Abstract
We studied the effect of hypertension and chronic hypoperfusion on brain parenchymal arteriole (PA) structure and function. PAs were studied isolated and pressurized from 18-wk-old Wistar-Kyoto (WKY18; n = 8) and spontaneously hypertensive stroke prone (SHRSP18; n = 8) and 5-wk-old prehypertensive (SHRSP5; n = 8) rats. In separate groups, unilateral common carotid artery occlusion (UCCAo) was performed for 4 wk to cause chronic hypoperfusion in 18-wk-old WKY (WKY18-CH; n = 8) and SHRSP (SHRSP18-CH; n = 8). UCCAo caused PAs to have significantly diminished myogenic tone (31 ± 3 vs. 14 ± 6% at 60 mmHg; P < 0.05) and reactivity to pressure from WKY18-CH vs. WKY18 animals. The effect of UCCAo was limited to normotensive animals, as there was little effect of chronic hypoperfusion on vascular reactivity or percent tone in PAs from SHRSP18 vs. SHRSP18-CH animals (53 ± 4 vs. 41 ± 3%; P > 0.05). However, PAs from SHRSP18 and SHRSP5 animals had significantly greater tone compared with WKY18, suggesting an effect of strain and not hypertension per se on PA vasoconstriction. Structurally, PAs from SHRSP18 and SHRSP5 animals had similar sized lumen diameters, but increased wall thickness and distensibility compared with WKY18. Interestingly, chronic hypoperfusion did not affect the structure of PAs from either WKY18-CH or SHRSP18-CH animals. Thus PAs responded to UCCAo with active vasodilation, but not structural remodeling, an effect that was absent in SHRSP. The increased tone of PAs from SHRSP animals, combined with lack of response to chronic hypoperfusion, may contribute to the propensity for ischemic lesions and increased perfusion deficit during hypertension.
Keywords: hypertension, cerebral arterioles, brain, chronic hypoperfusion, SHRSP
occlusive cerebral arterial disease and chronic hypoperfusion of the brain are common in adults with large and small vessel disease (SVD) that is associated with increased risk of stroke and cognitive impairment (1, 9, 33, 38). Several animal models have been developed to study chronic hypoperfusion of the brain that involves occlusion or stenosis of one or more carotid and/or vertebral arteries in normotensive animals (2, 4, 14, 32). Depending on the model used, various pathologies have been identified that may be relevant to human disease, including white matter lesions, blood-brain barrier permeability, neuroinflammation, and accelerated cognitive impairment (2, 4, 14, 32). These models have also shown that the cerebral vessels undergo an adaptive response to chronic hypoperfusion involving enlargement of collateral vessels (arteriogenesis) that can restore cerebral blood flow (CBF) to normal over a period of weeks (2, 14, 32). Arteriogenesis of primary and secondary collaterals in response to chronic hypoperfusion is thought to be protective of ischemic injury by enhancing CBF reserve and has been considered as a therapeutic intervention (21, 37). Indeed, enlargement of collaterals due to arteriogenesis after chronic hypoperfusion was shown to decrease infarct size in response to middle cerebral artery (MCA) occlusion (21, 37).
The process of arteriogenesis in response to chronic hypoperfusion occurs in collaterals through increased shear stress that triggers a cascade of events involving endothelial cell activation, recruitment of monocytes and polymorphonuclear cells, and release of various cytokines, chemokines, and growth factors, leading to vessel enlargement (3, 29). Arteriogenesis during chronic hypoperfusion is well-characterized in collaterals, where changes in shear stress are pronounced (30). However, how chronic hypoperfusion affects other segments of the cerebrovasculature that are not collaterals and thus do not experience large changes in shear stress, is less understood. In MCAs, bilateral common carotid artery occlusion (BCCAo) for 15 days caused modest structural remodeling that included hypotrophy of the vessel wall and diminished myogenic tone without a change in passive lumen diameter (22), suggesting adaptive responses to chronic hypoperfusion occur in large, noncollateral cerebral arteries as well.
Blood supply to the brain parenchyma is via penetrating and parenchymal arterioles (PAs) that branch off pial vessels at right angles and directly connect the pial surface vessels to the capillary bed (5). PAs are high-resistance vessels due to their long and relatively unbranched architecture with few anastomoses (5, 15). PAs have recently been shown to be the major site of autoregulatory vasodilation during unilateral common carotid artery occlusion (UCCAo) that maintains CBF during decreased cerebral perfusion pressure (36). Importantly, structural and functional changes in PAs are key features of ischemic cerebral SVD that undergo remodeling of the arteriolar wall and reduced lumen diameter, loss of autoregulation, and locally increased blood-brain barrier permeability that is thought to underlie white matter lesions and cognitive impairment (13, 27). SVD is also associated with chronic cerebral hypoperfusion that is pronounced in patients with carotid stenosis, aging, and hypertension (10, 38, 39). In fact, hypertension is considered a main risk factor for SVD, yet only a few studies have investigated the effect of chronic hypoperfusion on arteriogenesis in models of hypertension, and even fewer have studied PAs under these conditions. Jalal et al. (20) used spontaneously hypertensive stroke prone (SHRSP) rats combined with chronic hypoperfusion, and reported that BCCAo is lethal in these animals. In addition UCCAo in SHRSP causes white matter damage in both hemispheres, suggesting intolerance to chronic hypoperfusion during hypertension (20). Further, studies have shown arteriogenesis of both primary (circle of Willis vessels) and secondary (leptomeningeal anastomoses) collaterals in response to chronic hypoperfusion is impaired during hypertension (26, 34). However, the effect of chronic hypoperfusion on PAs in the setting of hypertension is less understood, but potentially important to understand, given the central role these vessels have in SVD.
In the present study, we investigated the effect of chronic hypoperfusion on PAs under normotensive and hypertensive conditions and measured changes in structural remodeling, vasoreactivity, and endothelial function. We hypothesized that chronic hypoperfusion causes adaptive changes in PAs to increase lumen diameter (outward remodeling and decreased tone) that is prevented during hypertension.
MATERIALS AND METHODS
Animals.
Experiments were performed using female Wistar-Kyoto (WKY) and SHRSP rats (Charles River) at age 5 or 18 wk. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Vermont and complied with the National Institutes of Health guidelines for care and use of laboratory animals. Rats were housed in the Animal Care Facility at the University of Vermont, an American Association for Accreditation of Laboratory Animal Care-accredited facility. Rats were maintained on a 12:12-h light-dark cycle and allowed food and water ad libitum. Animals (n = 8/group) were grouped as naive 5-wk-old SHRSP (SHRSP5), naive 18-wk-old SHRSP (SHRSP18), naive 18-wk-old WKY (WKY18), or 18-wk-old WKY (WKY18-CH) or SHRSP (SHRSP18-CH) with 4-wk chronic UCCAo.
Animal model of chronic UCCAo.
Chronic UCCAo was surgically induced at 14 wk of age and maintained for 4 wk until experimentation at 18 wk old in WKY and SHRSP animals. Animals were anesthetized by inhaled isoflurane (1.5-2%) in oxygen and air. A midline neck incision was placed, and the right common carotid artery and external carotid artery were exposed. The common carotid artery was permanently ligated with two 4-0 sterile silk sutures proximal to the bifurcation of the internal and external carotid arteries. Following surgery, animals received buprenorphine (0.05 mg/kg) subcutaneously near the incision site for pain.
Blood pressure measurements.
Blood pressures were measured noninvasively at 5 or 18 wk old by determining the tail blood volume with a volume pressure recording sensor and an occlusion tail cuff (Coda 8 system, Kent Scientific, Torrington, CT), as described previously (6). Briefly, animals were placed in individual holders, and both an occlusion cuff and a volume pressure recording sensor cuff were placed near the base of the tail. Volume pressure recording allowed the simultaneous measurement of six blood pressure parameters: systolic blood pressure, diastolic blood pressure, mean blood pressure, heart pulse rate, tail blood volume, and tail blood flow.
Preparation of isolated PAs and pressurized arteriograph.
Animals were quickly decapitated under isoflurane anesthesia, and the brain was removed and placed in cold, oxygenated physiological saline solution (PSS). PAs branching perpendicularly from the M1 and M2 regions of the right MCA and penetrating into the brain parenchyma were carefully dissected and mounted onto glass cannulas in an arteriograph chamber (Living Systems Instrumentation, Burlington, VT), as previously described (7). In animals that underwent UCCAo, PAs were taken from the side of occlusion.
Isolated arteriole myogenic tone, reactivity, and structural measurements.
PAs were dissected and mounted in an arteriograph chamber, pressurized to 20 mmHg, and equilibrated for 1 h to allow spontaneous development of myogenic tone. After the equilibration period, myogenic reactivity was assessed with stepwise increases in intravascular pressure up to 100 mmHg and recording lumen diameter once stable. Intravascular pressure was returned to 40 mmHg for the remainder of the experiment. NS309, a small- and intermediate-conductance calcium-activated K+ channel (SK/IK) agonist, was cumulatively added to the bath (10−8-10−5 M), and diameter measured at each dose. NS309 was washed out of the bath, and PAs were allowed to regain myogenic tone. A single dose of Nω-nitro-l-arginine (l-NNA, 0.1 mM), a nitric oxide synthase (NOS) inhibitor, was added to the bath and diameter measured once stable. Sodium nitroprusside (SNP), a nitric oxide (NO) donor, was cumulatively added to the bath (10−8-10−5 M) in the presence of l-NNA, and diameter measured at each concentration. At the conclusion of the experiment, PAs were superfused with papaverine (0.1 mM) and diltiazem (10 μM) in calcium-free PSS to obtain fully passive diameter and structural measurements.
Drugs and solutions.
All isolated vessel experiments were performed using a bicarbonate-based Ringer's PSS, the ionic composition of which was as follows (in mmol/l): NaCl 119.0, NaHCO3 24.0, KCl 4.7, KH2PO4 1.18, MgSO4 × 7H2O 1.17, CaCl2 1.6, EDTA 0.026, and glucose 5.5. PSS was made each week and stored without glucose at 4°C. Glucose was added to the PSS before each experiment. PSS was aerated with 5% CO2, 10% O2, and 85% N2 to maintain pH. Diltiazem was purchased from MP Biomedicals (Santa Ana, CA) and made up weekly to a 1 mM stock. Papaverine and l-NNA were purchased from Sigma (St. Louis, MO) and made weekly to 10 mM stocks. NS309 and SNP were purchased from Sigma, and aliquots of 10 mM stocks were frozen at −20°C until use.
Data calculations and statistical analysis.
Results are presented as means ± SE. Myogenic tone was calculated as a percent decrease in diameter from the fully relaxed diameter in calcium-free PSS with diltiazem and papaverine by the following equation: [1 − (φtone/φpassive)] × 100%, where φtone is the inner diameter of the vessel with tone, and φpassive is the inner diameter of the vessel in calcium-free PSS with diltiazem and papaverine. Percent constriction to l-NNA was calculated as a percent change in diameter from baseline by the following equation: [1 − (φdrug/φbaseline)] × 100%, where φdrug is the diameter of vessel in l-NNA, and φbaseline is the diameter before giving drug. Percent reactivity to SNP and NS309 was calculated from the following equation: [(φdose − φbaseline)/(φpassive − φbaseline)] × 100%, where φdose is the diameter at a specific concentration of drug. Percent distensibility was calculated for fully relaxed vessels in calcium-free PSS with diltiazem and papaverine at each pressure by the following equation: (φpressure − φ5mmHg)/(φ5mmHg) × 100%, where φpressure is the passive diameter at a specific pressure, and φ5mmHg is defined as the passive diameter at the lowest pressure (5 mmHg). Outer diameter (OD) was calculated using the equation ID + 2WT, where ID is the measured inner diameter, and WT is the measured wall thickness. Cross-sectional area (CSA) was calculated as π(OD/2)2 − π(ID/2)2.
Differences in blood pressure, active diameters, percent tone, percent constriction, percent reactivity, percent distensibility, and passive structural measurements were determined by one-way ANOVA with a post hoc Student-Newman-Keuls test for multiple comparisons. Two-way ANOVA was used to determine the effect of hypertension or UCCAo treatment and their interaction on passive structural characteristics. Differences were considered significant at P < 0.05.
RESULTS
Effect of hypertension on PA myogenic reactivity and tone.
To determine the influence of hypertension on PAs, we used SHRSP animals that were 18 wk old compared with age-matched normotensive WKY and prehypertensive 5 wk old SHRSP in an attempt to distinguish strain differences. Physiological parameters of WKY18, SHRSP18, and SHRSP5 animals are shown in Table 1. Systolic, diastolic, and mean blood pressures were significantly higher in SHRSP18 compared with WKY18. Blood pressures of SHRSP5 were significantly lower than those of SHRSP18 and similar to those of WKY18, confirming the prehypertensive state of these animals. The body weight of SHRSP5 was lower than that of all other groups, as expected.
Table 1.
Physiological characteristics of 18-wk-old WKY and SHRSP and 5-wk-old SHRSP animals
| WKY18 | SHRSP18 | SHRSP5 | |
|---|---|---|---|
| n | 8 | 8 | 8 |
| Body weight, g | 209 ± 4 | 198 ± 3 | 91 ± 2*† |
| Arterial blood pressure, mmHg | |||
| Systolic | 119 ± 3 | 161 ± 7*‡ | 127 ± 5 |
| Diastolic | 82 ± 3 | 122 ± 7*‡ | 86 ± 7 |
| Mean | 94 ± 3 | 135 ± 7*‡ | 100 ± 6 |
Values are means ± SE; n, no. of animals. WKY18, 18-wk-old Wistar-Kyoto rats; SHRSP18 and SHRSP5, 18- and 5-wk-old spontaneously hypertensive stroke prone rats, respectively.
P < 0.01 vs. WKY18;
P < 0.01 vs. SHRSP18; and
P < 0.01 vs. SHRSP5 by one-way ANOVA.
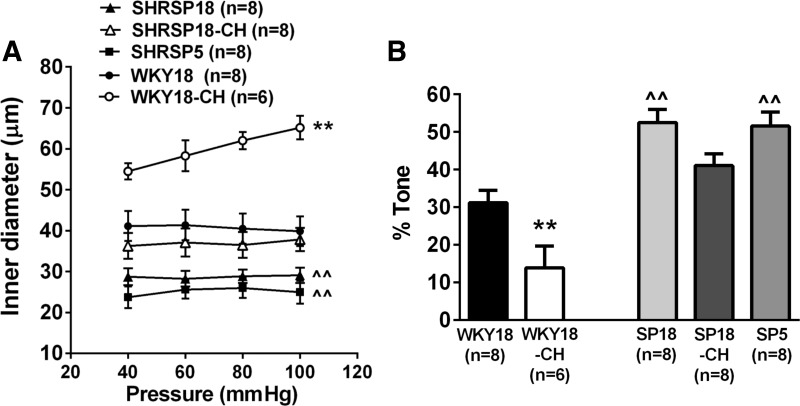
PAs from SHRSP18 had significantly smaller active inner diameters at all pressures studied compared with WKY18 (Fig. 1A). Active inner diameters of PAs from normotensive SHRSP5 were not different from those of SHRSP18 and significantly smaller vs. WKY18. The smaller active diameter of PAs from both SHRSP18 and SHRSP5 groups was at least partially due to increased pressure-induced tone in those vessels (Fig. 1B). Thus increased myogenic tone and smaller inner diameters of PAs were associated with the SHRSP strain and not hypertension per se. In addition, inner diameters of PAs from WKY18, SHRSP18, and SHRSP5 did not change appreciably with increasing pressure, demonstrating those vessels had myogenic reactivity.
Fig. 1.
Effect of hypertension and unilateral common carotid artery occlusion (UCCAo) on parenchymal arteriole (PA) myogenic activity. A: inner diameters vs. intravascular pressure of PAs from either 18-wk-old Wistar-Kyoto (WKY18) or 5- and 18-wk-old spontaneously hypertensive stroke prone (SHRSP5 and SHRSP18, respectively) rats that were naive, or from WKY18 and SHRSP18 rats that underwent 4 wk of UCCAo (WKY18-CH and SHRSP18-CH, respectively). PAs were taken from the ipsilateral side to occlusion. Both SHRSP5 and SHRSP18 animals had PAs with smaller lumen diameters compared with WKY18 at all pressures. These vessels displayed myogenic activity as diameter did not change with increased pressure. UCCAo caused increased diameters in PAs from WKY18-CH animals and diminished reactivity to pressure. There was little effect of UCCAo on PAs form SHRSP18-CH animals. B: percent myogenic tone of PAs from all groups of animals at 60 mmHg. Myogenic tone was increased in both SHRSP5 (SP5) and SHRSP18 (SP18) compared with WKY18 animals. UCCAo decreased tone only in WKY18-CH animals. Values are means ± SE; n, no. of rats. ^^P < 0.01 vs. WKY18. **P < 0.01 vs. all.
Effect of chronic hypoperfusion on PA myogenic reactivity and tone.
Chronic hypoperfusion by UCCAo for 4 wk decreased myogenic reactivity in PAs from WKY18-CH, such that diameters increased with increasing intravascular pressure (Fig. 1A). In fact, the slope of the diameter vs. pressure curves was minimal in WKY18, SHRSP18, SHRSP18-CH, and SHRSP5 (−0.02 ± 0.02, 0.01 ± 0.02, 0.03 ± 0.02, and 0.04 ± 0.04), but was significantly increased and positive in WKY18-CH (0.18 ± 0.05; P < 0.01 vs. all). In addition, they had significantly larger active diameters at all pressures studied compared with all other groups. PAs from WKY18-CH also had significantly less myogenic tone at 60 mmHg vs. all groups (Fig. 1B). In contrast, SHRSP18-CH had considerably less effect on PAs. Although PAs from SHRSP18-CH animals had inner diameters that were larger than those of SHRSP18, there was no statistical difference between these groups. Similarly, the percentage of tone was less in PAs from SHRSP18-CH compared with SHRSP18, but this was not statistically different. UCCAo was not performed on 5-wk-old SHRSP.
Effect of hypertension on PA reactivity to NO and SK/IK channel activation.
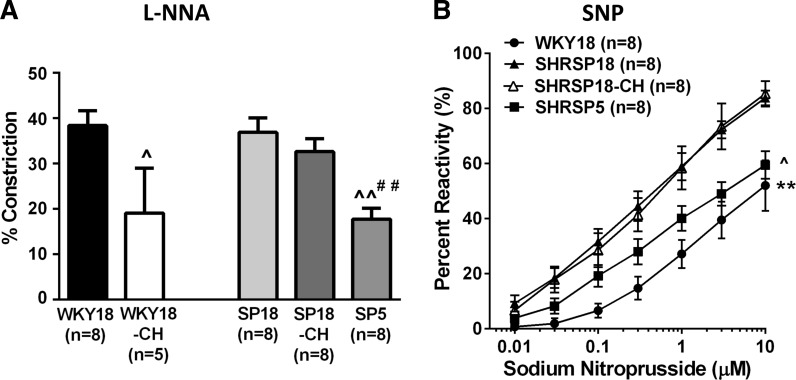
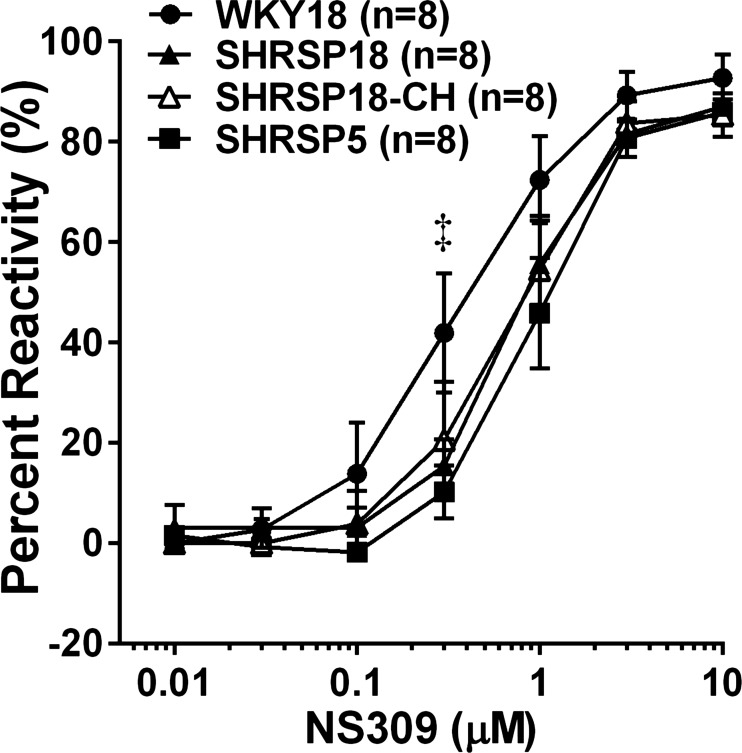
Figure 2A shows the percent constriction to l-NNA in PAs from all groups. All vessels constricted to NOS inhibition with l-NNA, demonstrating PAs have basal NO that inhibits tone in these vessels. The percent constriction was not different between WKY18 and SHRSP18; however, it was significantly decreased in PAs from SHRSP5. Figure 2B shows the reactivity to the NO donor SNP in PAs from all groups. Dilation to SNP was significantly increased in SHRSP18 compared with WKY18 and SHRSP5, demonstrating an effect of hypertension on sensitivity to SNP, but not strain. Figure 3 shows percent reactivity to the SK/IK channel opener NS309. All vessels dilated significantly to NS309, and there was no difference in reactivity between SHRSP5 and SHRSP18; however, PAs from WKY18 animals were modestly more sensitive to SK/IK channel activation that was significant at 0.3 μM.
Fig. 2.
Effect of hypertension and UCCAo on PA reactivity to nitric oxide. A: constriction of PAs to nitric oxide synthase inhibition with Nω-nitro-l-arginine (l-NNA; 10−4 M). All vessels constricted to nitric oxide synthase inhibition that was less in SHRSP5 (SP5) and after UCCAo in WKY18-CH animals. ^P < 0.05 vs. WKY18. ^^P < 0.01 vs. WKY18. ##P < 0.01 vs. SP18. B: percent reactivity as a measure of dilation to sodium nitroprusside (SNP). All vessels dilated to SNP that was greater in PAs from SHRSP18, regardless of UCCAo, compared with age-matched WKY18 and SHRSP5 animals. The dilation curve to SNP could not be performed on PAs from WKY18-CH animals, as they were already dilated. ^P < 0.05 vs. SHRSP18 and SHRSP18-CH. **P < 0.01 vs. all. Values are means ± SE; n, no. of rats.
Fig. 3.
Effect of hypertension and UCCAo on percent reactivity to NS309 in PAs. All vessels dilated to the small- and intermediate-conductance calcium-activated potassium channel activator NS309. PAs from WKY18 animals were modestly more sensitive to NS309 that was only significant at 3.0 μM. There was no difference in reactivity to NS309 in PAs from any of the SHRSP animals. A dilation curve to NS309 could not be performed on PAs from WKY18-CH animals as they were already dilated. Values are means ± SE; n, no. of rats. ‡P < 0.05 vs. SHRSP5.
Effect of chronic hypoperfusion on PA reactivity to NO and SK/IK activation.
Chronic hypoperfusion decreased constriction to l-NNA in PAs from WKY18 (WKY-CH) but not SHRSP (SHRSP-CH) (Fig. 2A). The constriction to l-NNA in PAs from SHRSP18-CH was unaffected by chronic hypoperfusion. Figure 2B shows the reactivity to SNP in PAs from all groups except WKY18-CH. In those vessels, the decrease in tone and substantial vasodilation did not allow for a dilatory curve to be performed. Chronic hypoperfusion of SHRSP had no effect on SNP dilation of PAs that was similar between SHRSP18 and SHRSP18-CH. Figure 3 shows the dilation of PAs to NS309. Again, dilation to NS309 was not done in WKY18-CH because these vessels were already dilated. There was no difference in reactivity to NS309 between SHRSP18 and SHRSP18-CH.
Effect of hypertension on PA structural remodeling.
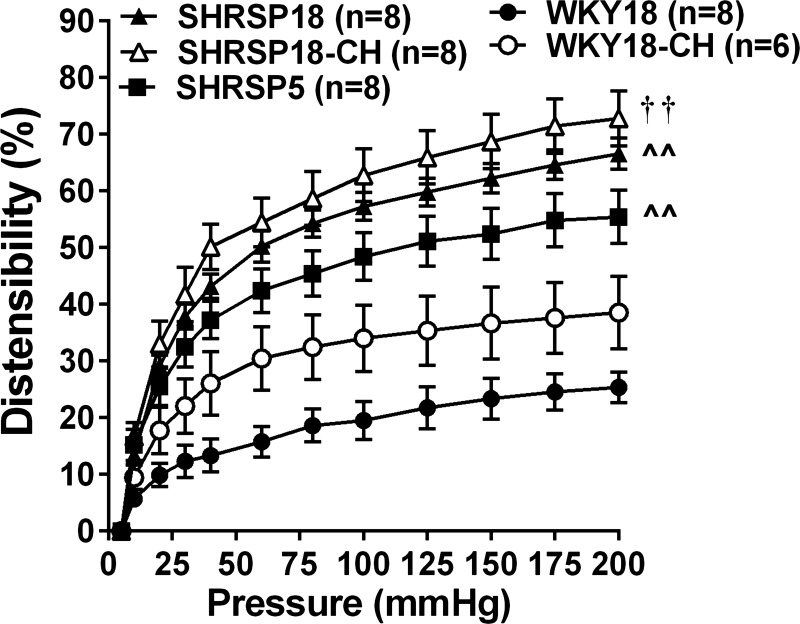
Structural characteristics of PAs from all groups are shown in Table 2. Structural measurements were compared at 5 and 60 mmHg to evaluate the effect of hypertension on PAs in an unpressurized state (5 mmHg), where differences in distensibility would not influence the diameters, and at a physiological pressure (60 mmHg). There were no statistical differences in inner or outer diameters at either 5 or 60 mmHg by one-way ANOVA between WKY18, SHRSP18, and SHRSP5. However, SHRSP5 had increased wall thickness compared with WKY18 at 5 mmHg, whereas SHRSP18 wall thickness was increased at 5 and 60 mmHg vs. WKY18. CSA was increased only in SHRSP18 at 60 mmHg, but not at 5 mmHg or in SHRSP5 vs. WKY18. Figure 4 shows the passive distensibility of PAs from all groups. Passive distensibility was significantly increased in both SHRSP18 and SHRSP5 compared with WKY18, suggesting a strain difference and not hypertension per se that affected vessel stiffness.
Table 2.
Effect of hypertension and chronic hypoperfusion on passive structural characteristics of parenchymal arterioles
| WKY18 | WKY18-CH | SHRSP18 | SHRSP18-CH | SHRSP5 | |
|---|---|---|---|---|---|
| n | 8 | 6 | 8 | 8 | 8 |
| ID5 | 48.2 ± 4.6 | 52.7 ± 2.8 | 40.9 ± 3.3 | 41.0 ± 3.2† | 38.4 ± 3.0 |
| ID60 | 55.2 ± 3.9 | 68.2 ± 2.6 | 61.5 ± 5.3 | 63.0 ± 4.7 | 54.0 ± 3.1 |
| OD5 | 62.2 ± 4.8 | 68.0 ± 2.6 | 59.4 ± 3.6 | 59.8 ± 4.1 | 55.9 ± 3.4 |
| OD60 | 67.8 ± 9.4 | 80.8 ± 2.6 | 77.5 ± 5.5 | 78.5 ± 5.2 | 67.3 ± 3.8 |
| WT5 | 7.00 ± 0.26 | 7.67 ± 0.42 | 9.25 ± 0.45§ | 9.39 ± 0.53‡ | 8.75 ± 0.31§ |
| WT60 | 6.33 ± 0.21 | 6.33 ± 0.33 | 8.00 ± 0.50§ | 7.75 ± 0.53‡ | 6.63 ± 0.42 |
| CSA5 | 1220 ± 123 | 1452 ± 89 | 1466 ± 132 | 1516 ± 185 | 1310 ± 124 |
| CSA60 | 1220 ± 187 | 1473 ± 99 | 1758 ± 176* | 1756 ± 211† | 1287 ± 145 |
Values are means ± SE; n, no. of animals. WKY18-CH and SHRSP18-CH, WKY18 and SHRSP18 rats with chronic hypoperfusion, respectively; ID5 and ID60, inner diameter at 5 or 60 mmHg, respectively; OD5 and OD60, outer diameter at 5 or 60 mmHg, respectively; WT5 and WT60, wall thickness at 5 or 60 mmHg, respectively; CSA5 and CSA60, cross-sectional area at 5 or 60 mmHg, respectively.
P < 0.05 vs. WKY18 and
P < 0.01 vs. WKY18 by one-way ANOVA.
P < 0.05 vs. WKY18-CH and
P < 0.01 vs. WKY18-CH by two-way ANOVA.
Fig. 4.
Effect of hypertension and UCCAo on passive distensibility of PAs. Distensibility was significantly increased in PAs from all groups of SHRSP animals vs. WKY18. UCCAo for 4 wk caused a nonsignificant increase in distensibility of PAs from WKY18-CH vs. WKY18 animals. There was no difference in distensibility of PAs from SHRSP18 vs. SHRSP18-CH; however, PAs from SHRSP5 animals were less distensible than from SHRSP18 and SHRSP18-CH. Values are means ± SE; n, no. of rats. ^^P < 0.01 vs. WKY18. ††P < 0.01 vs. WKY18-CH.
Effect of chronic hypoperfusion on PA structural remodeling.
Two-way ANOVA was used to determine the influence of hypertension and chronic hypoperfusion on PA remodeling in 18-wk-old animals only. Passive inner diameters of PAs at 5 mmHg were significantly smaller from SHRSP18-CH compared with WKY18-CH (Table 2). Similarly, wall thickness at 5 and 60 mmHg and CSA at 60 mmHg were significantly greater in PAs from SHRSP18-CH vs. WKY18-CH. However, despite smaller lumens and thicker walls of SHRSP18-CH compared with WKY18-CH, chronic hypoperfusion did not affect structural characteristics of PAs from either SHRSP or WKY, as there were no significant differences in any parameter between WKY18 vs. WKY18-CH or SHRSP18 vs. SHRSP18-CH. Passive distensibility was increased in WKY18-CH compared with WKY18, although this was not significantly different (Fig. 4). Again, although distensibility of PAs was increased in SHRSP18 and SHRSP18-CH compared with WKY, there was no effect of chronic hypoperfusion on distensibility in these vessels.
DISCUSSION
The major findings of this study were that 4 wk of UCCAo caused substantial vasodilation in PAs from WKY18 (WKY18-CH) animals, such that there was significantly decreased myogenic tone and reactivity to pressure. The effect of chronic hypoperfusion was largely prevented in SHRSP18 (SHRSP18-CH) animals, suggesting hypertension impaired this adaptive response to hypoperfusion. Interestingly, the effect of chronic hypoperfusion in WKY animals was limited to vasoactive responses, as there was no effect of UCCAo on passive structural characteristics (passive diameters, wall thickness, or distensibility) of PAs from either WKY18-CH or SHRSP18-CH animals compared with their naive counterparts. In addition, we found that PAs from SHRSP animals had smaller active lumen diameters that was due to increased myogenic tone and not structural changes in the vessel wall (i.e., inward remodeling). Lastly, prehypertensive SHRSP5 animals had PAs with similarly increased tone as hypertensive SHRSP18 animals, suggesting that it was the SHRSP strain and not hypertension per se that caused this effect.
PAs from normotensive WKY rats appeared to undergo an adaptive vasodilatory response to UCCAo. In fact, PAs from WKY18-CH animals had little myogenic tone and responded in a passive manner to increased intravascular pressure, suggesting loss of myogenic reactivity. Although we attempted to investigate underlying mechanisms by which vascular responses might be different, including dilation to NO and SK/IK channel activation, PAs from WKY18-CH animals were so potently dilated we could not perform dilation curves. We speculate that the smooth muscle from these vessels is in a hyperpolarized state, potentially due to changes in ion channel expression or activity, such as voltage-dependent or large-conductance calcium-activated K+ channels that can promote hyperpolarization of smooth muscle. While this vasodilatory response may be an important adaptation during UCCAo to preserve CBF to the brain parenchyma, the diminished reactivity to pressure would likely cause loss of local CBF autoregulation, one of the proposed mechanisms leading to white matter injury during cerebral ischemic SVD (13, 27, 36).
The adaptive vasodilatory response of PAs to UCCAo that was noted in WKY18-CH animals was not seen in SHRSP18-CH that had similar tone and reactivity as vessels from SHRSP18 animals. While it is not clear why PAs from SHRSP did not respond to UCCAo in the same manner as PAs from WKY, it is possible that there was an overall lack of adaptation in other (upstream) vessels as well, such as the collateral systems, and that CBF was not restored to normal in SHRSP18-CH animals. Although we did not measure CBF in either group of animals during the 28 days of UCCAo, studies have shown that hypertension prevents arteriogenesis of both circle of Willis and leptomeningeal anastomoses vessels in response to UCCAo, suggesting this may be the case (26, 34). In addition, Jalal et al. (20) reported that BCCAo in SHRSP animals was lethal, further supporting the concept that arteriogenesis of collaterals during chronic hypoperfusion is an important protective response of the cerebral circulation to maintain CBF that is impaired during hypertension. Although we do not know if the level of CBF during UCCAo was reduced in SHRSP compared with WKY, CBF has been shown to be decreased in male SHRSP at 20 wk of age (41), which would likely decrease further with UCCAo.
It is not entirely surprising that PAs from WKY18-CH or SHRSP18-CH did not undergo structural enlargement. The primary driver for arteriogenesis is increased shear stress that occurs in collaterals when blood flow is redirected during an occlusion (3, 29, 30). PAs are not collaterals, but end vessels that directly connect the pial circulation to the capillaries. Thus the stimulus for arteriogenesis may be absent in PAs, although this was not directly measured in this study. It is worth noting that, in studies using BCCAo, structural remodeling of noncollateral vessels has been found (21, 22, 32). Kim et al. (21) found that 4 wk of BCCAo caused enlargement of the posterior cerebral and communicating arteries, as well as increased size of parenchymal vessels on immunohistochemistry. Márquez-Martín et al. (22) found that 15 days of BCCAo caused decreased wall thickness and CSA, but not structural enlargement of MCAs. However, BCCAo does not engage collaterals to the same extent as UCCAo, and thus vascular remodeling in noncollateral vessels may be more related to ischemia during BCCAo than to changes in shear stress.
PAs from SHRSP18 had significantly increased tone and smaller lumen diameters actively compared with WKY18. Numerous other studies have shown that pial arteries and arterioles from spontaneously hypertensive and SHRSP rats have increased vasoconstriction compared with normotensive strains that is thought to be partially responsible for increased cerebrovascular resistance and shifting the CBF autoregulatory curve to higher pressures in those animals (17, 18). The increased tone in PAs from SHRSP18 animals does not appear to be related to diminished NO responsiveness, as constriction to NOS inhibition with l-NNA was not different from WKY18, but dilation to SNP was significantly increased. Thus PA smooth muscle from SHRSP18 appears more sensitive to NO, not less sensitive, as would be expected if the increased tone was due to a diminished effect of NO on inhibiting tone. PAs from SHRSP18 animals did have modestly diminished dilation to the SK/IK channel opener NS309. SK/IK channels are expressed only in cerebrovascular endothelium and are shown to be critical to the endothelium-derived hyperpolarization pathway (16). Although endothelium-derived hyperpolarization does not appear to oppose basal tone in large cerebral arteries, we have shown that SK/IK channels are basally active in PAs and inhibit tone (7). Thus impairment of SK/IK channel activity or the ability to promote hyperpolarization in PAs from SHRSP18 animals could contribute to the increased tone in those vessels.
Interestingly, we found that PAs from SHRSP18 animals did not have structurally smaller lumens, but did present with medial hypertrophy (thicker walls) and increased passive distensibility compared with WKY18. The structure of PAs in the brain has been studied extensively in SHRSP animals to understand cerebrovascular lesions and more recently the impact of hypertension on progressing SVD. Structural changes in PAs in SHRSP animals appear to depend on age, location, and diet. Schreiber et al. (31) performed a time course study of vascular pathology using MRI and histology in SHRSP animals from 12 to 42 wk of age and found that small vessel diameters were not different at any of these ages, except in the basal ganglia, where they were larger. A similar result was found in a study of PAs isolated from humans undergoing tumor resection, with and without essential hypertension (28). PAs from hypertensive patients had similar inner diameters, but increased wall-to-lumen ratios (28), a finding that is similar to the present study. However, other studies on SHRSP animals have found PAs with increased wall thickness, smaller lumen diameters, and fibrinoid degeneration of the arteriolar wall (11, 12, 19, 24, 25, 35, 40, 41). These pathologic findings are progressed with age and in animals that have the highest blood pressure or that are on a high-salt diet (8, 23, 35, 42). Thus it is possible that lack of inward remodeling in PAs from SHRSP18 in the present study was due to their relatively young age and modestly increased blood pressures that are lower in females than males. It is worth noting that increased wall thickness and distensibility were seen in PAs from both prehypertensive and hypertensive SHRSP, suggesting an effect of strain and not hypertension per se on vascular structure. In addition, because vascular lesions are progressive with age and degree of hypertension, there may be a genetic effect that makes the vasculature more sensitive to the effects of hypertension in SHRSP animals, as has been suggested previously (40).
PAs from SHRSP18 were compared with prehypertensive SHRSP5 animals to distinguish between the effects of hypertension vs. strain differences. We found that the majority of responses, including increased tone, reactivity to NS309, increased wall thickness, and distensibility were present in PAs from both hypertensive and prehypertensive SHRSP animals. While it is tempting to conclude that it was the strain and not hypertension that caused these effects in PAs, we did not have a control for young age, i.e., 5-wk-old WKY. However, it is important to consider that strain may have a significant influence on vascular reactivity and structure of the cerebrovasculature, independent of hypertension.
There are several limitations to this study that are worth considering. First, we did not measure CBF in any of animals, and, therefore, we do not know the relationship between PA vasoactivity, structure, and CBF during UCCAo or if the potent vasodilatory response in normotensive animals contributes to restoring CBF in those animals. Second, we did not measure changes in the structure and function of collaterals, especially the circle of Willis vessels that would be expected to undergo arteriogenesis and influence CBF. It is possible that there is an overall effect of hypertension that impairs arteriogenesis in both noncollateral and collateral vessels that is not specific for PAs. Third, the comparison of vessels from 18-wk-old animals to 5-wk-old animals was not straightforward, considering the young animals were considerably smaller in size and were still undergoing development. To better understand the influence of strain vs. hypertension on PAs, further studies are needed.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-045940 and R01 NS-093289, National Heart, Lung, and Blood Institute Grant PO1 HL-095488, the Totman Medical Research Trust, and the Cardiovascular Research Institute of Vermont.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.G.S. and S.-L.C. performed experiments; J.G.S., S.-L.C., and M.J.C. analyzed data; J.G.S., S.-L.C., and M.J.C. interpreted results of experiments; J.G.S. and M.J.C. prepared figures; J.G.S., S.-L.C., and M.J.C. drafted manuscript; J.G.S., S.-L.C., and M.J.C. edited and revised manuscript; J.G.S., S.-L.C., and M.J.C. approved final version of manuscript; M.J.C. conception and design of research.
REFERENCES
- 1.Adachi T, Kobayashi S, Yamaguchi S, Okada K. MRI findings of small subcortical “lacunar-like” infarction resulting from large vessel disease. J Neurol 247: 280–285, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab 23: 621–628, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389–395, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Choy M, Ganesan V, Thomas DL, Thornton JS, Proctor E, King MD, van der Weerd L, Gadian DG, Lythgoe MF. The chronic vascular and haemodynamic response after permanent bilateral common carotid occlusion in newborn and adult rats. J Cereb Blood Flow Metab 26: 1066–1075, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Sciences, 2009. [PubMed] [Google Scholar]
- 6.Cipolla MJ, DeLance N, Vitullo L. Pregnancy prevents hypertensive remodeling of cerebral arteries: a potential role in the development of eclampsia. Hypertension 47: 619–626, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke 40: 1451–1457, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle P. Dorsal cerebral collaterals of stroke-prone spontaneously hypertensive rats (SHRSP) and Wistar Kyoto rats (WKY). Anat Rec 218: 40–44, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Dufouil C, Godin O, Chalmers J, Coskun O, MacMahon S, Tzourio-Mazoyer N, Bousser MG, Anderson C, Mazoyer B, Tzourio C. Severe cerebral white matter hyperintensities predict severe cognitive decline in patients with cerebrovascular disease history. Stroke 40: 2219–2221, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O'Brien JT, Ince PG. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke 37: 1391–1398, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fredriksson K, Nordborg C, Kalimo H, Olsson Y, Johansson BB. Cerebral microangiopathy in stroke-prone spontaneously hypertensive rats. An immunohistochemical and ultrastructural study. Acta Neuropathol (Berl) 75: 241–252, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Fujita Y, Lin JX, Takahashi R, Tomimoto H. Cilostazol alleviates cerebral small-vessel pathology and white-matter lesions in stroke-prone spontaneously hypertensive rats. Brain Res 1203: 170–176, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82: 126–135, 2011. [DOI] [PubMed] [Google Scholar]
- 14.Guo H, Itoh Y, Toriumi H, Yamada S, Tomita Y, Hoshino H, Suzuki N. Capillary remodeling and collateral growth without angiogenesis after unilateral common carotid artery occlusion in mice. Microcirculation 18: 221–227, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 100: 1059–1064, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper SL, Bohlen HG. Microvascular adaptation in the cerebral cortex of adult spontaneously hypertensive rats. Hypertension 6: 408–419, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Hart MN, Heistad DD, Brody MJ. Effect of chronic hypertension and sympathetic denervation on wall/lumen ratio of cerebral vessels. Hypertension 2: 419–423, 1980. [DOI] [PubMed] [Google Scholar]
- 19.Henning EC, Warach S, Spatz M. Hypertension-induced vascular remodeling contributes to reduced cerebral perfusion and the development of spontaneous stroke in aged SHRSP rats. J Cereb Blood Flow Metab 30: 827–836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin loss associated with neuroinflammation in hypertensive rats. Stroke 43: 1115–1122, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Kim EH, Lee BI, Heo JH. Chronic cerebral hypoperfusion protects against acute focal ischemia, improves motor function, and results in vascular remodeling. Curr Neurovasc Res 5: 28–36, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Marquez-Martin A, Jimenez-Altayo F, Dantas AP, Caracuel L, Planas AM, Vila E. Middle cerebral artery alterations in a rat chronic hypoperfusion model. J Appl Physiol (1985) 112: 511–518, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mies G, Hermann D, Ganten U, Hossmann KA. Hemodynamics and metabolism in stroke-prone spontaneously hypertensive rats before manifestation of brain infarcts. J Cereb Blood Flow Metab 19: 1238–1246, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura T, Yamamoto E, Kataoka K, Yamashita T, Tokutomi Y, Dong YF, Matsuba S, Ogawa H, Kim-Mitsuyama S. Pioglitazone exerts protective effects against stroke in stroke-prone spontaneously hypertensive rats, independently of blood pressure. Stroke 38: 3016–3022, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Ogata J, Fujishima M, Tamaki K, Nakatomi Y, Ishitsuka T, Omae T. Vascular changes underlying cerebral lesions in stroke-prone spontaneously hypertensive rats. A serial section study. Acta Neuropathol (Berl) 54: 183–188, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Omura-Matsuoka E, Yagita Y, Sasaki T, Terasaki Y, Oyama N, Sugiyama Y, Todo K, Sakoda S, Kitagawa K. Hypertension impairs leptomeningeal collateral growth after common carotid artery occlusion: restoration by antihypertensive treatment. J Neurosci Res 89: 108–116, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9: 689–701, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Rizzoni D, De Ciuceis C, Porteri E, Paiardi S, Boari GE, Mortini P, Cornali C, Cenzato M, Rodella LF, Borsani E, Rizzardi N, Platto C, Rezzani R, Rosei EA. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens 27: 838–845, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 23: 1143–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Schierling W, Troidl K, Mueller C, Troidl C, Wustrack H, Bachmann G, Kasprzak PM, Schaper W, Schmitz-Rixen T. Increased intravascular flow rate triggers cerebral arteriogenesis. J Cereb Blood Flow Metab 29: 726–737, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber S, Bueche CZ, Garz C, Kropf S, Angenstein F, Goldschmidt J, Neumann J, Heinze HJ, Goertler M, Reymann KG, Braun H. The pathologic cascade of cerebrovascular lesions in SHRSP: is erythrocyte accumulation an early phase? J Cereb Blood Flow Metab 32: 278–290, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soria G, Tudela R, Marquez-Martin A, Camon L, Batalle D, Munoz-Moreno E, Eixarch E, Puig J, Pedraza S, Vila E, Prats-Galino A, Planas AM. The ins and outs of the BCCAo model for chronic hypoperfusion: a multimodal and longitudinal MRI approach. PLoS One 8: e74631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staekenborg SS, van Straaten EC, van der Flier WM, Lane R, Barkhof F, Scheltens P. Small vessel versus large vessel vascular dementia: risk factors and MRI findings. J Neurol 255: 1644–1651; discussion 1813–1814, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama Y, Yagita Y, Yukami T, Watanabe A, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, Mochizuki H, Kitagawa K. Granulocyte colony-stimulating factor fails to enhance leptomeningeal collateral growth in spontaneously hypertensive rats. Neurosci Lett 564: 16–20, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Tagami M, Nara Y, Kubota A, Sunaga T, Maezawa H, Fujino H, Yamori Y. Ultrastructural characteristics of occluded perforating arteries in stroke-prone spontaneously hypertensive rats. Stroke 18: 733–740, 1987. [DOI] [PubMed] [Google Scholar]
- 36.Tajima Y, Takuwa H, Kokuryo D, Kawaguchi H, Seki C, Masamoto K, Ikoma Y, Taniguchi J, Aoki I, Tomita Y, Suzuki N, Kanno I, Saeki N, Ito H. Changes in cortical microvasculature during misery perfusion measured by two-photon laser scanning microscopy. J Cereb Blood Flow Metab 34: 1363–1372, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, Yagita Y, Hori M. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke 39: 1875–1882, 2008. [DOI] [PubMed] [Google Scholar]
- 38.van der Flier WM, van Straaten EC, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 36: 2116–2120, 2005. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk EJ, Breteler MM, Schmidt R, Berger K, Nilsson LG, Oudkerk M, Pajak A, Sans S, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Launer LJ, Hofman A. The association between blood pressure, hypertension, and cerebral white matter lesions: cardiovascular determinants of dementia study. Hypertension 44: 625–630, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Yamori Y. Overview: studies on spontaneous hypertension-development from animal models toward man. Clin Exp Hypertens A 13: 631–644, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Yamori Y, Horie R. Developmental course of hypertension and regional cerebral blood flow in stroke-prone spontaneously hypertensive rats. Stroke 8: 456–461, 1977. [DOI] [PubMed] [Google Scholar]
- 42.Yamori Y, Horie R, Handa H, Sato M, Fukase M. Pathogenetic similarity of strokes in stroke-prone spontaneously hypertensive rats and humans. Stroke 7: 46–53, 1976. [DOI] [PubMed] [Google Scholar]