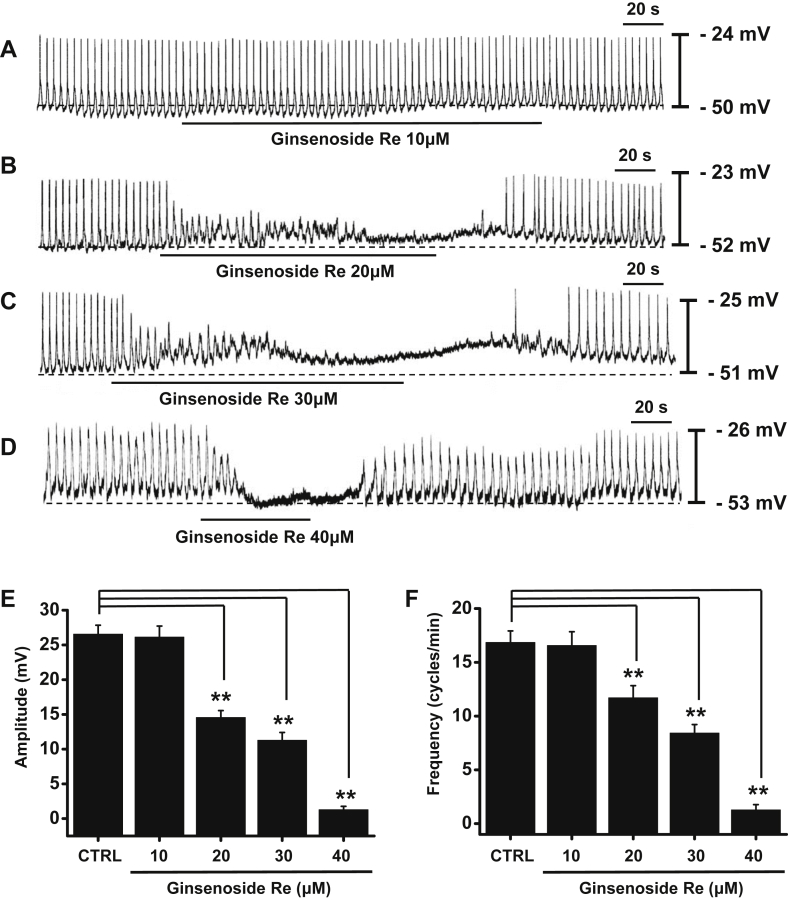
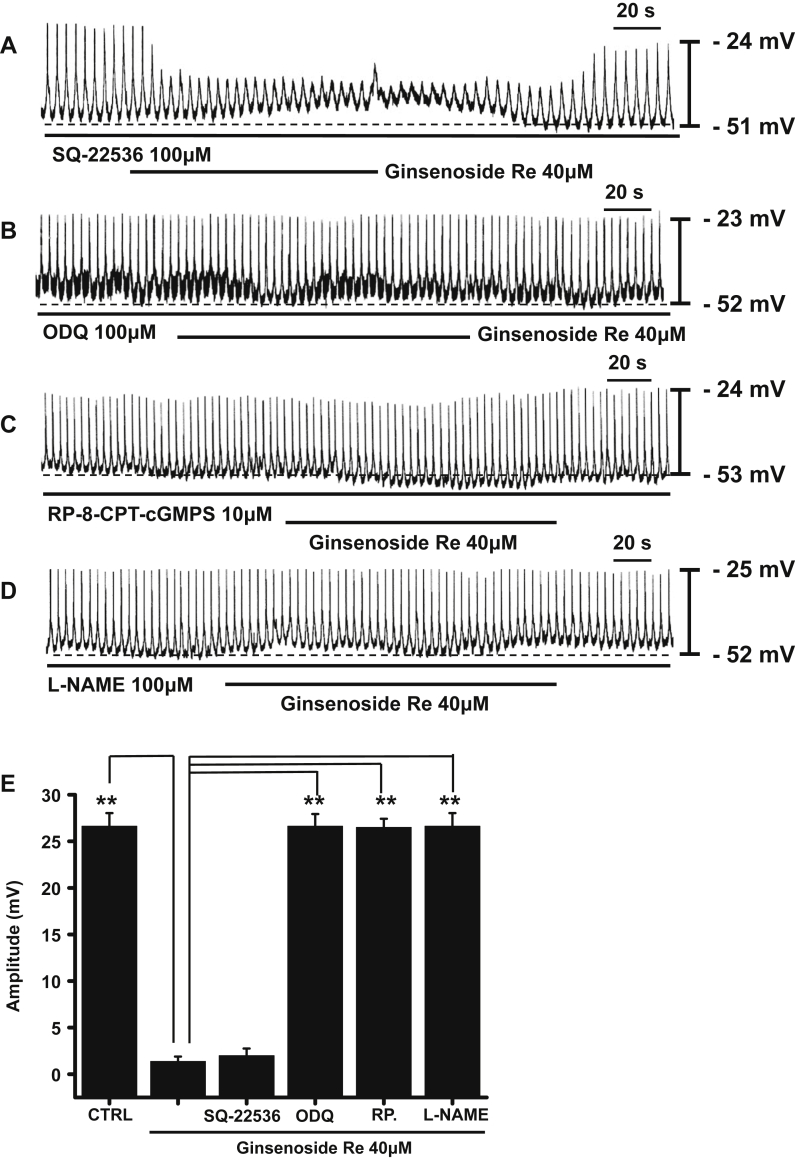
The ICCs formed network-like structures in the culture (12 h). Spontaneous rhythms were routinely recorded from cultured ICCs under current-clamp conditions. The ICCs within the networks displayed more robust electrical rhythms. Tissue-like spontaneous slow waves have been previously recorded from these cells [25], [26]. In the present study, cultured ICC clusters had a mean resting membrane potential of −52.2 ± 3.3 mV and produced electrical pacemaker activity at a frequency of 17.3 ± 2.3 cycles/min and an amplitude of 26.5 ± 2.2 mV (n = 55) at 30°C in the current-clamp mode (Fig. 2A). Ginsenoside Re (20–40μM) decreased the amplitude and the frequency of pacemaker activity in a concentration-dependent manner (Fig. 2B–2D). In the presence of GRe, pacemaker amplitudes were 26.1 ± 1.5 mV (n = 6) at 10μM; 14.5 ± 1.0 mV (n = 5) at 20μM; 11.2 ± 1.3 mV (n = 7) at 30μM; and 1.3 ± 0.5 mV (n = 8) at 40μM (Fig. 2E). The corresponding frequencies were 16.5 ± 1.2 cycles/min, 11.7 ± 1.1 cycles/min, 8.4 ± 0.7 cycles/min, and 1.2 ± 0.4 cycles/min (Fig. 2F). These results suggest that GRe inhibits the pacemaker activity of ICCs in a dose-dependent manner.
Fig. 2.
The effect of GRe on the pacemaker activity of cultured ICC clusters. (A–D) The pacemaker activity of interstitial cells of Cajal (ICCs) exposed to GRe (20–40μM) in the current-clamp mode (I = 0). Ginsenoside Re decreased the amplitude and frequency of the ICC pacemaker activity in a concentration-dependent manner. (E,F) The graphs summarize the responses to GRe. The bars represent the mean ± the standard deviation. ** p < 0.01. CTRL, control; GRe, ginsenoside Re; ICC, interstitial cells of Cajal.
3.2. The effect of potassium channel blockers on pacemaker activity inhibition by GRe
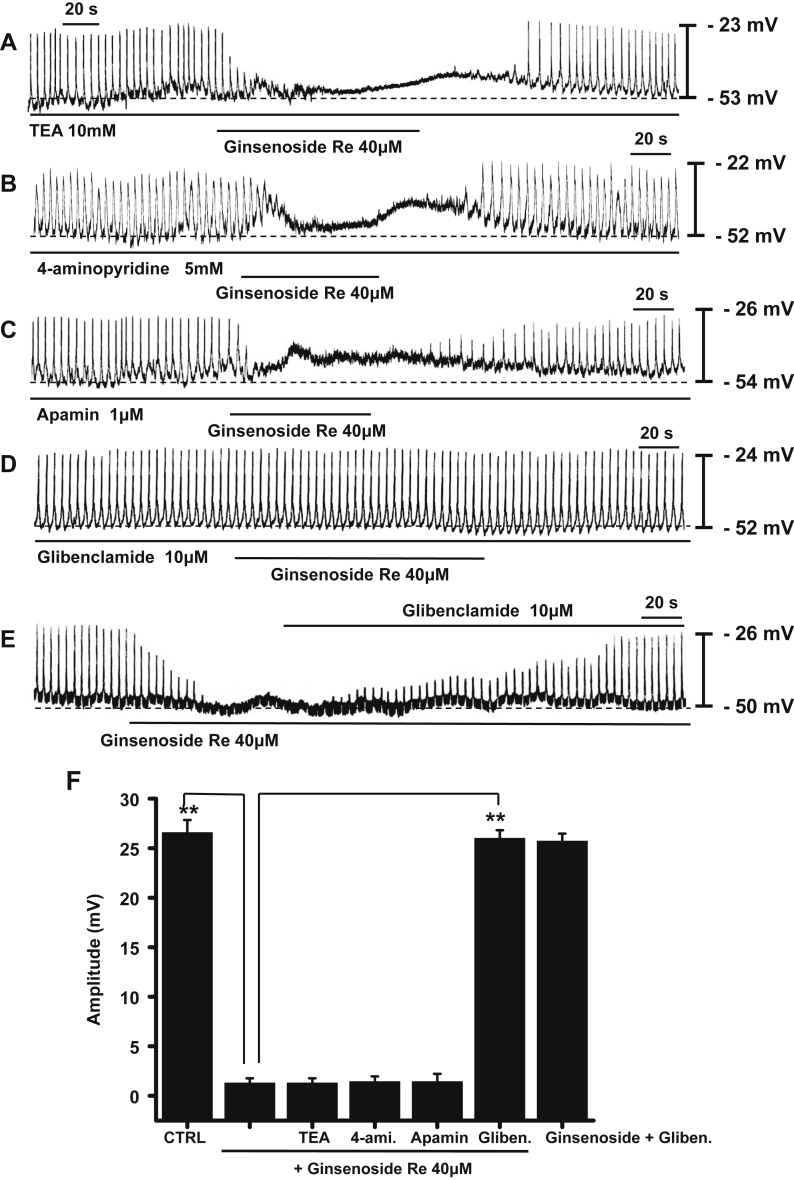
Various types of potassium channel blockers were used to identify the potassium channels that mediate GRe-induced pacemaker activity inhibition. Treatment of ICCs with the Ca2+-activated K+ channel blocker tetraethylammonium chloride (TEA; 10mM) had no effect on pacemaker activity. In the presence of TEA, GRe continued to inhibit pacemaker activity (n = 6; Fig. 3A). In addition, treatment with the transient voltage-dependent K+ channel blocker 4-aminopyridine (5mM) or the Ca2+-activated K+ channel blocker apamin (1 μM) had no effect on pacemaker activity. Ginsenoside Re inhibited pacemaker activity when co-treated with 4-aminopyridine or apamin (n = 6 for each; Fig. 3B and 3C, respectively). However, the ATP-sensitive K+ channel blocker glibenclamide (10μM) blocked pacemaker inhibition by GRe (n = 5, Fig. 3D and 3E), but it did not itself affect pacemaker activity. These results suggest GRe activates ATP-sensitive K+ channels in ICCs.
Fig. 3.
The effects of TEA, 4-aminopyridine, apamin, and glibenclamide on pacemaker activity inhibition by GRe in cultured ICC clusters. Pretreatment with (A) TEA (10mM), (B) 4-aminopyridine (5mM), or (C) apamin (1μM) did not affect the inhibitory effects of GRe. (D) Pretreatment with glibenclamide (10μM) blocked the inhibitory effects of GRe. (E) The GRe-induced inhibitory effects were reversed by adding glibenclamide. (F) The graph summarizes the responses to GRe in the presence of potassium channel blockers. The bars represent the mean ± the standard deviation. ** p < 0.01. 4-ami., 4-aminopyridine; CTRL, control; Gliben., glibenclamide; TEA, tetraethylammonium chloride.
3.3. Involvement of G proteins in pacemaker activity inhibition by GRe
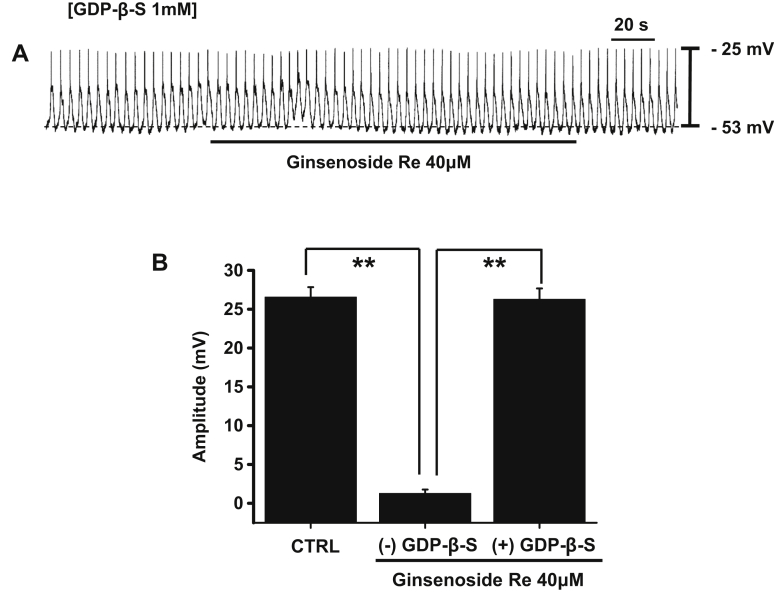
To investigate the signaling mechanisms involved and the role of G proteins during pacemaker activity inhibition by GRe, we added a nonhydrolysable guanosine 5′-diphosphate analogue, guanosine 5′-[β-thio]diphosphate (GDPβS; 1mM), which permanently inactivates GTP-binding proteins [27], [28], to the patch pipette solution. We found that GDPβS prevented pacemaker activity inhibition by GRe (n = 6; Fig. 4A); the pacemaker amplitudes were 26.3 ± 1.4 mV in the presence of GDPβS and 1.3 ± 0.5 mV in its absence (Fig. 4B). These results suggest that G proteins are involved in the inhibition of pacemaker activity by GRe.
Fig. 4.
The effects of GDPβS in the pipette on pacemaker activity inhibition by GRe in cultured ICC clusters. (A) The pacemaker activity of ICCs exposed to GRe in the presence of GDPβS (1mM) in the pipette solution. Under these conditions, the inhibitory effects of GRe on pacemaker activity were blocked. (B) The graph summarizes the responses to GRe in the presence of GDPβS in the pipette. The bars represent the mean ± the standard deviation. ** p < 0.01. CTRL, control; GDPβS, guanosine 5′-[β-thio]diphosphate; GRe, ginsenoside Re; ICC, interstitial cells of Cajal.
3.4. The involvement of guanylate cyclase, protein kinase G, and nitric oxide in the inhibition of pacemaker activity by GRe
To determine whether pacemaker activity inhibition by GRe is mediated by a cyclic nucleotide-dependent pathway, we administered the adenylate cyclase inhibitor SQ-22536 and the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) to the ICCs. Preincubation with SQ-22536 (100μM) for 10 min had no effect on pacemaker activity. In the presence of SQ-22536, GRe (40μM) still inhibited pacemaker activity (n = 6; Fig. 5A). However, ODQ (100μM) blocked pacemaker activity inhibition by GRe (n = 5; Fig. 5B).
Fig. 5.
The effects of SQ-22536 (an adenylate cyclase inhibitor), ODQ (a guanylate cyclase inhibitor), RP-8-CPT-cGMPS (a PKG inhibitor) and L-NAME (a nonselective nitric oxide synthase inhibitor) on pacemaker activity inhibition by GRe in cultured ICC clusters. (A) The pacemaker activity of ICCs exposed to GRe in the presence of SQ-22536 (100μM). SQ-22536 had no effect on the inhibition of pacemaker activity by GRe. The pacemaker activity of ICCs exposed to quercetin in the presence of (B) ODQ (100μM), (C) RP-8-CPT-cGMPS (10μM), or (D) L-NAME (100μM). The inhibitors ODQ, RP-8-CPT-cGMPS, and L-NAME blocked pacemaker activity inhibition by GRe. (E) The graph summarizes the response to GRe in the presence of SQ-22536, ODQ, RP-8-CPT-cGMPS, and L-NAME. The bars represent the mean ± the standard deviation. ** p < 0.01. CTRL, control; GRe, ginsenoside Re; ICC, interstitial cell of Cajal; L-NAME, L-NG-nitroarginine methyl ester; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PKG, protein kinase C; RP, RP-8-CPT-cGMPS.
To determine whether pacemaker activity inhibition by GRe was mediated by cGMP-dependent protein kinase G (PKG), we examined the effects of the PKG inhibitor RP-8-CPT-cGMPS. Preincubation of ICCs with RP-8-CPT-cGMPS (10μM) had no effect on ICC pacemaker activity. However, in the presence of RP-8-CPT-cGMPS, GRe failed to inhibit pacemaker activity (n = 5; Fig. 5C).
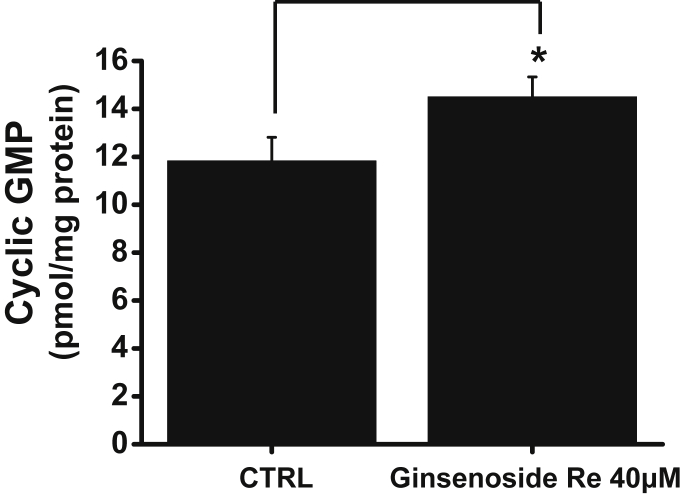
Nitric oxide (NO) activates soluble guanylyl cyclase, which results in the formation of cGMP and the activation of PKG [29]. Thus, to investigate whether pacemaker activity inhibition by GRe is mediated by NO, we treated ICCs with the nonselective nitric oxide synthase (NOS) inhibitor L-NG-nitroarginine methyl ester (L-NAME; 100μM). We found that L-NAME blocked pacemaker activity inhibition by GRe (n = 6; Fig. 5D). In addition, intracellular cGMP contents were measured under basal and GRe-stimulated conditions. Ginsenoside Re stimulated cGMP production (Fig. 6; control 11.8 ± 0.9 pmol/mg protein vs. ginsenoside Re at 14.5 ± 0.8 pmol/mg protein). These results suggest that cyclic GMP, PKG, and NO have roles in the inhibition of pacemaker activity by GRe.
Fig. 6.
The effect of cGMP production on pacemaker activity inhibition by GRe in cultured ICC clusters. Preincubation of the ICCs with GRe significantly stimulated cGMP production. The bars represent the mean ± the standard deviation. * p < 0.05. cGMP, cyclic guanosine monophosphate; CTRL, control; GRe, ginsenoside Re; ICC, interstitial cell of Cajal.
4. Discussion
This study was undertaken to determine the effect of GRe on GI motility by examining its effects on the pacemaker activity of ICCs of the murine small intestine. In these cells, GRe inhibited the pacemaker activity of ICCs via the ATP-sensitive K+ channel and the cGMP/NO-dependent pathway, which suggests that GRe may be a basis for developing novel spasmolytic agents intended to prevent or alleviate GI motility dysfunctions.
Ginsenoside Re has a variety of biological effects. For example, it regulates the intracellular redox state in C6 glioma cells [30], enhances serum specific immunoglobulin (Ig)G, IgG1, IgG2a, and IgG2b responses, and stimulates lymphocyte proliferation responses and the secretions of IFN-gamma and IL-5 [31]. In addition, its neurotrophic and neuroprotective effects enhance memory and learning [32]. Ginsenoside Re-induced intestinal regulation depends on the jejunal contractile state. The stimulatory effects of GRe on jejunal contractility are associated with cholinergic stimulation, but its inhibitory effects are associated with adrenergic activation and NO relaxing mechanisms [33]. Intestinal smooth muscles exhibit different tones, characterized by sustained rhythmic contractions driven by cycles of slow waves [34] that originate in the ICC network in the intestinal tract. Furthermore, ICCs express c-Kit, a tyrosine kinase receptor, which is possibly needed for spontaneous contraction [35], [36]. Xiong et al [37] report that imatinib, a potent inhibitor of c-Kit [38], blocks the GRe-induced regulation of jejunal contractility, which suggests that ICCs are required for GRe-induced intestinal regulation. In the present study, we examined for the first time, the effects of GRe on the pacemaker activity of the ICCs of the murine small intestine.
The ICCs act as the pacemaker cells of the GI tract by generating spontaneous pacemaker potentials and conducting slow waves into smooth muscle syncytium via electrical couplings to neighboring smooth muscle cells [16], [17], [19], which respond to slow wave depolarization by activating L-type Ca2+ channels [39]. Furthermore, the smooth muscle response is regulated by neural inputs, and excitatory and inhibitory enteric motor neurons are both closely associated with ICCs [15]. Thus, ICCs have an important role in the determination and regulation of GI motility [24].
In a previous study, ginseng total saponin depolarized ICC membranes in the current-clamp mode, and this depolarization was dependent on nonselective cation channels, external and internal Ca2+, and the phospholipase C (PLC) pathway [2]. In another study, the ginsenosides Rb1 and Rg3 had no effect on pacemaker activity, although ginsenoside Rf caused membrane depolarization. Application of a flufenamic acid nonselective cation channel blocker or a chloride channel blocker inhibited Rf-induced membrane depolarization, which indicated the involvement of internal or external Ca2+ and the phospholipase C (PLC) pathway in Rf-induced membrane depolarization. However, Rf-induced membrane depolarization was independent of G protein and protein kinase C [3]. Gintonin isolated from ginseng was recently found to activate ginseng-derived G protein-coupled (LPA) receptors [22], [23]. Furthermore, endogenous and exogenous gintonin activate LPA receptors in neuronal and non-neuronal cells; this activation affects cell survival, proliferation, migration, and induces morphological changes [23]. In addition, gintonin [24] was recently found to cause ICC membrane depolarization in the current-clamp mode, but this effect was blocked by Ki16425 (an LPA1/3 receptor antagonist) and by exogenous GDPβS. These effects of gintonin were dependent on PLC, the protein kinase C pathway, and on internal or external Ca2+ regulation. Furthermore, gintonin activated ANO1 channels, but not TRPM7 channels, and in vivo (at concentrations of 10–100 mg/kg, p.o.) significantly increased the intestinal transit rate in normal mice; it also increased GI motility in streptozotocin-induced diabetic mice in a dose-dependent manner [24].
The pacemaker activity of ICCs in the murine small intestine occurs primarily because of periodic activations of TRPM7 [16] or ANO1 channels [20]. Ginsenoside Rf has no effects on TRPM7 or ANO1 channels [3], but GTS and ginsenoside Rg3 block TRPM7 channels [40], [41]. However, the specific details of ion channel involvement during the upstroke and plateau phases of pacemaker potentials in the presence of GRe have not been elucidated. Thus, additional studies are required to identify which ion channels are involved and to determine more precisely the effects of GRe on pacemaker activity. In addition, GRe activated large-conductance Ca2+-activated K+ channels in the arterial smooth muscle cell line A10 in a dose-dependent manner. This GRe effect was inhibited by L-NIO, an endothelial NOS (eNOS) inhibitor. Nakaya et al [33] found SH-6 (an Akt inhibitor) and wortmannin (a PI3-kinase inhibitor) completely blocked the activation of large-conductance Ca2+-activated K+ channels by GRe, which suggests that GRe activates eNOS via a PI3-kinase/Akt-dependent mechanism. It is accordingly our intention to examine the relevance of this PI3-kinase/Akt-dependent mechanism with regard to ICC pacemaking activity.
In conclusion, we found that GRe reduced the amplitude and the frequency of the pacemaker activity of ICCs in a G protein-, cGMP-, PKG-, and NO-dependent manner via ATP-sensitive K+ channel activation. Our findings suggest that GRe is a drug development candidate for the treatment of GI spasms, pain, and transit disturbances associated with GI motility disorders
Conflicts of interest
The authors have no potential conflicts of interest to declare.
Acknowledgments
This work was supported by a National Research Foundation of Korea grant funded by the Korea government Ministry of Science, ICT and Future Planning (Gwacheon, Republic of Korea; grant number, 2014R1A5A2009936).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Nah S.Y. Ginseng: recent advances and trends. Korean J Ginseng Sci. 1997;21:1–12. [Google Scholar]
- 2.Kim H.S., Parajuli S.P., Yeum C.H., Park J.S., Jeong H.S., So I., Kim K.W., Jun J.Y., Choi S. Effects of ginseng total saponins on pacemaker currents of interstitial cells of Cajal from the small intestine of mice. Biol Pharm Bull. 2007;30:2037–2042. doi: 10.1248/bpb.30.2037. [DOI] [PubMed] [Google Scholar]
- 3.Han S., Kim J.S., Jung B.K., Han S.E., Nam J.H., Kwon Y.K., Nah S.Y., Kim B.J. Effects of ginsenoside on pacemaker potentials of cultured interstitial cells of Cajal clusters from the small intestine of mice. Mol Cells. 2012;33:243–249. doi: 10.1007/s10059-012-2204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito H., Tsuchiya M., Naka S., Takagi K. Effects of Panax ginseng root on conditioned avoidance response in rats. Jpn J Pharmacol. 1977;27:509–516. doi: 10.1254/jjp.27.509. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillis C.N. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8. doi: 10.1016/s0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa Y., Shiga Y., Hanyu N., Hashimoto Y., Mukai H., Nishikawa K., Nakamura T. Effect of Chinese herbal medicine on gastrointestinal motility and bowel obstruction. Jpn J Gastroenterol Surg. 1995;28:956–960. [Google Scholar]
- 8.Hashimoto K., Satoh K., Kase Y., Ishige A., Kubo M., Sasaki H., Nishikawa S., Kurosawa S., Yakabi K., Nakamura T. Modulatory effect of aliphatic acid amides from Zanthoxylum piperitum on isolated gastrointestinal tract. Planta Med. 2001;67:179–181. doi: 10.1055/s-2001-11513. [DOI] [PubMed] [Google Scholar]
- 9.Murata P., Hayakawa T., Satoh K., Kase Y., Ishige A., Sasaki H. Effects of Dai-kenchu-to, a herbal medicine, on uterine and intestinal motility. Phytother Res. 2001;15:302–306. doi: 10.1002/ptr.745. [DOI] [PubMed] [Google Scholar]
- 10.Sukrittanon S., Watanapa W.B., Ruamyod K. Ginsenoside Re enhances small-conductance Ca(2+)-activated K(+) current in human coronary artery endothelial cells. Life Sci. 2014;115:15–21. doi: 10.1016/j.lfs.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Lee S., Kim M.G., Ko S.K., Kim H.K., Leem K.H., Kim Y.J. Protective effect of ginsenoside Re on acute gastric mucosal lesion induced by compound 48/80. J Ginseng Res. 2014;38:89–96. doi: 10.1016/j.jgr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su X., Pei Z., Hu S. Ginsenoside Re as an adjuvant to enhance the immune response to the inactivated rabies virus vaccine in mice. Int Immunopharmacol. 2014;20:283–289. doi: 10.1016/j.intimp.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Jang H.J., Han I.H., Kim Y.J., Yamabe N., Lee D., Hwang G.S., Oh M., Choi K.C., Kim S.N., Ham J. Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem. 2014;62:2830–2836. doi: 10.1021/jf5000776. [DOI] [PubMed] [Google Scholar]
- 14.Huizinga J.D., Thuneberg L., Kluppel M., Malysz J., Mikkelsen H.B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 15.Sanders K.M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 16.Kim B.J., Lim H.H., Yang D.K., Jun J.Y., Chang I.Y., Park C.S., So I., Stanfield P.R., Kim K.W. Melastatin-type transient receptor potential channel 7 Is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Koh S.D., Jun J.Y., Kim T.W., Sanders K.M. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B.J., So I., Kim K.W. The relationship of TRP channels to the pacemaker activity of interstitial cells of Cajal in the gastrointestinal tract. J Smooth Muscle Res. 2006;42:1–7. doi: 10.1540/jsmr.42.1. [DOI] [PubMed] [Google Scholar]
- 19.Huizinga J.D., Zhu Y., Ye J., Molleman A. High conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002;123:1627–1636. doi: 10.1053/gast.2002.36549. [DOI] [PubMed] [Google Scholar]
- 20.Zhu M.H., Kim T.W., Ro S., Yan W., Ward S.M., Koh S.D., Sanders K.M. A Ca2+-activated Cl-conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–4918. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y., Mucci A., Huizinga J.D. Inwardly rectifying chloride channel activity in intestinal pacemaker cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G809–G821. doi: 10.1152/ajpgi.00301.2004. [DOI] [PubMed] [Google Scholar]
- 22.Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyo M.K., Choi S.H., Hwang S.H., Shin T.H., Lee B.H., Lee S.M., Lim Y.H., Kim D.H., Nah S.Y. Novel glycolipoproteins from ginseng. J Ginseng Res. 2011;35:92–103. [Google Scholar]
- 24.Kim B.J., Nam J.H., Kim K.H., Joo M., Ha T.S., Weon K.Y., Choi S., Jun J.Y., Park E.J., Wie J. Characteristics of gintonin-mediated membrane depolarization of pacemaker activity in cultured interstitial cells of Cajal. Cell Physiol Biochem. 2014;34:873–890. doi: 10.1159/000366306. [DOI] [PubMed] [Google Scholar]
- 25.Koh S.D., Sanders K.M., Ward S.M. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim B.J., Lee J.H., Jun J.Y., Chang I.Y., So I., Kim K.W. Vasoactive intestinal polypeptide inhibits pacemaker activity via the nitric oxide-cGMP-protein kinase G pathway in the interstitial cells of Cajal of the murine small intestine. Mol Cells. 2006;21:337–342. [PubMed] [Google Scholar]
- 27.Komori S., Kawai M., Takewaki T., Ohashi H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea pig ileal muscle. J Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogata R., Inoue Y., Nakano H., Ito Y., Kitamura K. Oestradiol-induced relaxation of rabbit basilar artery by inhibition of voltage-dependent Ca channels through GTP-binding protein. Br J Pharmacol. 1996;117:351–359. doi: 10.1111/j.1476-5381.1996.tb15198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moncada S., Palmer R.M.J., Higgs E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 30.Ng W.Y., Yang M.S. Effects of ginsenosides Re and Rg3 on intracellular redox state and cell proliferation in C6 glioma cells. Chin Med. 2008;3:1–8. doi: 10.1186/1749-8546-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song X., Chen J., Sakwiwatkul K., Li R., Hu S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int Immunopharmacol. 2010;10:351–356. doi: 10.1016/j.intimp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Christensen L.P., Jensen M. Biomass and content of ginsenosides and polyacetylenes in American ginseng roots can be increased without affecting the profile of bioactive compounds. J Nat Med. 2009;63:159–168. doi: 10.1007/s11418-008-0307-3. [DOI] [PubMed] [Google Scholar]
- 33.Nakaya Y., Mawatari K., Takahashi A., Harada N., Hata A., Yasui S. The phytoestrogen ginsenoside Re activates potassium channels of vascular smooth muscle cells through PI3K/Akt and nitric oxide pathways. J Med Invest. 2007;54:381–384. doi: 10.2152/jmi.54.381. [DOI] [PubMed] [Google Scholar]
- 34.Daigo Y., Takayama I., Ponder B.A., Caldas C., Ward S.M., Sanders K.M., Fujino M.A. Differential gene expression profile in the small intestines of mice lacking pacemaker interstitial cells of Cajal. BMC Gastroenterol. 2003;3:1–6. doi: 10.1186/1471-230X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamazawa T., Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cretoiu S.M., Simionescu A.A., Caravia L., Curici A., Cretoiu D., Popescu L.M. Complex effects of imatinib on spontaneous and oxytocin-induced contractions in human non-pregnant myometrium. Acta Physiol Hung. 2011;98:329–338. doi: 10.1556/APhysiol.98.2011.3.10. [DOI] [PubMed] [Google Scholar]
- 37.Xiong Y., Chen D., Lv B., Liu F., Yao Q., Tang Z., Lin Y. Effects of ginsenoside Re on rat jejunal contractility. J Nat Med. 2014;68:530–538. doi: 10.1007/s11418-014-0831-2. [DOI] [PubMed] [Google Scholar]
- 38.Wu T.J., Lee L.Y., Yeh C.N., Wu P.Y., Chao T.C., Hwang T.L., Jan Y.Y., Chen M.F. Surgical treatment and prognostic analysis for gastrointestinal stromal tumors (GISTs) of the small intestine: before the era of imatinib mesylate. BMC Gastroenterol. 2006;6:1–8. doi: 10.1186/1471-230X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanders K.M., Koh S.D., Ro S., Ward S.M. Regulation of gastrointestinal motility—insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9:633–645. doi: 10.1038/nrgastro.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim B.J., Nah S.Y., Jeon J.H., So I., Kim S.J. Transient receptor potential melastatin 7 channels are involved in ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic Clin Pharmacol Toxicol. 2011;109:233–239. doi: 10.1111/j.1742-7843.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim B.J. The role of ginseng total saponin in transient receptor potential melastatin type 7 channels. Animal Cells Syst (Seoul) 2012;16:376–384. [Google Scholar]