Abstract
Diet-induced obesity is often modeled by comparing mice fed high-fat diet (HFD), which is made from purified ingredients, vs. normal chow diet (NCD), which is a low-fat assemblage of relatively unrefined plant and animal products. The mechanism by which HFD promotes adiposity is complex but thought to involve low-grade inflammation and altered gut microbiota. The goal of this study was to investigate the extent to which HFD-induced adiposity is driven by fat content vs. other factors that differentiate HFD vs. NCD. Mice were fed NCD, HFD, or other compositionally defined diets (CDD), designed to mimic NCD and/or explore the role of HFD components. A range of metabolic parameters reflecting low-grade inflammation and adiposity were assayed. Relative to NCD, HFD, and to a lesser, but, nonetheless, significant extent, CDD induced increased adiposity, indicating both lipid content and other aspects of HFD are obesogenic. Moreover, HFD and CDD induced a rapid and marked loss of cecal and colonic mass. Such CDD-induced effects were not affected by adjusting dietary protein levels/types but could be largely eliminated by exchanging insoluble fiber (cellulose) for soluble fiber (inulin). Replacing cellulose with inulin in HFD also protected mice against decreased intestinal mass, hyperphagia, and increased adiposity. Such beneficial effects of inulin were microbiota dependent, correlated with elevated fecal short-chain fatty acid levels analyzed via 1H-NMR-based metabolomics and were partially recapitulated by administration of short-chain fatty acid. HFD-induced obesity is strongly promoted by its lack of soluble fiber, which supports microbiota-mediated intestinal tissue homeostasis that prevents inflammation driving obesity and metabolic syndrome.
Keywords: microbiota, short-chain fatty acids, low-grade inflammation, metabolic syndrome
the dramatic increased incidence of obesity, and its interrelated disorders, underscores the importance of understanding the pathophysiology of this disorder. While broad societal changes in diet are almost certainly central to the obesity epidemic, mechanisms by which changes in diet eventuate in obesity are complex. A commonly used means to model diet-induced obesity (DIO) in rodents is to administer an energy dense high-fat diet (HFD). While consumption of HFD likely results directly in extra calories that are efficiently converted to fat, the mechanisms that perpetuate and sustain continued excess caloric consumption are increasingly viewed to involve low-grade inflammation driven, in part, by gut microbiota (17). Specifically, HFD induces alteration of gut barrier function that results in translocation of lipopolysaccharide across the intestine (5, 19). Such lipopolysaccharide may drive low-grade proinflammatory gene expression that can desensitize signaling by insulin (i.e., insulin resistance) and other satiety signaling pathways. In support of this notion, DIO can be ameliorated in germ-free mice, by antibiotic treatment, or by absence of Toll-like receptor (TLR)-4 (3, 6, 22).
Most published studies, including ours (11, 23), that utilize HFD model of DIO examine differences between animals fed “rodent chow” to those fed HFD. However, these diets differ in a number of ways other than their fat content. Chow (e.g., LabDiet5001) is assembled from a broad array of partially processed and relatively unrefined plant and animal products. The components of chow are not well characterized and likely exhibit batch (lot), seasonal, and vendor-to-vendor variability. In contrast, HFD is a compositionally defined diet (CDD) manufactured from purified ingredients, each containing one main nutrient. Thus, as has been noted in the nutrition literature, studies seeking to define the role of HFD components in DIO ought to compare HFD to CDD that differ only in a limited number of specific components, especially percent fat content. Indeed, herein we report that two different commercial CDD, designed to match chow nutritionally and to be control diets for HFD, drove decreased intestinal mass and increased adiposity relative to mice fed chow. Investigation of the underlying mechanisms revealed that, in contrast to chow, HFD and control CDD failed to support intestinal health, correlating with their lack of soluble fiber. Addition of soluble fiber to CDD restored intestinal health and protected against HFD-induced adiposity. Such beneficial effects of inulin were dependent on the presence of a microbiota and correlated with increased production of short-chain fatty acids (SCFA). Thus we report that use of CDD ought to contain soluble fiber to maintain a healthy intestine, and that supplementation of various diets might further promote intestinal health that can protect against low-grade inflammation that drives adiposity.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6 mice were purchased from Jackson Laboratories. All mice were then housed at Georgia State University (Atlanta, GA) under institutionally approved protocols (Institutional Animal Care and Use Committee no. A14033). Mice were fed Purina rodent chow (cat. no. 5001) from LabDiets, unless another diet is specified.
Diets.
Diets were procured form Research Diets (New Brunswick, NJ) or Harlan (Indianapolis, IN). The composition of all diets used in this study was detailed in Tables 1 and 2. Fiber was added on a similar per kilocalorie basis in low- and high-fat diet formulas, and inulin was considered as 1.0 kcal/g, replacing an equal amount of kilocalories from starch (or maltodextrin in formulas with 60 kcal% fat). We considered cellulose to provide no calories.
Table 1.
Purified diets used in the study
| D12450B | D12492 | D13081101 | D13081104 | D11112212 | D11112213 | D11112208 | D11112209 | D11112210 | D11112211 | D13081102 | D13081103 | D14061501 | D14061502 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein source | Casein | Casein | Casein | Casein | Soy | Soy | Casein | Soy | Casein | Casein | Casein | Casein | Casein | Casein |
| Fiber source | Cellulose | Cellulose | Inulin | Inulin | Cellulose | Inulin | Mixed | Mixed | Cellulose | Inulin | Inulin | Inulin | Cellulose | Inulin |
| Kilocalories% fat | 10 | 60 | 10 | 60 | 15 | 15 | 15 | 15 | 15 | 15 | 10 | 10 | 45 | 45 |
| Protein, kcal% | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Carbohydrate, kcal% | 70 | 20 | 69 | 19 | 65 | 60 | 62 | 62 | 65 | 60 | 68 | 65 | 35 | 33 |
| Fat, kcal% | 10 | 60 | 10 | 60 | 15 | 15 | 15 | 15 | 15 | 15 | 10 | 10 | 45 | 45 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Kilocalories/gram | 3.8 | 5.2 | 3.9 | 5.3 | 3.5 | 3.6 | 3.5 | 3.5 | 3.5 | 3.6 | 3.8 | 3.5 | 4.5 | 4.6 |
| Ingredient | ||||||||||||||
| Casein (4 kcal/g), g | 200 | 200 | 200 | 200 | 0 | 0 | 200 | 0 | 200 | 200 | 200 | 200 | 200 | 200 |
| Soy protein (4 kcal/g), g | 0 | 0 | 0 | 0 | 200 | 200 | 0 | 200 | 0 | 0 | 0 | 0 | 0 | 0 |
| dl-Methionine (4 kcal/g), g | 0 | 0 | 0 | 0 | 3 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| l-Cystine (4 kcal/g), g | 3 | 3 | 3 | 3 | 0 | 0 | 3 | 0 | 3 | 3 | 3 | 3 | 3 | 3 |
| Corn starch (4 kcal/g), g | 315 | 0 | 302.5 | 0 | 387 | 337 | 362 | 362 | 387 | 337 | 290 | 265 | 72.8 | 72.8 |
| Maltodextrin 10 (4 kcal/g), g | 35 | 125 | 35 | 112.5 | 110 | 110 | 110 | 110 | 110 | 110 | 35 | 35 | 100 | 75 |
| Sucrose (4 kcal/g), g | 350 | 68.8 | 350 | 68.8 | 150 | 150 | 150 | 150 | 150 | 150 | 350 | 350 | 172.8 | 172.8 |
| Cellulose (0 kcal/g), g | 50 | 50 | 0 | 0 | 200 | 0 | 100 | 100 | 200 | 0 | 0 | 0 | 100 | 0 |
| Inulin (1 kcal/g), g | 0 | 0 | 50 | 50 | 0 | 200 | 100 | 100 | 0 | 200 | 100 | 200 | 0 | 100 |
| Soybean oil (9 kcal/g), g | 25 | 25 | 25 | 25 | 70 | 70 | 70 | 70 | 70 | 70 | 25 | 25 | 25 | 25 |
| Lard (9 kcal/g), g | 20 | 245 | 20 | 245 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 20 | 177.5 | 177.5 |
| RDI mineral mix (0 kcal/g), g | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 |
| Vitamin mix, V10001 (4 kcal/g), g | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Choline bitartrate (0 kcal/g), g | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total grams | 1,055.05 | 773.8 | 1,042.5 | 761.30 | 1,177.00 | 1,127.00 | 1,152.00 | 1,152.00 | 1,177.00 | 1,127.00 | 1,080.00 | 1,155.00 | 908.10 | 883.10 |
| Total kilocalories | 4,057 | 4,057 | 4,057 | 4,057 | 4,070 | 4,070 | 4,070 | 4,070 | 4,070 | 4,070 | 4,057 | 4,057 | 4,057 | 4,057 |
RDI, recommended daily intake.
Table 2.
Chow-based diets used in the study
| C11000 | C13062905 | C13062906 | C13062903 | C13062904 | C13887 | C13889 | |
|---|---|---|---|---|---|---|---|
| Protein source | Multiple | Multiple | Multiple | Multiple | Multiple | Multiple + added Casein | Multiple + added Soy |
| Fiber source | Multiple | Multiple + added cellulose | Multiple + added cellulose | Multiple + added inulin | Multiple + added inulin | Multiple | Multiple |
| Ingredient | |||||||
| Purina 5001, g | 1,000 | 900 | 800 | 900 | 800 | 850 | 850 |
| Casein, g | 0 | 0 | 0 | 0 | 0 | 150 | 0 |
| Soy protein, g | 0 | 0 | 0 | 0 | 0 | 0 | 150 |
| Cellulose, g | 0 | 100 | 200 | 0 | 0 | 0 | 0 |
| Inulin, g | 0 | 0 | 0 | 100 | 200 | 0 | 0 |
| FD&C red dye 40, g | 0 | 0.15 | 0.075 | 0 | 0 | 0 | 0 |
| FD&C blue dye 1, g | 0 | 0 | 0 | 0.15 | 0.075 | 0 | 0 |
| Total | 1,000 | 1,000.15 | 1,000.075 | 1,000.15 | 1,000.075 | 1,000 | 1,000 |
FD&C, Food, Drug & Cosmetic Colors.
Mice treatment.
Mice were fed with “normal” chow diet (NCD) of specified CDD for a period of 2 days to 12 wk. NCDs were autoclaved for experiments using germ-free mice. Body weights were measured every week and are expressed as a percentage gain compared with the initial body weight (day 0), defined as 100%. At the end of diet treatment, mice were fasted for 5 h. Mice were then euthanized, and colon length, colon weight, spleen weight, and adipose weight were measured. Organs were collected for downstream analysis.
Food intake measurement.
Groups of mice were placed in a clean cage with a known amount of food. Twenty-four hours later, the amount of remaining food was measured with the difference viewed as food intake per 24 h.
Antibiotic treatment.
Mice were placed on broad-spectrum antibiotics ampicillin (1.0 g/l) and neomycin (0.5 g/l) in drinking water for 4 wk (23). As ampicillin and neomycin are poorly absorbed, such treatment primarily affects only intestinal microbiota without direct systemic effects (15).
SCFA treatment.
Mice were put on drinking water supplemented with a mixture of SCFA (67.5 mM sodium acetate, 40 mM sodium butyrate, and 25.9 mM sodium propionate) for 21 days (21).
Germ-free experiments.
Germ-free C57BL/6 mice were kept under germ-free conditions in a Park Bioservices isolator in our germ-free mice facility. After 4 wk of diet feeding, mice were fasted for 5 h and then removed from the isolator to be euthanized immediately. Samples were collected as previously described.
NMR spectroscopy.
All 1H NMR spectra of fecal extracts were recorded at 298 K using a Bruker Avance III 600 MHz NMR spectrometer (operating at 600.08 MHz for proton, Bruker Biospin), equipped with an inverse cryogenic probe. A one-dimensional NMR spectrum was acquired for each of all samples, employing the first increment of NOESY pulse sequence (recycle delay-90°-t1-90°-tm-90°-acquisition) with a spoil gradient for water presaturation. The recycle delay of 2 s, t1 of 4 μs, and the mixing time (tm) of 100 ms were set. The 90° pulse length was adjusted to 10 μs. A total of 64 scans were collected into 32k data points for each spectrum with a spectral width of 20 ppm. For resonance assignment purposes, a series of two-dimensional NMR experiments (1H−1H correlation spectroscopy, 1H−1H total correlation spectroscopy, 1H−13C heteronuclear single quantum correlation spectroscopy, and 1H−13C heteronuclear multiple bond correlation spectroscopy) were carried out on the selected samples.
Spectral data processing and multivariate data analysis.
1H NMR spectra were corrected manually for phase and baseline distortions, and spectral region δ 0.5–9.5 was integrated into regions with equal width of 0.004 ppm (2.4 Hz) using AMIX software package (V3.8, Bruker-Biospin). Regions distorted were discarded. These regions are δ 4.45–5.20 for imperfect water saturation in both cecal and fecal extracts, and δ 1.15–1.23 and δ 3.62–3.69 for ethanol contaminations during the mouse dissection process. Each bucketed region was then normalized to the total sum of the spectral integrals to compensate for the overall concentration differences before statistical data analysis.
Multivariate data analysis was carried out with the SIMCAP+ software (version 13.0, Umetrics, Sweden). Principal component analysis was initially carried out on NMR data to generate an overview. Orthogonal projection to latent structure with discriminant analysis (OPLS-DA) was subsequently conducted using NMR data. The OPLS-DA models were validated using a sevenfold cross-validation method, and the quality of the model was described by the parameters R2X and Q2 values. To facilitate interpretation of the results, back-transformation (13) of the loadings generated from the OPLS-DA was performed before generating the loadings plots, which were color-coded with the Pearson linear correlation coefficients of variables (or metabolites) using an in-house developed script for MATLAB (The Mathworks, Natwick, MA). The color-coded correlation coefficient indicates the significance of the metabolite contribution to the class separation, with a “hot” color (e.g., red) being more significant than a “cold” color (e.g., blue). In this study, a cutoff value of |r| > 0.707 (r > 0.707 and r < −0.707) was chosen for correlation coefficient as significant based on the discrimination significance (P ≤ 0.05).
Statistical analysis.
Significance was determined using one-way ANOVA corrected for multiple comparisons with a Bonferroni test (GraphPadPrism software, version 6.01). Differences were noted as significant at P ≤ 0.05.
RESULTS
CDD are obesogenic and induce loss of intestinal mass.
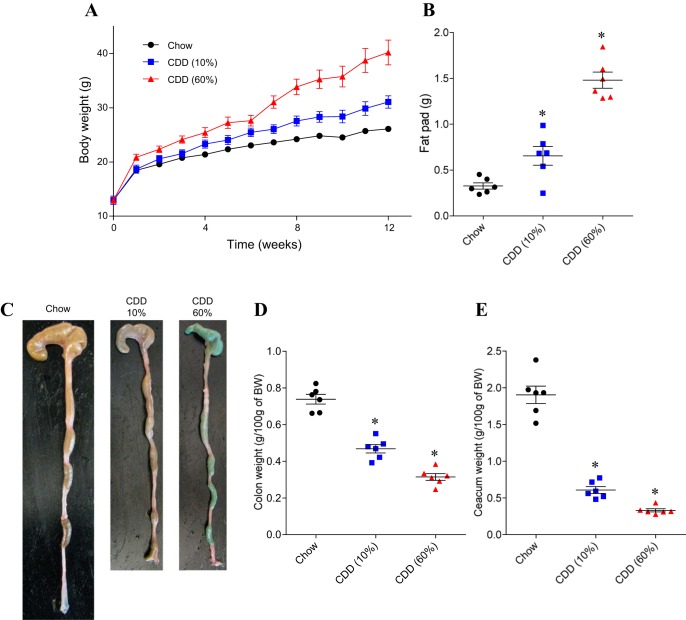
C57Bl/6 mice, in all cases herein fed ad libitum, were maintained on a NCD through the postweaning period (i.e., until 6 wk of age) so as to allow their mucosal immune system to mature and gut microbiota composition to stabilize (10, 14). Mice were then switched to one of two CDD, which are assembled from purified quality-controlled ingredients. One CDD contained 60% fat, i.e., HFD (HFD or CDD-60%), and has been widely utilized in research to promote obesity, while the other CDD contained 10% fat (CDD-10%) and can be viewed as an appropriate control diet for the HFD in that it is made from the same ingredients, albeit in different proportions and, moreover, is thought to be similar to chow in terms of its basic nutrient/calorie content. Relative to NCD-fed mice, mice fed HFD exhibited marked weight gain (Fig. 1A) that corresponded with development of epididymal fat pads about fourfold larger than that of NCD-fed animals (Fig. 1B). An intermediate result was observed with mice fed a CDD composed of 10% fat (CDD-10%). (Fig. 1, A and B). This pattern of results was consistently seen in multiple experiments and when both CDD were purchased from different vendor (data not shown). These results confirm that fat content is a key driver of the DIO phenotype, but also indicate that other differences between HFD and NCD contribute to HFD's promotion of obesity. Obesity is associated with, and promoted by, low-grade chronic inflammation. A correlate of low-grade intestinal inflammation is shortening of the colon, which is often accompanied by loss of cecal and colonic mass (24). Indeed, a striking consequence of feeding mice either HFD or control CDD was a dramatic loss of cecal and colonic tissue mass. Specifically, relative to chow-fed mice, the intestine of CDD-fed mice appeared thin and atrophied (Fig. 1C), which correlated with a shortening of the colon and loss of cecal and colonic mass that could be quantitated by weighing cecum (with contents) and colon (without contents) (Fig. 1, D and E). We reasoned that such gross effects on the intestine by CDD might be pivotal to their promotion of adiposity and, hence, sought to understand their cause.
Fig. 1.
Compositionally defined diets (CDD) are obesogenic and induce loss of intestinal mass. C57Bl/6 mice were maintained on the “normal” chow diet (NCD), or, at 4–5 wk of age, switched to the specified CDD for 12 wk (with 10 or 60% fat). A: body weight (BW) over time. B: epididymal fat pad weight. C: gross pictures of gut morphology. D: colon weight. E: cecum weight. Values are means ± SE of N = 6 mice per group. Significance was determined by Student's t-test. *P < 0.05.
CDD-induced changes are rapid and involve gut bacteria.
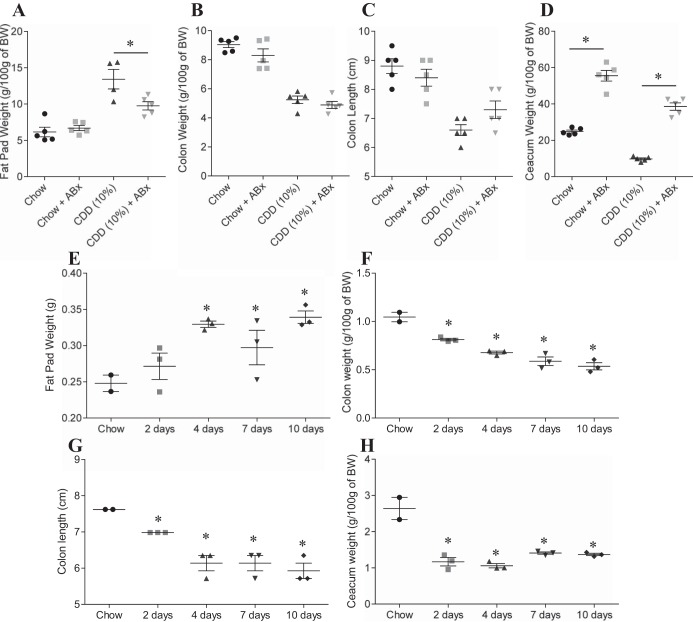
HFD-induced obesity is thought to involve interactions between the gut microbiota and host innate immune system that promotes low-grade inflammation. Consequently, the extent to which obesity is induced by HFD is reduced by antibiotic treatment (6). Analogously, the adiposity induced by low fat (10%) CDD-10% was significantly reduced by antibiotic treatment (Fig. 2A). Moreover, such amelioration of CDD-induced adiposity by antibiotic treatment was accompanied by a partial restoration of cecal and colonic tissue (Fig. 2, B–D). We hypothesized that these results might reflect that the CDD were increasing bacterial activation of MyD88 signaling, which is known to mediate a considerable portion of TLR signaling. However, the effects of CDD were only modestly reduced in MyD88-deficient mice, suggesting this pathway did not play a major role in mediating CDD's effect on the intestine and adipose tissue (Fig. 3). Next, we determined the time course with which CDD results in loss of intestinal mass. We observed that 2 days of feeding of CDD was sufficient to result in a dramatic loss of cecal mass (Fig. 2H) and a significant loss of colon mass/length, which continued to decline over the next several days (Fig. 2, F and G) and by 10 days was similar in magnitude to that seen in experiments where the diet was maintained for several weeks. A significant increase in adiposity was also observed over this relatively short time course (Fig. 2E). Together, these results suggest that CDD may alter gross intestinal morphology in a rapid manner that promotes microbiota-dependent, low-grade inflammation that promotes adiposity.
Fig. 2.
Microbiota involvement in protection of weight gain and intestinal mass loss induced by CDD. A–D: C57Bl/6 mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet and treated with ampicillin (1.0 g/l) and neomycin (0.5 g/l) in drinking water for 4 wk. A: epididymal fat pad weight. B: colon weight. C: colon length. D: cecum weight. E–H: C57Bl/6 mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet for 2–10 days. E: epididymal fat pad weight. F: colon weight. G: colon length. H: cecum weight. Values are means ± SE of N = 5 (A–D) or 3 (E–H) mice per group. Significance was determined by Student t-test. *P < 0.05. ABx, antibiotics.
Fig. 3.
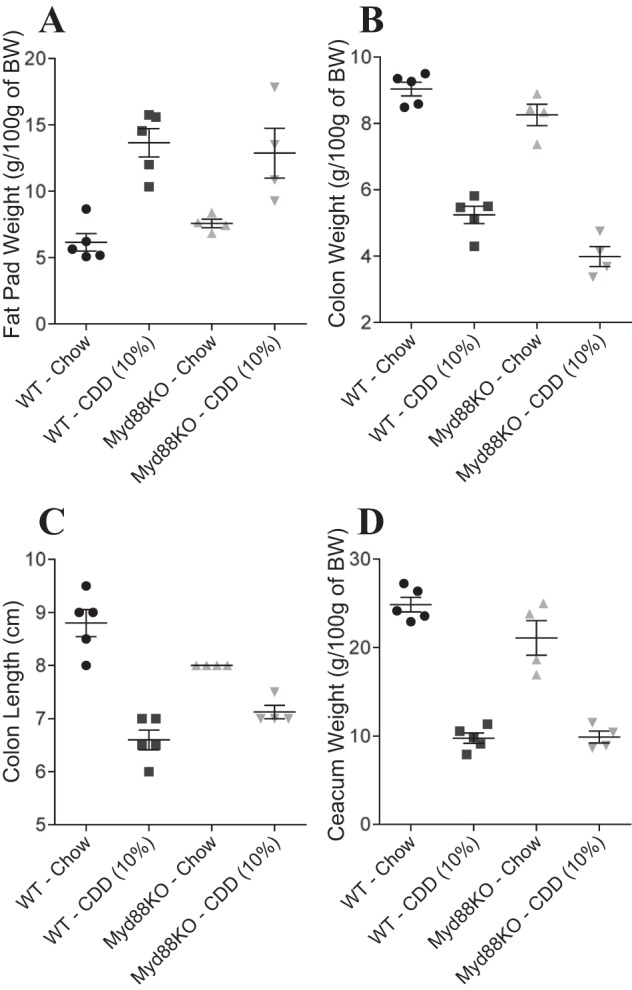
Myd88 involvement in weight gain and intestinal mass loss induced by CDD. C57Bl/6 WT or Myd88 knockout (KO) mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet for 12 wk. A: epididymal fat pad weight. B: colon weight. C: colon length. D: cecum weight. Values are means ± SE of N = 5 mice per group.
Dietary fiber content, but not protein, alters gut morphology alterations induced by CDD.
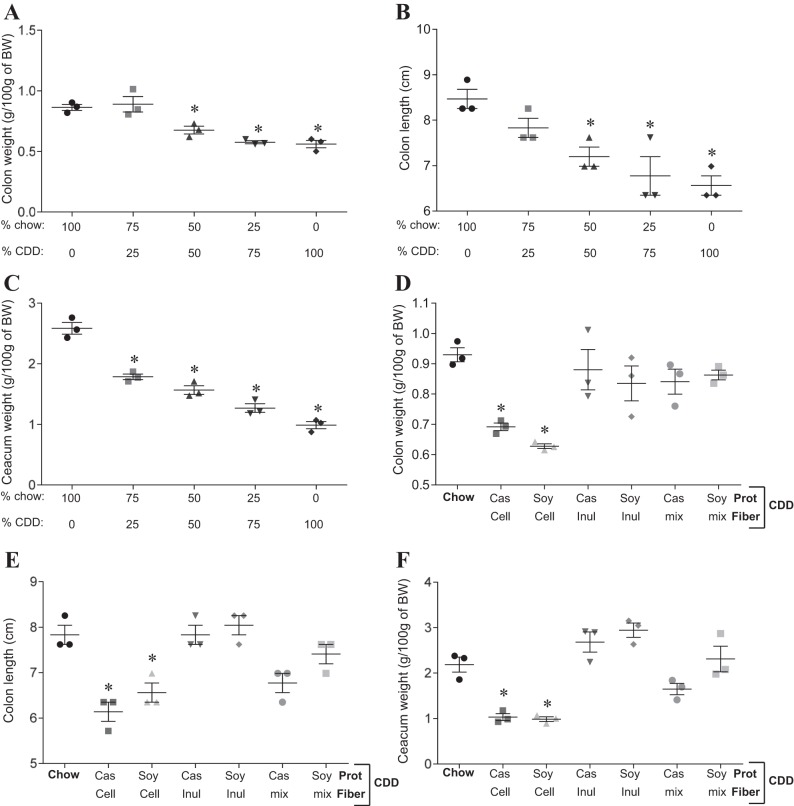
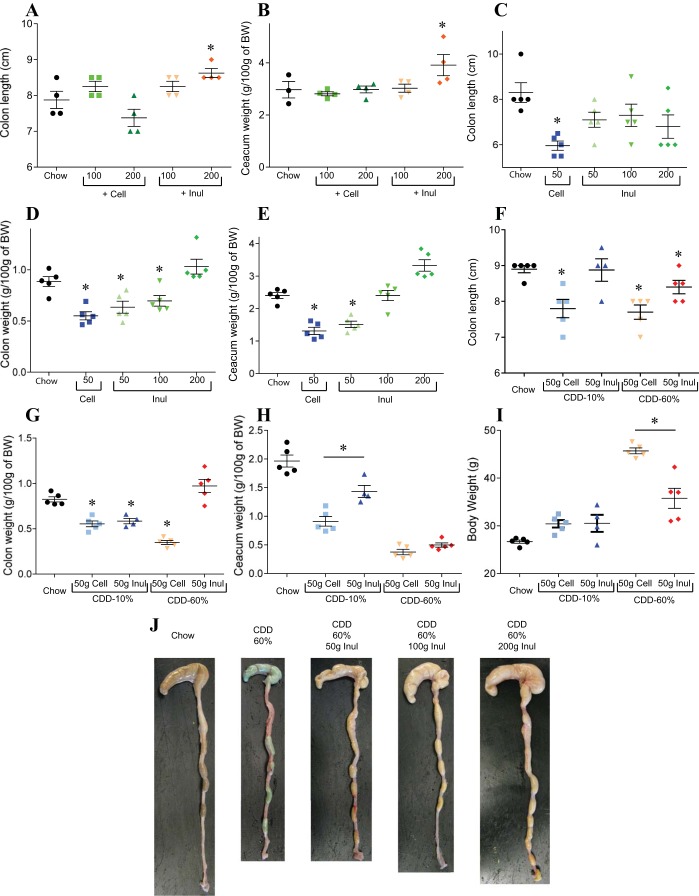
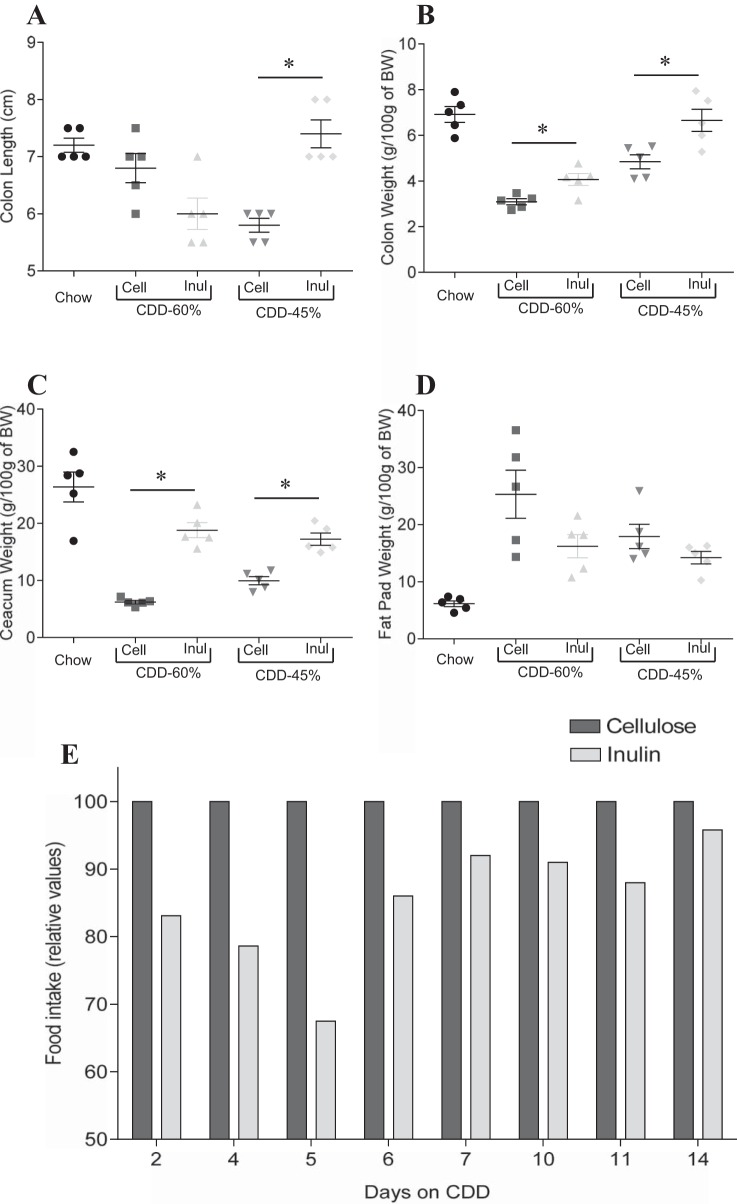
In light of our hypothesis that low-grade inflammation plays a key role in DIO, and that a healthy gut is necessary to protect the host against inflammation, we sought to better understand why CDD might result in loss of intestinal mass. First, to get a better sense of the extent to which this phenotype was driven by absence of a gut-maintaining component of NCD or presence of a gut-destroying component of CDD, we mixed the diets at a range of ratios of dietary protein or fibers. The loss of intestinal mass phenotype was proportional to the percentage of the diet composed of CDD, a result consistent with the possibility that the phenotype resulted from loss of a nutrient that was limiting in chow, or that it was a dose-dependent consequence of a component of CDD (Fig. 4, A–C). We first considered the possibility that the protein component of the CDD, namely purified animal protein casein, might be playing a role in the CDD-induced phenotype. Hence, we formulated diets in which the purified protein in CDD, casein, was replaced with plant-derived soy protein (plant protein source in NCD). This change did not significantly impact the CDD-induced loss of intestinal mass or its associated metabolic phenotypes (Fig. 4, D–F). Nor did addition of casein or soy to NCD alter these parameters relative to a NCD alone, thus arguing against a role for protein type and content (data not shown). Next, we considered the role of the fiber, which is present in NCD, albeit not in a defined amount. HFD, and therefore its matched low-fat control CDD, contain the insoluble fiber (resistant to bacterial fermentation) cellulose as a sole source of fiber. Exchanging cellulose for the soluble fiber (readily undergo bacterial fermentation) inulin largely reversed the deleterious effects of the CDD on the intestine (Fig. 5, F–I). This was true, regardless of whether the CDD had a protein source of casein or soy protein. Furthermore, mixing cellulose and a CDD containing both inulin and cellulose did not result in loss of gut mass, suggesting this phenotype may result from CDD's detriment of soluble fiber rather than the presence of cellulose per se. Next, we administered diets designed to investigate whether cellulose might have detrimental effects or if these results reflected that inulin was promoting gut health, as previously suggested (1, 7, 18). In support of this notion, addition of cellulose to chow resulted in only very modest loss of gut mass and did so only at relatively high concentrations (4× the fiber typically in CDD) (Fig. 5, A and B). Rather, in CDD, levels of inulin correlated well with maintenance of gut mass (Fig. 5, C–E), could correct loss of gut mass induced by CDD-10% or CDD-60% (Fig. 5, G–J), and addition of inulin to NCD boosted the level of intestinal mass relative to NCD alone (Fig. 5, A and B). The restoration of gut mass achieved by exchange of cellulose for inulin correlated with amelioration in body weight gain and adiposity induced by HFD (Fig. 6D). We hypothesized that the inulin's reduction in HFD would correlate with lower food consumption. However, the physical nature of the HFD makes this measure very difficult, as the diet crumbles and cannot be easily separated from cage bedding (we sought to avoid use of metabolic cages, which can be very stressful to mice and might impact feeding behavior). Hence, we designed a CDD with 45% fat (CDD-45%), which does not preclude food consumption measurements and contained cellulose or inulin as a fiber source. Analogous to the other CDD, inulin preserved gut mass and reduced adiposity relative to mice fed CDD-45% that contained cellulose (Fig. 6, A–D). Moreover, such reduction in adiposity in mice fed inulin CDD-45% correlated with reduced food consumption (Fig. 6E). Together, these results suggest that soluble fiber may be a limiting nutrient in NCD and that its complete absence in CDD contributes to DIO. Moreover, soluble fiber may be a means of maintaining gut health and preventing diseases driven by low-grade inflammation.
Fig. 4.
Fiber content, but not protein (Prot) content, alters gut morphology induced by CDD. C57Bl/6 mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet for 4 wk. A–C, percentage of diet composed of chow and CDD is shown. D–F: Prot and fiber components of the CDD are shown. A and D: colon weight. B and E: colon length. C and F: cecum weight. Cell, cellulose; Inul, inulin; Cas, casein. Values are means ± SE of N = 3 mice per group. Significance was determined by Student t-test. *P < 0.05.
Fig. 5.
Soluble fiber-supplemented CDD protect from gut morphology alterations. C57Bl/6 mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet for 4 wk. A and B: chow plus Cell or Inul. C–E: CDD plus Cell or Inul. F–I: chow and CDD with 10% and 60% fat plus Cell or Inul. A, C, and F: colon length. B, E, and H: cecum weight. C and F: colon length. D and G: colon weight. I: BW. J: gross pictures of gut morphology. Values are means ± SE of N = 4 (A and B) or 5 (C–J) mice per group. Significance was determined by Student t-test. *P < 0.05.
Fig. 6.
Soluble fiber-supplemented CDD protect from gut morphology alterations. C57Bl/6 mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet for 6 wk. A: colon length. B: colon weight. C: cecum weight. D: epididymal fat pad weight. E: food intake. Values are means ± SE of N = 5 mice per group. Significance was determined by Student t-test. *P < 0.05.
SCFA involvement in soluble fiber protection of gut morphology alterations.
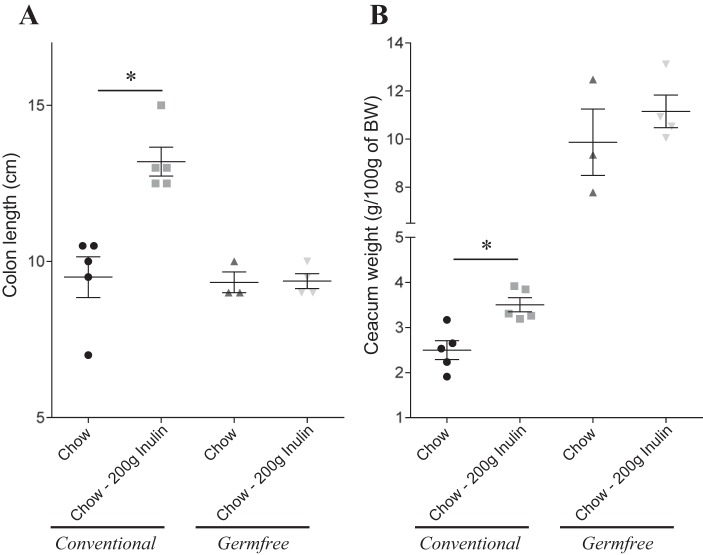
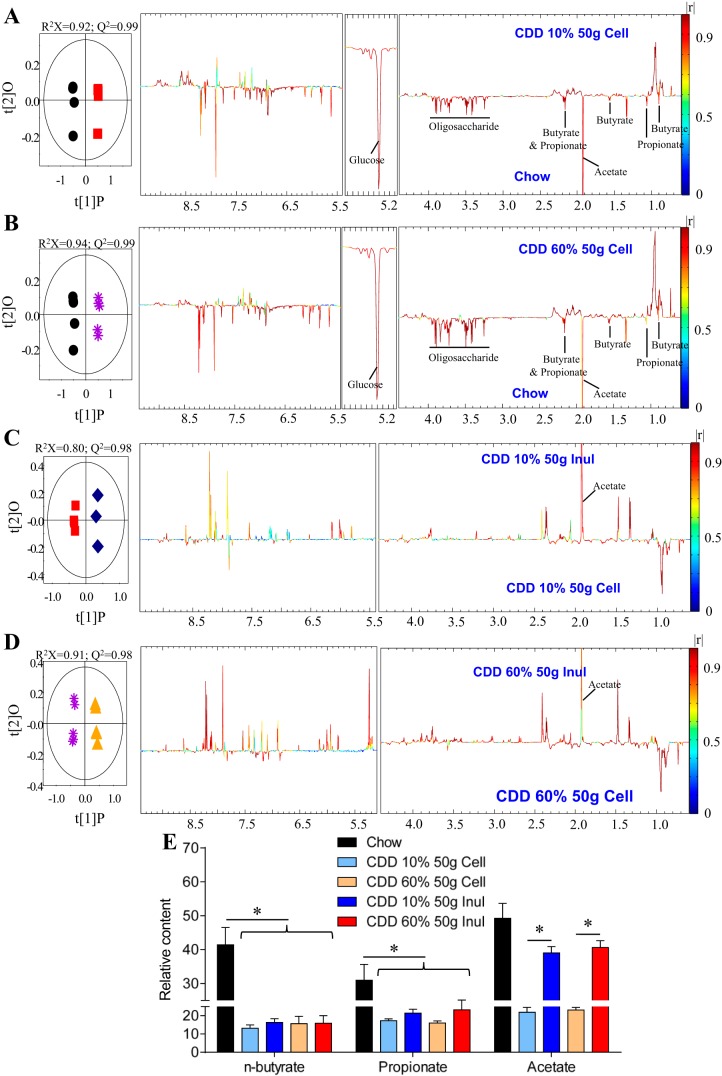
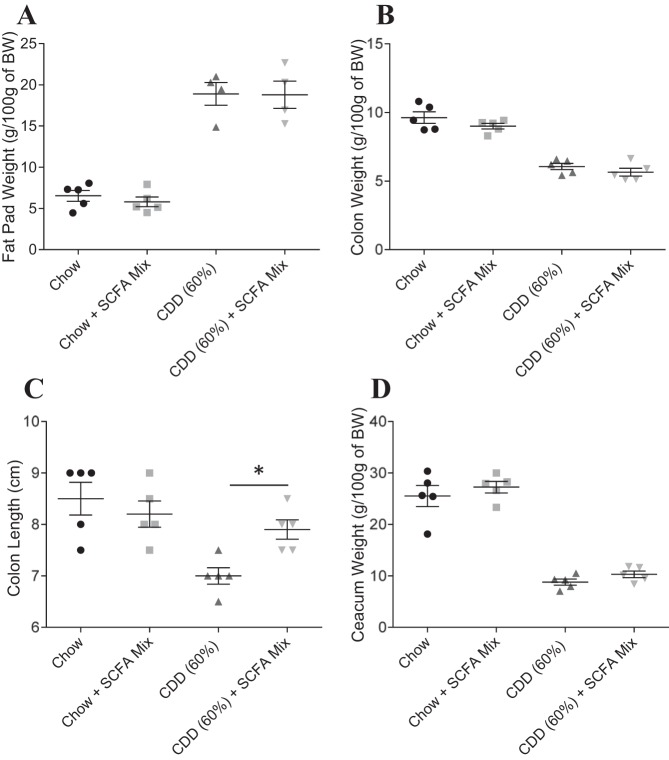
We next sought to better understand the mechanism by which inulin promotes gut health. First, we used a germ-free approach to investigate microbiota involvement. However, unlike chow, CDD cannot be autoclaved, and efforts to sterilize CDD by irradiation at typical doses (10–20 kGy) were not successfully achieved (i.e., germ-free mice fed an irradiated CDD developed high level of cultivable fecal bacteria; data not shown). Hence, we administered sterilized (i.e., autoclaved) inulin-supplemented NCD to conventional and germ-free mice and investigated if lack of a microbiota prevented inulin's ability to boost gut mass. We observed that inulin's ability to promote colon and cecum mass was completely absent in germ-free mice, indicating its promotion of gut health is likely microbiota dependent (Fig. 7). Microbiota-mediated production of SCFA is thought to be an important energy source for gut epithelia and which bacteria can readily generate from soluble but not insoluble fiber. Hence, we next measured levels of fecal SCFA via NMR, and we observed that, relative to NCD-fed mice, mice fed CDD, both CDD-10% and HFD-60%, exhibited reduced levels of butyrate, acetate, and propionate (Fig. 8, A, B, E). Exchanging the cellulose in these diets for inulin boosted SCFA levels (Fig. 8, C–E), supporting the notion that inulin promotes gut health via microbiota-mediated production of SCFA that protect against inflammation. To further examine this possibility, we administered SCFA to the drinking water of CDD-fed mice. Such SCFA treatment did not recapitulate all of inulin's beneficial effect, but attenuated CDD-induced colonic shortening, indicating partial protection against CDD-induced low-grade inflammation (Fig. 9). Together, these results indicate lack of microbiota-produced soluble fiber metabolites is pivotal to the maintenance of a healthy mouse intestine and suggest that SCFA production is one specific class of metabolites involved in this process.
Fig. 7.
Microbiota involvement in soluble fiber protection of gut morphology alterations. Conventional and germ-free C57Bl/6 mice were maintained on autoclaved NCD, or, at 4–5 wk of age, switched to the specified autoclaved diet for 4 wk. A: colon length. B: cecum weight. Values are means ± SE of N = 3–5 mice per group. Significance was determined by Student t-test. *P < 0.05.
Fig. 8.
NMR-based metabolomics reveal short-chain fatty acid (SCFA) involvement in soluble fiber-induced protection of gut morphology alterations. A–D: orthogonal projection to latent structure with discriminant analysis score plot (left) and correlation coefficient loading plot (right) showing the discrimination between 1H NMR spectra of fecal contents from mice fed with the indicated diet for 4 wk. A: NCD vs. CDD-10%. B: NCD vs. CDD-60%. C: CDD-10% vs. CDD-10% + 200 g Inul. D: CDD-60% vs. CDD-60% + 200 g Inul. E: relative content of n-butyrate, propionate, and acetate in mice fed with the indicated diet for 4 wk. Values are means ± SE of N = 5 mice per group. Significance was determined by Student t-test. *P < 0.05.
Fig. 9.
SCFA involvement in soluble fiber protection of gut morphology alterations. C57Bl/6 mice were maintained on the NCD, or, at 4–5 wk of age, switched to the specified diet and treated with SCFA mix (67.5 mM sodium acetate, 40 mM sodium butyrate, and 25.9 mM sodium propionate) in drinking water for 3 wk. A: epididymal fat pad weight. B: colon weight. C: colon length. D: cecum weight. Values are means ± SE of N = 5 mice per group. Significance was determined by Student t-test. *P < 0.05.
DISCUSSION
Societal changes in food production and diet are presumed to be major contributors to the epidemic of obesity and its associated diseases. In particular, the increased availability and consumption of foods rich in fat and calories is thought to be a major factor underlying the epidemic of obesity and its related diseases; i.e., metabolic syndrome. In support of this notion, ad libitum feeding of a HFD to mice rapidly increases and recapitulates and/or promotes many features of metabolic syndrome. Consequently, administration of HFD has been widely used to investigate the pathophysiology and potential treatments of metabolic syndrome. Such studies indicate that HFD-induced metabolic disease may be driven, in part, by altered host-microbiota interactions that drive low-grade inflammation (8). Yet, how the HFD alters such events remains largely unknown. Even the relative role of the HFD's fat content vs. the numerous other ways by which this diet differs from the NCD to which it is typically experimentally compared have not been well defined. Herein, we observed that, while fat content is an important determinant of the extent to which HFD promotes adiposity, other determinants of this diet are pivotal in driving low-grade inflammation and, consequently, likely contribute to the HFD-induced phenotype. Specifically, we observed that, among mice fed NCD vs. a range of CDD, lack of soluble fiber correlated with cecal and colonic atrophy that resulted in microbiota-dependent promotion of adiposity. The notion of diet-induced, microbiota-dependent increase in adiposity is in accord with the more general hypothesis that a broad range of genetic and nongenetic factors that result in disturbance of the microbiota can promote metabolic disease (9–11). Consequently, that addition of exogenous soluble fiber (i.e., inulin) protected against both gut atrophy and HFD-induced adiposity suggests that increasing dietary soluble fiber intake may have broad applicability in ameliorating metabolic disease.
Dietary fiber intake, which has long been appreciated as an effective means to treat and prevent constipation, is increasingly recognized for its association with metabolic health. Consequently, US Department of Agriculture dietary guidelines encourage consumption of foods rich in fibers, but do not distinguish between soluble and insoluble fibers. Yet our results herein that a soluble fiber, namely inulin, but not an insoluble one, namely cellulose, promoted bowel mass and attenuated the obesogenic effects of a HFD suggest a major distinction in the ability of such fibers to promote health. The ability of inulin to suppress adiposity by CDD diets high in fat content correlated with reduced food intake, which seems highly likely to have been a factor in reducing adiposity. That such ability was observed relative to CDD containing cellulose argues against the notion that such actions reflect simple addition of dietary bulk, but, rather, suggest a key difference in how such fibers are metabolized. Gut microbiota readily metabolize soluble fiber into SCFA, notably butyrate, which is known to serve as a major fuel source for colonic epithelia and have an array of anti-inflammatory properties, including promotion of regulatory T cells (2, 16, 20). Accordingly, we and others have observed near complete absence of SCFAs in germ-free mice and, herein, observed that the ability of inulin to increase gut mass was absolutely dependent on the presence of a gut microbiota, suggesting that SCFA production might contribute to inulin's beneficial effects observed in our model. SCFA-mediated nourishment of the epithelium can be envisaged to result in enhanced barrier function that might reduce exposure of immune cells to bacterial products that would drive inflammatory gene expression and, consequently, phenotypes associated with gut inflammation. However, that addition of SCFA, following a regimen reported by others to support gut regulatory T-cell homeostasis (2, 16, 20), only partially recapitulated the gut protective effects exhibited by inulin, suggesting that SCFA production may be but one mechanism by microbiota metabolism of inulin protection against gut atrophy (21).
A pivotal observation that drove this study is that, relative to NCD, the ill-defined conglomerate of food scraps (grains and animal by-products) that most rodents in biomedical research are fed, CDD (with cellulose) assembled from characterized ingredients results in rapid gut atrophy, which seems likely to play a role in the obesogenic potential of such diets. Specifically, considering that intestinal cell proliferation protects the gut from a range of inflammatory challenges and HFD-induced obesity involves gut barrier dysfunction and subsequent microbiota activation of proinflammatory gene expression, we envisage that such gut atrophy results in increased bacterial induction of low-grade inflammation that can promote adiposity (10, 23). That some indexes of CDD-induced low-grade inflammation, particularly colon shortening, were ameliorated in MyD88-deficient mice suggests a role for TLR-mediated detection of bacterial products in driving such low-grade inflammation, which is in accord with work of Cani and colleagues that TLR-4 drives HFD-induced metabolic syndrome (5). Yet, that such amelioration was only partial indicates that other pathways are also likely involved. Given that a large portion of gene expression induced by TLR agonists is in fact MyD88-independent (4), additional work will be needed to define other signaling pathways that contribute to CDD-induced phenotypes. Moreover, the state of low-grade inflammation induced by CDD is not itself well defined, in that CDD induced only very modest increases in myeloperoxidase and, moreover, did not associate with clear increases in fecal lipocalin-2, which we have found can reliably mark low-grade inflammation in chow-fed mice (12). We hypothesize that this reflects that fecal lipocalin-2 is largely produced by epithelial cells, thus obviating its utility as a marker in states wherein inflammation may have resulted from gut atrophy. Hence, in future work, it will be important to better define the low-grade inflammatory state that results from CDD feeding and to determine how this state influences, and is influenced by, gut microbiota.
In any case, that such phenotypes could be largely prevented by addition of inulin suggests the CDD induces gut atrophy as a result of its lack of soluble fiber. However, it is important to note that, at present, we cannot rule out the possibility that the CDD causes gut atrophy by another mechanism and that inulin simply protects against this phenotype by a mechanism not related to its underlying cause (Fig. 10). Regardless, the ability of inulin to promote gut mass when added to a wide variety of diets may make it an important ingredient in biomedical research. Specifically, the ill-defined nature of NCD, its consequent, inherent variability (vendors and different facilities/countries), and, perhaps most importantly, the inability to precisely manipulate its components greatly hinder its utility in a broad range of experimental approaches, especially those related to macro/micronutrient metabolism and host metabolic phenotype. Yet the dramatic deleterious effects of commonly used CDD on gut health, irrespective of fat content, suggest that such diets are not a good alternative to NCD. In contrast, as shown herein, CDD containing inulin remedy this concern and may serve as a good choice of control diet that would be a better mimic of NCD but yet retain tractability.
Fig. 10.
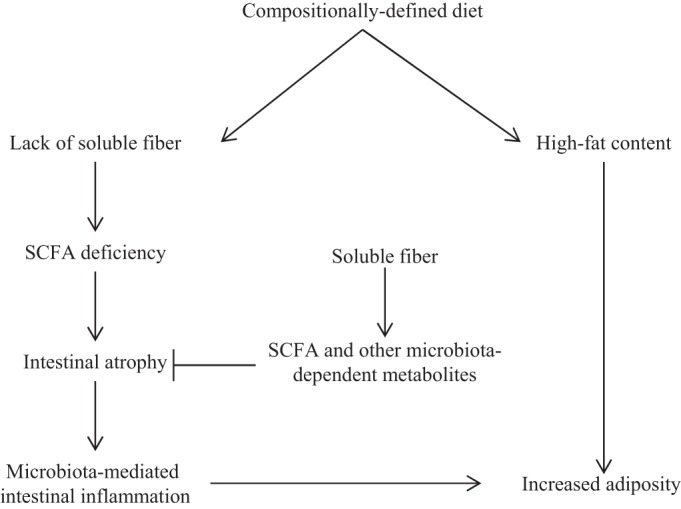
Proposed mechanism.
In conclusion, supplementation of a broad range of mouse diets with inulin promotes gut mass and protects against DIO by a microbiota-dependent mechanism, albeit one whose understanding is far from complete. If our observations were to prove applicable to humans, it would suggest encouraging consumption of foods with high soluble fiber content may be a means to combat the epidemic of metabolic disease. Moreover, addition of inulin, and perhaps other soluble fibers to processed foods, including calorically-rich obesogenic foods, may be a means to ameliorate their detrimental effects.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK099071 and DK083890. B. Chassaing is a recipient of the Research Fellowship award from the Crohn's and Colitis Foundation of America.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.C., J.P.M.-B., M.P., E.U., M.R., A.D.P., M.V.-K., and A.T.G. conception and design of research; B.C., J.P.M.-B., and L.Z. performed experiments; B.C. and A.T.G. analyzed data; B.C., J.P.M.-B., M.P., E.U., M.R., A.D.P., M.V.-K., and A.T.G. interpreted results of experiments; B.C. prepared figures; B.C. and A.T.G. drafted manuscript; B.C., J.P.M.-B., M.P., E.U., M.R., A.D.P., M.V.-K., and A.T.G. edited and revised manuscript; B.C., J.P.M.-B., M.P., E.U., M.R., L.Z., A.D.P., M.V.-K., and A.T.G. approved final version of manuscript.
REFERENCES
- 1.Adam CL, Williams PA, Dalby MJ, Garden K, Thomson LM, Richardson AJ, Gratz SW, Ross AW. Different types of soluble fermentable dietary fibre decrease food intake, body weight gain and adiposity in young adult male rats. Nutr Metab (Lond) 11: 36, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104: 979–984, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjorkbacka H, Fitzgerald KA, Huet F, Li X, Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT, Freeman MW. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics 19: 319–330, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr 60: 567–572, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3: 279–288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho FA, Aitken JD, Vijay-Kumar M, Gewirtz AT. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annu Rev Physiol 74: 177–198, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–96, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147: 1363–1377, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7: e44328, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloarec O, Dumas ME, Trygg J, Craig A, Barton RH, Lindon JC, Nicholson JK, Holmes E. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in 1H NMR spectroscopic metabonomic studies. Anal Chem 77: 517–526, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zarate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158: 705–721, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrier L, Berard F, Debrauwer L, Chabo C, Langella P, Bueno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol 168: 1148–1154, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol 29: 415–445, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Juskiewicz J, Zdunczyk Z. Effects of cellulose, carboxymethylcellulose and inulin fed to rats as single supplements or in combinations on their caecal parameters. Comp Biochem Physiol A Mol Integr Physiol 139: 513–519, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7: e47713, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araujo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 56: 1986–1998, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis 13: 856–864, 2007. [DOI] [PubMed] [Google Scholar]