Abstract
Mitochondrial dysfunction causes oxidative stress and cardiomyopathy. Oxidative stress also is a side effect of dideoxynucleoside antiretrovirals (NRTI) and is observed in NRTI-induced cardiomyopathy. We show here that treatment with the NRTI AZT {1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione} modulates cardiac gene expression epigenetically through production of mitochondrially derived reactive oxygen species. Transgenic mice with ubiquitous expression of mitochondrially targeted catalase (MCAT) and C57Bl/6 wild-type mice littermates (WT) were administered AZT (0.22 mg/day po, 35 days), and cardiac DNA and mRNA were isolated. In AZT-treated WT, 95 cardiac genes were differentially expressed compared with vehicle-treated WTs. When MCAT mice were treated with AZT, each of those 95 genes reverted toward the expression of vehicle-treated WTs. In AZT-treated WT hearts, Mthfr [5,10-methylenetetrahydrofolate reductase; a critical enzyme in synthesis of methionine cycle intermediates including S-adenosylmethionine (SAM)], was overexpressed. Steady-state abundance of SAM in cardiac extracts from AZT-treated MCAT mice increased 60% above that of vehicle-treated MCAT. No such change occurred in WT. AZT caused hypermethylation (47%) and hypomethylation (53%) of differentially methylated DNA regions in WT cardiac DNA. AZT-treated MCAT heart DNA exhibited greater hypermethylation (91%) and less hypomethylation (9%) compared with vehicle-treated MCAT controls. The gene encoding protein kinase C-α displayed multifocal epigenetic regulation caused by oxidative stress. Results show that mitochondrially derived oxidative stress in the heart hinders cardiac DNA methylation, alters steady-state abundance of SAM, alters cardiac gene expression, and promotes characteristic pathophysiological changes of cardiomyopathy. This mechanism for NRTI toxicity offers insight into long-term side effects from these commonly used antiviral agents.
Keywords: AZT, epigenetics, mitochondria, oxidative stress, s-adenosylmethionine
cardiomyopathy (CM) is an intrinsic weakening of the heart causing reduced pump function and congestive heart failure (5). Among the etiologies for CM are ischemia, infection, and chemical or drug toxicity (30). Nucleoside reverse transcriptase inhibitors (NRTI), are important components of current human immunodeficiency virus (HIV)-1 therapies, particularly important to resource-poor HIV/ acquired immunodeficiency syndrome (AIDS) populations (23). Among the NRTI compounds, zidovudine {AZT, 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione, azidothymidine}, stavudine {d4T, 1-[(2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl]-5-methyl-1,2,3,4-tetrahydropyrimidine-2,4-dione}, and others exhibit mitochondrial toxicities to heart, liver, and skeletal muscle (25, 26).
We articulated, tested, and continue to refine a hypothesis to unravel mechanisms of NRTI toxicity that emanates from our “pol γ hypothesis” that was formulated in the 1990s (24). The pol γ hypothesis suggests that the toxic mechanism of NRTIs directly relate to competitive and noncompetitive inhibition of the mitochondrial polymerase-γ and decreased ATP synthesis through decreased abundance of mitochondrially encoded polypeptides in the electron transport chain. (27). We showed that mitochondrial dysfunction from NRTIs increased reactive oxygen species (ROS) within the affected cell and amplified mitochondrial dysfunction in a feed-forward way (20). We prevented or attenuated NRTI toxicity both in vitro with exogenous metalloporphyrin antioxidants administration and in vivo with genetically engineered murine overexpression of mitochondrially targeted antioxidant enzymes (20, 21, 41).
Because of the increased awareness of NRTI toxic mechanisms and unprecedented failures in clinical trials of NRTIs for HIV/AIDS that showed no preclinical mitochondrially targeted toxicity, more attention is directed toward the aging population with HIV/AIDS, where cumulative NRTI side effects may result in long-term tissue damage (1, 4, 29). In support of this, muscle biopsies from patients treated long term with NRTIs exhibited reduced mitochondrial function that worsened over the duration of NRTI treatment (37). Large-scale deletions and single nucleotide mutations in mtDNA were observed in the muscle biopsies, which suggested the perpetuation of mitochondrial dysfunction through reduced mtDNA integrity.
DNA methylation epigenetically modulates gene expression (11, 36, 40). Methylation of cytosine in CpG results from activity of DNA methyltransferases that use S-adenosylmethionine (SAM) as the requisite methyl donor. We and others previously showed that changes in cardiac DNA methylation in CM correlate with gene expression changes (17, 19, 31–33). Moreover, CM in humans is associated with mitochondrial dysfunction and oxidative stress, which are characteristics of AZT toxicity and are documented in murine studies (15, 16, 18, 23).
We investigated the role of AZT in DNA methylation and its relationship to gene expression changes in the heart of AZT-treated mice. Utilizing genetically engineered mice expressing a mitochondrial-targeted catalase (MCAT), epigenetic regulation by AZT-induced mitochondrial-derived ROS was elucidated (7–9). AZT causes cardiac gene expression changes, altered DNA methylation, and changed SAM abundance. These results support and correlate mechanistically with pathophysiological changes of CM that we reported previously (20). In those studies, AZT caused CM to develop in wild-type mice (WT), while MCAT was able to prevent that CM. Results here show MCAT mice were protected epigenetically from AZT-induced cardiomyopathy by enhanced ROS scavenging, altered gene expression, increased DNA methylation, and higher SAM abundance.
MATERIALS AND METHODS
General.
Mitochondrial-targeted overexpressing catalase mice (termed MCAT) were from breeder stock from Peter Rabinovich, Department of Pathology, University of Washington (7–9). All mice were housed at Emory University in accordance with the Institutional Animal Care and Use Committee protocols in an Association for Assessment and Accreditation of Laboratory Animal Care-certified vivarium according to the National Institutes of Health guidelines. MCAT mice were bred with WT C57Bl/6 mice (Jackson Labs, Bar Harbor, ME). Mouse pups were weaned at 3 wk and genotyped, and experiments employed mice 8–12 wk of age. For genotyping, genomic DNA was extracted from mouse tail clippings, and genotype was determined by PCR.
For antiretroviral treatment, mice were administered AZT [0.22 mg/day in 1% carboxymethyl cellulose (CMC)] or 1% CMC vehicle control by gavage once daily for 35 days. After 35 days, mice underwent cardiac physiological, histological, and molecular analyses according to our previously published protocols (20). All biomolecular analyses used left ventricle (LV) tissue sections.
Myocardial gene expression analysis.
Gene expression was analyzed as previously described (17, 19). RNA was extracted from hearts from at least three mice in each treatment group with the Qiagen Fibrous Tissue RNeasy kit (Qiagen, Valencia, CA). Total RNA was used to synthesize double-stranded cDNA with the SuperScript Double-Stranded cDNA Synthesis Kit (Invitrogen/Life Technologies, Grand Island, NY). cDNA was labeled with Cy3 and hybridized to a 12 × 135 kb mouse expression array (Roche, Indianapolis, IN) overnight at 42°C. Expression arrays were washed and scanned with a Roche Nimblegen MS200 scanner. Images were analyzed by Nimblescan software as directed by the manufacturer, including robust multiarray average normalization and generation of expression data. Mean expression values were generated for each cDNA analyzed, and a P value was determined by a Student's t-test using pair-wise analysis of AZT-treated and vehicle-treated cohorts. Significance was defined operationally by a 1.5-fold change in gene expression and a P < 0.05 value on the t-test.
Methylated DNA immunoprecipitation of cardiac DNA.
DNA was extracted as previously described with a MagNAPure DNA Extraction System (Roche) (17). Total cellular DNA from four murine hearts of each treatment group was individually quantitated and sonicated to obtain an average DNA fragment size of 200–500 bp. The sonicated DNA was enriched for methylated fragments with the MethylCollector Ultra kit (Active Motif, Carlsbad, CA) following the manufacturer's directions whereupon both the methylated and input DNA were amplified by whole genome amplification (Sigma-Aldrich, St. Louis, MO). For DNA methylation analysis, Roche Nimblegen 2.1M Deluxe Promoter Arrays were utilized (Roche). Following the manufacturer's instructions, the DNA was labeled with Cy5 and Cy3 dyes to distinguish methylated and input DNA. Arrays were allowed to hybridize overnight at 42°C. Arrays were washed and scanned with a Roche Nimblegen MS200 scanner. Images were analyzed with Nimblescan software from the manufacturer. This included normalizing methylated DNA abundance to the input DNA abundance and peak identification using a modified ACME algorithm, and this method resulted in a final analysis that included a peak score of the detected methylated DNA peak and annotation of the probe location (Gene Expression Omnibus accession number: GSE54896) (38). DNA was discriminated for differential methylation statistically by Student's t-test of the peak scores via pair-wise analysis. Significance was defined as peaks with P < 0.05 and peak score difference>1.0.
SAM quantitation.
Frozen (−80°C) LV heart samples from experimental mice were weighed and deproteinated with the Deproteinizing Sample Preparation Kit (Biovision, Milpitas, CA) according to manufacturer's methods. For SAM quantitation, a SAM fluorescence assay was utilized (Mediomics, St. Louis, MO). Cardiac SAM abundance was quantitated against an external standard according to the manufacturer's protocols. Samples were normalized to tissue wet weight. At least three samples were analyzed for each treatment group.
Statistical analysis.
Quantitation for microarray results used methods described above. R programming software with Bioconductor was used for microarray analyses. All remaining statistics used GraphPad Prism (GraphPad, La Jolla, CA) and employed either a Student's t-test (P < 0.05) for two sample comparison, a one-way ANOVA with a Tukey post hoc test (P < 0.05), or a two-way ANOVA with a Bonferroni post hoc test (P < 0.05). A Grubbs' outlier test identified statistical outliers. Sample numbers are noted with each data set above, and an n ≥ 3 was used for all experiments.
RESULTS
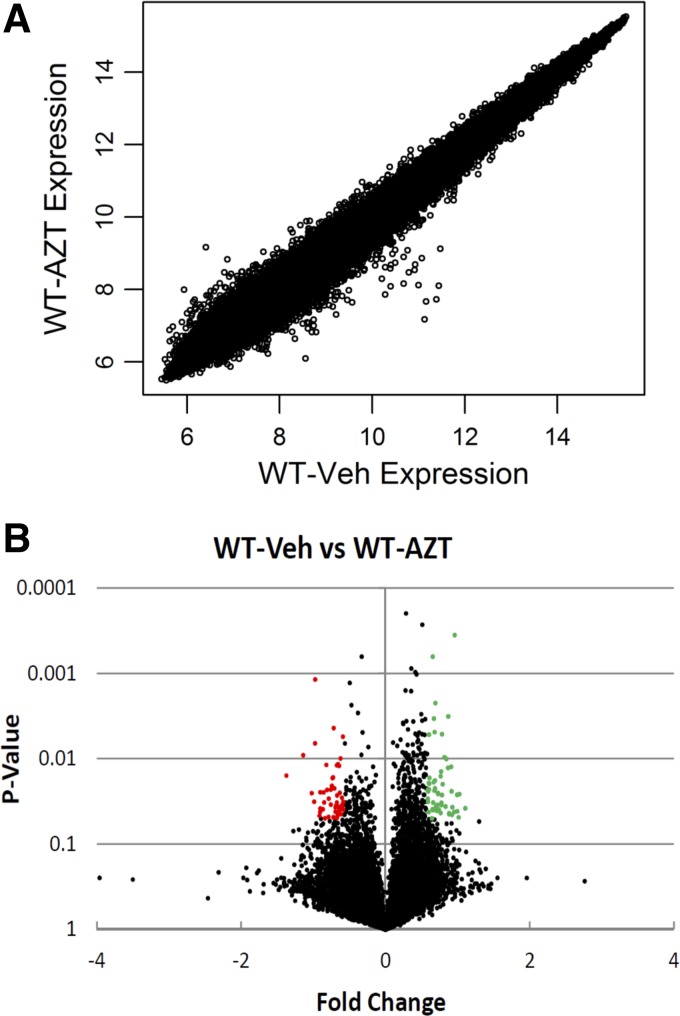
Experiments utilized gene expression arrays to identify expression changes in 17,869 cardiac genes caused by AZT. Using operationally defined significance (≥1.5-fold change in expression and a P < 0.05), we found 95 genes to be differentially expressed in the hearts of C57Bl/6 WT mice that were treated with AZT compared with vehicle controls (Supplemental Table S1).1 Of these identified genes, 46 were upregulated and 49 were downregulated (Fig. 1, A and B).
Fig. 1.
1-[(2R,4S,5S)-4-55 azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione, azidothymidine (AZT)-induced gene expression changes. Wild-type mice (WT) were treated with AZT or vehicle (Veh) for 35 days (0.22 mg/day via gavage). RNA was isolated from left ventricle tissue, and cDNA was hybridized to an expression array. A: expression results from vehicle and AZT-treated WT mice. The Log2 gene expression for each gene was plotted. B: Log2 fold change and P value for gene expression results of AZT-treated WT mice to vehicle only. Green dots denote genes significantly overexpressed following AZT treatment; red dots denote genes significantly repressed following AZT.
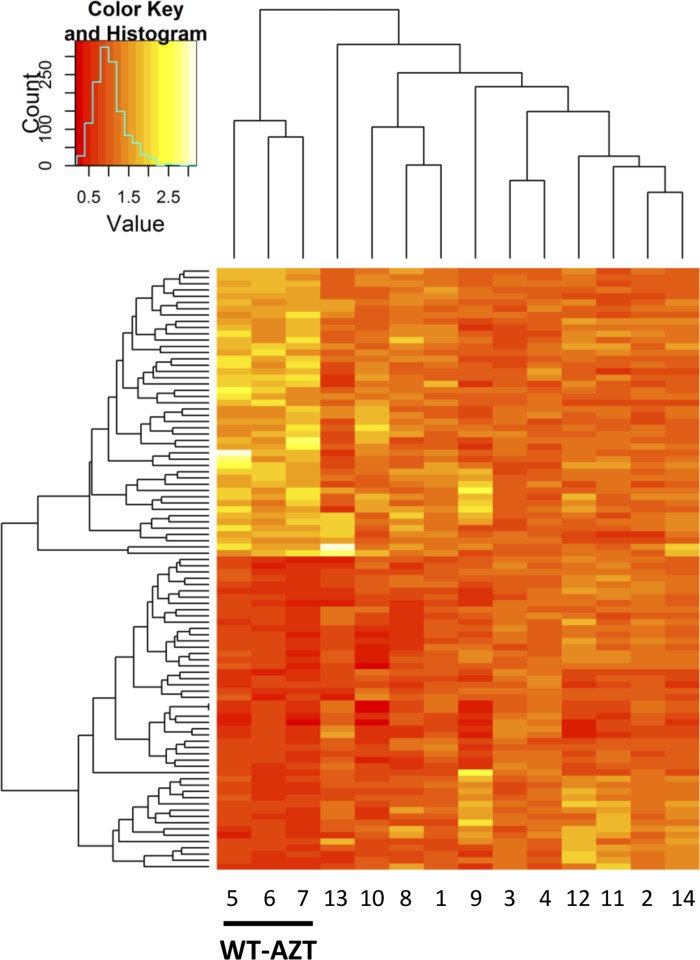
When myocardial gene expression of the 95 genes was interrogated in the same way in the hearts of the MCAT mouse, expression of all of the same 95 genes in AZT-treated MCAT mice improved and more closely resembled the expression pattern found in hearts of WT untreated mice (Fig. 2). Hierarchical clustering was effective to group and identify AZT-treated WT mice based on the 95 differentially expressed genes. The remaining experimental groups could not be distinguished further (Fig. 2). Since our previous studies showed that MCAT was protective from cardiac dysfunction caused by AZT treatment, it was logical to consider the expression of these 95 genes as pathophysiologically significant in CM from AZT.
Fig. 2.
Differentially expressed genes. Heat-map with hierarchical clustering of the 95 differentially expressed genes in all 4 treatment groups. In WT mice, 46 genes were upregulated and 49 were downregulated by AZT. AZT treatment in mitochondrially targeted catalase (MCAT) mice did not induce similar changes, with MCAT showing protection against AZT-induced gene expression changes. Hierarchical clustering identified the 3 WT AZT-treated mice but could not discern the remaining groups. Samples 1–4, WT vehicle treated; samples 5–7, WT AZT treated; samples 8–10, MCAT vehicle treated; samples 11–14, MCAT AZT treated. Each lane represents a single sample analyzed on a single array.
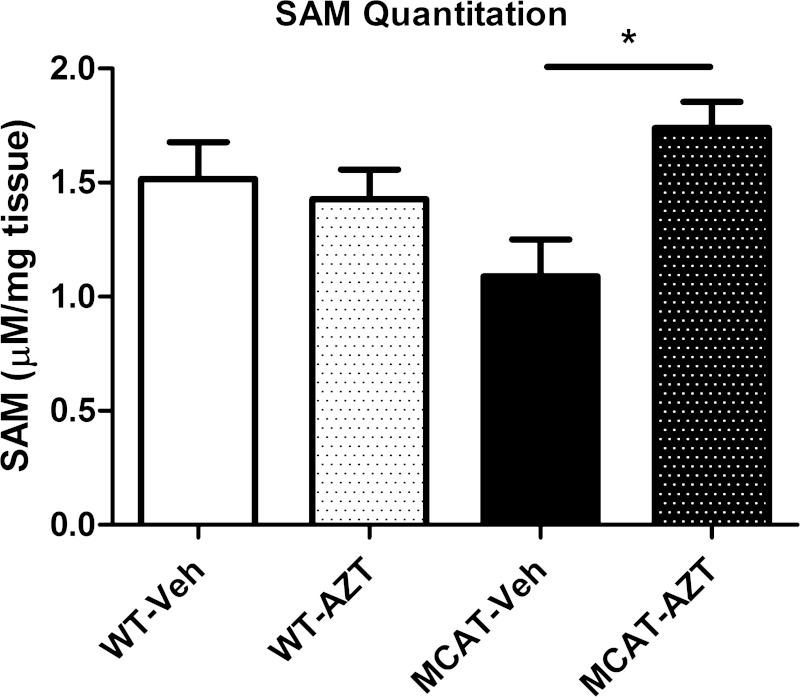
Transcription factors (e.g., oxr1 and SP4), phosphorylation pathway proteins (e.g., Pik3r1), and mitochondrial proteins (e.g., GPAT2 and Mrpl3) were among the cardiac genes that exhibited altered expression levels with AZT treatment. Expression of one gene, 5,10-methylene tetrahydrofolate reductase (Mthfr), exhibited a 67% increase with AZT treatment of WTs (Supplemental Table S1). Mthfr overexpression may serve as compensation for altered SAM synthesis and abundance. Quantitation of steady-state abundance of SAM revealed no change in its abundance in the hearts of vehicle-treated and AZT-treated WT mice (Fig. 3). A 60% increase in SAM abundance was observed in AZT-treated MCAT mice compared with vehicle-treated MCAT controls. Results suggest that AZT treatment elicits an increase in SAM that is prevented by AZT-associated oxidative stress.
Fig. 3.
S-adenosylmethionine (SAM). SAM was quantitated from deproteinated left ventricle tissue samples. SAM concentration (μM) was normalized to weight of input tissue (mg). SAM levels did not change following AZT treatment in WT mice compared with vehicle controls. SAM levels increased following AZT treatment in MCAT mice compared with vehicle controls (*P < 0.05).
SAM is the requisite methyl donor in reactions for DNA methylation driven by DNA methyltransferases. Because we identified changes in gene expression, we investigated whether SAM served as a modulator of DNA methylation to regulate cardiac gene expression. DNA methylation microarrays were employed to probe DNA promoters −5 kb to +1 kb of gene transcriptional start sites. This approach identified regions of differential DNA methylation using 2.1 M probes, and the analysis algorithm creates peak scores that identify DNA hypermethylated and hypomethylated promoter regions (17, 38).
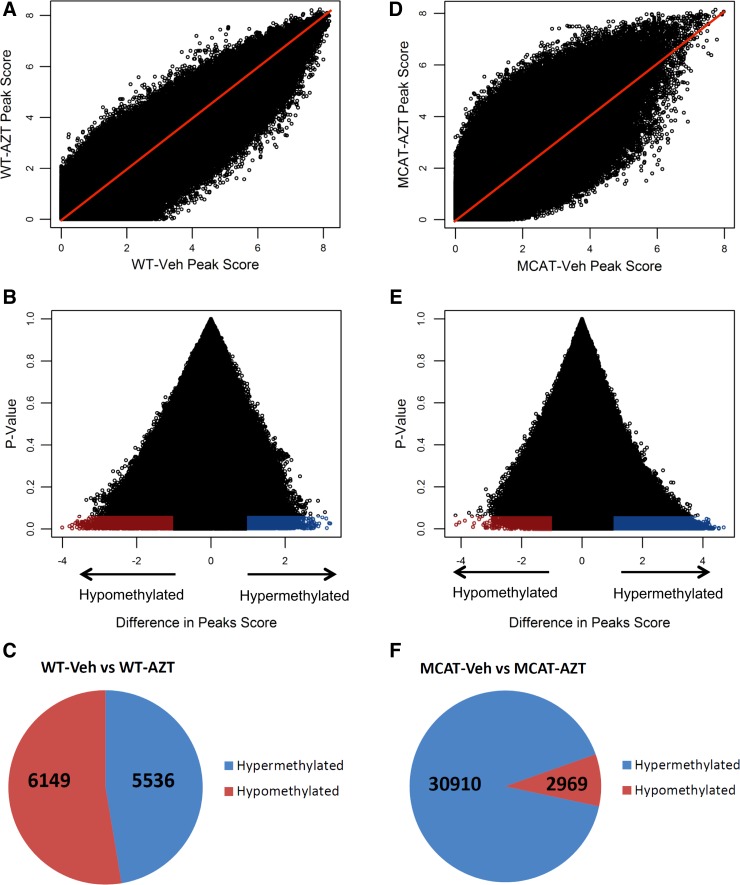
By analyzing differences in peak scores, we identified 11,685 differentially methylated DNA regions between AZT treated and vehicle-treated WT mice (Fig. 4, A and B). This represented a distribution of 53% of peaks that were hypomethylated while 47% were hypermethylated (Fig. 4C). When MCAT vehicle-treated and AZT-treated groups were compared, 33,879 differentially methylated DNA regions were identified (Fig. 4, D and E). Of these latter peaks, 91% were hypermethylated and 9% were hypomethylated (Fig. 4F). Together, these results indicate that mitochondrially derived ROS prevents DNA methylation following AZT treatment and is concordant with SAM abundance.
Fig. 4.
DNA methylation microarray analysis. DNA methylation changes were analyzed by oligonucleotide microarrays. Using a computational algorithm, we gave a peak score to each of 2.1 M probe regions. A: comparison of DNA methylation peak scores between WT vehicle- and AZT-treated mice. We identified 11,685 differentially methylated peaks. Red line shows the 1:1 peak score ratio (i.e., unchanged DNA methylation). B: peak score changes (WT AZT treated peak score minus WT vehicle treated peak score) plotted to P values. Regions were identified as hypermethylated (blue) or hypomethylated (red). C: number of hypermethylated and hypomethylated regions significantly changed by AZT in WT mice. D: comparison of DNA methylation peak scores between MCAT vehicle and AZT-treated mice. We identified 33,879 differentially methylated peaks. Red line shows the 1:1 peak score ratio. E: peak score changes (MCAT AZT treated peak score minus MCAT vehicle treated peak score) plotted to P values. Regions were identified as hypermethylated (blue) or hypomethylated (red). F: number of hypermethylated and hypomethylated regions significantly changed by AZT in MCAT mice.
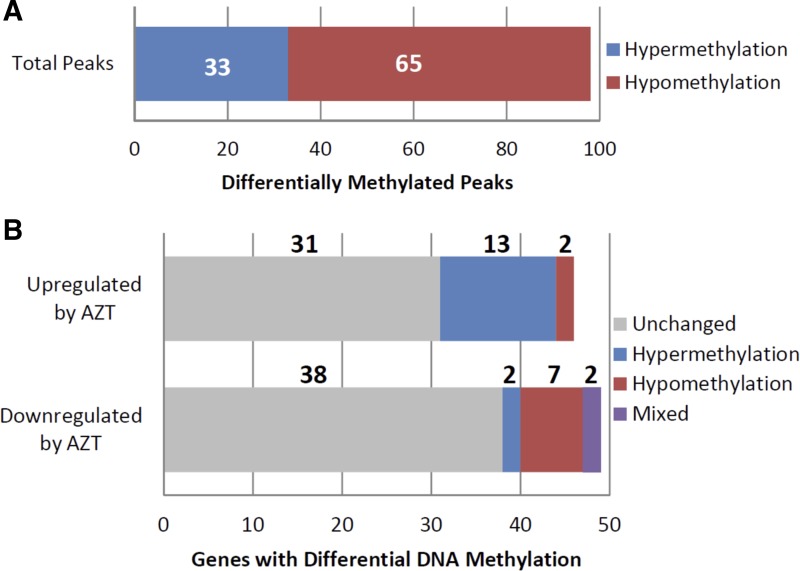
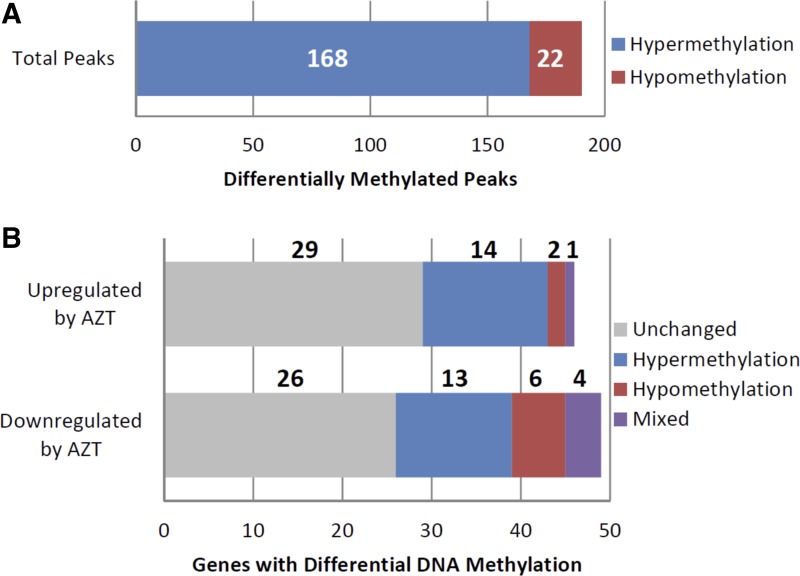
Of the 95 genes differentially expressed between AZT-treated and vehicle-treated control WT mice, 26 gene promoters displayed 98 individual, differentially methylated DNA regions. The number of identified DNA methylation peaks per gene promoter ranged from a single peak up to 17 peaks (Supplemental Table S2). Of these 98 peaks, 33 showed increased DNA methylation, while 65 displayed reduced DNA methylation (Fig. 5A). The directional change in DNA methylation was largely gene specific: nine genes displayed only hypermethylated regions, 15 displayed only hypomethylated regions, and two displayed both hyper- and hypomethylated regions (Fig. 5B). The differential methylated peaks were not necessarily sequential within the gene promoter; they did appear to be distinct regions based on the analytical methods used here. When those 95 genes were analyzed for changes in DNA methylation in MCAT mice following AZT or vehicle-only treatment, we identified 40 gene promoters that displayed 190 individual differentially methylated DNA regions. The number of identified DNA methylation peaks per gene promoter again ranged widely from a single peak to 31 (Supplemental Table S2). Of these 190 peaks, 168 showed increased DNA methylation while 22 displayed reduced DNA methylation (Fig. 6A). The directional change in DNA methylation was largely gene specific as was seen in the WT mice (Fig. 6B).
Fig. 5.
DNA methylation changes in wild-type AZT-treated mice. WT AZT mouse DNA methylation was compared with vehicle-treated controls to identify significant changes in DNA methylation in the 95 genes identified as differentially expressed. A: of the differentially expressed genes, 98 regions displayed differential DNA methylation. Of these, 65 were hypomethylated and 33 were hypermethylated. B: of the 46 genes upregulated by AZT treatment, 13 gene promoters were hypomethylated, 2 were hypermethylated, and 31 displayed no change following AZT treatment. Of the 49 genes downregulated by AZT, 2 gene promoters were hypomethylated, 7 were hypermethylated, 2 displayed both hypermethylated and hypomethylated regions, and 38 displayed no change following AZT treatment.
Fig. 6.
DNA methylation changes in MCAT AZT-treated mice. MCAT AZT mouse DNA methylation was compared with vehicle-treated MCAT controls to identify significant changes in DNA methylation in the 95 genes identified as differentially expressed. A: of the differentially expressed genes, 190 regions displayed differential DNA methylation. Of these, 168 were hypermethylated and 22 were hypomethylated. B: of the 46 genes upregulated by AZT, 14 gene promoters were hypermethylated, 2 were hypomethylated, 2 displayed both hypermethylated and hypomethylated regions, and 29 displayed no change following AZT treatment. Of the 49 genes downregulated by AZT, 13 gene promoters were hypermethylated, 6 were hypomethylated, 4 displayed both hypermethylated and hypomethylated regions, and 26 displayed no change following AZT treatment.
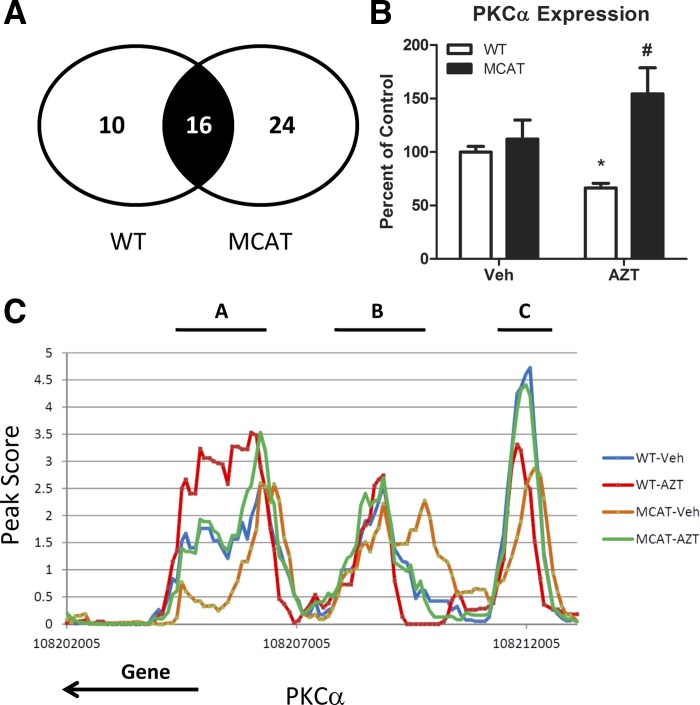
We cross-analyzed the 98 regions differentially methylated by AZT in WT mice and the 190 regions differentially methylated by AZT in MCAT mice. There were 16 genes that were common to both sets (Fig. 7A); however, in no instance did the differentially methylated DNA regions in one set align to the same differentially methylated DNA region in the other set. In some cases, differentially methylated promoter regions provided complex control over gene expression; this would result in a combination of different DNA regions that regulate gene expression changes.
Fig. 7.
DNA methylation regulation. A: 16 genes were differentially methylated following AZT in WT and MCAT paired studies. Among these genes were PKCα. B: gene expression of PKCα from paired microarray analysis. Microarray expression for each biological replicate is normalized to vehicle-treated WT expression. *P < 0.05 compared with WT-Veh, #significant interaction (P < 0.05 by 2-way ANOVA) between MCAT and AZT. C: DNA methylation peak scores of PKCα with respect to genomic location for all 4 treatment groups. Each peak score represents the average of 4 arrays for each treatment group. Regions A, B, and C denote the 3 differentially methylated regions in the gene promoter. Note that PKCα lies on the negative strand, so the gene proceeds from the transcriptional start site to the left.
We analyzed protein kinase C-alpha (PKCα) DNA methylation to determine how those changes correlate with expression changes (Fig. 7B). While no change in PKCα expression was observed between vehicle-treated WT or MCAT mice, microarray expression results showed AZT significantly decreased PKCα expression in WT mice. This effect was attenuated by MCAT. Analysis of PKCα promoter showed significant changes in DNA methylation (Fig. 7C). One region (labeled “A”) showed significant differential methylation between WT AZT-treated and vehicle-treated mice, one region (labeled “B”) showed significant differential methylation between MCAT AZT-treated and vehicle-treated mice, and the most distal region (labeled “C”) from the PKCα displayed differential methylation by AZT in both mice. When combined with expression results, the DNA methylation changes suggest that hypermethylation in regions A and B are associated with reduced gene expression, while region C does not correlate with gene expression. Coordination of DNA methylation changes in regions A and B were linked to regulation of PKCα expression following AZT. These regions were differentially methylated by AZT-induced ROS and are associated with gene expression changes.
DISCUSSION
Our previous work identified oxidative stress to be a critical component in the development of CM in AZT-treated mice (20). In those studies, pathophysiological changes in cardiac mass and structure were identified following 35 days of AZT treatment, and these changes could be attenuated or prevented by enhanced ROS scavenging by MCAT (7–9). MCAT was shown to reduce mitochondrial-derived oxidative stress, though studies have not identified gene expression changes that occur as a result of AZT-induced oxidative stress. Mitochondrial dysfunction has been implicated in CM development, with decreased mtDNA abundance, decreased energy production, and increased oxidative stress implicated in pathophysiological changes (34, 35). Here, we determined the role that mitochondrial-derived ROS have in determining the gene expression and epigenetic profile in the heart. Our results show that mitochondrial-derived ROS significantly alters gene expression of 95 genes, and enhanced ROS scavenging by MCAT is able to protect against these ROS-induced gene expression changes.
Our results further show that mitochondrial-derived ROS prevents DNA methylation that will occur following AZT-induced cardiotoxicity, and increased ROS scavenging allows for DNA to become hypermethylated following AZT treatment. Of note, expression of Mthfr, which connects folate metabolism to the methionine cycle, exhibited a 67% increase following AZT treatment of WT mice (Supplemental Table S1). Overexpression of Mthfr may compensate for changes in SAM production in AZT-treated cells, leading to alterations in SAM-related reactions such as DNA methylation (22, 44).
Cardiac DNA methylation serves as an important pathological marker of heart failure in humans. Our own studies and those of others have implicated changes in DNA methylation in the etiology of heart failure (17, 19, 31–33). Because mitochondrial dysfunction has been observed in human congestive heart failure, a mechanism similar to the one observed here for altering DNA methylation may play a key role in human disease. One limitation of our study is that whole LV cardiac tissue was used to identify epigenetic changes. Cardiac tissue contains multiple cell types (cardiomyocytes, fibroblasts, etc.) that each contain different DNA methylation profiles. This may dilute important DNA methylation changes and obscure pathophysiologically relevant epigenetic modulation in cardiomyocytes. Further research is required to separate these cell types and identify cell-specific DNA methylation profiles.
The mechanism by which mitochondrial-derived ROS reduces DNA methylation remains incompletely understood. One hypothesis is that increased ROS may reduce SAM that in turn reduces available substrates for new DNA methylation to occur, DNA methylation that was present before the ROS damage would be maintained. A second hypothesis is that increased ROS reduces 5-methylcytosine directly. This would require DNA methyltransferases to remethylate DNA at the expense of SAM. In either case, SAM steady-state abundance correlates with DNA methylation status following oxidative stress. Because we detected increased SAM abundance in hearts of MCAT mice (which are protected from AZT-induced oxidative stress and which would not be subjected to ROS-induced DNA damage), our data support the first hypothesis more strongly.
The subcellular mechanism that invokes mitochondrial ROS-induced DNA methylation changes may not be tissue or drug specific. Other therapeutic compounds, such as the anthracycline antineoplastic doxorubicin, are believed to cause cardiotoxicity through oxidative stress (39). Accordingly, changes in DNA methylation could help explain how cumulative doses of doxorubicin increase risk of CM (42). AZT induces mitochondrial dysfunction in skeletal muscle and liver; however, its mechanism may involve depletion of mtDNA and promotion of oxidative stress due to depletion of polypeptides for electron transport (6, 12, 25, 28). Understanding how different compounds and different tissues are affected by mitochondrial ROS-associated epigenetic changes may be important to explain different tissue effects and toxicities. Our work here suggests that a more comprehensive view of mitochondrial toxicity of many drugs would require epigenetic analyses.
We showed that SAM steady-state abundance in heart tissue following AZT treatment is dependent on oxidative stress. Because SAM is a requisite methyl donor for numerous biochemical reactions, it is reasonable to assume that biochemical reactions requiring SAM will also be affected by mitochondria-derived oxidative stress, including other epigenetic factors requiring SAM. It should be noted that histone methylation requires SAM as the methyl group donor. Interestingly we recently documented changes in specific histone methylation modifications in cardiac DNA extracted from cardiomyopathic human hearts (17). Changes in histone methylation in the heart may also help explain why not all differentially expressed genes were associated with DNA methylation changes (see Figs. 5 and 6). Interplay of a variety of epigenetic factors (DNA methylation, histone modifications, miRNA, etc.) may control the expression of these 95 genes, and SAM steady-state abundance may be crucial in determining how these genes are expressed based on its role as a methyl donor.
Our finding of DNA methylation of PKCα gene highlights the complexity of epigenetic control of gene expression. We chose PKC because of it has been implicated in the development of CM, and altered levels of PKC have been reported in CM (2, 3, 10, 13). In this case, we identified two regions of the PKCα gene promoter (Fig. 7C, regions A and B) that are important in regulating PKCα expression. For region A, hypermethylation reduces gene expression, which explains why PKCα promoter hypermethylation reduces gene expression below that found in vehicle-treated controls. For region B of the promoter, hypermethylation reduces its expression, which explains why PKCα in vehicle-treated MCAT mice was lower than their AZT-treated cohorts. This combination of DNA methylation in these two DNA regions essentially is linked to PKCα expression, and it is yet to be defined how PKCα is regulated in CM (10). Genetically engineered mice may shed further light on this problem. Some genetic models implicate higher PKCα activity in hypertrophic CM, while others suggest it plays no role (2, 3, 13). Our data support a decreased expression of PKCα in AZT-induced CM, though as we have seen in our previous studies, there may be additional regulation of protein activity posttranscriptionally (19).
Lastly, this study underscores clinical concerns about long-term cardiovascular side effects of antiretroviral therapy in HIV/AIDS. This is supported by discovery of somatic mtDNA mutations and deletions in striated muscle of HIV/AIDS patients (37). As the free radical theory of aging includes mitochondrial free radical generation as a key contributor to the aging process, it is likely that increased ROS from NRTIs and aging will compound to cause further changes in DNA methylation that will impact gene expression (14, 43). Because therapy with antiviral NRTIs appears to be lifelong, it is yet to be determined when and if the DNA methylation changes are reversible upon termination of AZT treatment in patients with HIV/AIDS.
GRANTS
Supported by National Institutes of Health Grants 1R01DA-030996 and 1R56HL-125062 to W. Lewis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A.K. and W.L. conception and design of research; C.A.K., Z.J., E.J.F., R.R., and T.L. performed experiments; C.A.K. and E.J.F. analyzed data; C.A.K. and W.L. interpreted results of experiments; C.A.K. prepared figures; C.A.K. and W.L. drafted manuscript; C.A.K. and W.L. edited and revised manuscript; C.A.K. and W.L. approved final version of manuscript.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondrial toxicity in HAART: an overview of in vitro evidence. Curr Pharm Des 17: 2130–2144, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Braz JC, Bueno OF, De Windt LJ, Molkentin JD. PKC alpha regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase1/2 (ERK1/2). J Cell Biol 156: 905–919, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med 10: 248–254, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 354: 1112–1115, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Cotran R, Kumar V, Collins T. Robbins Pathologic Basis of Disease. Philadelphia, PA: WB Saunders, 1999, p. 1425. [Google Scholar]
- 6.d'Amati G, Lewis W. Zidovudine causes early increases in mitochondrial ribonucleic acid abundance and induces ultrastructural changes in cultured mouse muscle cells. Lab Invest 71: 879–884, 1994. [PubMed] [Google Scholar]
- 7.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 108: 837–846, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai DF, Rabinovitch P. Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy 7: 917–918, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119: 2789–2797, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorn GW 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 115: 527–537, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duygu B, Poels EM, da Costa Martins PA. Genetics and epigenetics of arrhythmia and heart failure. Front Genet 4: 219, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerschenson M, Erhart SW, Paik CY, St. Claire MC, Nagashima K, Skopets B, Harbaugh SW, Harbaugh JW, Quan W, Poirier MC. Fetal mitochondrial heart and skeletal muscle damage in Erythrocebus patas monkeys exposed in utero to 3′-azido-3′-deoxythymidine. AIDS Res Hum Retroviruses 16: 635–644, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, Bodi I, Liggett SB, Schwartz A, Dorn GW 2nd. Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res 93: 1111–1119, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc 20: 145–147, 1972. [DOI] [PubMed] [Google Scholar]
- 15.Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr, Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W, Tian R. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab 18: 239–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res 106: 1541–1548, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koczor CA, Lee EK, Torres RA, Boyd A, Vega JD, Uppal K, Yuan F, Fields EJ, Samarel AM, Lewis W. Detection of differentially methylated gene promoters in failing and nonfailing human left ventricle myocardium using computation analysis. Physiol Genomics 45: 597–605, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koczor CA, Torres RA, Fields E, Qin Q, Park J, Ludaway T, Russ R, Lewis W. Transgenic mouse model with deficient mitochondrial polymerase exhibits reduced state IV respiration and enhanced cardiac fibrosis. Lab Invest 93: 151–158, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koczor CA, Torres RA, Fields EJ, Boyd A, He S, Patel N, Lee EK, Samarel AM, Lewis W. Thymidine kinase and mtDNA depletion in human cardiomyopathy: epigenetic and translational evidence for energy starvation. Physiol Genomics 45: 590–596, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohler JJ, Cucoranu I, Fields E, Green E, He S, Hoying A, Russ R, Abuin A, Johnson D, Hosseini SH, Raper CM, Lewis W. Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Lab Invest 89: 782–790, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler JJ, Hosseini SH, Cucoranu I, Hoying-Brandt A, Green E, Johnson D, Wittich B, Srivastava J, Ivey K, Fields E, Russ R, Raper CM, Santoianni R, Lewis W. Murine cardiac mtDNA: effects of transgenic manipulation of nucleoside phosphorylation. Lab Invest 89: 122–130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laing ME, Cummins R, O'Grady A, O'Kelly P, Kay EW, Murphy GM. Aberrant DNA methylation associated with MTHFR C677T genetic polymorphism in cutaneous squamous cell carcinoma in renal transplant patients. Br J Dermatol 163: 345–352, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med 1: 417–422, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Drug Discov 2: 812–822, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Lewis W, Gonzalez B, Chomyn A, Papoian T. Zidovudine induces molecular, biochemical, and ultrastructural changes in rat skeletal muscle mitochondria. J Clin Invest 89: 1354–1360, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis W, Grupp IL, Grupp G, Hoit B, Morris R, Samarel AM, Bruggeman L, Klotman P. Cardiac dysfunction occurs in the HIV-1 transgenic mouse treated with zidovudine. Lab Invest 80: 187–197, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Lewis W, Simpson JF, Meyer RR. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circ Res 74: 344–348, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Lynx MD, Bentley AT, McKee EE. 3′-Azido-3′-deoxythymidine (AZT) inhibits thymidine phosphorylation in isolated rat liver mitochondria: a possible mechanism of AZT hepatotoxicity. Biochem Pharmacol 71: 1342–1348, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med 333: 1099–1105, 1995. [DOI] [PubMed] [Google Scholar]
- 30.Mestroni L, Gilbert EM, Lowes BD, Bristow MR. Dilated cardiomyopathy. In: Hurst's The Heart, edited by Fuster V, O'Rourke RA, Walsh RA, Poole-Wilson P. New York: McGraw Hill, 2008, p. 803–821. [Google Scholar]
- 31.Movassagh M, Choy MK, Goddard M, Bennett MR, Down TA, Foo RS. Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One 5: e8564, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Movassagh M, Choy MK, Knowles DA, Cordeddu L, Haider S, Down T, Siggens L, Vujic A, Simeoni I, Penkett C, Goddard M, Lio P, Bennett MR, Foo RS. Distinct epigenomic features in end-stage failing human hearts. Circulation 124: 2411–2422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Movassagh M, Vujic A, Foo R. Genome-wide DNA methylation in human heart failure. Epigenomics 3: 103–109, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Neubauer S. Cardiac magnetic resonance spectroscopy. Curr Cardiol Rep 5: 75–82, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med 356: 1140–1151, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Papait R, Greco C, Kunderfranco P, Latronico MV, Condorelli G. Epigenetics: a new mechanism of regulation of heart failure? Basic Res Cardiol 108: 361, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Payne BA, Wilson IJ, Hateley CA, Horvath R, Santibanez-Koref M, Samuels DC, Price DA, Chinnery PF. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet 43: 806–810, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scacheri PC, Crawford GE, Davis S. Statistics for ChIP-chip and DNase hypersensitivity experiments on NimbleGen arrays. Metho Enzymol 411: 270–282, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Sterba M, Popelova O, Vavrova A, Jirkovsky E, Kovarikova P, Gersl V, Simunek T. Oxidative stress, redox signaling, and metal chelation in anthracycline cardiotoxicity and pharmacological cardioprotection. Antioxid Redox Signal 18: 899–929, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Medi 34: 883–901, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Velsor LW, Kovacevic M, Goldstein M, Leitner HM, Lewis W, Day BJ. Mitochondrial oxidative stress in human hepatoma cells exposed to stavudine. Toxicol Appl Pharmacol 199: 10–19, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 91: 710–717, 1979. [DOI] [PubMed] [Google Scholar]
- 43.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner AS, Boyarskikh UA, Voronina EN, Mishukova OV, Filipenko ML. Methylenetetrahydrofolate reductase C677T and methionine synthase A2756G polymorphisms influence on leukocyte genomic DNA methylation level. Gene 533: 168–172, 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.