Abstract
The accessible genetics and extensive skeletal musculature of the zebrafish make it a versatile and increasingly used model for studying muscle contraction. We here describe the development of an in vivo assay for measuring the contractile force of intact zebrafish at the larval stage. In addition, as proof of applicability, we have used this assay to quantify contractile strength of zebrafish larvae in a morphant model of deranged rbfox function. Average maximum tetanic (180 Hz) whole body forces produced by wild-type larvae at 2, 3, 4, and 5 days postfertilization amounted to 3.0, 7.2, 9.1, and 10.8 mN, respectively. To compare at potentially different stages of muscle development, we developed an immunohistological assay for empirically determining the cross-sectional area of larval trunk skeletal muscle to quantify muscle-specific force per cross-sectional area. At 4-5 days postfertilization, specific force amounts to ∼300 mN/mm2, which is similar to fully developed adult mammalian skeletal muscle. We used these assays to measure contractile strength in zebrafish singly or doubly deficient for two rbfox paralogs, rbfox1l and rbfox2, which encode RNA-binding factors shown previously to modulate muscle function and muscle-specific splicing. We found rbfox2 morphants produce maximal tetanic forces similar to wild-type larvae, whereas rbfox1l morphants demonstrate significantly impaired function. rbfox1l/rbfox2 morphants are paralyzed, and their lack of contractile force production in our assay suggests that paralysis is a muscle-autonomous defect. These quantitative functional results allow measurement of muscle-specific phenotypes independent of neural input.
Keywords: contraction, rbfox1, rbfox2, zebrafish, muscle
in skeletal muscle research, zebrafish is becoming an increasingly used model system for studying structure and function. The vertebrate zebrafish lends itself to the study of muscle for several reasons: 1) transgenic and mutant models are relatively easy and inexpensive to generate; 2) a large part of the zebrafish larva is composed of skeletal muscle; and 3) transparency during early development facilitates fluorescence/luminescence-based in vivo studies (14). These features of the zebrafish model provide opportunities to answer questions not easily addressed in other species.
To date, there exist many zebrafish models that recapitulate human muscular and neuromuscular disease (for reviews, see Refs. 10, 15, 18, 26). However, most studies on zebrafish muscle do not directly assess actual muscle contractility. Typically, muscle functionality in live zebrafish larvae is extrapolated or indirectly derived from recording movements of anesthetized larvae embedded in agarose, or from observation of swimming behavior (6, 11, 16). These assays give valuable information about the general effect of induced mutations on movement, yet do not address effects specific to muscles because of the nervous system input required for stimulus response (30). Thus developing new assays in which muscle-specific effects on movement can be assayed independently of neural influence/input are important new tools to study zebrafish models of neuromuscular disease (25).
Recently, muscle physiology assays have been applied to zebrafish models to improve skeletal muscle characterization (4). Force data generated by the skeletal muscle during these assays are distributed per cross-sectional area (CSA) of the fish larvae to give muscle-specific force. Some groups have used fixed or decapitated larval preparations to rule out neural input, but these approaches are limited by constraints of ex vivo preparations, such as the loss of regulatory systems (2, 4, 16). A more recent approach measures contractile force in live larvae; however, it may underestimate the true force-generating capacity of larval skeletal muscle by overestimating the actual muscle CSA (30).
To enhance current techniques, we developed an assay to assess the contractility of larval zebrafish skeletal muscle in vivo using a muscle physiology approach to measure force produced, distributed per empirically determined muscle CSA. As proof of concept, we measure the forces produced by live wild-type larvae at developmental time points and the CSA of representative wild-type larvae and demonstrate that there is an increase in force-generating capacity of muscle until 3 days postfertilization (dpf). Before this investigation, we did not previously know whether electrophysiological stimulation would be sufficient to induce contractions in rbfox-depleted muscle. To demonstrate the sensitivity of the assay to discriminate between muscle phenotypes, we characterize muscle function of the previously established morphant model of deranged muscle splicing, rbfox1l, rbfox2, and double-morphant larvae, and show that rbfox1l and rbfox1l/2 knockdown, but not rfbox2 single knockdown, negatively affects muscle function. Previous work had shown that these two zebrafish rbfox genes function partially redundantly, with loss of skeletal muscle-specific rbfox1l function causing defects in skeletal muscle fiber organization, and loss of both genes causing severe fiber defects and complete paralysis (8). However, since rbfox2 is expressed more broadly, including in neural tissue, it was not clear whether paralysis in rbfox1l/rbfox2 double morphants was muscle autonomous. Because the assay described here directly measures contractile force without requiring neural input, we used it to assess contractile defects in rbfox-depleted larvae and found that the defects observed are consistent with skeletal muscle-autonomous rbfox function.
MATERIALS AND METHODS
Zebrafish husbandry.
Wild-type (AB) adult zebrafish were maintained in an institutional facility at 28°C with a light-dark cycle of 14:10 h. Before contractile experiments, embryos were maintained in system water in an incubator at ∼28.5°C until they reached the desired stage. Adult fish and larvae were used and maintained according to protocols approved by the Institutional Animal Care and Use Committee at The Ohio State University.
Morpholinos and injections.
Morpholinos (MOs) were designed and injected as described previously (8). Briefly, 6 ng of splice-blocking MO against either rbfox1l or rbfox2 or 3 ng of both MO (to keep the total 6 ng, same as the other groups to avoid a potential impact of dose) were injected into zebrafish embryos at the one-cell stage.
Muscle contractile analysis.
Embryos and larvae were raised to the desired stage and then anesthetized in 0.02% wt/vol tricaine buffered with Tris·HCl in Krebs-Henseleit (KH) solution containing (in mM) 118 NaCl, 5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, 10 glucose, and 2 CaCl2. During initial optimization of the assay, anesthetic concentration was varied. Anesthetized larvae were transferred to a heated KH bath maintained at ∼28.5°C and mounted on a custom-built setup, with a force transducer grasping the tail and a hook through the head to displace the body length. The larvae were progressively stretched to achieve optimum length, or the point at which twitch amplitude after stimulation for 4 ms at 180 Hz no longer increased with length. The force at optimum length was recorded as twitch force for the larva. The larvae were then allowed to rest for 10 min. Further experiments were conducted at this length. After the rest period, larvae were stimulated at trains of 250-ms duration at 20, 50, 80, 120, 150, and 180 Hz. Larvae were unstimulated for 2 min after each train of stimuli. Force tracings were collected at a rate of 1 kHz by a custom-designed application in LabView. The maximum force attained in twitches or tetanus at each frequency was taken to represent force attained at that frequency in milli-Newtons. Experiments performed on larvae were confined to the period between the hour at which they reached a new DPF and 6 h following this time point to minimize variation due to normal developmental progression (i.e., fish denoted 2 dpf are 48–54 h postfertilization). Rbfox morphant genotypes were staggered during contractile experiments, also to account for development during experimentation.
Cryosectioning.
Zebrafish were raised under standard housing conditions to the desired stage. Embryos and larvae were anesthetized with tricaine, fixed in 4% cold paraformaldehyde overnight at 4°C, rinsed in PBS, and then shaken in 43% wt/vol sucrose in PBS for 3 h at room temperature. Larvae were then rinsed in optimum cutting temperature and positioned in optimum cutting temperature blocks and frozen on dry ice. Blocks were cut in transverse 20- to 25-μm sections, and sections from anterior to the yolk sac to the end of the yolk tube were retained. Slides were frozen at −20°C until staining.
Histology.
Slides of zebrafish transverse sections were rinsed and then incubated in PBS and 0.5% Triton-X for 15 min. Slides were washed in PBS and blocked in 3% BSA for 4 h at room temperature. Sections were incubated in A410.25 anti-myosin heavy chain mouse monoclonal IgG2A (DSHB, 1:1,000) in 3% BSA overnight. They were rinsed over 2 h and incubated briefly in PBS and 0.1% Triton-X. Slides were washed and then incubated in goat anti-mouse Alexa Fluor 568 (Thermo Fisher Scientific; 1:200) in PBS and 0.1% Triton-X for 2 h at room temperature. Afterward, sections were rinsed and incubated in 4,6-diamidino-2-phenylindole (1:1,000) for 10 min. After rinsing and mounting, slides were stored in the dark at 4°C until imaging.
Imaging.
Sixteen-bit images were obtained using MetaMorph (Molecular Devices) on an Ando Spinning Disc Confocal Microscope (Oxford Instruments) at ×20 with a NikonNeo camera; laser wavelength and intensity were at 561 nm and 100%, respectively. Images were exposed for 1 s on a single plane in Z at which muscle area was greatest. Sections with tissue damage that would interfere with CSA determination were not included in the analysis.
Image analysis.
In MetaMorph, images were thresholded to include muscle fluorescence. A program function drew regions around thresholded objects. A calibration factor based on the camera, microscope objective, and pixel size was used to calculate the area of the regions, including trunk skeletal muscle. Regions from the same section image were summed. The two sections with the largest combined trunk muscle area from each larva were selected to determine average CSA, since the thickest part of skeletal muscle contributes the most power to contraction. For muscle-to-body area ratio analyses, sections with intact tissue were traced manually to include whole body area. Yolk and gut regions of larvae were ignored, as these vary widely over the length of the organism.
Statistical analyses.
Contractile data (twitch force or tetanic force at 180 Hz), muscle-to-body ratio, and muscle CSA were analyzed using one-way (for simply multigroup comparison) or two-way ANOVA (for comparison of DPF and genotype, with interaction) and the Holm-Bonferroni post hoc test. Results in both instances were considered significant when two-tailed P ≤ 0.05.
RESULTS
Establishment of an in vivo contractile assay.
A custom experimental setup was designed to measure the contractile force of larval zebrafish body muscle. Anesthetized larvae are held between a force transducer gripping the tail and a hook that extends the animal in a KH-containing bath (Fig. 1, A and B). The distance between the hook insertion point in the head and the point at which the force transducer grips the tail varies, depending on the age of the larvae, and ranges from ∼1.5 mm at 2 dpf to ∼4 mm at 5 dpf. Parallel platinum electrodes in the bath on either side of the larva deliver stimuli from a customized program in LabView (National Instruments). This same program receives and records data from the force transducer. Representative traces of raw data obtained with the setup for a 2-dpf embryo are shown in Fig. 1, C and D. This approach measures the contractile force across the whole zebrafish body, which contains a high percentage of skeletal muscle relative to other nonmuscle tissues at these stages.
Fig. 1.
Development of muscle contraction assay. A: live larvae were anesthetized with tricaine and placed in a Krebs-Henseleit (KH) bath. Platinum electrodes on either side delivered the desired stimuli. B: image of a live larvae suspended between the force transducer (left) and the length displacement hook (right). C and D: representative traces of force generated at 20 and 120 Hz, respectively, by a 2-day postfertilization (dpf) wild-type (WT) larva. y-Axes were measured in volts (sensitivity set at 10 mN/V), and x-axes in milliseconds.
Direct contractile assessment of developing wild-type zebrafish larvae.
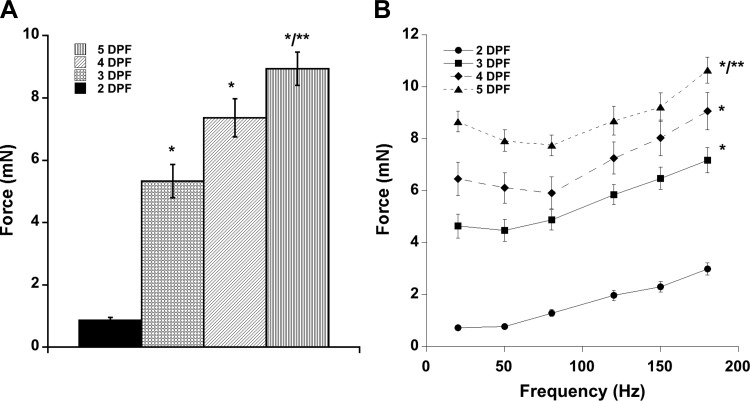
We first used this assay to determine the force generated by zebrafish skeletal muscle during embryonic and larval development and to establish conditions and parameters for analyzing mutants. We measured the contractile force of skeletal muscle in developing wild-type zebrafish larvae from 2 to 5 dpf. We found the optimal length (the length at which contractile force no longer increased) of individual embryos and larvae by single-twitch stimulation and then measured contractile force for increasing frequencies, ultimately leading to fully fused tetani, at this length. As expected, we observed that whole larval skeletal muscle contractile force increases significantly with developmental time and stimulation frequency (Fig. 2). At 2 dpf, maximal larval contractile forces were 0.72 ± 0.11 mN at 20 Hz, 0.77 ± 0.09 mN at 50 Hz, 1.29 ± 0.13 mN at 80 Hz, 1.97 ± 0.20 mN at 120 Hz, 2.31 ± 0.20 mN at 150 Hz, and 2.99 ± 0.24 mN at 180 Hz (Fig. 2B). By 5 dpf, whole larval maximal tetanic forces reached 10.8 ± 0.49 mN at a stimulation frequency of 180 Hz (Fig. 2B). We also measured contractile forces at individual twitches. Maximal twitch forces were 0.86 ± 0.09 mN for 2 dpf, 5.33 ± 0.54 mN for 3 dpf, 7.4 ± 0.6 mN for 4 dpf, and 9.1 ± 0.4 mN for 5 dpf larvae (Fig. 2A). Twitch and tetanic force increased with developmental age, as expected.
Fig. 2.
Force generation by WT zebrafish larvae. Live larvae were anesthetized with tricaine and stimulated at various frequencies. A: absolute force generated at each developmental time point by twitch contractions. B: tetanic forces generated by WT larvae at each developmental time point. Values are means ± SE. Significant difference determined (twitch and 180-Hz tetani) by one-way ANOVA with Bonferroni post hoc analysis, P < 0.05 vs. *2 dpf and **3 dpf.
CSA determination to calculate muscle-specific force.
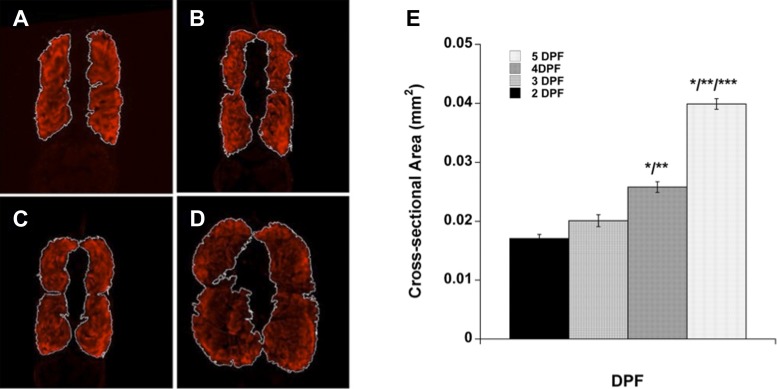
Muscle contractile force is proportional to the muscle CSA, which changes as an animal develops. Therefore, once we measured contractile forces in developing zebrafish embryos and larvae, we sought to attribute these measurements to muscle-specific CSA to account for changes in muscle contractile force due to growth. Assays developed to directly measure zebrafish skeletal muscle contractile force are generally unable to accurately report muscle-specific force, since the approximated CSA used to determine the size of the fish includes other anatomical structures, such as the neural tube, yolk, and spinal cord. We thus needed to empirically determine muscle CSA and did so using immunofluorescence for muscle-specific markers on tissue sections (Fig. 3). We observed that muscle CSA increased with developmental age and was greatest at the posterior end of the yolk tube before tapering gradually. Sections with the largest fluorescent muscle areas were selected to represent the CSA for a developmental stage because the largest section of muscle contributes the most to the power of contraction (Fig. 3, Table 1). Additionally, we determined the ratio of muscle to total body CSA (excluding yolk) by comparing the stained muscle area to the whole body area to facilitate future studies of embryonic and larval muscle function by providing a normalization factor for each developmental stage to allow for meaningful comparisons between stages. We also examined muscle CSA-to-body CSA ratio in all intact wild-type tissue sections and observed that this proportion is not consistent along the anterior-posterior axis of the larval body. Differences between developmental time points in these proportions reach statistical significance in wild-type larvae, as one might expect, due to differential body growth of muscle vs. nonmuscle tissues along the anterior-posterior axis (Table 1). We conclude from these data that muscle area-to-body area ratios along the axis of the larva or embryo cannot be used to approximate muscle CSA, except at sections with the largest muscle area.
Fig. 3.
Cross-sectional area of WT larval muscle. Paraformaldehyde-fixed larvae were sectioned transversely and stained, and then the two sections with the largest muscle area from each fish were used to represent cross-sectional area of muscle. Representative sections of 2 dpf (A), 3 dpf (B), 4 dpf (C), and 5 dpf (D) larvae are shown. E: cross-sectional area of muscle for each developmental stage. Values are means ± SE. Significant difference determined by one-way ANOVA with Bonferroni post hoc analysis, P < 0.05 vs. *2 dpf, **3 dpf, and ***4 dpf.
Table 1.
CSA of muscle and ratios of muscle to whole body area
| Largest Muscle Sections |
All Muscle Sections |
|||||
|---|---|---|---|---|---|---|
| Genotype | dpf | CSA, mm2 | Muscle-to-body area ratio | n | Muscle-to-body area ratio | n |
| Wild type | 2 | 0.0171 ± 0.0007 | 0.600 ± 0.009 | 6 | 0.562 ± 0.002 | 24 |
| 3 | 0.0201 ± 0.001 | 0.622 ± 0.006* | 8 | 0.603 ± 0.003 | 16 | |
| 4 | 0.0258 ± 0.0009* | 0.625 ± 0.019 | 7 | 0.641 ± 0.001 | 61 | |
| 5 | 0.0399 ± 0.0009* | 0.722 ± 0.055* | 4 | 0.656 ± 0.002 | 44 | |
| rbfox1l | 2 | 0.0175 ± 0.0006 | 0.557 ± 0.003 | 8 | 0.498 ± 0.0008 | 83 |
| 3 | 0.0250 ± 0.0007* | 0.666 ± 0.009* | 4 | 0.594 ± 0.002 | 42 | |
| 4 | 0.0290 ± 0.0006* | 0.681 ± 0.008 | 5 | 0.599 ± 0.001 | 75 | |
| rbfox2 | 2 | 0.0167 ± 0.001 | 0.541 ± 0.004 | 8 | 0.51 ± 0.0008 | 56 |
| 3 | 0.0291 ± 0.001* | 0.691 ± 0.007* | 7 | 0.607 ± 0.0009 | 77 | |
| 4 | 0.0336 ± 0.002* | 0.685 ± 0.017 | 5 | 0.64 ± 0.001 | 67 | |
| rbfox1l/2 | 2 | 0.0139 ± 0.002 | 0.527 ± 0.015 | 8 | 0.511 ± 0.0009 | 73 |
| 3 | 0.0249 ± 0.0006* | 0.601 ± 0.051* | 7 | 0.571 ± 0.0008 | 68 | |
| 4 | 0.0359 ± 0.003* | 0.627 ± 0.005 | 8 | 0.523 ± 0.0008 | 109 | |
Values are means ± SE; n, no. of larvae. Paraformaldehyde-fixed larvae were sectioned transversely and stained, and then the two sections with the largest muscle area from each fish were used to represent cross-sectional area (CSA) of muscle. Sample sizes of sections analyzed are listed. Two-way ANOVA on the largest muscle CSA indicated days postfertilization (dpf), but neither genotype nor interaction between dpf and genotype was significant.
P < 0.05 vs. 2 dpf.
Effect of development on muscle-specific force of wild-type larvae.
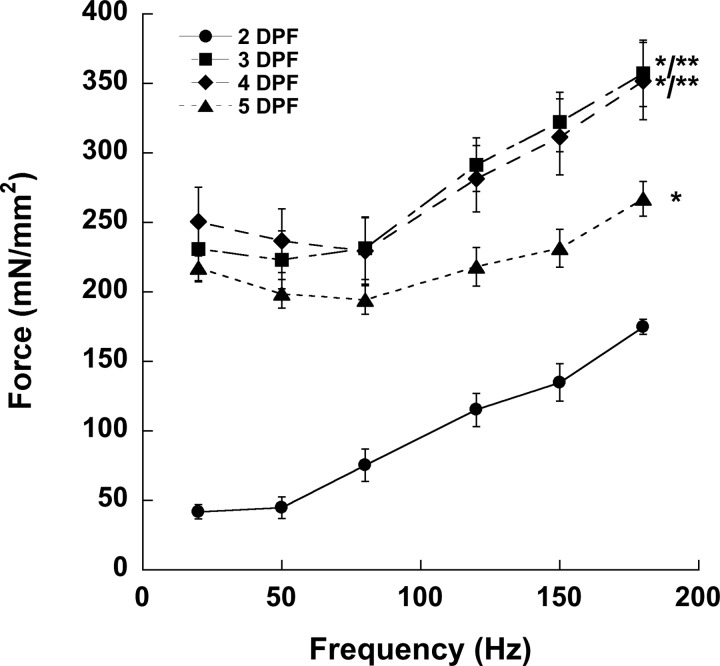
We sought to demonstrate the ability of our assay to discriminate between different zebrafish embryonic and larval skeletal muscle forces by examining wild-type individuals. Forces produced by wild-type larvae at 2, 3, 4, and 5 dpf were normalized to the muscle CSA that we determined empirically (Table 1) to calculate muscle-specific force. Although forces generated by 2-dpf larvae at every frequency were significantly lower than at other time points, 3-dpf larvae generated forces that were not significantly lower than 4- and 5-dpf larvae at 20, 50, and 80 Hz and no different than 4-dpf larvae at 120, 150, and 180 Hz. These measurements suggest that, by 3 dpf, zebrafish larval skeletal muscle is fully competent. However, high-frequency muscle forces in 5-dpf larvae begin to decrease around 120 Hz and become significantly lower than the forces generated by 3- and 4-dpf fish at the same frequencies (Fig. 4). Larval muscle generally begins to tetanize at 120 Hz, and we observed that 5-dpf fish generated contractile forces of such magnitude during stimulation that their tissue began to tear slightly. We believe that tearing is responsible for the decrease in muscle-specific force generated at higher frequencies in 5-dpf larvae and conclude that force measurements in larvae ≥5 dpf should only be investigated using this technique at frequencies below those that initiate tearing (<120 Hz).
Fig. 4.
Force generation by WT zebrafish larvae. Live larvae were anesthetized with tricaine and stimulated at various frequencies. Whole forces generated at each developmental time point at twitch and tetanic frequencies depicted in Fig. 2 were distributed per muscle cross-sectional area listed in Table 1. Values are means ± SE. Significant difference determined by one-way ANOVA with Bonferroni post hoc analysis, P < 0.05 vs. *2 dpf and **3 dpf.
Muscle force is differentially affected in fish depleted of rbfox functions.
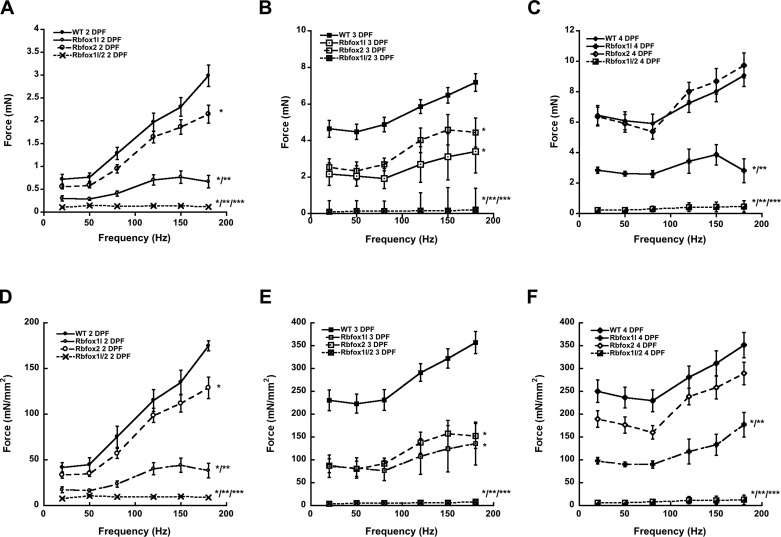
Having established the ability of our assay to measure larval contractile force in vivo, we sought to test its discriminatory potential by measuring forces in the previously established rbfox1l, rbfox2, and rbfox1l/2 morphants (8). Rbfox genes encode RNA binding factors that mediate splicing programs in skeletal muscle, cardiac muscle, and neural cell lineages in mammals (17, 22, 29, 31). Zebrafish carry at least two rbfox paralogs that are expressed in skeletal muscle, rbfox1l and rbfox2 (8). Rbfox1l knockdown results in mild muscle defects, and rbfox2 knockdown has no overt muscle phenotype; however, knockdown of both rbfox1l and rbfox2 results in severe myofibrillar disorganization and muscle paralysis, suggesting partial functional redundancy of these two rbfox genes (8). Because rbfox1l is expressed exclusively in muscle, and because neuromuscular junctions appear largely normal in rbfox1l/2 double morphants, we hypothesized that rbfox genes function muscle autonomously; however, since rbfox2 is expressed more broadly, including in neuronal tissues, rbfox could function muscle nonautonomously as well. To examine the role of rbfox1l and rbfox2 in muscle force generation in larval zebrafish, we generated morphant larvae, characterized muscle CSA, and assessed skeletal muscle force generation in vivo at 2, 3, and 4 dpf (Figs. 5 and 6). As expected, rbfox2 morphant fish produced essentially wild-type forces at each developmental stage, except for a slight dip at 3 dpf that was ameliorated by 4 dpf (Fig. 6, B and C). Although rbfox1l morphant muscles contracted, they produced significantly less whole force than those of rbfox2 morphant larvae or wild-type larvae at each stage (Fig. 6, A–C), supporting that rbfox1l is required for functional muscle development and demonstrating that our assay can detect changes in muscle physiology associated with more subtle muscle morphological defects. Finally, rbfox1l/2 morphants were unable to generate force above background at any developmental point or frequency examined (Fig. 6, A–C), consistent with the complete paralysis phenotype and supporting a muscle-autonomous role for rbfox genes.
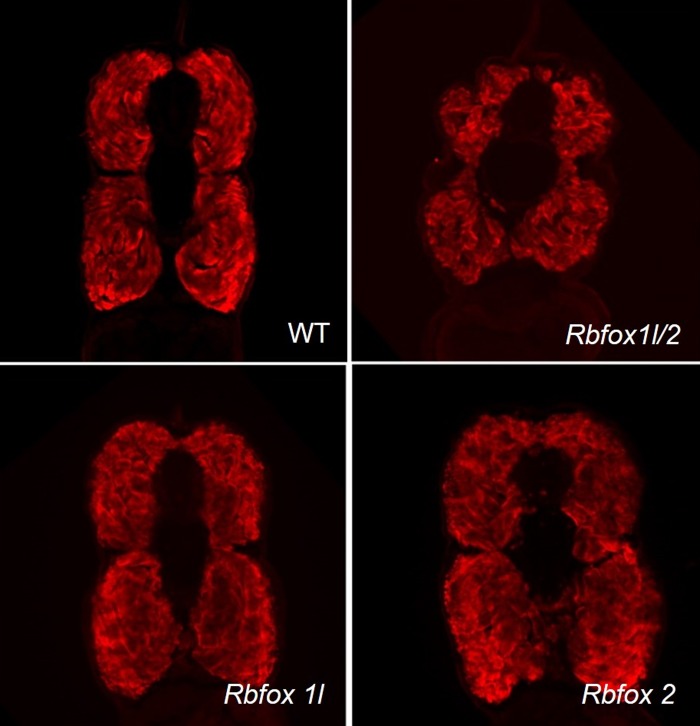
Fig. 5.
Skeletal muscle morphology in rbfox morphants. Transverse sections of 4-dpf morphant larvae were stained for α-myosin heavy chain. Representative sections from roughly the same anatomical location are shown for each morphant condition.
Fig. 6.
Characterization of muscle function in rbfox morphants. Live morphant larvae were anesthetized with tricaine and stimulated at various frequencies. Muscle-specific force generated at each developmental time point at twitch and tetanic frequencies shown in Fig. 4 were distributed per muscle cross-sectional area listed in Table 1. A–C: forces generated by rbfox morphant larvae at 2, 3, and 4 dpf, respectively. D–F: muscle-specific force by rbfox morphant larvae at 2, 3, and 4 dpf, respectively. Values are means ± SE. Significant difference (180-Hz data) determined by two-way ANOVA with Bonferroni post hoc analysis, P < 0.05 vs. *WT, **rbfox2, and ***rbfox1l.
To better represent the force generation due to muscle strength in each morphant, we determined muscle CSA for rbfox1l, rbfox2, and rbfox1l/2 morphant larvae. Sectioning analyses of the largest muscle areas demonstrated that muscle CSA were similar in wild-type and morphant larvae (Table 1). Two-way ANOVA indicated that the factor DPF was significant (P < 0.001) (muscle-to-body ratio increases with age), but the factor genotype was not quite significant (P = 0.052), and the interaction between these factors was not significant (P = 0.221). Thus overall, rbfox genetic perturbations did not affect muscle CSA. However, rbfox2 morphants at 3 dpf and rbfox1l/2 morphants at 4 dpf did have a slightly decreased CSA. The difference in 4-dpf larvae could be due to defects in myofibrillar and sarcomere assembly that we previously described (8). Moreover, we observed a marked difference in the shape of rbfox1l/2 morphant muscle sections at 4 dpf, which were lobular and less compact than wild-type or single rbfox morphant larval muscle sections (Fig. 5). Regardless, the muscle-to-body area ratio for rbfox morphants at each stage was similar to their wild-type counterparts, suggesting that this proportion can be used to estimate muscle CSA (Table 1).
Once we obtained the muscle CSA for single and double rbfox morphants, we calculated the muscle-specific force generated at each developmental time point by normalizing the total force by the muscle CSA (Fig. 6, D–F). Two-way ANOVA on tetanic force at 180 Hz indicated that both the factor DPF (P < 0.001) and the factor genotype (P < 0.001) were significant, as well as their interaction (P < 0.005). This attribution of force to muscle did not change the relationship between different morphant groups' muscle force production as it did for wild-type larvae. The same trends of decreased force in rbfox1l and double morphant embryos and larvae can be observed when comparing muscle-specific contractile force, which confirms that the effects of rbfox knockdown on muscle function are not due to larval growth, but to muscle dysfunction (Fig. 6). Rbfox2 morphants were capable of generating similar contractile force as wild type at each frequency for each developmental time point, except at 3 dpf (Fig. 6, B and D). At 2, 3, and 4 dpf, both rbfox1l and rbfox1l/2 morphants generated forces significantly below their wild-type counterparts at each frequency (Fig. 6, D–F). The muscle-specific forces generated by these morphants support the conclusion that rbfox1l is important for muscle development and function. Furthermore, combined knockdown of rbfox1l and rbfox2 has a negative synergistic effect, indicating that rbfox2 partially compensates for loss of rbfox1l function in muscle (Fig. 6) (8).
DISCUSSION
Here we report on the development and application of an assay to measure the contractility of larval zebrafish muscle in vivo at late embryonic and early larval stages during a period of robust muscle development. We have combined this assessment with the empirical determination of larval muscle CSA at each of these stages, allowing for calculation of muscle-specific force. Our data show that muscle-specific force reaches its maximum per area already at 4 dpf. As proof of our assay's ability to characterize zebrafish models, we have quantified the muscle-specific contractile effects upon rbfox1l and rbfox2 knockdown in larval zebrafish. Knockdown of rbfox1l results in decreased muscle force production at all time points, whereas rbfox2 knockdown only minimally affects function, and only at the 3-dpf time point. Combined knockdown of rbfox1l and rbfox2 leads to paralysis and inability to generate contractile force at all time points examined.
The muscle forces obtained in our assay are generally higher than those measured in previous similar studies (2, 4, 16, 30, 32), which we attribute to four main factors. First, we noticed that any damage to the body, even to nonmuscle regions such as the head, decreased the force of contraction measured in our assay due to hemorrhaging. It appears that live animals were capable of greater contractile force, which at least partially explains why measured forces in decapitated preparations are smaller (2, 4, 16). Second, we noted that electrode placement was integral to capturing contractile potential of larval skeletal muscle. The skin and other tissues of zebrafish larvae act as electrical insulators, preventing the more interior tissue from exposure to the full strength of the electrical field. Third, the electrical field strength is the voltage applied divided by the distance between the electrodes, so electrodes placed too far from the larval body fail to produce an electric field in the tissue sufficient to fully excite the skeletal muscle (20). Having tried several different stimulators, we noticed that several stimulators do not allow a sufficiently high amperage to be delivered, even at the maximal voltage of most field-stimulating units (near 20 V); sufficient amperage is required to fully stimulate the muscle to contract. Fourth, the use of KH solution, which is >10 times more conductive than typical fish water (700-1,000 μS/cm), allows for better conductance of the stimulation field. Therefore, we optimized electrode placement, used KH solution, and used a stimulator that allows for the delivery of a sufficiently large excitation pulse to induce total muscle excitation and maximum force generation possible.
Compared with mammalian skeletal muscle, the muscle-specific forces obtained in zebrafish larvae are very similar. For example, in mouse extensor digitorum longus muscles, located near the tibia, tetanic forces are also typically in the 200–400 mN/mm2 range (3, 12, 19, 24, 27). Thus for future studies on muscle contractile forces in zebrafish, inclusion of a wild-type group where forces are obtained at or near the levels reported in this study would ensure the quality of the assessment is not hampered by technical limitations.
During our investigation, we observed that tetanic contractions in older larvae, beginning at 4 dpf but most evident at 5 dpf, result in tears (sometimes near head, sometimes in body or tail) that decrease the force-generating capacity of skeletal muscle. The isometric mode of contraction experimentally induced in our studies may generate forces in the area with the largest cross section that well exceeds the limit the tissue in smaller areas can bear. In future studies, it may be necessary to investigate only twitch forces at these stages to obtain a more accurate measure of muscle strength. We did not investigate the 5-dpf time point in rbfox larvae partly for this reason, and partly because MOs injected at the one-cell stage of development tend to lose activity after 2 dpf. Moreover, rbfox1l/2 double morphant larvae begin to develop cardiac edema at 2 dpf, and their mortality rate increases. In addition to high mortality rates affecting the number of larvae we were able to study, the pericardial edema typical of these morphants hampered our ability to mount 4-dpf rbfox1l/2 morphants to our rig for analysis. Therefore, we were limited in our study size by the ages that could be studied and by the number of living rbfox1l/2 morphants.
Important to the study of live zebrafish larvae is the use of anesthetic to immobilize larvae and to limit discomfort for the animals. In our experiments, we used tricaine (MESAB-22) to anesthetize larvae for in vivo study of skeletal muscle contraction. There have been several studies suggesting that tricaine, which acts on sodium channels (7), actually decreases muscle excitability and contraction force (13, 28, 30) due to a specific action on neural sodium channels in larval zebrafish when used at low concentrations (≤0.02% wt/vol) (1). In early experiments we varied tricaine concentrations and observed that prolonged exposure (over 1 h) can affect the performance of muscle (data not shown). However, at lower concentrations and short exposure times (30 min), we did not observe a decrease in generated muscle force.
For unambiguous determination of muscle-specific force in developing zebrafish larvae, it is important to accurately determine the muscle CSA of zebrafish larvae at specific time points that will be tested, since these fish develop rapidly and CSA changes over developmental time. We determined muscle CSA as well as the ratio of muscle to body area for zebrafish larvae, facilitating estimation of muscle CSA in future studies. The ratio of muscle to body area can be applied to elliptical measurements of zebrafish larvae (excluding the yolk and yolk tube). Specific attribution of force to muscle facilitates comparison between fiber types, animal systems, and disease models to understand differences in contractility. By assigning muscle-specific force to larval zebrafish muscle, we were able to determine that contractile strength in these animals is similar to that produced by whole preparations of mouse skeletal muscles (1, 3, 9, 12, 21, 23, 24, 27). Comparisons of muscle-specific force will facilitate study of drug effects, disease, and genetic manipulation in various vertebrate model systems. In addition, this quantitative analysis illustrates that development of all components required for maximal muscle-specific force is completed by 4 dpf, including sarcomere assembly, t-tubule development, and sarcoplasmic reticulum maturation.
To assess muscle-specific zebrafish larval contractility, we characterized the effect of rbfox1l and rbfox2 knockdown on muscle contractility. Rbfox1l and rbfox2 are paralogs that regulate alternative splicing in muscle (and possibly neural tissues for the more broadly expressed rbfox2 paralog) (17). When knocked down simultaneously, depletion of rbfox1l and rbfox2 function results in complete paralysis (8). We confirmed that rbfox1l and rbfox2 are important for development of functional, contractile skeletal muscle at 2, 3, and 4 dpf. Rbfox1l morphants generated significantly lower contractile forces than wild-type counterparts at 20, 50, 80, 120, 150, and 180 Hz, indicating that, of the two rbfox proteins investigated, rbfox1l may be more important for muscle development or organization. On the other hand, rbfox2 morphants generated contractile forces similar to wild-type counterparts at 2 and 4 dpf. However, forces generated by rbfox2 morphants were significantly lower than wild type and no different from rbfox1l morphants at 3 dpf. Although we did not determine the cause for this effect, it suggests that the period from 3 dpf to 4 dpf may be an important time point in the development of functional zebrafish skeletal muscle. This idea is supported by the fact that we observed a change in functional competency in wild-type larval skeletal muscle between 3 and 4 dpf (Fig. 2).
With the development of genome editing techniques in zebrafish, there has been a general shift away from the use of antisense MOs in zebrafish studies (30). However, validated MOs are still extremely useful tools for generating a penetrant cohort of embryos depleted of gene function, especially if the phenotype is not readily apparent by morphology. A number of well-established MO controls are routinely used to control for off-target effects (5). In this study, we utilized rbfox splice-blocking MOs validated in our laboratory's previous studies (8). For example, we used the lowest effective MO dose (3 ng per embryo) that causes complete paralysis in double morphants, gives reproducible results, and minimizes pericardial edema and axial curvature that interfere with muscle force measurements. Furthermore, our laboratory's previous work shows that coinjection of splice-blocking rbfox MOs with spliced rbfox2 mRNAs (nontargetable by splice-blocking MOs) completely rescues the knockdown phenotype, again demonstrating MO specificity. In that study, we also used an independent second set of MOs that targeted a different exon for each rbfox gene (exon 3 5'SS sbMO vs. exon 5 3'SS sbMO), which elicited the same phenotype as the MO pair used in the present study (8). Therefore, we conclude that defects in muscle force generation observed in rbfox morphants accurately reflect functional depletion of rbfox1l and rbfox2. Future confirmation awaits recovery of induced rbfox null alleles.
In summary, we report here a method to directly assess skeletal muscle contractility in live zebrafish larvae aged 2–5 dpf and determine the muscle-specific force. As proof that our assay accurately describes muscle function, we have characterized muscle-specific forces in rbfox single and double morphants and have described a functional muscle defect consistent with the observed contractile dysfunction.
GRANTS
This study was supported by P30 core National Institute of Neurological Disorders and Stroke Grant P30 NS045758-06 (C. E. Beattie, P. M. L. Janssen), and by a pilot grant from the Ohio State University Center for Muscle Health and Neuromuscular Disorders (J. P. Davis, S. L. Amacher, C. E. Beattie, P. M. L. Janssen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.L.M., T.L.G., J.P.D., C.E.B., S.L.A., and P.M.J. conception and design of research; B.L.M. and N.R. performed experiments; B.L.M., N.R., and P.M.J. analyzed data; B.L.M., T.L.G., J.P.D., C.E.B., S.L.A., and P.M.J. interpreted results of experiments; B.L.M. and C.E.B. prepared figures; B.L.M. and P.M.J. drafted manuscript; B.L.M., T.L.G., J.P.D., C.E.B., S.L.A., and P.M.J. edited and revised manuscript; B.L.M., T.L.G., J.P.D., C.E.B., S.L.A., and P.M.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Benjamin Canan for help with statistical analysis.
REFERENCES
- 1.Beastrom N, Lu H, Macke A, Canan BD, Johnson EK, Penton CM, Kaspar BK, Rodino-Klapac LR, Zhou L, Janssen PM, Montanaro F. mdx(5cv) mice manifest more severe muscle dysfunction and diaphragm force deficits than do mdx mice. Am J Pathol 179: 2464–2474, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrasekar G, Arner A, Kitambi SS, Dahlman-Wright K, Lendahl MA. Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish. Neurotoxicol Teratol 33: 752–764, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekharan K, Yoon JH, Xu Y, deVries S, Camboni M, Janssen PM, Varki A, Martin PT. A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci Transl Med 2: 42ra54, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dou Y, Andersson-Lendahl M, Arner A. Structure and function of skeletal muscle in zebrafish early larvae. J Gen Physiol 131: 445–453, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisen JS, Smith JC. Controlling morpholino experiments: don't stop making antisense. Development 135: 1735–1743, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Fero K, Bergeron SA, Horstick EJ, Codore H, Li GH, Ono F, Dowling JJ, Burgess HA. Impaired embryonic motility in dusp27 mutants reveals a developmental defect in myofibril structure. Dis Model Mech 7: 289–298, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazier DT, Narahashi T. Tricaine (MS-222): effects on ionic conductances of squid axon membranes. Eur J Pharmacol 33: 313–317, 1975. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher TL, Arribere JA, Geurts PA, Exner CR, McDonald KL, Dill KK, Marr HL, Adkar SS, Garnett AT, Amacher SL, Conboy JG. Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions. Dev Biol 359: 251–261, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grose WE, Clark KR, Griffin D, Malik V, Shontz KM, Montgomery CL, Lewis S, Brown RH, Janssen PM, Mendell JR, Rodino-Klapac LR. Homologous recombination mediates functional recovery of dysferlin deficiency following AAV5 gene transfer. PLoS One 7: e39233, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyon JR, Steffen LS, Howell MH, Pusack TJ, Lawrence C, Kunkel LM. Modeling human muscle disease in zebrafish. Biochim Biophys Acta 1772: 205–215, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Hao LT, Duy PQ, Jontes JD, Wolman M, Granato M, Beattie CE. Temporal requirement for SMN in motoneuron development. Hum Mol Genet 22: 2612–2625, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heller KN, Montgomery CL, Janssen PM, Clark KR, Mendell JR, Rodino-Klapac LR. AAV-mediated overexpression of human α7 integrin leads to histological and functional improvement in dystrophic mice. Mol Ther 21: 520–525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herr VD, Sonnenburg DC, Courogen PM, Fiamengo SA, Downes H. Muscle weakness during tricaine anesthesia. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 110: 289–296, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Ingham PW. The power of the zebrafish for disease analysis. Hum Mol Genet 18: R107–R112, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Kawahara G, Guyon JR, Nakamura Y, Kunkel LM. Zebrafish models for human FKRP muscular dystrophies. Hum Mol Genet 19: 623–633, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M, Andersson-Lendahl M, Sejersen T, Arner A. Knockdown of desmin in zebrafish larvae affects interfilament spacing and mechanical properties of skeletal muscle. J Gen Physiol 141: 335–345, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li YI, Sanchez-Pulido L, Haerty W, Ponting CP. RBFOX and PTBP1 proteins regulate the alternative splicing of micro-exons in human brain transcripts. Genome Res 25: 1–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin YY. Muscle diseases in the zebrafish. Neuromuscul Disord 22: 673–684, 2012. [DOI] [PubMed] [Google Scholar]
- 19.Martin PT, Xu R, Rodino-Klapac LR, Oglesbay E, Camboni M, Montgomery CL, Shontz K, Chicoine LG, Clark KR, Sahenk Z, Mendell JR, Janssen PM. Overexpression of Galgt2 in skeletal muscle prevents injury resulting from eccentric contractions in both mdx and wild-type mice. Am J Physiol Cell Physiol 296: C476–C488, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell W, Sundararajan R. Electric field distribution in biological tissues for various electrode configurations-A FEMLAB study. In: Proceedings of the COMSOL Multiphysics User's Conference 2005 Boston. Berlington, MA: COMSOL Multiphysics, 2005. [Google Scholar]
- 21.Murray JD, Canan BD, Martin CD, Stangland JE, Rastogi N, Rafael-Fortney JA, Janssen PM. The force-temperature relationship in healthy and dystrophic mouse diaphragm; implications for translational study design. Front Physiol 3: 422, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedrotti S, Giudice J, Dagnino-Acosta A, Knoblauch M, Singh RK, Hanna A, Mo Q, Hicks J, Hamilton S, Cooper TA. The RNA-binding protein Rbfox1 regulates splicing required for skeletal muscle structure and function. Hum Mol Genet 24: 2360–2374, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterson JM, Kline W, Canan BD, Ricca DJ, Kaspar B, Delfín DA, DiRienzo K, Clemens PR, Robbins PD, Baldwin AS, Flood P, Kaumaya P, Freitas M, Kornegay JN, Mendell JR, Rafael-Fortney JA, Guttridge DC, Janssen PM. Peptide-based inhibition of NF-κB rescues diaphragm muscle contractile dysfunction in a murine model of Duchenne muscular dystrophy. Mol Med 17: 508–515, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfín DA, Janssen PM, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in Duchenne muscular dystrophy mice. Circulation 124: 582–588, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramesh T, Lyon AN, Pineda RH, Wang C, Janssen PM, Canan BD, Burghes AH, Beattie CE. A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis Model Mech 3: 652–662, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravenscroft G, Laing NG, Bönnemann CG. Pathophysiological concepts in the congenital myopathies: blurring the boundaries, sharpening the focus. Brain 138: 246–268, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR, Mendell JR. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med 5: 45, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rombough PJ. Ontogenetic changes in the toxicity and efficacy of the anaesthetic MS222 (tricaine methanesulfonate) in zebrafish (Danio rerio) larvae. Comp Biochem Physiol A Mol Integr Physiol 148: 463–469, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Runfola V, Sebastian S, Dilworth FJ, Gabellini D. Rbfox proteins regulate tissue-specific alternative splicing of Mef2D required for muscle differentiation. J Cell Sci 128: 631–637, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sloboda DD, Claflin DR, Dowling JJ, Brooks SV. Force measurement during contraction to assess muscle function in zebrafish larvae. J Vis Exp 77: 50539, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Zhang Z, Fregoso O, Krainer AR. Mechanisms of activation and repression by the alternative splicing factors RBFOX1/2. RNA 18: 274–283, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Telfer WR, Nelson DD, Waugh T, Brooks SV, Dowling JJ. Neb: a zebrafish model of nemaline myopathy due to nebulin mutation. Dis Model Mech 5: 389–396, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]