Abstract
Butyrate, a key short-chain fatty acid metabolite of colonic luminal bacterial action on dietary fiber, serves as a primary fuel for the colonocytes, ameliorates mucosal inflammation, and stimulates NaCl absorption. Absorption of butyrate into the colonocytes is essential for these intracellular effects. Monocarboxylate transporter 1 (MCT1) plays a major role in colonic luminal butyrate absorption. Previous studies (Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. Adv Immunol 121: 91–119, 2014.) showed decreased MCT1 expression and function in intestinal inflammation. We have previously shown (Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Am J Physiol Gastrointest Liver Physiol 290: G30–G35, 2006.) impaired butyrate absorption in human intestinal epithelial Caco-2 cells due to decreased MCT1 level at the apical cell surface following enteropathogenic E. coli (EPEC) infection. Current studies, therefore, examined the potential role of probiotic Lactobacilli in stimulating MCT1-mediated butyrate uptake and counteracting EPEC inhibition of MCT1 function. Of the five species of Lactobacilli, short-term (3 h) treatment with L. acidophilus (LA) significantly increased MCT1-mediated butyrate uptake in Caco-2 cells. Heat-killed LA was ineffective, whereas the conditioned culture supernatant of LA (LA-CS) was equally effective in stimulating MCT1 function, indicating that the effects are mediated by LA-secreted soluble factor(s). Furthermore, LA-CS increased apical membrane levels of MCT1 protein via decreasing its basal endocytosis, suggesting that LA-CS stimulation of butyrate uptake could be secondary to increased levels of MCT1 on the apical cell surface. LA-CS also attenuated EPEC inhibition of butyrate uptake and EPEC-mediated endocytosis of MCT1. Our studies highlight distinct role of specific LA-secreted molecules in modulating colonic butyrate absorption.
Keywords: SCFA, Caco-2, probiotics, pCMBS, endocytosis
the short-chain fatty acids (SCFA) are produced by bacterial fermentation of dietary fiber and are efficiently absorbed by the colonic epithelial cells. Butyrate, a key SCFA, serves as the major and preferred metabolic substrate for colonocytes, providing 60–70% of energy requirements necessary for their proliferation and differentiation (13, 40). As such, colonocytes of germ-free mice, deficient in SCFAs, are highly energy deprived, as indicated by decreased expression of key mitochondrial enzymes involved in energy metabolism (14). Butyrate also promotes colonic mucosal integrity (40), inhibits inflammatory pathways (43), and induces NaCl absorption (1), hence eliciting antidiarrheal effects. Besides, interest in SCFAs has recently been rekindled for their emerging roles in the regulation of metabolism (14, 24), barrier function (31), autophagy (14), and immune responses (25, 30).
Earlier reports in ulcerative colitis (UC) patients suggested that a major consequence of reduction in intracellular SCFA oxidation results in metabolic starvation and mucosal atrophy. In fact, UC has been suggested to be a local starving disease in the colon, associated with a decrease in nutrient availability (16, 23). Butyrate oxidation deficiency was mainly observed in the inflamed mucosa of patients with active disease, and not with quiescent UC, suggesting that impaired butyrate oxidation is not a primary defect, but is rather a consequence of defect in luminal butyrate absorption (41, 42).
SCFA absorption may involve passive diffusion of the undissociated form and specific carrier-mediated transport of SCFA anions (1, 17, 40). Previous studies from our laboratory and others suggested monocarboxylate transporter isoform 1 (MCT1) to play a key role in mediating H+-coupled absorption of human colonic luminal butyrate (22, 33, 34). Various studies have also shown differential regulation of MCT1 to impact colonic epithelial health. In fact, downregulation of MCT1 expression and/or activity has been reported in mucosal inflammation (41, 42), colon cancer (12, 19), and in response to infection by enteropathogenic E. coli (4), an important human enteric pathogen causing infantile diarrhea. On the other hand, various studies showed upregulation of MCT1 expression and/or activity in response to various agents, such as luminal leptin (9), somatostatin (36), butyrate (7, 11), and via nutrient-sensing mechanisms (6). In this report, we have demonstrated upregulation of MCT1 activity by the bioactive soluble factors secreted by the probiotic Lactobacillus acidophilus (LA) via increasing the levels of the transporter at the apical cell surface. These factors also alleviated enteropathogenic E. coli-induced inhibition of MCT1 activity.
MATERIALS AND METHODS
Materials.
Caco-2 cells and probiotic Lactobacilli species were obtained from American Type Culture Collection (ATCC; Manassas, VA). 14C-butyrate was obtained from American Radiochemicals, p-chloromercuri-benzene sulfonate (pCMBS) was purchased from Sigma-Aldrich (St. Louis, MO); sulfo-NHS-SS-biotin was obtained from Thermo Scientific (Rockford, IL).
Cell culture.
Caco-2 cells were grown at 37°C in an atmosphere of 5% CO2. Cells were maintained in DMEM with 4.5 g/l glucose, 50 kU/l penicillin, 5 mg/l streptomycin, 2 mg/l gentamycin, and 20% fetal bovine serum. Butyrate uptake studies were performed using fully differentiated cells grown for 12–14 day postplating on 24-well plastic supports or on 0.4 μM polycarbonate membrane filters in 12-mm inserts.
Bacterial culture and preparation of conditioned culture supernatant.
The following Lactobacilli species, with ATCC strain numbers given in parentheses, were grown in Mann-Rogosa-Sharpe broth (Difco Laboratories, Detroit, MI) for 24 h at 37°C without shaking: LA (4357), Lactobacillus rhamnosus (53103), L. plantarum (LP) (14917), L. casei (LC) (393), and L. reuteri (23272). The cultures were then centrifuged at 3,000 g × 10 min at 4°C. The supernatant, filtered through a 0.22-μm filter (Millex, Millipore, Billerica, MA) to sterilize and remove all bacterial cells, was designated as conditioned culture supernatant (CS). For treating the cell monolayers, the bacterial pellet was washed with DMEM/F-12 media (Invitrogen, Carlsbad, CA) containing 5 mg/l mannose and resuspended in the same media. Heat-killed bacteria were prepared by resuspending pellets and heating to 95°C for 20 min.
Enteropathogenic E. coli culture and infection of cells.
The enteropathogenic E. coli (EPEC) strain used in this study was wild-type EPEC strain E2348/69 (generously provided by Dr. Gail Hecht of the Department of Medicine, Loyola University, Maywood, IL). Strains were grown overnight in Milleva Luria Broth media. On the day of experiment, an aliquot of the overnight culture was inoculated in an appropriate volume of serum and antibiotic-free medium supplemented with 0.5% mannose. Bacteria were grown to midlog phase (optical density at 600 nm = 0.4). The culture was spun down and resuspended in the same volume of fresh media. Cell monolayers were then infected at a multiplicity of infection of 1:100. After infection for the desired time, media were removed, and cell monolayers were washed with PBS.
Measurement of 14C-butyrate uptake.
Apical uptake of 14C-butyrate was measured as described previously (6), in the presence or absence of pCMBS, a specific inhibitor of MCT1. Uptake values were calculated as nanomoles of 14C-butyrate per milligram protein per 5 min.
Cell surface biotinylation and immunoblotting.
Cell surface biotinylation was performed using sulfo-NHS-SS-biotin (Thermo Scientific, Rockford, IL) (0.5 g/l) in borate buffer (in mmol/l: 154 NaCl, 7.2 KCl, 1.8 CaCl2, 10 H3BO3, pH 9.0), as previously described (18). Labeling was allowed to proceed for 60 min at 4°C to prevent endocytosis and internalization of antigens. After immunoprecipitation of biotinylated antigens with neutravidin agarose, biotinylated proteins were released by boiling in Laemmli buffer containing dithiothreitol, subjected to SDS-PAGE, and then probed with anti-MCT1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The surface MCT1 was compared with total cellular MCT1, as determined by immunoblotting of the solubilized cell extract.
Endocytic internalization assay by reversible cell-surface biotinylation.
To measure the extent of endocytosis, Caco-2 cells were plated at a density of 1 × 105 cells on six-well plates. Cell surface was labeled with sulfo-NHS-SS-biotin (1.5 mg/ml; Pierce, Rockford, IL) in borate buffer (in mM: 154 NaCl, 7.2 KCl, 1.8 CaCl2, 10 H3BO3, pH 9.0) at 4°C for 60 min. Following surface biotinylation, cells were incubated with the CS of LA (LA-CS) at 37°C for 3 h, followed by 30 min incubation with or without EPEC. Immediately after treatment, cells were rinsed with ice-cold 1× PBS twice at 4°C. Surface biotin was cleaved by 150 mM GSH in 1× PBS, and the biotinylated freshly endocytosed proteins were protected from cleavage by GSH. Cells were solubilized in RIPA buffer, and biotinylated proteins were retrieved and assayed for endocytosed MCT1, as described above.
Statistical analysis.
All experiments were performed in triplicate on four to five separate sets. Data were analyzed by ANOVA using GraphPad Prism software. Results shown are means ± SE; P ≤ 0.05 was considered statistically significant.
RESULTS
LA and LC stimulate MCT1-mediated butyrate uptake.
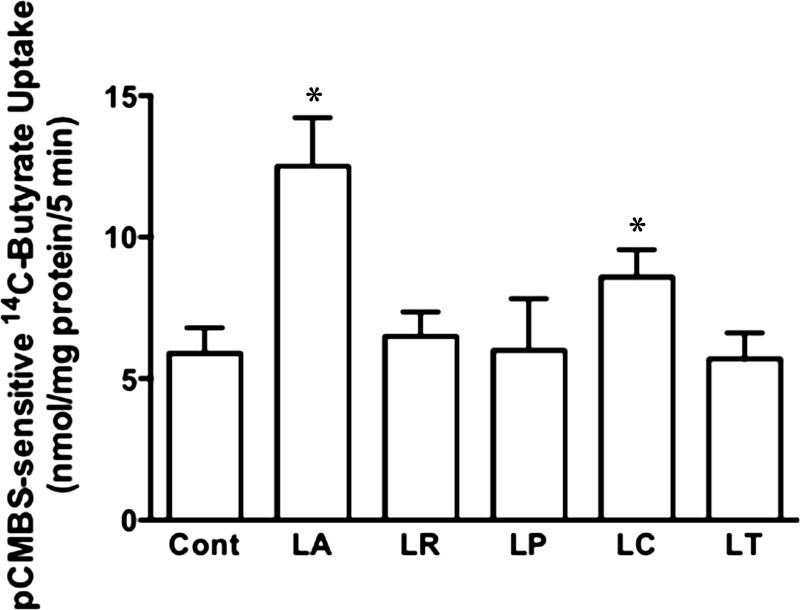
In the initial set of studies, we used five species of Lactobacilli, as outlined in materials and methods, to examine their short-term treatment effects on MCT1-mediated butyrate uptake in postconfluent Caco-2 cell monolayers. Of the five species, LA and LC significantly stimulated pCMBS-sensitive butyrate uptake, whereas L. rhamnosus, LP, and L. reuteri had no effects (Fig. 1). Since LA showed maximal stimulatory effects, all subsequent studies were performed with this species. A time course experiment showed that LA did not affect pCMBS-sensitive butyrate uptake at 1 h, but by 2 h, butyrate uptake increased essentially to the level at 3 h (data not shown). A 3-h time point was used to treat cells in subsequent studies. We also compared the effects of LA added apically to Caco-2 monolayers grown on 0.4-μm polycarbonate membrane filters in 12-mm transwell inserts and obtained similar effects results on pCMBS-sensitive butyrate uptake (data not shown).
Fig. 1.
Short-term treatment with L. acidophilus (LA) and L. casei (LC) stimulates monocarboxylate transporter 1 (MCT1)-mediated butyrate uptake in Caco-2 cells. Postconfluent Caco-2 cells were treated with various Lactobacillus species (overnight cultures diluted to 600-nm absorbance = 0.2) for 3 h. Cont, control; LR, L. rhamnosus; LP, L. plantarum; LT, L. reuteri. MCT1 function [p-chloromercuri-benzene sulfonate (pCMBS)-sensitive 14C-butyrate uptake] was determined and calculated as nmol butyrate·mg protein−1·5 min−1. Values are means ± SE; n = 5. *Different from control, P ≤ 0.05.
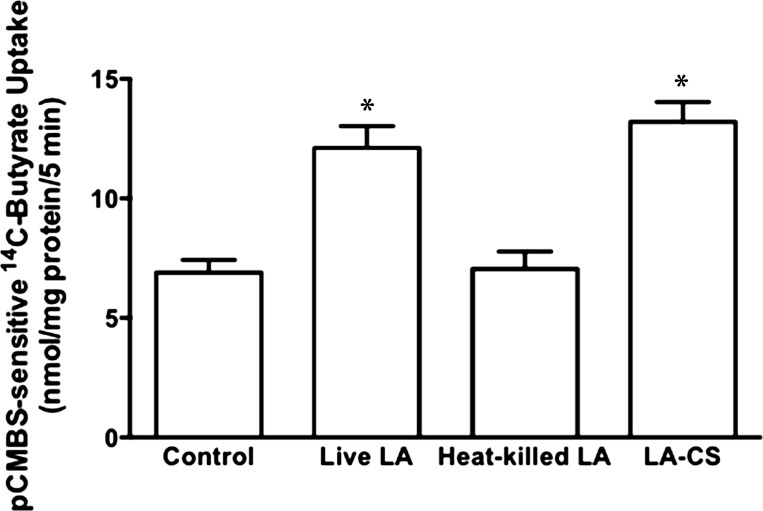
CS of LA, but not heat-killed bacteria, mimics the effects of live bacteria on pCMBS-sensitive butyrate uptake.
We next examined whether the effects of LA on butyrate uptake required live organisms. Unlike live LA, however, the heat-killed bacteria did not stimulate apical butyrate uptake in Caco-2 cells (Fig. 2). Various earlier studies have shown that bacteria-free CS of certain probiotic bacteria could show beneficial effects similar to live bacteria on host intestinal epithelial cells (3, 44, 45). We, therefore, tested the effects of LA-CS prepared as described in materials and methods, diluted 1:10 in DMEM/F-12 and pH adjusted to 7.4, on apical butyrate uptake. LA-CS stimulated butyrate uptake to the same extent, compared with control, as that of live bacteria (Fig. 2). Therefore, we used LA-CS instead of live LA for our subsequent studies. We have also determined LA-CS effects on MCT1 function in T-84 cells, another human intestinal epithelial cell line, and observed significant increase in pCMBS-sensitive 14C-butyrate uptake following LA-CS treatments for 3 h (in nmol butyrate·mg protein−1·5 min−1: control, 1.62 ± 0.08 vs. LA-CS, 2.90 ± 0.26; P ≤ 0.05). These results suggest that LA-CS effects in stimulating MCT1-mediated butyrate uptake are not cell line specific.
Fig. 2.
Culture supernatant of LA, but not heat-killed bacteria, mimics the effects of live bacteria on MCT1 function: postconfluent Caco-2 cells were treated with live or heat-killed LA (overnight cultures diluted to 600-nm absorbance = 0.2), or bacteria-free culture supernatant of LA (LA-CS) (diluted 1:10 in DMEM/F-12). MCT1 function (pCMBS-sensitive 14C-butyrate uptake) was calculated as nmol butyrate·mg protein−1·5 min−1. Values are means ± SE; n = 3. *Different from control, P ≤ 0.05.
LA-CS increases Vmax of MCT1-mediated butyrate uptake and causes a decrease in Km.
To examine the mechanisms of butyrate enhancement of MCT1 function, we performed kinetic analysis of LA-CS effects on butyrate uptake. Effects of LA-CS on pCMBS-sensitive 14C-butyrate uptake were measured at different concentrations (0.5–15 mM) of cold butyrate added to the uptake buffer, and kinetic parameters Km and Vmax were calculated using GraphPad Prism software. As shown in Table 1, LA-CS treatment caused significant increase in Vmax of MCT1-mediated butyrate uptake and a decrease in Km. Increased Vmax could imply increased MCT1 levels at the cell surface, whereas decreased Km suggests increased affinity of MCT1 for the substrate.
Table 1.
LA-CS increases Vmax and decreases Km of pCMBS-sensitive 14C-butyrate uptake in Caco-2 cells
| Treatments | Km, mM | Vmax, nmol·mg protein−1·5 min−1 |
|---|---|---|
| Control | 4.17 ± 0.04 | 9.10 ± 0.13 |
| LA-CS | 2.17 ± 0.03* | 14.27 ± 0.11* |
Values are means ± SE of 3 independent experiments. LA-CS, conditioned culture supernatant of Lactobacillus acidophilus.
Different from control, P ≤ 0.05.
LA-CS increases surface MCT1 expression via decreasing endocytic internalization.
We next utilized cell surface biotinylation to measure apical membrane levels of MCT1 in response to LA-CS treatments. Consistent with increased function, LA-CS treatment (3 h) significantly increased apical membrane MCT1 levels (Fig. 3A). The plasma membrane level of a protein is governed by the rates of its endocytic internalization and exocytic recycling pathways for its membrane insertion. To examine whether LA-CS-mediated increase in apical membrane MCT1 is via decreasing basal endocytosis of MCT1, we performed endocytic internalization assay to measure endocytosed MCT1 fraction in the presence or absence of LA-CS. As shown in Fig. 3B, LA-CS significantly decreased basal MCT1 endocytosis, suggesting that increased cell surface MCT1, at least partially, is due to decreased endocytosis in response to LA-CS treatments.
Fig. 3.
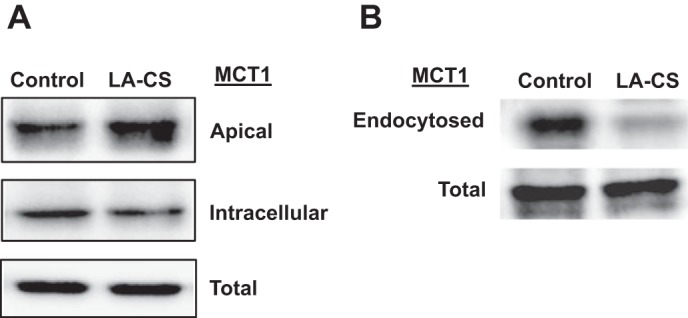
LA-CS increases apical surface MCT1 level via decreasing endocytic internalization. A: Caco-2 monolayers (14-day postplating) were treated with LA-CS for 3 h, and apical membrane MCT1 levels were measured by cell surface biotinylation. B: following surface biotinylation, Caco-2 monolayers were incubated with LA-CS at 37°C for 3 h, and endocytosed MCT1 fractions were measured by reversible biotinylation, as described in materials and methods. Representative blots of 3 independent experiments are shown in A and B.
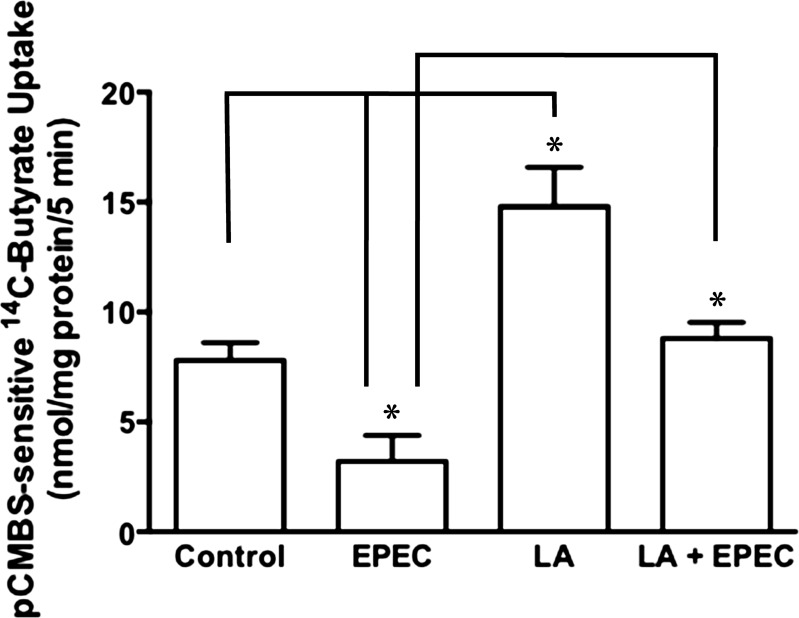
LA-CS attenuates EPEC inhibition of butyrate uptake.
Our earlier studies showed that infection of Caco-2 cells with EPEC for short term (30–120 min) significantly inhibited butyrate uptake (4). Since probiotics are known to counteract pathogen infection via varied mechanisms, we examined whether LA-derived soluble factors could block the inhibitory effects of EPEC infection on butyrate uptake. Caco-2 cells were pretreated with LA-CS for 3 h, followed by additional 30-min treatments with or without EPEC infection. The 30-min time point for EPEC infection was chosen to minimize the adverse effects of EPEC infection for longer times on cell viability and tight junction permeability. EPEC infection for 30 min substantially inhibited MCT1-mediated butyrate uptake (Fig. 4), as was also shown in our previous studies (4). On the other hand, LA-CS also attenuated EPEC-induced inhibition of MCT1-mediated butyrate uptake.
Fig. 4.
LA-CS attenuates enteropathogenic E. coli (EPEC) inhibition of butyrate uptake. Caco-2 cells were pretreated with LA-CS for 3 h, followed by additional 30-min treatments with or without EPEC infection. MCT1 function (pCMBS-sensitive 14C-butyrate uptake) was determined and calculated as nmol butyrate·mg protein−1·5 min−1. Values are means ± SE; n = 3. *Different between groups, P ≤ 0.05.
LA-CS attenuates EPEC-induced decrease in cell surface MCT1.
Our earlier studies have shown that EPEC inhibition of butyrate uptake was associated with decreased MCT1 levels on the apical cell surface (4). Therefore, we examined whether LA-CS could counteract EPEC-induced internalization of surface MCT1. As shown in Fig. 5A, LA-CS alone increased the level of surface MCT1 and also blocked the EPEC-induced decrease in surface MCT1.
Fig. 5.
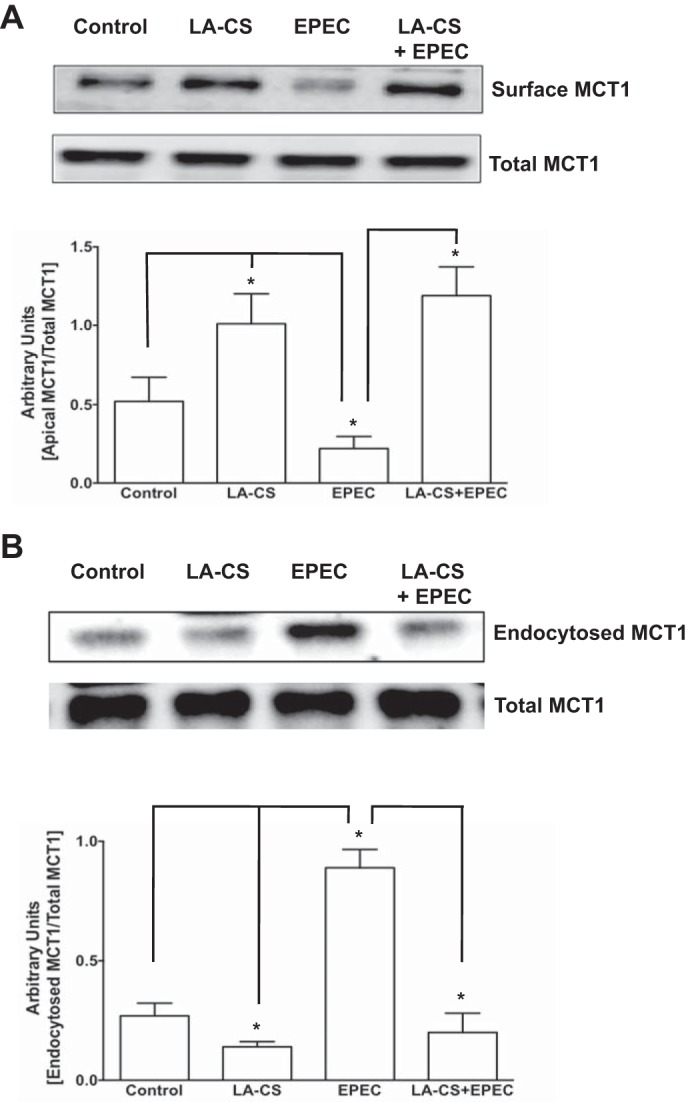
LA-CS attenuates EPEC-induced decrease in cell surface MCT1 via counteracting EPEC-induced MCT1 endocytosis. A: postconfluent Caco-2 monolayers were pretreated with LA-CS for 3 h, followed by additional 30-min treatments with or without EPEC infection. Apical membrane MCT1 levels were measured by cell-surface biotinylation. Top: the band intensities of apical vs. total MCT1 in different groups. Bottom: densitometric analysis of band intensities. B: following surface biotinylation, Caco-2 monolayers were incubated with LA-CS at 37°C for 3 h, followed by additional 30-min treatments with or without EPEC infection. Endocytosed MCT1 fractions were measured by reversible biotinylation, as described in materials and methods. Top: the band intensities of endocytosed vs. total MCT1 in different groups. Bottom: densitometric analysis of band intensities. Representative blots of 3 independent experiments are shown in A and B. *Different between groups, P ≤ 0.05.
LA-CS attenuated EPEC-induced MCT1 endocytosis.
To examine the mechanisms of LA-CS alleviation of EPEC-induced decrease in surface MCT1, we performed endocytic internalization studies utilizing reversible biotinylation. As shown in Fig. 5B, EPEC infection increased the endocytosed fraction of MCT1 compared with control. LA-CS not only decreased basal endocytosis, but also counteracted EPEC-induced endocytosis of MCT1.
DISCUSSION
SCFAs are produced in the colonic lumen via the action of specific gut microbiota on dietary fiber. Of the SCFAs, butyrate has a prominent role at the colonic level, as it is the primary fuel for the colonocytes, and its oxidation is involved in various important metabolic processes in the colon (16). Besides being the energy nutrient, butyrate also ameliorates mucosal inflammation (40), exerts antidiarrheal effects by stimulating electrolyte and fluid absorption (1), and enhances mucosal barrier integrity (40). Many of these beneficial effects of butyrate contributing to the maintenance of colonic epithelial homeostasis, however, are concentration dependent and require cellular absorption of butyrate. As such, efficient absorption of butyrate is of fundamental importance to colonic epithelial health. Indeed, disturbed energy homeostasis observed in chronically inflamed mucosa of inflammatory bowel disease patients has been attributed to impaired absorption of butyrate (41, 42). In this regard, studies from our laboratory and others have shown MCT1 to play a major role in the absorption of colonic luminal butyrate (17, 22, 33, 34). Impaired butyrate absorption secondary to downregulation of MCT1 has been reported in inflammation (41, 42) and colon cancer (12, 19). We have also shown inhibition of MCT1 function in response to infection by EPEC (4), a food-borne pathogen causing early diarrhea, more particularly in children. Therefore, agents that upregulate MCT1 activity and/or correct MCT1 dysfunction could be of therapeutic value for intestinal inflammatory disorders associated with impaired butyrate absorption. In the present report, we have shown short-term effects of the probiotic LA in stimulating butyrate uptake in Caco-2 cells via increasing the level of MCT1 at the apical membrane. Probiotic bacterial species are known to contribute to intestinal homeostasis via exerting several positive effects on epithelial cell functions. For example, they are known to alleviate mucosal inflammation, improve barrier function, inhibit pathogen adherence, and modulate mucosal immune functions (8, 15, 29, 37). Pioneering previous studies from our laboratory have demonstrated distinct proabsorptive effects of Lactobacilli and Bifidobacteria to alleviate inflammation-associated diarrheal disorders by virtue of their ability to modulate electrolyte absorption (5, 27, 32, 38, 39). Our laboratory's recent studies have also shown LP stimulation of Na+-coupled butyrate uptake in rat intestinal epithelial IEC-6 cells via upregulation of sodium-coupled MCT1 (SMCT1) expression (2). SMCT1 is a relatively recently characterized transport protein implicated in Na+-coupled transport of butyrate across colonic luminal membrane (21). However, our studies in human intestinal Caco-2 cells or apical membrane vesicles prepared from human colonic mucosa of organ donors did not exhibit Na+-dependent butyrate uptake (2), although these models still showed H+-gradient-coupled 14C-butyrate uptake (22). Therefore, it seemed logical to us to investigate the effects of probiotic Lactobacilli species on MCT1 activity. Interestingly, LP showing long-term effects in increasing SMCT1 expression and function in IEC-6 cells, and mouse colon, as reported earlier (2), had no effects on MCT1 function. On the other hand, LA, another species of Lactobacilli, increased MCT1 function via increasing the level of MCT1 at the apical cell surface. Thus the effects of probiotics on these two SCFA transporters appeared to be species specific and to involve distinct mechanisms. Additionally, heat-killed LA did not affect MCT1 function, whereas bacteria-free LA-CS had the same stimulatory effects on MCT1 function as that of live LA, suggesting the role of LA-secreted soluble factors in mediating the beneficial effects on MCT1 function. Although some protective effects of probiotics require direct interactions of epithelial cells with live bacteria, various earlier and recent reports have shown that even the cellular components of these agents, or their secreted soluble effector molecules, may just be as effective and considerably safer for the host (3, 20, 35, 44, 45).
Short-term regulation of a transporter protein may involve its altered membrane abundance or changes in kinetic properties, such as substrate affinity or interaction with other molecules (28). LA-CS-induced activation of MCT1 function in polarized Caco-2 cells was kinetically manifested by increased Vmax and decreased Km. Increased Vmax suggests that LA-CS-induced increase in the absorptive capacity of MCT1 was due to its increased apical membrane abundance, which was also supported by our cell surface biotinylation studies. Endocytic internalization studies further suggested that increased apical membrane localization of MCT1 could, at least partially, be due to LA-CS inhibition of basal MCT1 endocytosis. The observed decrease in Km in response to LA-CS treatments could indicate increased affinity of MCT1 for butyrate. However, potential alternative mechanisms of LA-CS effects, such as phosphorylation/dephosphorylation of MCT1, could also account for the observed effects on the Km. Furthermore, altered interactions of MCT1 with CD147, an auxillary glycoprotein known to play critical role in membrane targeting of MCT1 (26), need to be examined to account for LA-CS enhancement of apical membrane levels of MCT1.
Our results showed that LA-CS not only stimulates MCT1 function and membrane expression, but also counteracts EPEC inhibition of MCT1 function and EPEC-mediated endocytic internalization of MCT1 from apical cell surface. Counteracting enteric infections by probiotics involve varied mechanisms, such as competitive exclusion, secretion of anti-microbial compounds, and induction of host epithelial signaling pathways (10, 29). Since bacteria-free LA-CS itself could counteract EPEC inhibition of MCT1 activity and live LA was not required, the effects were presumably not due to pathogen exclusion, but rather via induction of signaling events that modulated MCT1 intracellular trafficking mechanisms. Our laboratory's earlier studies (5) showed the role of phosphatidylinositol 3-kinase/Rac1 small GTPase signaling and involvement of lipid rafts in LA-CS-induced increase in apical membrane level of SLC26A3, the intestinal epithelial apical membrane Cl−/HCO3− exchanger protein. These and other relevant signaling events could also play important roles in mediating LA-CS effects on MCT1 endocytosis and recycling under basal conditions or in response to EPEC infection.
Based on the multiple beneficial effects on gut epithelial health and impact on overall systemic health, butyrate is currently being considered as an important nutrient. This is further supported by evidence from clinical trials that adding resistant starch (source of butyrate) increases efficacy of oral rehydration solution to ameliorate diarrhea (1). Therefore, maintaining optimal availability of butyrate to colonic epithelial cells via manipulation of production and absorption of butyrate appears to be critical for a healthy colon. Therefore, our studies showing positive effects of probiotic-secreted molecules on the functionality of MCT1, a major player in mediating cellular entry of luminally produced butyrate, have great implications in colonic epithelial health and integrity. Furthermore, precise molecular identification of the LA-secreted soluble factor(s) will be of critical importance for designing novel probiotic-based therapeutic strategies for intestinal inflammatory diseases associated with impaired SCFA absorption.
GRANTS
These studies were supported by Bill & Melinda Gates Foundation Grant OPP1058288 (A. Borthakur); National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016, DK-81858, DK-92441 (P. K. Dudeja), and DK-71596 (W. A. Alrefai); and the Department of Veteran Affairs Grants BX 002011 (P. K. Dudeja) and BX 000152 (W. A. Alrefai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.K. and A.B. performed experiments; A.K., W.A.A., and A.B. analyzed data; A.K. and A.B. prepared figures; W.A.A., A.B., and P.K.D. interpreted results of experiments; W.A.A., A.B., and P.K.D. edited and revised manuscript; A.B. and P.K.D. conception and design of research; A.B. drafted manuscript; A.B. and P.K.D. approved final version of manuscript.
REFERENCES
- 1.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 72: 297–313, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Borthakur A, Anbazhagan AN, Kumar A, Raheja G, Singh V, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus plantarum counteracts TNF-α-induced downregulation of SMCT1 expression and function. Am J Physiol Gastrointest Liver Physiol 299: G928–G934, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borthakur A, Bhattacharyya S, Kumar A, Anbazhagan AN, Tobacman JK, Dudeja PK. Lactobacillus acidophilus alleviates platelet-activating factor-induced inflammatory responses in human intestinal epithelial cells. PLoS One 8: e75664, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borthakur A, Gill RK, Hodges K, Ramaswamy K, Hecht G, Dudeja PK. Enteropathogenic Escherichia coli inhibits butyrate uptake in Caco-2 cells by altering the apical membrane MCT1 level. Am J Physiol Gastrointest Liver Physiol 290: G30–G35, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borthakur A, Priyamvada S, Kumar A, Natarajan AA, Gill RK, Alrefai WA, Dudeja PK. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol 303: G1126–G1133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: Involvement of NF-kappaB pathway. J Cell Biochem 103: 1452–1463, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect 44: 1–8, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco2-BBE. J Biol Chem 277: 28182–28190, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Corr SC, Hill C, Gahan CG. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv Food Nutr Res 56: 1–15, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Cuff MA, Lambert DW, Shirazi-Beechey SP. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J Physiol 539: 361–371, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly K, Cuff MA, Fung F, Shirazi-Beechey SP. The importance of colonic butyrate transport to the regulation of genes associated with colonic tissue homoeostasis. Biochem Soc Trans 33: 733–735, 2005. [DOI] [PubMed] [Google Scholar]
- 13.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 13: 517–526, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gareau MG, Sherman PM, Walker WA. Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7: 503–514, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gassull MA. Review article: the intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther 24, Suppl 3: 90–95, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Intestinal anion absorption. In: Physiology of the Gastrointestinal Tract (5th Ed), edited by Johnson LR. Amsterdam: Elsevier, 2012, sect. V, chapt. 67, p. 1819–1848. [Google Scholar]
- 18.Gill RK, Borthakur A, Hodges K, Turner JR, Clayburgh DR, Saksena S, Zaheer A, Ramaswamy K, Hecht G, Dudeja PK. Mechanism underlying inhibition of intestinal apical Cl/OH exchange following infection with enteropathogenic E. coli. J Clin Invest 117: 428–437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab 14: 994–1008, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Guo S, Guo Y, Ergun A, Lu L, Walker WA, Ganguli K. Secreted metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL-1beta-induced inflammation: a transcription profiling analysis. PLoS One 10: e0124549, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci 78: 2419–2425, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104–119, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7: 2839–2849, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw 14: 277–288, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19: 3896–3904, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Hecht C, Priyamvada S, Anbazhagan AN, Alakkam A, Borthakur A, Alrefai WA, Gill RK, Dudeja PK. Probiotic bifidobacterium species stimulate human SLC26A3 gene function and expression in intestinal epithelial cells. Am J Physiol Cell Physiol 307: C1084–C1092, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mertl M, Daniel H, Kottra G. Substrate-induced changes in the density of peptide transporter PEPT1 expressed in Xenopus oocytes. Am J Physiol Cell Physiol 295: C1332–C1343, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Oelschlaeger TA. Mechanisms of probiotic actions: a review. Int J Med Microbiol 300: 57–62, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8: 80–93, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 139: 1619–1625, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J Physiol 513: 719–732, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification of a monocarboxylate transporter isoform type 1 (MCT1) on the luminal membrane of human and pig colon. Biochem Soc Trans 26: S120, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz L, Hevia A, Bernardo D, Margolles A, Sanchez B. Extracellular molecular effectors mediating probiotic attributes. FEMS Microbiol Lett 359: 1–11, 2014. [DOI] [PubMed] [Google Scholar]
- 36.Saksena S, Theegala S, Bansal N, Gill RK, Tyagi S, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms underlying modulation of monocarboxylate transporter 1 (MCT1) by somatostatin in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 297: G878–G885, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva MJ, Carneiro MB, dos Anjos Pultz B, Pereira Silva D, Lopes ME, dos Santos LM. The multifaceted role of commensal microbiota in homeostasis and gastrointestinal diseases. J Immunol Res 2015: 321241, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh V, Kumar A, Raheja G, Anbazhagan AN, Priyamvada S, Saksena S, Jhandier MN, Gill RK, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus attenuates downregulation of DRA function and expression in inflammatory models. Am J Physiol Gastrointest Liver Physiol 307: G623–G631, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh V, Raheja G, Borthakur A, Kumar A, Gill RK, Alakkam A, Malakooti J, Dudeja PK. Lactobacillus acidophilus upregulates intestinal NHE3 expression and function. Am J Physiol Gastrointest Liver Physiol 303: G1393–G1401, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119, 2014. [DOI] [PubMed] [Google Scholar]
- 41.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis 16: 684–695, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Down-regulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology 133: 1916–1927, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Vieira EL, Leonel AJ, Sad AP, Beltrao NR, Costa TF, Ferreira TM, Gomes-Santos AC, Faria AM, Peluzio MC, Cara DC, Alvarez-Leite JI. Oral administration of sodium butyrate attenuates inflammation and mucosal lesion in experimental acute ulcerative colitis. J Nutr Biochem 23: 430–436, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 303: G32–G41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan F, Polk DB. Characterization of a probiotic-derived soluble protein which reveals a mechanism of preventive and treatment effects of probiotics on intestinal inflammatory diseases. Gut Microbes 3: 25–28, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]