Abstract
This Synthesis highlights a series of recent studies that has systematically interrogated age-related deficits in cold-induced skin vasoconstriction. In response to cold stress, a reflex increase in sympathetic nervous system activity mediates reductions in skin blood flow. Reflex vasoconstriction during cold exposure is markedly impaired in aged skin, contributing to the relative inability of healthy older adults to maintain core temperature during mild cold stress in the absence of appropriate behavioral thermoregulation. This compromised reflex cutaneous vasoconstriction in healthy aging can occur as a result of functional deficits at multiple points along the efferent sympathetic reflex axis, including blunted sympathetic outflow directed to the skin vasculature, reduced presynaptic neurotransmitter synthesis and/or release, and altered end-organ responsiveness at several loci, in addition to potential alterations in afferent thermoreceptor function. Arguments have been made that the relative inability of aged skin to appropriately constrict is due to the aging cutaneous arterioles themselves, whereas other data point to the neural circuitry controlling those vessels. The argument presented herein provides strong evidence for impaired efferent sympathetic control of the peripheral cutaneous vasculature during whole body cold exposure as the primary mechanism responsible for attenuated vasoconstriction.
Keywords: cold stress, hypothermia, skin blood flow, autonomic control
the physiological responses to whole body cold exposure are complex and require the integration of reflex neural signals leading to and originating from the central nervous system, local neurovascular control mechanisms, and appropriate end-organ responsiveness to sympathetic stimuli to protect against convective heat loss in cold environments. The sympathetic nervous system mediates the initial thermoregulatory response to cold exposure—peripheral cutaneous vasoconstriction (52, 62)—to maintain body core temperature and prevent hypothermia.
The cutaneous vasoconstrictor response to cold exposure is markedly impaired in healthy older adults (33, 53, 92, 133, 149), and even during mild cold stress (e.g., ambient temperature = 22°C), older adults exhibit a relative inability to defend body core temperature in the absence of appropriate behavioral thermoregulation or metabolic heat production via shivering (33). Impaired reflex cutaneous vasoconstriction in older adults is apparent even when accounting and controlling for age-related differences in body composition and behavioral thermoregulation that may influence cutaneous vasoconstrictor responsiveness (32, 33). This attenuated reduction in skin blood flow during cooling in older adults leads to excessive heat loss that, if sustained in duration, could result in hypothermia (26, 33, 83, 149). In the United States, exposure to environmental cold is responsible for approximately twice the number of deaths per year than those attributed directly to hyperthermia (e.g., heat stroke) (9), with adults older than 65 years accounting for more than half of all hypothermia-related deaths each year (29, 102). Thus older adults, even in the absence of overt vascular disease or other clinical conditions affecting skin blood flow, are the most susceptible subset of the population to environmental cold exposure. Because the global population of aged individuals is rapidly growing (151a), an increasingly larger subset of the population will be vulnerable to the risks imposed by temperature extremes, which continue to increase in both frequency and severity (47, 101).
The risk factors contributing to morbidity and mortality from environmental cold exposure are undoubtedly multifaceted and include nonphysiological factors such as drug intoxication, altered mental status, social isolation, and socioeconomic status (37a, 78, 102). However, regardless of the cause of prolonged exposure to cold, it is the pronounced age-related impairment in systemic cutaneous vasoconstriction that ultimately determines the resultant physiological and pathological sequelae contributing to increased morbidity and mortality risk in the aged. Therefore, research aimed at elucidating the mechanisms underlying aberrant regulation of the cutaneous circulation during cold exposure in older adults is clinically relevant.
The influence of primary human aging on the various mechanisms mediating reflex cutaneous vasoconstriction has previously been reviewed (22, 71, 72, 84). So why is there a need for this “Synthesis” review? Experimental paradigms to date have focused on distinct individual neural, neurotransmitter, receptor, vascular smooth muscle, or endothelial mechanisms. Multiple sites of age-related alterations have been identified, yet a thorough higher-level analysis of the entire integrated response to cold stress has not been carried out. Do older men and women not appropriately decrease skin blood flow in the cold due to faulty “wiring” (neural) or aged “plumbing” (vascular)? As such, the purpose of this brief Synthesis is to update the literature with mechanistic data from recent studies that have systematically interrogated age-related alterations in sympathetic control of the peripheral cutaneous vasculature during whole body cold exposure. Specifically, we highlight a series of studies suggesting that changes in the neural circuitry responsible for sensing, integrating, and effecting thermoregulatory vasomotor function are responsible for the impaired cutaneous vasoconstrictor response to cold stress in aging. These alterations in “wiring” are distinct from, and supersede, downstream “plumbing” impairments resulting from age-related vascular dysfunction. We have attempted to provide a succinct, yet comprehensive, current interpretation of this area of ongoing research. However, because of space constraints, we have cited a number of reviews in an attempt to direct the reader to additional research in this field.
THERMOREGULATORY CONTROL OF SKIN BLOOD FLOW
Reflex cutaneous vasoconstriction.
The cutaneous circulation is critical for human thermoregulation. In thermoneutral environments, skin blood flow, assessed via venous occlusion plethysmography, typically approximates 30 ml·min−1·100 g−1 of skin (121, 123). This circulation is overperfused relative to its metabolic rate; however, this is beneficial for thermoregulatory function because major reductions in cutaneous blood flow, such as occur with the vasoconstrictor response to skin surface cooling (reductions of ∼3-4°C from a thermoneutral skin temperature of ∼33–34°C), do not threaten the skin with ischemia until the stimulus, and the response, are extreme. The human cutaneous vasculature is innervated by the autonomic nervous system (120), and it is sympathetic adrenergic nerve fibers that mediate cutaneous blood vessel tone during normothermia (core temperature ∼37°C and skin temperature ∼33–34°C), as well the vasoconstriction that occurs in response to cold exposure (77). Surgical sympathectomy (55, 57) or experimental blockade of neurotransmitter release from adrenergic nerve terminals (82, 149) abolished the cutaneous vasoconstrictor response to whole body cooling, clearly demonstrating the critical role of the sympathetic nervous system for an appropriate vascular response.
The sympathetic thermoregulatory reflexes responsible for maintaining body core temperature during cold exposure are activated when mean skin temperature decreases below a thermoneutral temperature (∼34°C). Such decreases in mean skin temperature are sensed by peripheral thermosensors in the skin, and this information is integrated with afferent sensory input from cold-sensitive neurons in the anterior hypothalamic-preoptic area (13, 55), ultimately eliciting peripheral cutaneous vasoconstriction (see Fig. 1). During skin surface cooling to ∼29.5–30°C (33), skin blood flow decreases to a physiological minimum, after which further cooling will not induce additional vasoconstriction (121). This reflex cutaneous vasoconstriction is graded in accordance with stimulus intensity (i.e., decreased whole body mean skin temperature) (72), and during severe cold stress, skin blood flow can approach zero (77, 121).
Fig. 1.
Simplified schematic summary of the central and peripheral mechanisms involved in the thermoregulatory responses to cold exposure. For details, see text. POA, preoptic area; RMR, rostral medullary raphe; IML, intermediolateral cell column; DRG, dorsal root ganglion; NE, norepinephrine; NPY, neuropeptide Y; ATP, adenosine triphosphate; ROCK, Rho-kinase; MLC, myosin light chain; P, phosphate.
Generally, relatively small changes in sympathetic vasoconstrictor activity drive the decreases in skin blood flow necessary to maintain body core temperature, and it is only during extreme or prolonged cold exposure that shivering and nonshivering thermogenesis and the resultant increases in metabolic heat production become important for thermoregulation (16, 23, 69, 76, 123). Although decreases in skin temperature can also occur in response to decreases in internal temperature, core cooling in the absence of skin cooling is typically only observed during specialized medical procedures (20, 89, 151).
Central and peripheral sympathetic organization and pathways.
A brief overview of neural organization is necessary for the understanding of the reflex sympathetic responses to cold exposure (summarized in Fig. 1). In cold environments, sensory signals from cutaneous thermoreceptors ascend through the dorsal horn. Centrally, the preoptic area, located in the rostral pole of the hypothalamus, is thought to be critical for thermoregulation, because it receives and integrates afferent sensory input from thermosensitive neurons in the periphery and the spinal cord (106, 143). Specifically, the median preoptic nucleus appears to be a major preoptic area site that receives afferent cutaneous cool-sensitive inputs (107). Descending signal pathways from the preoptic area regulate the activity of neurons in sympathoexcitatory areas fundamental to the efferent thermoregulatory responses. The sympathetic premotor neurons controlling thermoregulatory vasomotor function are located in the rostral medullary raphe region and, to a lesser extent, in the rostroventrolateral medulla (100, 106, 143, 144). These premotor neurons convey impulses from the brain stem to the spinal cord and make synaptic contacts with sympathetic preganglionic neurons.
The cell bodies of preganglionic sympathetic neurons are located in the intermediolateral column of all thoracic and the two upper lumbar segments of the spinal cord. Preganglionic neurons are cholinergic, thinly myelinated, and conduct at velocities up to 15 m/s. They exit the spinal cord in the ventral roots and white rami to synapse in ipsilateral paravertebral ganglia in the sympathetic chain or bilaterally in prevertebral ganglia, typically located near large blood vessels (30). Stimulation of preganglionic neurons results in activation of postganglionic neurons and subsequent end-organ responses.
Postganglionic neurons are primarily adrenergic, unmyelinated, and conduct at velocities ranging between 0.5 and 1.0 m/s. They innervate target organs, including the heart and the vasculature; most fibers project via gray rami and peripheral nerves to the effectors in the periphery (30). In humans, postganglionic sympathetic vascular nerves generally cause vasoconstriction. Upon discharge or “firing” of sympathetic nerves, norepinephrine (NE; the primary neurotransmitter) is released from sympathetic varicosities, diffuses and binds to postjunctional α-adrenergic receptors, and evokes vascular smooth muscle contraction and thus vasoconstriction (114). In addition to NE, sympathetic neurons also release cotransmitters, including neuropeptide Y (NPY) and adenosine triphosphate (ATP), that contribute to the full vasoconstrictor response (77). During cooling, parallel signaling pathways within the vascular smooth muscle cells themselves, including RhoA/Rho-kinase (ROCK) and angiotensin II, also contribute to cutaneous vasoconstriction (94, 95).
Methods of measuring sympathetic control of the cutaneous vasculature.
Given the relative lack of an appropriate animal model in which to study thermoregulatory control of skin blood flow, the vast majority of the research to date has been performed in humans. Reflex cutaneous vasoconstriction can be experimentally examined by measuring whole body cooling-induced decreases in skin blood flow at a site where local skin temperature has been artificially maintained at thermoneutrality (34°C). The cutaneous vasoconstriction that occurs at those local sites is solely attributed to sympathetic reflex mechanisms, because any potential confounding effects of localized decreases in skin temperature (i.e., locally mediated vasoconstriction) are experimentally eliminated. Cutaneous vasoconstriction is commonly assessed with laser Doppler flowmetry, which is used to measure dynamic changes in laser Doppler flux over a small area of skin in response to vasoreactive stimuli (73, 77). To examine the specific mechanisms mediating cutaneous vasoconstriction, intradermal microdialysis is commonly used. This minimally invasive technique permits the bidirectional exchange of small molecular weight substances through a porous cellulose membrane, which permits continuous drug delivery to a small localized area of skin while simultaneously obtaining a measurement of skin blood flow via laser Doppler flowmetry. Thus laser Doppler flowmetry can be coupled with intradermal microdialysis to pharmacologically probe the signaling pathways and molecular mediators of cutaneous microvascular function contributing to vascular smooth muscle contraction during whole body cooling (77, 138).
The technique of microneurography, originally developed by Hagbarth and Vallbo (65), allows for the direct recording of efferent sympathetic outflow from peripheral nerve fibers (34). Multiunit postganglionic sympathetic nerve activity occurs as bursts of impulses separated by silent periods of varying duration; the bursts occur in different temporal patterns and in responses to differing stimuli/maneuvers in skin and muscle nerve fascicles (34, 35), allowing for the reliable identification of skin (SSNA) and muscle sympathetic nerve activity (MSNA). SSNA bursts may contain vasoconstrictor, vasodilator, sudomotor, and/or pilomotor impulses; during whole body cooling, SSNA is predominantly adrenergic vasoconstrictor in origin (11). In addition to thermoregulatory challenges, SSNA is also highly responsive to arousal stimuli and psychological stress (24). Given the methodological challenges associated with the analysis and interpretation of SSNA, including the lack of cardiac rhythmicity, the irregular shape and bursting pattern, and the potential for multiple different types of impulses to be contained within a single burst of activity, relatively few studies have examined efferent SSNA during cold stress in older adults.
Deficient skin vasoconstriction in aging.
With primary human aging, reflex cutaneous vasoconstriction in response to cooling is impaired. On average, the reduction in skin blood flow during whole body cooling is attenuated by ∼50% in healthy aged humans (60–90 years) compared with young healthy adults (71) (Fig. 2). This deficit has been documented and characterized in detail in a multitude of thermoregulatory studies employing various experimental paradigms to induce cold stress and measure skin blood flow (33, 53, 59, 71, 83, 85, 92–95, 107a, 118, 133, 149). Cumulatively, these studies demonstrate that reflex cutaneous vasoconstriction is markedly impaired in aged skin and suggest that pronounced cutaneous vasomotor dysfunction is widespread in older populations.
Fig. 2.

Reflex vasoconstriction in response to whole body cooling (i.e., a decrease in mean skin temperature from 34°C to 30.5°C) is severely attenuated in older (n = 51; 60–90 yr) compared with young (n = 41; 18–30 yr) humans. Reductions in skin blood flow are expressed as normalized cutaneous vascular conductance (e.g., percentage change from baseline). Tsk, skin temperature. *P < 0.05 vs. Young.
Compromised thermoregulatory vasoconstriction in aged skin is likely due to functional impairments at multiple points along the efferent neural reflex axis, including alterations in sympathetic outflow, axonal biosynthesis, release of NE, and cotransmitter function, as well as functional mechanistic impairments within the vasculature itself (Table 1). The next sections of this Synthesis discuss putative sites within the neural reflex pathway and within the cutaneous vessels at which age-related changes occur.
Table 1.
Potential loci for impairments in cold-induced cutaneous vasoconstriction in aging
| Potential Site of Impairment | Age-Related Alteration | References |
|---|---|---|
| Neural Reflex Axis | ||
| Afferent Thermosensitivity | ? (likely ↓) | – |
| Central Integration | ? | – |
| Efferent SSNA | ↓ | (58, 59) |
| Neuronal NE Synthesis/Release | ↓ | (92, 93, 133, 146, 149) |
| Sympathetic Cotransmitters | ↓ | (149) |
| Vascular Function | ||
| Adrenergic Responsiveness | ⇆ | (59, 148, 158) |
| Second-Messenger Pathways | ||
| RhoA/ROCK | ↑ | (94) |
| Angiotensin II | ↑ | (95) |
POTENTIAL LOCI FOR DEFICIENT SKIN VASOCONSTRICTION IN AGING
Efferent skin sympathetic outflow.
During whole body cooling, decreases in mean skin temperature to 30.5°C resulted in a robust increase in efferent skin sympathetic nerve activity (SSNA) in young adults (28, 58, 59, 124) (Fig. 3). These increases in SSNA during cooling were closely correlated with reductions in skin blood flow (59), confirming that it is primarily an adrenergic vasoconstrictor neural stimulus during cold stress that evokes reflex peripheral vasoconstriction in young skin (11). However, as shown in Fig. 3, the SSNA response to whole body cooling was substantially reduced in healthy older adults (>∼60 yr) (58, 59). This diminished efferent sympathetic outflow directed to the skin contributes substantially to the age-related attenuation in reflex cutaneous vasoconstriction and the resultant relative inability of older adults to adequately reduce skin blood flow during whole body cold exposure (59).
Fig. 3.

Original records of integrated skin sympathetic nerve activity (SSNA; A), as well as group summary data (B), illustrating that whole body cold stress [i.e., gradual decreases in mean skin temperature (Tsk) from 34.0°C to 30.5°C] elicited robust and progressive increases in SSNA (ΔSSNA total activity) in young, but not older, adults. *P < 0.05 vs. older; †P < 0.05 vs. mean Tsk 34.0°C. [Figure adapted with permission from John Wiley and Sons Publishing (59).]
To further examine potential age-related alterations in the central ability to elicit skin sympathetic outflow, Greaney et al. (59) additionally assessed SSNA during the nonthermoregulatory stimulus of mental stress at both thermoneutrality and also during whole body cooling. Aged adults exhibited robust increases in SSNA during mental stress superimposed on whole body cooling (59). These data suggest that a central inability to elicit further increases in SSNA does not explain the lack of an increase in skin sympathetic outflow during cooling in healthy aging. Age-related deficits in efferent SSNA during whole body cooling are instead likely the result of alterations in either afferent signaling from cutaneous thermoreceptors or central integration of converging afferent signals or both. It is important to note that the lack of increase in efferent sympathetic outflow during whole body cooling in older adults appears confined to neural traffic directed to the skin circulation, because the MSNA response to acute cold stress was augmented in older adults, likely contributing to altered blood pressure regulation (61). These differential findings regarding sympathetic outflow during cold stress in older adults are perhaps not surprising given the functional selectivity and differentiation of sympathetic nerve traffic (24).
Sympathetic neurotransmitters.
As noted above, the vasoconstrictor response to whole body cooling is entirely dependent on sympathetic stimulation of perivascular nerves and the subsequent release of sympathetic transmitters (135, 137, 149). In young adults, the adrenergic neurotransmitter NE mediates ∼60% of the vasoconstrictor response during graded whole body cooling, because selective postsynaptic antagonism of both α- and β-adrenergic receptors in the cutaneous circulation reduced, but did not eliminate, vasoconstriction (82, 135, 136, 149). Therefore, the remaining ∼40% of the vasoconstrictor response is mediated by nonadrenergic sympathetic cotransmitters.
NPY and ATP, the two most likely nonadrenergic cotransmitters, are produced and coreleased from perivascular nerve endings with NE in multiple vascular beds (14, 17–19, 49, 66, 98). NPY, which acts postjunctionally through Y1 receptors, modulates cutaneous blood flow in animal models (67, 105). A role for NPY in reflex cutaneous vasoconstriction in humans was initially established by Stephens et al. (137), who demonstrated that intradermal microdialysis administration of BIBP-3226 (an antagonist for the NPY peptide fragment) blunted cold stress-induced cutaneous vasoconstriction. Furthermore, when both NPY and adrenergic inhibition were combined, the cutaneous vasoconstrictor response to cooling was abolished (137), suggesting that both NPY and NE play critical additive roles in reflex cutaneous vasoconstriction. This notion is strengthened by multiple studies indicating a synergistic effect of NPY on adrenergic receptor function in a number of tissues, including human skin (48, 54, 66, 98, 115, 116, 135, 137, 155).
In contrast to the aforementioned findings that NPY contributes to reflex vasoconstriction in young skin (137), in our hands, local administration of BIBP-3226 did not affect any portion of the reflex cutaneous vasoconstrictor response to whole body cooling in young adults (146), arguing against a functional role for NPY as a sympathetic cotransmitter during cold stress. This observed lack of an effect of NPY blockade during whole body cooling was surprising, especially given the evidence supporting a role for NPY as a neurotransmitter in the cutaneous vasculature. The reason(s) for the apparent differences regarding NPY function in the human cutaneous vasculature is not entirely clear, but may be due to methodological differences between studies, specifically the rate of skin surface cooling, which can influence axonal release of NPY. Higher nerve stimulation frequencies promote NPY release (98, 132, 155); therefore, because higher rates of skin cooling elicit greater increases in skin sympathetic outflow (124), the faster cooling rate used by Stephens and colleagues (137) may have yielded greater increases in SSNA sufficient to facilitate the release of the large dense core vesicles that contain NPY. Conversely, the slower rate of cooling used in our laboratory (146), a cooling rate designed to elicit progressive gradual declines in mean skin temperature without altering core temperature or inducing shivering (71, 149, 157), may be insufficient to stimulate the release of NPY from adrenergic nerve terminals. Future research aimed at determining the functional importance of NPY as a sympathetic cotransmitter in the cutaneous vasculature during cold exposure is therefore warranted.
ATP is also costored with NE in small vesicles in sympathetic adrenergic nerves (17, 18, 98) and is therefore a plausible sympathetic cotransmitter candidate for reflex cutaneous vasoconstriction. Cold-induced neurogenic vasoconstriction of canine cutaneous veins appears to be mediated primarily via purinergic sympathetic transmission (51). This cooling-induced reduction in skin blood flow was mediated by the release of ATP from sympathetic nerves and the subsequent stimulation of presynaptic purinergic receptors on sympathetic nerve terminals, facilitating the release of NE and resulting in vasoconstriction (86). In humans, ATP-induced vasoconstriction in thermoregulatory vascular beds occurs in response to low-frequency electrical nerve stimulation (14, 115, 117) and lower body negative pressure (142) and is mediated by P2X purinergic receptors located on vascular smooth muscle cells. However, given the methodological challenges associated with inhibiting ATP in humans, in addition to the multiple sites of action for ATP on both the endothelium and vascular smooth muscle cells and the potential for ATP to cause either constriction or dilation depending upon the purinergic receptor subtype activated, a role for ATP in the sympathetic control of the cutaneous vasculature during whole body cooling has not yet been determined.
Age-related impairments in the sympathetic control of reflex cutaneous vasoconstriction include alterations in the relative contribution of both adrenergic neurotransmitter- and nonadrenergic cotransmitter-mediated effects (71, 72, 77). As noted above, in young adults, reflex cutaneous vasoconstriction is mediated by the neural release of both NE and sympathetic cotransmitters (135, 137, 149). However, in healthy older adults, the cotransmitter-mediated portion of cutaneous vasoconstriction is functionally absent (149) (Fig. 4); therefore, older adults instead rely entirely on an impaired adrenergic mechanism to elicit reductions in skin blood flow during cold exposure (83, 149). The decrements in both NE- and cotransmitter-mediated constriction are due, at least in part, to age-related reductions in transmitter synthesis and release (27, 42).
Fig. 4.

Cotransmitter-mediated portion of reflex cutaneous vasoconstriction is functionally absent in healthy older adults. When adrenergic vasoconstriction was blocked (Y+P, yohimbine + propranolol), significant, yet attenuated, reflex vasoconstriction was noted in young adults; however, the vasoconstrictor response during whole body cooling was completely abolished in older adults. Cutaneous vasoconstriction was completely absent in both groups when sympathetic release of axonal contents was blocked (BT, bretylium tosylate). *P < 0.05 vs. baseline; †P < 0.05 vs. control; ‡P < 0.05 vs. young. [Figure adapted with permission from John Wiley and Sons Publishing (149).]
Presynaptic neurotransmitter synthesis and release.
NE synthesis in the presynaptic nerve terminal is dependent upon the activity of tyrosine hydroxylase (TH), the rate-limiting enzyme in prejunctional catecholamine biosynthesis that catalyzes the hydroxylation of tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA) (81, 88, 103). The cofactor tetrahydrobiopterin (BH4), which is found throughout neural and vascular tissue, is essential for the functional activity of TH. Additionally, BH4 is a powerful reductant; it maintains TH in its active ferrous form by reducing the iron moiety of TH, thereby priming TH for catalytic reaction (46, 81, 88, 150, 152). Consequently, NE biosynthesis requires adequate BH4 bioavailability. Both de novo synthesis and the recycling pathways for BH4 production are highly sensitive to oxidation, such that BH4 bioavailability decreases with increased oxidant stress. Indeed, the induction of oxidant stress in cultured sympathetic neurons decreased BH4 concentrations by ∼90%, resulting in a significant reduction in catecholamine synthesis (96). Primary aging, even in the absence of pathology, is associated with a systemic net increase in reactive oxygen and nitrogen species, as a result of both increased production and decreased clearance (36, 90, 130), ultimately leading to oxidant damage and attendant pathologies. This pro-oxidant cellular environment depletes intraneuronal BH4 bioavailability in aged skin (36, 90), which limits TH activity and consequent NE synthesis, thereby functionally contributing to impaired sympathetic control of cutaneous vasoconstriction during cold stress (92, 93, 133).
The potential for blunted NE synthesis and subsequent axonal release from sympathetic nerves to contribute to impaired reflex cutaneous vasoconstriction has been extensively examined in a series of studies designed to functionally restore peripheral NE bioavailability to the peripheral vasculature (92, 93, 133). Local administration of exogenous BH4 to the cutaneous vasculature via intradermal microdialysis improved reflex vasoconstriction in aged skin to the extent that the decrease in skin blood flow during cooling was not different from that noted in young adults (92, 93). Additionally, intradermal microdialysis administration of BH4 in aged skin restored the cutaneous vasoconstrictor response to tyramine, a monoamine that evokes NE release from storage vesicles in postganglionic sympathetic nerve terminals (92, 93). Furthermore, when the amino acid substrate for TH, l-tyrosine, was locally administered to the cutaneous vascular bed, reflex vasoconstriction was also improved in older adults, but there was no additive effect of combining BH4 and tyrosine (93). Collectively, the data from this stepwise series of studies suggest that reductions in NE synthesis, due to decreases in both functional substrate (l-tyrosine) and critical cofactor (BH4) for TH, contribute to attenuated sympathetically mediated cutaneous vasoconstriction.
Recently, a study was performed in which acute oral administration of pharmaceutical BH4 (sapropterin) restored reflex cutaneous vasoconstriction in older adults (133). Presumably, this restoration occurred by augmenting NE biosynthesis and storage in the perivascular nerve terminals, thereby allowing for greater NE release during the sympathetic stimulation that accompanies progressive decreases in mean skin temperature. Interestingly, emerging evidence indicates that oral sapropterin may also centrally modulate efferent sympathetic outflow (113). However, future studies directly investigating the potential for strategies targeted at increasing efferent SSNA to similarly restore reflex cutaneous vasoconstriction in healthy aging are necessary. Nevertheless, the collective evidence suggests that the attenuated cutaneous vasoconstrictor response to cold exposure in older adults is due, at least in part, to a reduced rate of neurotransmitter synthesis and release from sympathetic nerve endings and consequent lower synaptic concentration of NE.
POSTJUNCTIONAL AND VESSEL RESPONSES
Vascular smooth muscle responsiveness.
As noted above, sympathetic cotransmitter mechanisms are functionally absent in the cutaneous vasculature of healthy older adults (149) and they instead rely solely on (impaired) noradrenergic mechanisms for vasoconstriction. Consequently, reductions in cutaneous adrenergic sensitivity and altered end-organ responsiveness could conceivably also contribute to the relative inability of the sympathetic nervous system to evoke adequate peripheral vasoconstriction during whole body cooling in older skin. Peripheral cutaneous vascular responsiveness to adrenergic neurotransmitters or nonadrenergic cotransmitters is typically examined by using graded intradermal microdialysis administration of the transmitter of interest (NE, NPY, or ATP) to the cutaneous vasculature at thermoneutrality (skin temperature of ∼33–34°C). In this experimental approach, upstream alterations in efferent sympathetic outflow and transmitter synthesis/release are bypassed, allowing for the direct assessment of cutaneous vasoconstriction in response to the given pharmacological stimulus.
The generally accepted notion has been that exogenous NE-mediated cutaneous vasoconstriction was blunted in older adults (148). However, more recent evidence appears to refute these earlier findings, demonstrating instead that the sensitivity to exogenous NE (quantified as the logEC50 of a NE dose-response curve) was remarkably similar between young and older adults when assessed in the skin of both the forearm (158) and the lateral calf (59). Although the reason(s) for the contrasting conclusions between studies are not entirely clear, methodological differences such as study protocol, mathematical modeling, and statistical analyses may have contributed. When the data previously collected in our laboratory (148) were reanalyzed using sigmoidal dose-response curves with variable slope and generated using four-parameter nonlinear regression modeling (59, 60, 156, 158), no age-related differences in cutaneous vascular responsiveness to exogenous NE were observed (young: −6.00 ± 0.23 vs. older: −5.72 ± 0.69%ΔCVCbase, P = 0.66), which is consistent with aforementioned more recent findings (59, 158). Although maximal vasoconstriction in response to exogenous NE, an additional parameter obtained from a statistical analysis of the sigmoidal dose-response curves, was blunted in older adults (59, 148), this only occurs at supraphysiological doses of NE (e.g., 10−2 M) that far exceed those that occur during whole body cooling (53, 149). Thus altered end-organ responsiveness to adrenergic stimuli likely does not contribute to impaired peripheral cutaneous vasoconstriction during cold exposure in healthy aging.
Although vascular α-adrenergic receptor expression is downregulated with aging in rodent models (97), this has not been tested in the human cutaneous microvasculature. Therefore, because older adults have a functionally absent nonadrenergic contribution (149), coupled with profoundly attenuated increases in efferent skin sympathetic outflow during cooling (58, 59), reflex cutaneous vasoconstriction remains impaired, even in the face of preserved cutaneous vascular responsiveness to NE itself. Furthermore, during whole body cooling—a physiological stimulus that evokes endogenous NE release—the slope of the relation between cutaneous sympathetic outflow and skin blood flow was similar between young and older adults (59) (Fig. 5). These functional data lend further support to the concept that the relative inability of older adults to decrease skin blood flow during whole body cold stress is not a result of a diminished ability of the cutaneous vasculature to respond to an increase in efferent SSNA. Rather, these data suggest that the vascular transduction of SSNA to reductions in skin blood flow during whole body cold stress is preserved in older adults. Taken together, the maintained ability of the aged cutaneous vasculature to respond to a given pharmacological adrenergic stimulus (exogenous NE), as well as to a given physiological increase in SSNA during cooling (albeit in a reduced operating range), suggests that the deficits in reflex cutaneous vasoconstriction characteristic of healthy human aging result from functional impairments within the neural reflex axis and not peripheral vascular sensitivity to adrenergic stimuli.
Fig. 5.
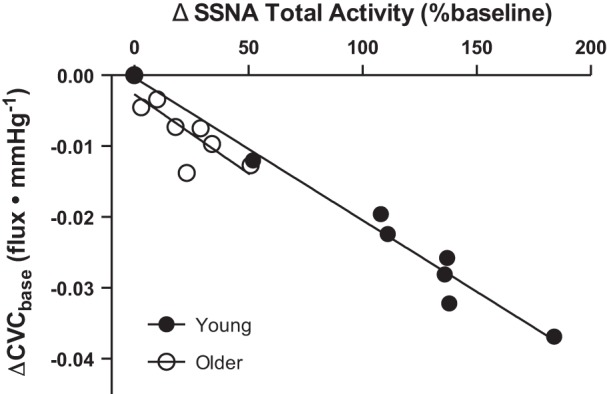
There is a significant relation between the change in SSNA (ΔSSNA Total Activity) and cutaneous vascular conductance (ΔCVC) during whole body cooling in both young (●) and older adults (○); thus the impaired efferent sympathetic response to cooling observed in aging was linearly related to impairments in reflex cutaneous vasoconstriction. However, the slope of the relation was similar between age groups, suggesting that the relative inability of older adults to decrease skin blood flow during whole body cooling is not a result of diminished sensitivity of the reflex response. [Figure adapted with permission from John Wiley and Sons Publishing (59).]
Relatively little research has been targeted at examining potential age-related alterations in cutaneous vascular sensitivity to NPY and ATP. Exogenous NPY-mediated venoconstriction in the dorsal hand was attenuated in older adults (91). Although this may influence sympathetic control of the venous circulation with advancing age, a direct role for altered NPY responsiveness in the cutaneous microvasculature of aged adults requires additional research. This has been methodologically challenging because of the large molecular weight and complex protein interactions of the NPY molecule. Similarly, ATP-mediated vasoconstriction in isolated mesenteric arteries was profoundly reduced in old rats (87). To date, no studies have examined the cutaneous vascular responsiveness to exogenous ATP in aged humans. Paradoxically, ATP, which is coreleased with NE from multiple nerve types in humans and animals (17, 18, 43, 154), also causes cutaneous vasodilation (44). Therefore, the vascular effects of ATP are complex, and its precise role in contributing to age-related decrements in cutaneous vasoconstriction during cold exposure has yet to be fully elucidated.
Insights from the skeletal muscle circulation.
As noted above, although the precise mechanisms controlling skin sympathetic vascular transduction remain to be fully elucidated, some insights might be gained by examining the molecular mechanisms mediating sympathetic vascular transduction in the skeletal muscle circulation. Spontaneous bursts of MSNA evoke α-adrenergic receptor-mediated decreases in vascular conductance in large arteries in young adults, suggesting that increased transmission of NE across the synaptic cleft causes the corresponding vasomotor response (50). The magnitude of the vasoconstrictor response was graded with the preceding MSNA burst pattern and burst cluster size (50); speculatively, these burst variants may represent the ability of the central nervous system to modulate the rate and amount of NE release (80, 110) as a means to ensure an appropriate vasomotor response. Furthermore, studies have reported a blunted rise in blood pressure after a spontaneous burst of MSNA in older adults (140, 153), suggesting potential age-related impairments in sympathetic vascular transduction to resistance arterioles. Thus age-related changes in the ability of MSNA to modulate vascular tone may contribute to altered blood pressure regulation during cold stress in older adults (61, 68, 157); however, this requires further study to extrapolate these findings to the skin circulation.
Preserved vascular adrenergic sensitivity with healthy aging appears to be limited to the cutaneous vasculature. Previous studies in aged humans report reduced postjunctional adrenergic responsiveness in the skeletal muscle circulation (31, 39, 40, 70), a vascular bed critical for blood pressure regulation. Perhaps surprisingly, the contribution of sympathetic α-adrenergic-mediated vasoconstriction in the forearm was reduced with age (39), despite the progressive age-related increases in basal MSNA (109, 141). The reduction in vascular adrenergic sensitivity in aging occurs in response to both exogenous administration of NE (70) and to endogenous NE release evoked via tyramine (39). Interestingly, augmented adrenergic vasoconstriction has been documented to be primarily responsible for decreases in resting leg blood flow in older adults (41); however, whether this reflects less of an attenuation in adrenergic sensitivity in the leg muscle vasculature is not completely understood. These age-related reductions in sympathetic vasoconstrictor responsiveness in the skeletal muscle circulation have yet to be examined during whole body cold stress. This is clinically important because such functional alterations may have direct implications for impaired thermal-cardiovascular integration in the aged population.
Second-messenger signaling pathways.
During cold exposure, multiple parallel second-messenger signaling pathways increase intracellular calcium in smooth muscle cells, subsequently causing vascular smooth muscle contraction. These intracellular signaling pathways thereby mediate cutaneous vasoconstriction during skin cooling, and function separately from, as well as in concert with, the sympathetic control mechanisms that occur primarily via adrenergic receptor activation. The Rho-kinase (ROCK) pathway is a proconstrictor vascular control mechanism that serves as an important alternative mediator of reflex cutaneous vasoconstriction during whole body cooling. ROCK pathways remain functionally intact, and may even be upregulated, in aged skin (94, 147).
ROCK can be stimulated directly by NE and by mitochondrial-derived superoxide, as occurs during localized skin cooling (2, 7). Once activated, ROCK elicits vascular smooth muscle contraction by two distinct mechanisms: 1) inhibition of myosin light chain phosphatase and maintenance of myosin light chain phosphorylation without additional calcium influx (calcium sensitization) and 2) translocation of α2c-adrenergic receptors from the Golgi apparatus to the cell membrane (2, 25, 74). The cutaneous vasoconstrictor response to mild skin cooling was attenuated by the local inhibition of the ROCK signaling pathway; this attenuation was noted in both young and older adults (94). However, during sustained whole body cold stress (i.e., a decrease in mean skin temperature to 30.5°C), ROCK inhibition blunted vasoconstriction only in older adults and had no effect in young adults (94). ROCK inhibition also blunted pharmacologically induced vasoconstriction (exogenous NE) in aged skin (94). Collectively, these data suggest that older adults rely to a greater extent on ROCK-dependent signaling pathways to elicit reflex cutaneous vasoconstriction. Interestingly, phenylephrine, an α-adrenergic agonist, stimulates superoxide production (79), which subsequently elicits ROCK-mediated vasoconstriction (75). Thus the globalized age-related increases in oxidant stress may tonically increase ROCK activity and responsiveness to adrenergic receptor activation, thereby contributing to the alteration in vascular signaling pathway function during cooling in older adults; however, this potential requires further study. Despite activation of this additional intracellular signaling pathway during whole body cooling-induced decreases in skin temperature, reflex vasoconstriction remains impaired in older adults, even in the face of greater activation of ROCK-dependent mechanisms during cooling.
The human cutaneous circulation also expresses a renin angiotensin system for the production and activation of angiotensin II (134). Angiotensin II participates in cutaneous vasomotor function via activation of AT1 receptors (139), which activate downstream intracellular targets, including ROCK (15, 64, 126). Aging is associated with increased AT1 receptor density (45, 129) and sensitivity (8, 160), and given the increased reliance on ROCK-dependent mechanisms to elicit reflex vasoconstriction in older adults, age-related alterations in angiotensin II function within the cutaneous vasculature, representing an additional potential mediator of reflex vasoconstriction. A recent study demonstrated that AT1 receptor inhibition attenuated reflex vasoconstriction during whole body cooling in older, but not young, adults (95). Furthermore, exogenous angiotensin II elicited greater vasoconstriction in older adults, a response that was abolished during ROCK inhibition (95). Cumulatively, these data suggest that endogenous angiotensin II contributes importantly to reflex cutaneous vasoconstriction via a ROCK-mediated mechanism in healthy older adults, presumably functioning to support the severely compromised adrenergic-mediated vasoconstriction during whole body cooling.
From a thermoregulatory standpoint, increased ROCK-mediated cutaneous vasoconstriction is likely beneficial in mitigating excessive heat loss during cold exposure in older adults. However, it is important to note that this may be occurring at the expense of microvascular function. Indeed, multiple cardiovascular diseases are characterized by increased ROCK activity (12, 38, 99, 119, 122, 126, 131). Therefore, increased ROCK activation during cold exposure in older adults may signal a pathogenic shift toward preclinical signaling mechanisms indicative of globalized vascular dysfunction.
CONCLUSIONS AND FUTURE DIRECTIONS
A longstanding paradigm has been that aged arterioles in human skin are less capable of constricting during body cooling. Clearly there are subtle changes in the vessels themselves (e.g., alterations in second-messenger signaling, including a greater reliance on ROCK-dependent pathways) that may contribute to this deficit. However, a series of sequential investigations performed over that past decade has shown that the age-related impairment in sympathetic control of reflex cutaneous vasoconstriction can be attributed to decrements in 1) efferent sympathetic outflow directed to the skin, 2) a functional loss of sympathetic cotransmitters, and 3) reduced sympathetic biosynthesis and release of NE, occurring through decreased BH4 and tyrosine bioavailability. Collectively, these findings suggest that neural deficits, likely arising from altered afferent signaling from cutaneous thermoreceptors or the central processing of converging inputs at the level of the hypothalamus, or both, contribute to altered sympathetic control of the cutaneous microvasculature during whole body cold stress and the resultant reductions in reflex cutaneous vasoconstriction in healthy older adults.
There is little direct physiological evidence of afferent thermoregulatory impairments with aging; however, behavioral thermoregulatory alterations are apparent. For instance, the elderly have a decreased ability to perceive cold, indicative of reduced thermal sensitivity (53, 108, 145). Consistent with this notion, in older adults, the core temperature threshold at which the thermoregulatory vasoconstrictor response is initiated is reduced (53). Collectively, an age-related decrease in thermal sensitivity provides indirect support for the concept that the afferent arm of the reflex arc controlling cold-induced vasoconstriction is impaired in human aging. Several factors have been hypothesized to account for age-related changes in thermal sensitivity, including age-related reductions in the density of sensory epidermal nerve fibers (10, 112), skin vascularity (21), and transmission properties of the peripheral nervous system (63). To date, no reports have directly examined age-related changes in cold-sensitive afferent fibers. Alterations in the skin afferent signals mediating reflex cutaneous vasoconstriction during cold stress are methodologically challenging to precisely quantify in humans, because there are few experimental approaches to reduce, or abolish, sensory feedback. The role of muscle afferent sensory fibers in eliciting the neurocardiovascular responses to exercise have been probed using lumbar intrathecal fentanyl to block the central project of μ-opioid-receptor sensitive group III/IV fibers during lower limb dynamic exercise (1, 37); however, this highly invasive experimental paradigm has not yet been used to examine thermosensitive afferent fibers.
One recent study tested the contribution of skin thermoreceptors to the cardiovascular adjustments that occur in response to heat stress in humans. This was achieved by comparing the cardiovascular responses to heating of sensate skin in able-bodied adults to heating of insensate skin in the legs of adults with a thoracic spinal cord injury (128). In this elegant experimental design, the central nervous system in the thoracic spinal cord injury group does not receive afferent input indicative of elevated skin temperatures from the insensate region. Heat stress altered several indices of cardiovascular function in these individuals, suggesting that skin thermoreceptors contribute, as least in part, to the cardiovascular adjustments to thermal challenges (128). Whether a similar role exists during cold exposure or whether there are alterations in afferent sensory nerve fiber function with healthy aging remains unknown.
The central circuitry responsible for integrating and processing thermal stimuli has been almost exclusively obtained from experiments using rodents (106, 107). The preoptic area appears critical for the central integration of afferent signals and the generation of efferent command signals that coordinate the peripheral thermoregulatory response (106). Recently, fMRI studies in humans have reported that body cooling activates the parabrachial nucleus and rostral medullary raphe (100, 125), supporting involvement of similar central circuitry to the human thermoregulatory system. Potential age-related changes in central brain function during whole body cold stress have not yet been performed.
PERSPECTIVES AND SIGNIFICANCE
Given the significant increase in cardiovascular and thermoregulatory risk during environmental cold exposure in older adults (29, 104, 111, 127, 159), understanding the mechanisms underlying impaired thermal-cardiovascular integration in this population is clinically important. There are a multitude of clinically significant disease states that increase in severity with aging and likely further influence sympathetic regulation of cutaneous vasoconstriction. Advancing age is the primary risk factor for the development of cardiovascular disease (56); therefore, understanding the neural mechanisms mediating impaired reflex cutaneous vasoconstriction during cold exposure in healthy older adults provides novel insight for elucidating potential alterations in sympathetic neural control of the vasculature in cardiovascular pathophysiology.
GRANTS
The primary research funding from the authors' laboratories that contributed to this Synthesis was National Heart, Lung, and Blood Institute Grant (NHLBI) HL120471-01 (to J.L.G.), National Institute on Aging Grant AG007004-23 (to W.L.K.), and NHLBI Grant HL093-238-04 (to L.M.A.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.G., L.M.A., and W.L.K. conception and design of research; J.L.G., L.M.A., and W.L.K. performed experiments; J.L.G., L.M.A., and W.L.K. analyzed data; J.L.G., L.M.A., and W.L.K. interpreted results of experiments; J.L.G. prepared figures; J.L.G. drafted manuscript; J.L.G., L.M.A., and W.L.K. edited and revised manuscript; J.L.G., L.M.A., and W.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of colleagues whose research contributed to this synthesis. We also acknowledge time and effort expended by all the volunteer subjects who participated in research in our laboratories.
REFERENCES
- 1.Amann M, Sidhu SK, Weavil JC, Mangum TS, Venturelli M. Autonomic responses to exercise: group III/IV muscle afferents and fatigue. Auton Neurosci 188: 19–23, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res 94: 1367–1374, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol 289: H243–H250, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Barrett-O'Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE, Sander M, Richardson RS, Wray DW. Angiotensin II potentiates alpha-adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124: 413–422, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Berko J, Ingram DD, Saha S, Parker JD. Deaths attributed to heat, cold, and other weather events in the United States, 2006–2010. Natl Health Stat Reports 76: 1–15, 2014. [PubMed] [Google Scholar]
- 10.Besne I, Descombes C, Breton L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol 138: 1445–1450, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vasoconstrictor outflow in human cutaneous nerves. J Physiol 306: 537–552, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA 101: 9121–9126, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol (1985) 100: 1347–1354, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Bradley E, Law A, Bell D, Johnson CD. Effects of varying impulse number on cotransmitter contributions to sympathetic vasoconstriction in rat tail artery. Am J Physiol Heart Circ Physiol 284: H2007–H2014, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Bregeon J, Loirand G, Pacaud P, Rolli-Derkinderen M. Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am J Physiol Cell Physiol 297: C1062–C1070, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Brengelmann GL, Savage MV. Temperature regulation in the neutral zone. Ann NY Acad Sci 813: 39–50, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Burnstock G. Purinergic cotransmission. Brain Res Bull 50: 355–357, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G, Knight GE, Greig AV. Purinergic signaling in healthy and diseased skin. J Invest Dermatol 132: 526–546, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Campos JM, Paniagua P. Hypothermia during cardiac surgery. Best practice & research. Clin Anaesthesiol 22: 695–709, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Cerimele D, Celleno L, Serri F. Physiological changes in ageing skin. Br J Dermatol 122, Suppl 35: 13–20, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Charkoudian N. Mechanisms and modifiers of reflex induced cutaneous vasodilation and vasoconstriction in humans. J Appl Physiol (1985) 109: 1221–1228, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc Sport Sci Rev 28: 108–112, 2000. [PubMed] [Google Scholar]
- 24.Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Comprehens Physiol 4: 825–850, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol 278: H1075–H1083, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Collins KJ, Dore C, Exton-Smith AN, Fox RH, MacDonald IC, Woodward PM. Accidental hypothermia and impaired temperature homoeostasis in the elderly. Br Med J 1: 353–356, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connat JL, Busseuil D, Gambert S, Ody M, Tebaldini M, Gamboni S, Faivre B, Quiquerez AL, Millet M, Michaut P, Rochette L. Modification of the rat aortic wall during ageing; possible relation with decrease of peptidergic innervation. Anat Embryol 204: 455–468, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Cui J, Sathishkumar M, Wilson TE, Shibasaki M, Davis SL, Crandall CG. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol 290: H1601–H1609, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Curriero FC, Heiner KS, Samet JM, Zeger SL, Strug L, Patz JA. Temperature and mortality in 11 cities of the eastern United States. Am J Epidemiol 155: 80–87, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998. [DOI] [PubMed] [Google Scholar]
- 32.DeGroot DW, Havenith G, Kenney WL. Responses to mild cold stress are predicted by different individual characteristics in young and older subjects. J Appl Physiol (1985) 101: 1607–1615, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Degroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol 292: R103–R108, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. [DOI] [PubMed] [Google Scholar]
- 35.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972. [DOI] [PubMed] [Google Scholar]
- 36.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol 586: 1161–1168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dempsey JA, Blain GM, Amann M. Are type III-IV muscle afferents required for a normal steady-state exercise hyperpnoea in humans? J Physiol 592: 463–474, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37a.Department of Health and Human Services. Hypothermia-related deaths—United States, 2003–2004. MMWR Morb Mortal Wkly Rep 54: 173–175, 2005. [PubMed] [Google Scholar]
- 38.Didion SP, Lynch CM, Baumbach GL, Faraci FM. Impaired endothelium-dependent responses and enhanced influence of Rho-kinase in cerebral arterioles in type II diabetes. Stroke 36: 342–347, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional alpha-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol 536: 977–983, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donoso V, Gomez CR, Orriantia MA, Perez V, Torres C, Coddou C, Nelson P, Maisey K, Morales B, Fernandez R, Imarai M, Huidobro-Toro JP, Sierra F, Acuna-Castillo C. The release of sympathetic neurotransmitters is impaired in aged rats after an inflammatory stimulus: a possible link between cytokine production and sympathetic transmission. Mech Ageing Dev 129: 728–734, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dowdall MJ, Boyne AF, Whittaker VP. Adenosine triphosphate. A constituent of cholinergic synaptic vesicles. Biochem J 140: 1–12, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duff F, Patterson GC, Shepherd JT. A quantitative study of the response to adenosine triphosphate of the blood vessels of the human hand and forearm. J Physiol 125: 581–589, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duggan J, Kilfeather S, O'Brien E, O'Malley K, Nussberger J. Effects of aging and hypertension on plasma angiotensin II and platelet angiotensin II receptor density. Am J Hypertens 5: 687–693, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91: 1025–1043, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO. Climate extremes: observations, modeling, impacts. Science 289: 2068–2074, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Edvinsson L, Ekblad E, Hakanson R, Wahlestedt C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol 83: 519–525, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept 8: 225–235, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC 2nd, Wray DW, Davis MJ, Fadel PJ. The role of alpha-adrenergic receptors in mediating beat-by-beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591: 3637–3649, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flavahan NA, Vanhoutte PM. Sympathetic purinergic vasoconstriction and thermosensitivity in a canine cutaneous vein. J Pharmacol Exp Therap 239: 784–789, 1986. [PubMed] [Google Scholar]
- 52.Fox RH, Edholm OG. Nervous control of the cutaneous circulation. Br Med Bull 19: 110–114, 1963. [DOI] [PubMed] [Google Scholar]
- 53.Frank SM, Raja SN, Bulcao C, Goldstein DS. Age-related thermoregulatory differences during core cooling in humans. Am J Physiol Regul Integr Comp Physiol 279: R349–R354, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Villalon AL, Padilla J, Fernandez N, Monge L, Martinez MA, Gomez B, Dieguez G. Effect of neuropeptide Y on the sympathetic contraction of the rabbit central ear artery during cooling. Pflügers Arch 440: 548–555, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Gibbins IL, Jobling P, Morris JL. Functional organization of peripheral vasomotor pathways. Acta Physiol Scand 177: 237–245, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 129: e28–e292, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldstein DS, Bonner RF, Zimlichman R, Zahn TP, Cannon RO 3rd, Rosing DR, Stull R, Keiser HR. Indices of sympathetic vascular innervation in sympathectomized patients. J Auton Nerv Syst 15: 309–318, 1986. [DOI] [PubMed] [Google Scholar]
- 58.Grassi G, Seravalle G, Turri C, Bertinieri G, Dell'Oro R, Mancia G. Impairment of thermoregulatory control of skin sympathetic nerve traffic in the elderly. Circulation 108: 729–735, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Impaired increases in skin sympathetic nerve activity contribute to age-related decrements in reflex cutaneous vasoconstriction. J Physiol 593: 2199–2211, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Lack of limb or sex differences in the cutaneous vascular responses to exogenous norepinephrine. J Appl Physiol (1985) 117: 1417–1423, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol (1985) 117: 648–657, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green HD, Kepchar JH. Control of peripheral resistance in major systemic vascular beds. Physiol Rev 39: 617–686, 1959. [DOI] [PubMed] [Google Scholar]
- 63.Guergova S, Dufour A. Thermal sensitivity in the elderly: a review. Ageing Res Rev 10: 80–92, 2011. [DOI] [PubMed] [Google Scholar]
- 64.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nature Med 16: 183–190, 2010. [DOI] [PubMed] [Google Scholar]
- 65.Hagbarth KE, Vallbo AB. Pulse and respiratory grouping of sympathetic impulses in human muscle-nerves. Acta Physiol Scand 74: 96–108, 1968. [DOI] [PubMed] [Google Scholar]
- 66.Han S, Yang CL, Chen X, Naes L, Cox BF, Westfall T. Direct evidence for the role of neuropeptide Y in sympathetic nerve stimulation-induced vasoconstriction. Am J Physiol Heart Circ Physiol 274: H290–H294, 1998. [DOI] [PubMed] [Google Scholar]
- 67.Heath ME. Neuropeptide Y and Y1-receptor agonists increase blood flow through arteriovenous anastomoses in rat tail. J Appl Physiol (1985) 85: 301–309, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol (1985) 107: 1076–1082, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Himms-Hagen J. Nonshivering thermogenesis. Brain Res Bull 12: 151–160, 1984. [DOI] [PubMed] [Google Scholar]
- 70.Hogikyan RV, Supiano MA. Arterial alpha-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol Endocrinol Metab 266: E717–E724, 1994. [DOI] [PubMed] [Google Scholar]
- 71.Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (1985) 109: 1538–1544, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci 15: 718–739, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol (1985) 105: 370–372, 2008. [DOI] [PubMed] [Google Scholar]
- 74.Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of alpha2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells. Mol Pharmacol 60: 1195–1200, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol 287: H1495–H1500, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Johnson JM, Brengelmann GL, Hales JR, Vanhoutte PM, Wenger CB. Regulation of the cutaneous circulation. Fed Proc 45: 2841–2850, 1986. [PubMed] [Google Scholar]
- 77.Johnson JM, Minson CT, Kellogg DL Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Comprehens Physiol 4: 33–89, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Jurkovich GJ. Environmental cold-induced injury. S urg Clin North Am 87: 247–267, viii, 2007. [DOI] [PubMed] [Google Scholar]
- 79.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007. [DOI] [PubMed] [Google Scholar]
- 80.Kahan T, Pernow J, Schwieler J, Wallin BG, Lundberg JM, Hjemdahl P. Noradrenaline release evoked by a physiological irregular sympathetic discharge pattern is modulated by prejunctional alpha- and beta-adrenoceptors in vivo. Br J Pharmacol 95: 1101–1108, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaufman S. Establishment of tetrahydrobiopterin as the hydroxylase cofactor and a review of some recent studies in man. Psychopharmacol Bull 14: 38–40, 1978. [PubMed] [Google Scholar]
- 82.Kellogg DL Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol Heart Circ Physiol 257: H1599–H1606, 1989. [DOI] [PubMed] [Google Scholar]
- 83.Kenney WL, Armstrong CG. Reflex peripheral vasoconstriction is diminished in older men. J Appl Physiol (1985) 80: 512–515, 1996. [DOI] [PubMed] [Google Scholar]
- 84.Kenney WL, Munce TA. Invited review: aging and human temperature regulation. J Appl Physiol (1985) 95: 2598–2603, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Khan F, Spence VA, Belch JJ. Cutaneous vascular responses and thermoregulation in relation to age. Clin Sci (Lond) 82: 521–528, 1992. [DOI] [PubMed] [Google Scholar]
- 86.Koganezawa T, Ishikawa T, Fujita Y, Yamashita T, Tajima T, Honda M, Nakayama K. Local regulation of skin blood flow during cooling involving presynaptic P2 purinoceptors in rats. Br J Pharmacol 148: 579–586, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Konishi C, Naito Y, Ohara N. Age-related changes in adenosine 5'-triphosphate-induced constriction of isolated, perfused mesenteric arteries of rats. Life Sci 64: 1265–1273, 1999. [DOI] [PubMed] [Google Scholar]
- 88.Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem 67: 443–462, 1996. [DOI] [PubMed] [Google Scholar]
- 89.Kurz A. Physiology of thermoregulation. Best practice & research. Clin Anaesthesiol 22: 627–644, 2008. [DOI] [PubMed] [Google Scholar]
- 90.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278: 22546–22554, 2003. [DOI] [PubMed] [Google Scholar]
- 91.Lambert ML, Callow ID, Feng QP, Arnold JM. The effects of age on human venous responsiveness to neuropeptide Y. Br J Clin Pharmacol 47: 83–89, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lang JA, Holowatz LA, Kenney WL. Local tetrahydrobiopterin administration augments cutaneous vasoconstriction in aged humans. J Physiol 587: 3967–3974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lang JA, Holowatz LA, Kenney WL. Localized tyrosine or tetrahydrobiopterin supplementation corrects the age-related decline in cutaneous vasoconstriction. J Physiol 588: 1361–1368, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lang JA, Jennings JD, Holowatz LA, Kenney WL. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol 297: H1792–H1797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lang JA, Kolb KE. Angiotensin II type I receptor blockade attenuates reflex cutaneous vasoconstriction in aged but not young skin. Am J Physiol Heart Circ Physiol 308: H1215–H1220, 2015. [DOI] [PubMed] [Google Scholar]
- 96.Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem 86: 774–783, 2003. [DOI] [PubMed] [Google Scholar]
- 97.Li YF, Cao XJ, Bai XY, Lin SP, Shi ST. Change of expression of renal alpha1-adrenergic receptor and angiotensin II receptor subtypes with aging in rats. Aging Clin Exp Res 22: 123–128, 2010. [DOI] [PubMed] [Google Scholar]
- 98.Lundberg JM. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, adenosine triphosphate, amino acids and nitric oxide. Pharmacol Rev 48: 113–178, 1996. [PubMed] [Google Scholar]
- 99.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res 93: 884–888, 2003. [DOI] [PubMed] [Google Scholar]
- 100.McAllen RM, Farrell M, Johnson JM, Trevaks D, Cole L, McKinley MJ, Jackson G, Denton DA, Egan GF. Human medullary responses to cooling and rewarming the skin: a functional MRI study. Proc Natl Acad Sci USA 103: 809–813, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McMichael AJ, Woodruff RE, Hales S. Climate change and human health: present and future risks. Lancet 367: 859–869, 2006. [DOI] [PubMed] [Google Scholar]
- 102.Meiman J, Anderson H, Tomasallo C. Hypothermia-related deaths–Wisconsin, 2014, and United States, 2003–2013. MMWR Morb Mortal Wkly Rep 64: 141–143, 2015. [PMC free article] [PubMed] [Google Scholar]
- 103.Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol 50: 238–246, 2007. [DOI] [PubMed] [Google Scholar]
- 104.Morabito M, Modesti PA, Cecchi L, Crisci A, Orlandini S, Maracchi G, Gensini GF. Relationships between weather and myocardial infarction: a biometeorological approach. Int J Cardiol 105: 288–293, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Morris JL. Selective constriction of small cutaneous arteries by NPY matches distribution of NPY in sympathetic axons. Regul Pept 49: 225–236, 1994. [DOI] [PubMed] [Google Scholar]
- 106.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nature Neurosci 11: 62–71, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107a.National Center for Health Statistics. In: Health, United States, 2005: With Chartbook on Trends in the Health of Americans. Hyattsville, MD: U. S. Dept. of Health and Human Services, 2005. [PubMed] [Google Scholar]
- 108.Natsume K, Ogawa T, Sugenoya J, Ohnishi N, Imai K. Preferred ambient temperature for old and young men in summer and winter. Int J Biometeorol 36: 1–4, 1992. [DOI] [PubMed] [Google Scholar]
- 109.Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol 267: H344–H353, 1994. [DOI] [PubMed] [Google Scholar]
- 110.Ninomiya I, Malpas SC, Matsukawa K, Shindo T, Akiyama T. The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre- and postganglionic fibers in anesthetized cats. J Auton Nerv Syst 45: 139–147, 1993. [DOI] [PubMed] [Google Scholar]
- 111.Panagiotakos DB, Chrysohoou C, Pitsavos C, Nastos P, Anadiotis A, Tentolouris C, Stefanadis C, Toutouzas P, Paliatsos A. Climatological variations in daily hospital admissions for acute coronary syndromes. Int J Cardiol 94: 229–233, 2004. [DOI] [PubMed] [Google Scholar]
- 112.Panoutsopoulou IG, Wendelschafer-Crabb G, Hodges JS, Kennedy WR. Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology 72: 1205–1210, 2009. [DOI] [PubMed] [Google Scholar]
- 113.Park J, Liao P, Sher S, Lyles RH, Deveaux DD, Quyyumi AA. Tetrahydrobiopterin lowers muscle sympathetic nerve activity and improves augmentation index in patients with chronic kidney disease. Am J Physiol Regul Integr Comp Physiol 308: R208–R218, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piascik MT, Soltis EE, Piascik MM, Macmillan LB. Alpha-adrenoceptors and vascular regulation: molecular, pharmacologic and clinical correlates. Pharmacol Therap 72: 215–241, 1996. [DOI] [PubMed] [Google Scholar]
- 115.Racchi H, Irarrazabal MJ, Howard M, Moran S, Zalaquett R, Huidobro-Toro JP. Adenosine 5'-triphosphate and neuropeptide Y are co-transmitters in conjunction with noradrenaline in the human saphenous vein. Br J Pharmacol 126: 1175–1185, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Racchi H, Schliem AJ, Donoso MV, Rahmer A, Zuniga A, Guzman S, Rudolf K, Huidobro-Toro JP. Neuropeptide Y Y1 receptors are involved in the vasoconstriction caused by human sympathetic nerve stimulation. Eur J Pharmacol 329: 79–83, 1997. [DOI] [PubMed] [Google Scholar]
- 117.Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation 84: 1–14, 1991. [DOI] [PubMed] [Google Scholar]
- 118.Richardson D, Tyra J, McCray A. Attenuation of the cutaneous vasoconstrictor response to cold in elderly men. J Gerontol 47: M211–214, 1992. [DOI] [PubMed] [Google Scholar]
- 119.Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36: 2251–2257, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 86: 1309–1379, 2006. [DOI] [PubMed] [Google Scholar]
- 121.Rowell L. Human Circulation Regulation during Physical Stress. New York, NY: Oxford University Press, 1986. [Google Scholar]
- 122.Sato M, Tani E, Fujikawa H, Kaibuchi K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res 87: 195–200, 2000. [DOI] [PubMed] [Google Scholar]
- 123.Savage MV, Brengelmann GL. Control of skin blood flow in the neutral zone of human body temperature regulation. J Appl Physiol (1985) 80: 1249–1257, 1996. [DOI] [PubMed] [Google Scholar]
- 124.Sawasaki N, Iwase S, Mano T. Effect of skin sympathetic response to local or systemic cold exposure on thermoregulatory functions in humans. Auton Neurosci 87: 274–281, 2001. [DOI] [PubMed] [Google Scholar]
- 125.Seifert F, Maihofner C. Representation of cold allodynia in the human brain—a functional MRI study. NeuroImage 35: 1168–1180, 2007. [DOI] [PubMed] [Google Scholar]
- 126.Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 92: 411–418, 2003. [DOI] [PubMed] [Google Scholar]
- 127.Sheth T, Nair C, Muller J, Yusuf S. Increased winter mortality from acute myocardial infarction and stroke: the effect of age. J Am Coll Cardiol 33: 1916–1919, 1999. [DOI] [PubMed] [Google Scholar]
- 128.Shibasaki M, Umemoto Y, Kinoshita T, Kouda K, Ito T, Nakamura T, Crandall CG, Tajima F. The role of cardiac sympathetic innervation and skin thermoreceptors on cardiac responses during heat stress. Am J Physiol Heart Circ Physiol 308: H1336–H1342, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siebers MJ, Goodfriend TL, Ball D, Elliott ME. Analysis of angiotensin II binding to human platelets: differences in young and old subjects. J Gerontol 45: B42–47, 1990. [DOI] [PubMed] [Google Scholar]
- 130.Sindler AL, Devan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol (1985) 116: 463–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith CJ, Santhanam L, Alexander LM. Rho-Kinase activity and cutaneous vasoconstriction is upregulated in essential hypertensive humans. Microvasc Res 87: 58–64, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smyth L, Bobalova J, Ward SM, Mutafova-Yambolieva VN. Neuropeptide Y is a cotransmitter with norepinephrine in guinea pig inferior mesenteric vein. Peptides 21: 835–843, 2000. [DOI] [PubMed] [Google Scholar]
- 133.Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin augments reflex vasoconstriction in aged human skin through noradrenergic mechanisms. J Appl Physiol (1985) 115: 1025–1031, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steckelings UM, Wollschlager T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol 13: 148–154, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Stephens DP, Aoki K, Kosiba WA, Johnson JM. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol 280: H1496–H1504, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Stephens DP, Bennett LA, Aoki K, Kosiba WA, Charkoudian N, Johnson JM. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol 282: H264–H272, 2002. [DOI] [PubMed] [Google Scholar]
- 137.Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol 287: H1404–H1409, 2004. [DOI] [PubMed] [Google Scholar]
- 138.Stern MD, Lappe DL, Bowen PD, Chimosky JE, Holloway GA Jr, Keiser HR, Bowman RL. Continuous measurement of tissue blood flow by laser-Doppler spectroscopy. Am J Physiol Heart Circ Physiol 232: H441–H448, 1977. [DOI] [PubMed] [Google Scholar]
- 139.Stewart JM, Taneja I, Glover J, Medow MS. Angiotensin II type 1 receptor blockade corrects cutaneous nitric oxide deficit in postural tachycardia syndrome. Am J Physiol Heart Circ Physiol 294: H466–H473, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sugiyama Y, Matsukawa T, Shamsuzzaman AS, Okada H, Watanabe T, Mano T. Delayed and diminished pressor response to muscle sympathetic nerve activity in the elderly. J Appl Physiol (1985) 80: 869–875, 1996. [DOI] [PubMed] [Google Scholar]
- 141.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taddei S, Pedrinelli R, Salvetti A. Sympathetic nervous system-dependent vasoconstriction in humans. Circulation 82: 2061–2067, 1990. [DOI] [PubMed] [Google Scholar]
- 143.Tanaka M, McKinley MJ, McAllen RM. Preoptic-raphe connections for thermoregulatory vasomotor control. J Neurosci 31: 5078–5088, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphe in thermoregulatory vasomotor control in rats. J Physiol 540: 657–664, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Taylor NA, Allsopp NK, Parkes DG. Preferred room temperature of young vs aged males: the influence of thermal sensation, thermal comfort, and affect. J Gerontol 50: M216–221, 1995. [DOI] [PubMed] [Google Scholar]
- 146.Thompson CS. Aging and efferent control of thermoregulatory cutaneous vasoconstriction. In: Intercollege Graduate Degree Program in Physiology. The Graduate School: The Pennsylvania State University, 2005. [Google Scholar]
- 147.Thompson CS, Holowatz LA, Kenney WL. Attenuated noradrenergic sensitivity during local cooling in aged human skin. J Physiol 564: 313–319, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thompson CS, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol 288: R1108–R1113, 2005. [DOI] [PubMed] [Google Scholar]
- 149.Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol 558: 697–704, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thony B, Calvo AC, Scherer T, Svebak RM, Haavik J, Blau N, Martinez A. Tetrahydrobiopterin shows chaperone activity for tyrosine hydroxylase. J Neurochem 106: 672–681, 2008. [DOI] [PubMed] [Google Scholar]
- 151.Torossian A. Survey on intraoperative temperature management in Europe. Eur J Anaesthesiol 24: 668–675, 2007. [DOI] [PubMed] [Google Scholar]
- 151a.United Nations Department of Economic and Social Affairs Population Division. World Population Ageing, 1950–2050. New York: United Nations, 2002, p. 483. [Google Scholar]
- 152.Urano F, Hayashi N, Arisaka F, Kurita H, Murata S, Ichinose H. Molecular mechanism for pterin-mediated inactivation of tyrosine hydroxylase: formation of insoluble aggregates of tyrosine hydroxylase. J Biochem 139: 625–635, 2006. [DOI] [PubMed] [Google Scholar]
- 153.Vianna LC, Hart EC, Fairfax ST, Charkoudian N, Joyner MJ, Fadel PJ. Influence of age and sex on the pressor response following a spontaneous burst of muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 302: H2419–H2427, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.von Kugelgen I, Allgaier C, Schobert A, Starke K. Co-release of noradrenaline and ATP from cultured sympathetic neurons. Neuroscience 61: 199–202, 1994. [DOI] [PubMed] [Google Scholar]
- 155.Wahlestedt C, Hakanson R, Vaz CA, Zukowska-Grojec Z. Norepinephrine and neuropeptide Y: vasoconstrictor cooperation in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol 258: R736–R742, 1990. [DOI] [PubMed] [Google Scholar]
- 156.Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol (1985) 111: 1703–1709, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol 298: R1627–R1633, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wilson TE, Monahan KD, Short DS, Ray CA. Effect of age on cutaneous vasoconstrictor responses to norepinephrine in humans. Am J Physiol Regul Integr Comp Physiol 287: R1230–R1234, 2004. [DOI] [PubMed] [Google Scholar]
- 159.Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, Hymer H, Wichmann HE, Peters A. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120: 735–742, 2009. [DOI] [PubMed] [Google Scholar]
- 160.Wray DW, Nishiyama SK, Harris RA, Richardson RS. Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension 51: 1611–1616, 2008. [DOI] [PubMed] [Google Scholar]



